Introduction
Family with sequence similarity 107 (FAM107)
proteins are conserved beyond species: they are found in mammals,
Xenopus, fish and Drosophila. They are characterized
by a common N-terminal domain of unknown function (DUF1151) with no
homology match to other functional conserved domains. Mammals have
two genes, FAM107A and FAM107B, respectively encoding the proteins
of 144 amino acids (aa) and 131 aa (http://www.uniprot.org/uniprot). C-terminal variable
regions of FAM107 carry a coiled-coil domain that has been
identified in many nuclear proteins including transcription
factors, suggesting a role of FAM107 in regulating gene
transcription. Analysis of protein-protein interactions revealed
that FAM107A and FAM107B interact, respectively, with
transcriptional adaptor 2α and 3α (Tada2α and Tada3α) (1–3). In
fact, Tada2α and Tada3α are reciprocal binding partners and core
proteins of the histone acetyltransferase (HAT) complex, suggesting
that FAM107 family proteins can modulate the structure and function
of HAT complexes that are involved in chromatin structure
modification for gene transcription and acetylation of many
proteins (4,5).
Because of epigenetic silencing, FAM107A is
downregulated or deleted from cancer of many types such as
non-small cell lung, renal cell, and prostate cancers and
astrocytoma (6–10). FAM107A has been regarded as a
candidate of tumor suppressor gene (TSG) because of its decreased
expression in cancer and because of the fact that introduction of
FAM107A suppresses cancer cell proliferation and induces apoptosis
(7,10–13).
In contrast, it was recently reported that FAM107A is highly
expressed in the invasive component of gliomas and that it drives
tumor invasion by functioning as a new cytoskeletal crosslinker
that regulates focal adhesion dynamics and cell movement (14,15).
Consequently, the physiological role and function of FAM107A as a
TSG remain controversial.
In humans, FAM107B protein is encoded by the gene on
chromosome 10p13, sharing 65% sequence similarity with FAM107A in
their N-terminal DUF1151 regions. We previously investigated
histopathological localization and functions of FAM107B in
gastrointestinal cancers, and designated it as heat-shock-inducible
tumor small protein (HITS) (16)
based on its unique expression pattern and biological properties in
cancer cells and its low molecular weight (18 kDa) among a family
of heat-shock proteins (HSPs) (17–19).
Similar to FAM107A, HITS expression is decreased or absent in
gastric and colorectal cancers; moreover, it inhibits tumor cell
proliferation in vitro (16), which contrasts with previously
described oncogenic activities of other HSPs such as HSP70 and
HSP90 (20–24).
In this study, we performed immunohistochemical
examination of HITS expression in cancer tissues of the multiple
organs spotted on tissue microarrays that include clinical and
histopathological information of the respective tumor samples.
Consistent with the hypothesis that HITS is a tumor suppressor
protein in gastrointestinal cancer (16), our current study demonstrated that
loss of HITS expression is commonly observed in cancers of multiple
organs and involved in tumor proliferation.
Materials and methods
Tissue microarrays
Several types of human tissue microarrays including
normal, malignant, and metastatic formats (US Biomax, Inc.,
Rockville, MD) were used to examine HITS expression in cancer and
to compare the results with clinical and histopathologic
characteristics of the tumors (Table
I). The high-density multiple organ tumor and normal tissue
microarray (MC5003) was used for the initial screening of HITS
expression. This microarray includes 18 types of tumor (20
cases/each tumor type) and normal tissues (5 cases/each organ) with
TNM classification and pathology grades of the tumors. According to
results of HITS expression in MC5003 tissue microarray and
statistical analysis, additional analysis of individual organs was
used with the following tissues microarrays. BR1503 is a breast
cancer tissue microarray that includes TNM classification and
pathology grades of the respective tumor spots and the levels of
immunohistochemical expression of human epidermal growth factor
receptor 2 (HER2), estrogen receptor (ER), progesterone receptor
(PR), p53 and Ki-67. BRM961 is a breast cancer tissue microarray
including the spots of primary and matched metastatic tumors in
lymph nodes and adjacent normal breast tissues. TH807 includes
tissue spots of various thyroid diseases. CR602 includes the tissue
spots of uterine cervical diseases [cervical cancer, cervical
intraepithelial neoplasia (CIN), inflammation] and adjacent normal
tissues, and TE803 includes those of testis diseases (testicular
benign and malignant tumors).
 | Table IFormats of the tissue microarray. |
Table I
Formats of the tissue microarray.
| Tissue
microarraya | Organ | Specificity |
|---|
| MC5003 | Multiple | High-density
multiple organ tumor and normal tissue microarray, containing 18
types of tumor (20 spots/type) and normal controls (5
spots/type) |
| BRM961 | Breast | Breast carcinoma
metastatic tissue microarray, containing 96 spots of breast cancer,
with 72 matched metastatic breast cancer (in lymph nodes) and 24
normal tissue |
| BR1503 | Breast | Breast carcinoma
tissue microarray (including TNM and pathology grade, with IHC
results of HER2, ER, PR, P53 and Ki-67), containing 6 spots of
normal tissue, 6 fibroadenoma, 4 cystosarcoma phyllodes, 14
intraductal carcinoma, 120 invasive ductal carcinoma |
| TH807 | Thyroid | Thyroid disease
spectrum tissue microarray, 10 spots of each types (papillary
carcinoma, follicular carcinoma, metastatic carcinoma, adenoma,
goiter, thyroiditis, cancer adjacent normal and normal tissue) |
| CR602 | Uterine cervix | Uterine cervical
disease spectrum tissue microarray, containing 30 spots of uterine
cervix tumor, 10 spots of CIN, 10 spots of inflammation and10
adjacent normal tissue |
| TE803 | Testis | Testis disease
spectrum (testicular cancer progression) tissue microarray,
containing 26 spots of seminoma, 12 embryonal carcinoma, 10 yolk
sac tumor, 7 teratoma, 4 tuberculosis, 6 atrophy, 10 adjacent
normal tissue and 5 normal tissue |
Antibodies
To generate a polyclonal anti-human HITS antibody,
rabbits were immunized with keyhole limpet hemocyanin
(KLH)-conjugated polypeptides (MAEPDYIEDDNPE) as an antigen
(16). The antibody was purified
from the anti-serum using affinity chromatography against the same
polypeptides cross-linked to agarose beads, and verified its
specificity by flow cytometry of immunostaining and western blot
analysis. Antibodies to β-actin (Santa Cruz Biotechnology, Santa
Cruz, CA), proliferating cell nuclear antigen (PCNA) and survivin
(Dako A/S, Glostrup, Denmark) were purchased.
Immunohistochemistry
Expression and localization of HITS, PCNA and
survivin in paraffin sections of tissues and microarrays were
examined using immunohistochemical staining, as conducted in a
previous study (25).
Representative paraffin sections and tissue microarrays placed on
silanized slides were treated by microwaving in the citrate buffer
to unmask antigens. Then they were incubated with 0.3%
H2O2 in methanol and with 10% normal goat
serum for blocking endogenous peroxidase activity and nonspecific
antibody binding. The pretreated sections were incubated with the
respective antibodies (1:500 dilution each) overnight at 4°C with
subsequent serial incubation with the biotinylated goat anti-rabbit
IgG (Vector Laboratories Inc., Burlingame, CA) and standard
avidin-biotinperoxidase complex (ABC). Cell nuclei were
counterstained with hematoxylin.
Scores for HITS expression and
statistical analysis
We assigned grades 0–4 to microscopic evaluation of
HITS expression in tissues according to the signal intensity and
nuclear localization of HITS (Fig.
1). Grade 0, no staining in cytoplasm and nucleus; grade 1,
borderline cytoplasmic staining but no apparent nuclear
localization; grade 2, weak nuclear staining; grade 3, moderate
nuclear staining; grade 4, strong nuclear staining.
Scores of HITS grading in the respective groups of
tissue specimens were expressed as means ± standard deviation (SD).
The mean scores of HITS expression of the two groups were compared
using two-sided Mann-Whitney U tests. For comparison of the scores
of more than two groups, the significance of differences among the
data was determined using one-way ANOVA followed by Scheffe’s F
post hoc test. Correlation between scores of HITS expression and
the clinical and pathological parameters of the tumors was
determined using the nonparametric Spearman’s rank correlation
analysis. Values of P<0.05 were considered significant.
In breast cancer, desmoplastic reaction, also called
reactive fibrosis, is characterized by mobilization of fibroblasts
and deposition of abundant collagen as a stromal response to an
invasive carcinoma. The HITS expression scores for breast cancers
with and without desmoplastic stromal reaction were compared
statistically.
In vivo tumorigenicity assays
We previously established a human uterine
cervical-cancer-derived HeLa Tet-On advanced cell line (Clontech
Laboratories, Inc., Mountain View, CA) transduced with a mock or
Tet-inducible HITS gene (16).
Cells (1×106) of each HeLa cell line were inoculated
subcutaneously to two opposite sites of flank in each Scid mouse
(n=14; Charles River Laboratories Japan Inc., Yokohama, Japan).
These mice were divided into two groups and treated or not treated
with doxycycline (1.5 mg/ml; Clontech Laboratories Inc.) dissolved
in drinking water in light-protected bottles, respectively, for 8
weeks. Following treatment, all mice were euthanized. Tumors
removed from mice were weighed and fixed in neutral-buffered 10%
formalin and embedded in paraffin for histopathologic and
immunohistochemical examination. Tumor formation in mice with (n=7)
or without (n=7) doxycycline administration was expressed as
average weight ± SD. The statistically significant difference in
tumor formation between the groups was determined using a two-sided
Mann-Whitney U test, for which P<0.05 was considered
significant. We repeated this experiment twice to confirm
reproducibility. The animal study was conducted according to the
Guidelines for Experimental Animals at Kanazawa Medical University
and the National Guidelines for Animal Usage (http://www.lifescience.mext.go.jp/policies) in
Japan.
Results
Screening for the expression of HITS in
multiple tissues
The high-density multiple organ tumor and normal
tissue array (MC5003) including tumors of 18 types along with
corresponding normal tissues was used for immunohistochemical
screening for the expression of HITS. Scores of HITS expression in
normal (n=5) and cancer tissues (n=20) in each organ are added up
separately, expressed as mean ± SD, and compared statistically
using the Mann-Whitney U test. Despite the small number of samples
in each group, lower expression of HITS in tumor tissues than their
normal counterparts was frequent to a significant degree in cancers
of the thyroid, breast, colon, and uterine cervix (Table II). Scores of HITS expression in
cancer tissues seemed lower than their normal counterparts in the
lung, stomach, and testis, although no statistically significant
differences were found among them. In contrast, in the tumors of
the liver, pancreas, ovary, and urinary tracts including the
kidney, bladder, and prostate, scores of HITS expression in cancer
tissues seemed slightly higher than those in the corresponding
normal tissues, although the intensity of HITS expression in these
organs was very low, even in normal tissues (Table II and Fig. 1).
 | Table IIImmunohistochemical screening for the
expression of HITS in multiple organs and tissues. |
Table II
Immunohistochemical screening for the
expression of HITS in multiple organs and tissues.
| Normala (n=5) | Cancera (n=20) | P-valueb |
|---|
| Brain | 2.20±1.30 | 2.70±1.34 | 0.43 |
| Head and neck | 2.80±1.10 | 2.67±0.84 | 0.61 |
| Thyroid | 3.80±0.45 | 1.30±0.73 | <0.01 |
| Lung | 3.00±1.00 | 2.25±1.12 | 0.18 |
| Breast | 4.00±0.00 | 2.40±1.19 | <0.01 |
| Esophagus | 2.20±1.30 | 1.55±1.10 | 0.28 |
| Stomach | 2.33±1.15 | 1.31±1.08 | 0.13 |
| Colon | 3.20±0.45 | 1.42±0.69 | <0.01 |
| Liver | 0.60±0.55 | 0.75±0.44 | 0.51 |
| Pancreas | 1.20±0.45 | 1.58±0.96 | 0.51 |
| Uterus | 1.60±1.34 | 1.84±1.12 | 0.42 |
| Uterine cervix | 3.80±0.45 | 1.68±0.89 | <0.01 |
| Ovary | 1.60±1.14 | 1.80±1.01 | 0.80 |
| Kidney | 0.80±0.45 | 1.35±1.42 | 0.64 |
| Bladder | 2.00±1.41 | 2.75±1.12 | 0.24 |
| Prostate | 0.80±0.84 | 1.63±1.21 | 0.15 |
| Testis | 3.60±0.55 | 2.21±1.23 | 0.06 |
| Soft tissue | 3.40±0.55 | 3.35±0.88 | 0.82 |
| Skin | 3.00±0.00 | 2.40±1.18 | 0.29 |
| Lymph node | 2.20±0.84 | 1.70±0.92 | 0.23 |
Through screening of the MC5003 tissue array, we
selected four organs of the breast, thyroid, testis and uterine
cervix for the next step of investigation of detailed pathological
analysis and statistical analysis using cancer tissue arrays of the
respective organs to explore HITS expression in the tumors
(Table I).
HITS expression in breast cancer
Because HITS expression was high in normal breast
tissues (Table II and Fig. 1), average scores of HITS expression
summed up in the normal tissues spotted on the three
tissue-microarrays MC5003, BR1503 and BRM961 were 3.17±0.98. The
mean score of HITS expression in cancer tissues was 1.50±1.08,
which was significantly lower than in normal tissues (p<0.001).
Decreased HITS expression was common to the two representative
histological types of breast cancers, invasive ductal and lobular
carcinomas (Fig. 2A). Comparison
of cancers with different stromal reactions revealed that the
breast cancers associated with desmoplastic reaction exhibited
significantly higher HITS expression than those without this
stromal reaction (Fig. 2B and C).
Cystosarcoma phyllodes is a rare, usually large, fast growing
breast tumor that arises from periductal cells of the breast; it is
regarded as a borderline malignancy (26). This tumor showed an intermediate
score of HITS expression between normal and carcinoma tissues
(Fig. 2A and D).
The relation between tumor progression and HITS
expression was examined according to the TNM classification. In the
results of statistical analysis presented in Table III, the HITS expression scores were
lower in accordance with advances in the TNM stages of tumors
(rs=-0.19, p=0.01). They were inversely correlated with the T-value
(primary tumors) but not with the N-value (lymph node metastasis).
To support this, the intensity of HITS expression showed no
difference between the primary and metastatic lymph node tumors in
the same patients (1.37±1.17 and 1.23±0.94, respectively, p=0.40)
(Fig. 2B). No relation to the
histological grade of tumor differentiation was found (Table III).
 | Table IIIExpression of HITS for breast
cancer. |
Table III
Expression of HITS for breast
cancer.
| Parameter | HITS mean ± SD
(n) | rs | P-value |
|---|
| TNM stagea | | −0.19 | 0.01 |
| I | 1.71±0.91 (14) | | |
| IIa | 1.68±1.15 (92) | | |
| IIb | 1.27±1.07 (45) | | |
| IIIa | 1.47±1.22 (19) | | |
| IIIb | 1.07±0.80 (15) | | |
| Ta | | −0.23 | <0.01 |
| 1 | 1.71±0.91 (14) | | |
| 2 | 1.67±1.17
(126) | | |
| 3 | 1.03±0.87 (31) | | |
| 4 | 1.07±0.55 (15) | | |
| Na | | −0.05 | 0.48 |
| 0 | 1.55±1.10
(132) | | |
| 1 | 1.34±1.09 (38) | | |
| 2,3 | 1.63±1.20 (16) | | |
| ERb | | 0.02 | 0.82 |
| − ∼ + | 1.43±1.04 (88) | | |
| ++ ∼ +++ | 1.37±1.02 (43) | | |
| PRb | | −0.22 | <0.05 |
| − ∼ + | 1.55±1.02 (87) | | |
| ++ ∼ +++ | 1.14±1.00 (44) | | |
| TP53b | | −0.06 | 0.47 |
| − ∼ + | 1.45±1.08 (85) | | |
| ++ ∼ +++ | 1.34±0.93 (46) | | |
| HER2b | | 0.23 | <0.01 |
| − ∼ + | 1.27±1.20 (41) | | |
| ++ ∼ +++ | 1.48±0.94 (90) | | |
| Ki-67b | | 0.33 | <0.001 |
| − ∼ + | 1.27±1.28 (30) | | |
| ++ ∼ +++ | 1.46±0.94
(102) | | |
| Histological
gradec | | 0.006 | 0.93 |
| 1 | 2.17±1.17 (6) | | |
| 2 | 1.38±1.07
(134) | | |
| 3 | 1.68±1.09 (31) | | |
| Agec | | 0.13 | 0.07 |
| <49 | 1.36±1.08
(105) | | |
| >50 | 1.64±1.08 (90) | | |
Although the most important prognostic factors are
the tumor size, histological grade and lymph node stage (27), the importance of several molecular
markers in breast cancer has been of considerable interest
recently, not only as prognostic markers, but also as predictors of
response to therapy (28). For
further analysis of correlation between HITS expression and other
pathological parameters, we exploited the tissue microarray BR1503,
which includes data of immunohistochemical expression of HER2, ER,
PR, p53 and Ki-67 in addition to those of TNM stages and pathology
grade (Table I). Statistical
analysis revealed that the score of HITS expression was correlated
positively with the expressions of HER2 (rs=0.23, p<0.01) and
Ki-67 (rs=0.33, p<0.001), but inversely with PR expression
(rs=-0.22, p<0.05) (Table III).
For example, the average score of HITS expression in cancers
showing PR- and HER2+++ was 2.16±0.99 (n=25), although the score of
HITS expression in the two cases with PR+++ and HER2- was grade 0
(p= 0.02) (Fig. 2E). Among other
pathological parameters, age has been shown to be a prognostic
factor: young patients (<35 years) with breast cancer have a
poorer prognosis (29). The
present study showed an apparent trend in the increase of HITS
scores in cancer tissue in accordance with age, although it was not
statistically significant (rs=0.13, p=0.07) (Table III). Means of HITS expression
scores in normal breast tissues were greater than 3.0 in all
generations (data not shown).
HITS expression in the thyroid, uterine
cervical and testicular diseases
In analysis of the thyroid disease tissue microarray
(TH807), the HITS expression intensity clearly distinguished
thyroid cancer (papillary and follicular carcinomas) from normal
tissue, thyroiditis, diffuse goiter, and adenoma (Fig. 3A and B). Consistent with the
results obtained for breast cancer, the HITS expression level was
similar in primary tumor and lymph node metastasis in the same
patients, but inversely correlated with the T-values of TNM grading
(Fig. 3C and D).
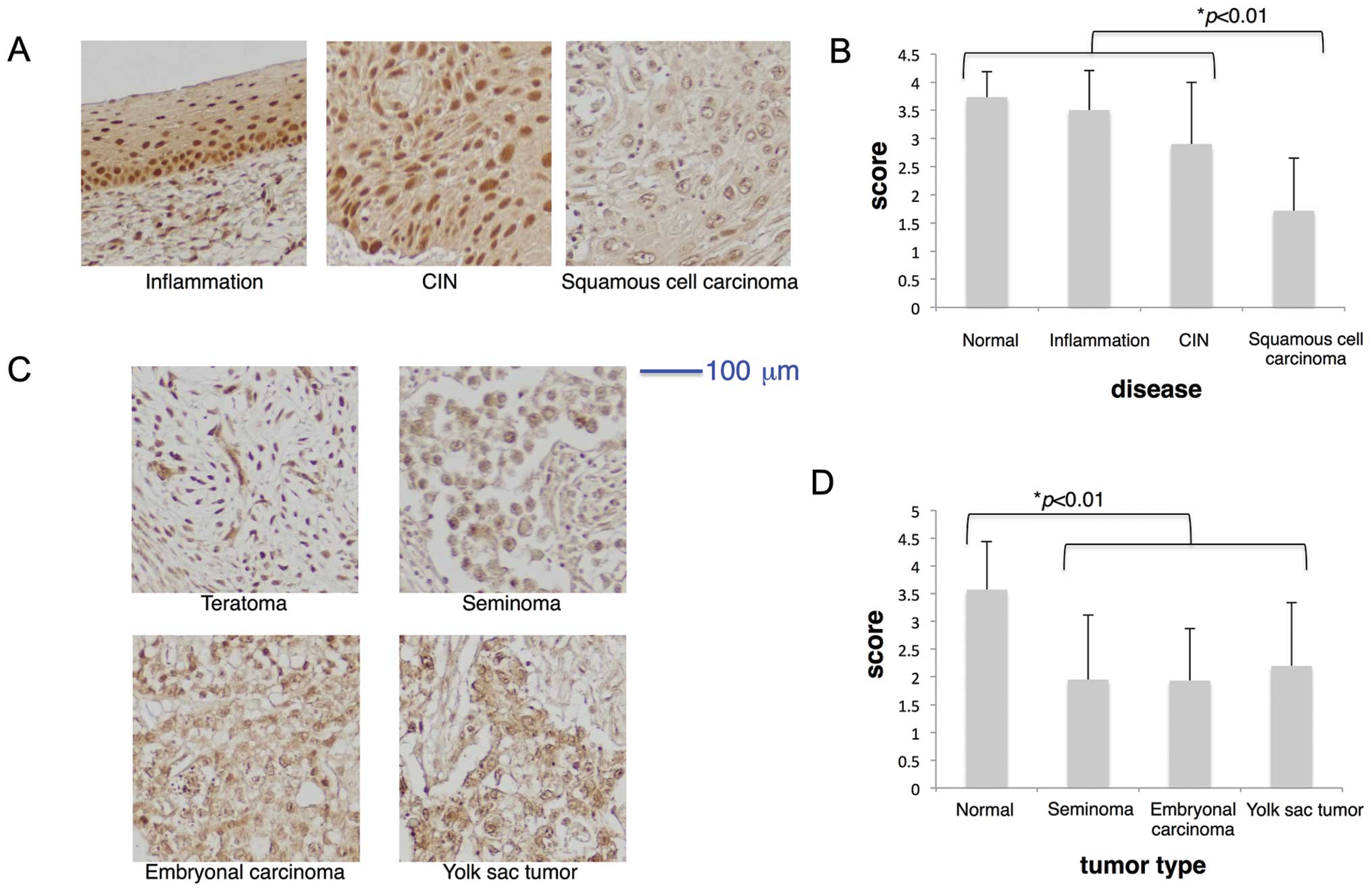
Regarding analysis of the uterine cervical disease
tissue microarray (CR602), HITS expression was found in cervical
epithelial cell nuclei in inflammation and CIN, but it was markedly
decreased in invasive squamous cell carcinoma (Fig. 4A and B). Infection of human
papilloma virus (HPV) is known as an inducer of cervical cancers
because of strong causal relations between HPV infection, CIN and
invasive carcinoma (30). Because
some lesions among those of CIN progress to invasive cancer in 10
to 20 years (31), our results
suggest that HITS expression is lost during the progression of CIN
toward invasive carcinoma.
Analysis of the testis disease tissue microarray
(TE803) showed that, similar to normal breast, thyroid and uterine
cervix tissues, HITS expression was high in normal testis (average
score >3.5) (Table II, Figs. 1 and 4D). Tumors of the testis, including germ
cell tumors (seminoma) and embryonic tumors (embryonal carcinoma
and yolk sac tumor), showed significantly lower expression of HITS
than either normal testis or benign teratoma (Fig. 4C and D). No significant difference
was found in expression levels of HITS between seminoma and
non-seminomatous tumors (embryonal carcinoma and yolk sac
tumor).
Inducible expression of HITS in vivo
reduces tumor size
Based on the immunohistochemical analysis of breast
and thyroid cancer tissue microarrays showing that intensity of
HITS expression is inversely correlated with the T-value of TNM
grading, i.e. primary tumor size, we hypothesized that tumor
proliferation might be inhibited by ectopic or forced expression of
HITS, which functions as a tumor suppressor protein. Previously, we
reported that tetracycline-inducible expression (Tet-ON) of HITS in
cancer cells diminished proliferation in response to growth factors
such as epidermal growth factor, fibroblast growth factor and fetal
calf serum (16). To investigate
the role of HITS in tumor proliferation in vivo,
HITS-transduced and mock-transduced human cervical cancer HeLa
cells were transplanted subcutaneously in the flank of Scid mice.
Then their tumor formation status was observed for 8 weeks with or
without induction of HITS by doxycycline administration in drinking
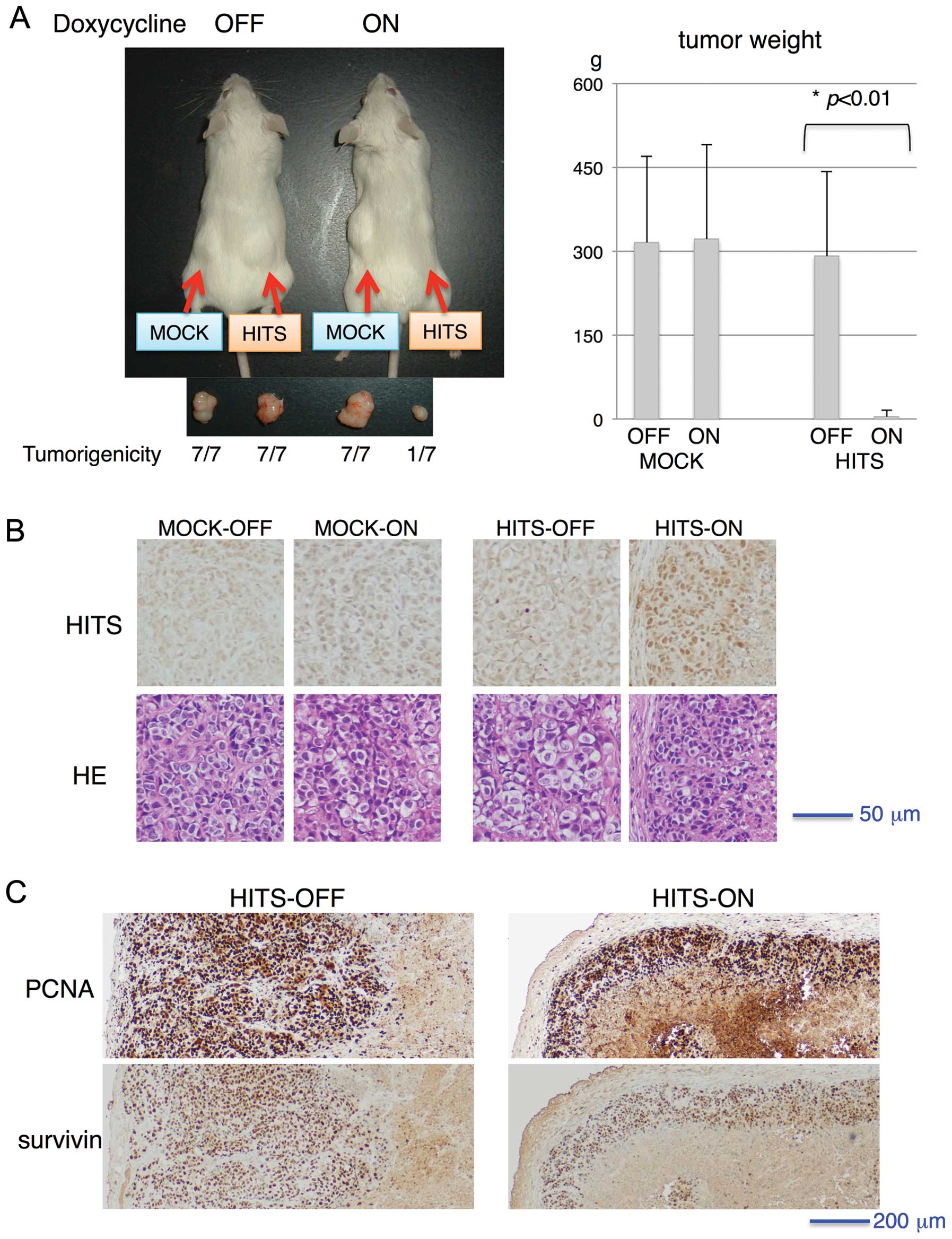
water. As portrayed in Fig. 5A,
all mock-transduced cells developed tumors, whereas the
Tet-inducible HITS-transduced cells generated tumors in the absence
of HITS induction, indicating the suppressive role of HITS for
tumor growth. The HITS-induced tumors, although very rarely
generated (only one out of seven) and small (Fig. 5A), were processed for histo-logical
examination. Regarding pathological examination of the tumors,
HITS-induced tumors showed no morphologically distinct gross or
microscopic differences except for tumor size (Fig. 5A and B). Immunohistochemical
staining of PCNA and survivin showed no apparent difference in
intensities between tumors with and without HITS induction,
although the area of positively stained cells surrounding central
necrosis was much less in HITS-induced tumors than in non-induced
tumors, which might reflect the minimal size of tumor formation
(Fig. 5C).
Discussion
In this study, we clarified that loss of HITS
expression in cancer is common among organs in which the expression
level of HITS was high in respective normal tissues. Statistical
analysis of data obtained from tissue microarray examination
revealed the relevance of HITS expression scores to several
pathological parameters, especially to primary tumor size (Table III and Fig. 3D). Forced induction of HITS in
cancer cells inhibited the proliferation of tumor xenografts in
rodents. These results suggest that HITS might be a tumor
suppressor in breast, thyroid, uterine cervix and testis as well as
stomach and colon (16).
Both FAM107A (TU3A/DRR1) and FAM107B (HITS) are
expected to be tumor suppressors because their expressions are
often decreased in cancer cells; moreover, their introduction
suppresses cancer cell growth (6,7,10,16,32).
HITS possesses a conserved domain of DUF1151 in the N-terminal
region, as does FAM107A. However the tissue distribution and the
physiological function of HITS are distinctive. First, HITS is
expressed in various tissues including digestive, respiratory,
genital, and lymphoid organs (Table
II and Fig. 1) (16). In contrast, FAM107A is expressed
predominantly in the nervous system, especially in neurons, but not
in astrocytes or in oligodendrocytes (13,14).
Regarding subcellular localization, both HITS and FAM107A are
localized in the cell nucleus because of a nuclear localization
signal in the center of the protein sequences (16). Second, the HITS gene carries the
promoter region providing heat-shock transcription factor 1 (HSF1)
binding sites and the transcription of HITS is amplified by
heat-shock or hyperthermia treatment (16). This is a salient distinguishing
feature of HITS because other HSPs and HSF1 are regarded as having
oncogenic activities in many respects (22–24,33–37).
In our studies, similarly to FAM107A, ectopic or forced expression
of HITS in cancer cells inhibited tumor growth in vitro and
in vivo (Fig. 5) (16). Consequently, HITS is a potential
tumor suppressor protein with a unique feature of its
transcriptional induction by heat-shock stimulation. It is expected
to be useful for tumor diagnosis and for monitoring therapeutic
effects of hyperthermia.
A previous study by the authors found that the level
of HITS expression in gastrointestinal cancer cells was lower than
in normal epithelial cells, although its expression pattern and
intensity varied among cancers of different histological types
(16). Expression of HITS was
decreased during the process of colorectal adenoma-to-carcinoma
sequence, and also decreased in intestinal-type gastric
adenocarcinomas, but not in diffuse type adenocarcinomas. HITS
expression was positive in both stomach and colon mucinous
adenocarcinomas. In the present study, tissue microarray analysis
revealed that loss of HITS expression in cancer was commonly
observed in several organs in a histopathological-type-specific
manner. HITS expression was decreased in the prevalent histologic
types of breast cancer, invasive ductal, and lobular carcinomas
compared with normal breast ducts (Fig. 2A). Statistical analysis elucidated
that HITS expression became lower in accordance with breast cancer
progression on TNM staging (Table
III). It is particularly interesting that, among the factors
determining TNM stages, HITS expression scores were inversely
correlated with the T-value but not with the N-value. Inverse
association with the T-value was also found in thyroid cancer
(Fig. 3D).
HITS expression was significantly higher in cancers
with a desmoplastic stromal reaction (Fig. 2B and C), similar to diffuse-type
gastric cancers with scirrhous stromal reaction that exhibited much
higher HITS expression than intestinal-type cancers (16). In further statistical analysis with
other pathological parameters of breast cancer, the score of HITS
expression correlates positively with expression of HER2 and Ki-67,
and inversely with PR expression (Table III and Fig. 2E). These molecular markers play
important roles in carcinogenesis and tumor progression in breast
cancers (38–40). Expression levels of HER2, ER and PR
are well-established markers for predicting anti-tumor effects of
trastuzumab (Herceptin), a therapeutic monoclonal antibody to HER2
and of endocrine therapy, respectively (41,42).
Scores of HITS expression are apparently high in HER2 positive,
Ki-67 positive, PR negative, and desmoplastic reaction-positive
breast cancer, which was thought to be an aggressive phenotype
presenting increased risk of disease recurrence and shortened
survival (43,44).
Invasive ductal carcinoma of the breast is
classified histologically into two subtypes: pure invasive ductal
carcinoma (IDC) and IDC associated with ductal carcinoma in
situ (IDC/DCIS) (40). Pure
IDC is morphologically, pathologically, and genetically distinct
and has a worse prognosis than IDC/DCIS, suggesting that they might
be different biological and clinical tumor entities (33,45–47).
HER2 amplification and Ki-67 expression were significantly higher
in pure IDC cases than in IDC/DCIS, although the PR expression in
pure IDC was significantly lower than in IDC/DCIS. The ER
expression and p53 expression showed no statistically significant
differences between the two histological subtypes (40). The correlation of HITS expression
with the molecular characteristics as described above suggests that
the distinct staining pattern of HITS can surrogate the reported
difference in molecular characteristics between pure IDC and
IDC/DCIS, thereby enabling differential diagnosis of these tumor
subtypes with different clinical outcomes.
Considering our results that HITS expression was
lost during the course of tumor progression in T-value of TNM
grading (Table III and Fig. 3D), it is speculated on one hand
that HITS expression decreases during the long process from
pre-neoplastic or earlier neoplastic lesions such as DCIS in
breast, intestinal metaplasia in stomach, tubular adenoma in colon
and CIN in uterine cervix to invasive cancers. On the other hand,
HITS expression is preserved in aggressive types of cancers, such
as scirrhous-type gastric and breast cancers, which are
characterized by distinct genetic alterations and rapid growth or
invasions (48,49). Because forced expression of HITS
inhibited cancer cell proliferation in response to growth factors
and tumor xenograft growth (Fig.
5) (16), we assume that HITS
expression influences the primary tumor growth per se during
tumor development, but that it does not influence the invasion or
metastasis, e.g., tumor spread such as scirrhous-type or lymph-node
metastasis.
A clear boundary of HITS expression levels was
apparent between cancer and non-cancerous lesions e.g.
inflammation, benign tumor, and precancerous lesions in thyroid,
cervical uterine and testicular tissues (Figs. 3 and 4). Diagnosis of malignancy in thyroid
tumors is a difficult task performed using a combination of
pathologic examination, blood tests including those of thyroid
hormones, and image diagnostic methods such as computed tomography,
ultrasound sonography, and scintigraphy. Fine needle aspiration
cytology (FNAC) is a useful method to differentiate benign and
malignant nodules. However, the sensitivity and specificity of FNAC
has been reported, respectively, as 65–98% and 73–100% (50). Our statistical analysis revealed
that HITS expression levels in both papillary and follicular types
of thyroid carcinoma were significantly lower than in non-cancerous
lesions such as adenoma, goiter, and thyroiditis (Fig. 3), suggesting the possibility of
using immunohistochemical examination of HITS expression to
complement the routine pathology diagnosis of thyroid cancer.
Persistent HPV infection is the primary cause of CIN
and invasive cervical cancer. CIN encompasses a continuum of
morphologic changes arising in the basal layer of the stratified
squamous epithelium of the transformation zone. The mean age of
women with CIN is about 15 years younger than the age of those with
invasive cancer, suggesting a slow progression of CIN to invasive
carcinoma (31). Our result that
the HITS expression level was significantly different between CIN
and invasive cervical cancer (Fig.
4) indicates that the HITS expression was lost during a course
of CIN progression to invasive carcinoma following HPV
infection.
The molecular mechanism underlying the biological
functions of FAM107 remains unclear. The C-terminal variable
regions of FAM107 with a coiled-coil domain are expected to be
involved in regulating gene transcription. Recently, it was
reported that a point mutation in the C-terminal region of HITS
(chromosome10:14603968 C•G→T•A transition) is frequently observed
in the primary tumor, brain metastasis and especially in xenograft
in genomic analyses of basal-like breast cancer (51). The protein sequence of the
C-terminal region is unique to HITS, suggesting its role in
tumorigenicity through the transcriptional regulation of oncogenes
and tumor-suppressor genes. In contrast, C-terminal coiled-coil
domain of FAM107A was reported to associate with and organize the
actin and microtubular cytoskeletons and that these associations
are essential for focal adhesion (FA) disassembly and cell invasion
(14). In fact, FAM107A is highly
expressed in the invasive component of gliomas and drives invasion
as a new cytoskeletal crosslinker that regulates FA dynamics and
cell movement. As expected from the difference of C-terminal
sequences between HITS and FAM107A, our preliminary experiments
showed no association of HITS with the actin cytoskeleton or
regulation of FA dynamics either in neural cells or cancer cells
(unpublished observation). The function and molecular interaction
of the N-terminal conserved domain (DUF1151) of FAM107 remain to be
elucidated, but might play an important role in interacting with
other proteins to transduce cellular signals and modulate gene
transcription in common with FAM107 family proteins as candidate
tumor suppressors.
In conclusion, our study clarified HITS as a
potential tumor suppressor belonging to HSPs and as a useful marker
for pathological diagnosis for use in cases since expression is
often lost in carcinomas in various tissues in
histopathological-type-specific manner, and involved in suppression
of tumor growth.
Abbreviations:
|
FAM107
|
family with sequence similarity
107
|
|
HITS
|
heat-shock-inducible tumor small
protein
|
|
HSP
|
heat-shock protein
|
|
TSG
|
tumor suppressor gene
|
|
DUF
|
domain of unknown function
|
|
HAT
|
histone acetyltransferase
|
|
ER
|
estrogen receptor
|
|
PR
|
progesterone receptor
|
|
HER2
|
human epidermal growth factor receptor
2
|
|
IDC
|
invasive ductal carcinoma
|
|
DCIS
|
ductal carcinoma in situ
|
|
CIN
|
cervical intraepithelial neoplasia
|
|
HPV
|
human papilloma virus
|
|
PCNA
|
proliferating cell nuclear antigen
|
|
FNAC
|
fine needle aspiration cytology
|
|
FA
|
focal adhesion
|
Acknowledgements
We thank Atsuko Asaka, Kazumi Tanaka
and Yumiko Hoshiba for their technical assistance. This study was
supported by Grants-in-Aid for Scientific Research from the
Japanese Ministry of Education, Culture, Sports, Science and
Technology; Collaborative Research (C2011-1) of Kanazawa Medical
University.
References
|
1
|
Rual JF, Venkatesan K, Hao T, et al:
Towards a proteome-scale map of the human protein-protein
interaction network. Nature. 437:1173–1178. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ewing RM, Chu P, Elisma F, et al:
Large-scale mapping of human protein-protein interactions by mass
spectrometry. Mol Syst Biol. 3:892007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stelzl U, Worm U, Lalowski M, et al: A
human protein-protein interaction network: a resource for
annotating the proteome. Cell. 122:957–968. 2005.PubMed/NCBI
|
|
4
|
Wang YL, Faiola F, Xu M, Pan S and
Martinez E: Human ATAC Is a GCN5/PCAF-containing acetylase complex
with a novel NC2-like histone fold module that interacts with the
TATA-binding protein. J Biol Chem. 283:33808–33815. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Frijters R, Fleuren W, Toonen EJ, et al:
Prednisolone-induced differential gene expression in mouse liver
carrying wild type or a dimerization-defective glucocorticoid
receptor. BMC Genomics. 11:3592010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamato T, Orikasa K, Fukushige S, Orikasa
S and Horii A: Isolation and characterization of the novel gene,
TU3A, in a commonly deleted region on 3p14.3-->p14.2 in renal
cell carcinoma. Cytogenet Cell Genet. 87:291–295. 1999.PubMed/NCBI
|
|
7
|
Wang L, Darling J, Zhang JS, et al: Loss
of expression of the DRR 1 gene at chromosomal segment 3p21.1 in
renal cell carcinoma. Genes Chromosomes Cancer. 27:1–10. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
van den Boom J, Wolter M, Blaschke B,
Knobbe CB and Reifenberger G: Identification of novel genes
associated with astrocytoma progression using suppression
subtractive hybridization and real-time reverse
transcription-polymerase chain reaction. Int J Cancer.
119:2330–2338. 2006.
|
|
9
|
Vanaja DK, Ballman KV, Morlan BW, et al:
PDLIM4 repression by hypermethylation as a potential biomarker for
prostate cancer. Clin Cancer Res. 12:1128–1136. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Q, Zhao XY, Bai RZ, et al: Induction
of tumor inhibition and apoptosis by a candidate tumor suppressor
gene DRR1 on 3p21.1. Oncol Rep. 22:1069–1075. 2009.PubMed/NCBI
|
|
11
|
Kholodnyuk ID, Kozireva S, Kost-Alimova M,
Kashuba V, Klein G and Imreh S: Down regulation of 3p genes, LTF,
SLC38A3 and DRR1, upon growth of human chromosome 3-mouse
fibrosarcoma hybrids in severe combined immunodeficiency mice. Int
J Cancer. 119:99–107. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao XY, Liang SF, Yao SH, et al:
Identification and preliminary function study of Xenopus
laevis DRR1 gene. Biochem Biophys Res Commun. 361:74–78. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Asano Y, Kishida S, Mu P, Sakamoto K,
Murohara T and Kadomatsu K: DRR1 is expressed in the developing
nervous system and downregulated during neuroblastoma
carcinogenesis. Biochem Biophys Res Commun. 394:829–835. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Le PU, Angers-Loustau A, de Oliveira RMW,
et al: DRR drives brain cancer invasion by regulating
cytoskeletal-focal adhesion dynamics. Oncogene. 29:4636–4647. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schmidt MV, Schulke JP, Liebl C, et al:
Tumor suppressor down-regulated in renal cell carcinoma 1 (DRR1) is
a stress-induced actin bundling factor that modulates synaptic
efficacy and cognition. Proc Natl Acad Sci USA. 108:17213–17218.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakajima H, Ishigaki Y, Xia QS, et al:
Induction of HITS, a newly identified family with sequence
similarity 107 protein (FAM107B), in cancer cells by heat shock
stimulation. Int J Oncol. 37:583–593. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jameel A, Skilton RA, Campbell TA, Chander
SK, Coombes RC and Luqmani YA: Clinical and biological significance
of HSP89 alpha in human breast cancer. Int J Cancer. 50:409–415.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takayama S, Reed JC and Homma S:
Heat-shock proteins as regulators of apoptosis. Oncogene.
22:9041–9047. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Foster CS, Dodson AR, Ambroisine L, et al:
Hsp-27 expression at diagnosis predicts poor clinical outcome in
prostate cancer independent of ETS-gene rearrangement. Br J Cancer.
101:1137–1144. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ciocca DR, Clark GM, Tandon AK, Fuqua SA,
Welch WJ and McGuire WL: Heat shock protein hsp70 in patients with
axillary lymph node-negative breast cancer: prognostic
implications. J Natl Cancer Inst. 85:570–574. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nanbu K, Konishi I, Mandai M, et al:
Prognostic significance of heat shock proteins HSP70 and HSP90 in
endometrial carcinomas. Cancer Detect Prev. 22:549–555. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jolly C and Morimoto RI: Role of the heat
shock response and molecular chaperones in oncogenesis and cell
death. J Natl Cancer Inst. 92:1564–1572. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rohde M, Daugaard M, Jensen MH, Helin K,
Nylandsted J and Jaattela M: Members of the heat-shock protein 70
family promote cancer cell growth by distinct mechanisms. Genes
Dev. 19:570–582. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Whitesell L and Lindquist SL: HSP90 and
the chaperoning of cancer. Nat Rev Cancer. 5:761–772. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mai W, Kawakami K, Shakoori A, et al:
Deregulated GSK3(beta) sustains gastrointestinal cancer cells
survival by modulating human telomerase reverse transcriptase and
telomerase. Clin Cancer Res. 15:6810–6819. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Belkacemi Y, Bousquet G, Marsiglia H, et
al: Phyllodes tumor of the breast. Int J Radiat Oncol Biol Phys.
70:492–500. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Woodward WA, Strom EA, Tucker SL, et al:
Changes in the 2003 American Joint Committee on Cancer staging for
breast cancer dramatically affect stage-specific survival. J Clin
Oncol. 21:3244–3248. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pusztai L, Cristofanilli M and Paik S: New
generation of molecular prognostic and predictive tests for breast
cancer. Semin Oncol. 34:S10–S16. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kollias J, Elston CW, Ellis IO, Robertson
JF and Blamey RW: Early-onset breast cancer - histopathological and
prognostic considerations. Br J Cancer. 75:1318–1323. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bosch FX and de Sanjose S: Chapter 1:
Human papillomavirus and cervical cancer - burden and assessment of
causality. J Natl Cancer Inst Monogr. 3–13. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kivlahan C and Ingram E: Papanicolaou
smears without endocervical cells. Are they inadequate? Acta Cytol.
30:258–260. 1986.PubMed/NCBI
|
|
32
|
Awakura Y, Nakamura E, Ito N, Kamoto T and
Ogawa O: Methylation-associated silencing of TU3A in human cancers.
Int J Oncol. 33:893–899. 2008.PubMed/NCBI
|
|
33
|
Park K, Han S, Kim HJ, Kim J and Shin E:
HER2 status in pure ductal carcinoma in situ and in the intraductal
and invasive components of invasive ductal carcinoma determined by
fluorescence in situ hybridization and immunohistochemistry.
Histopathology. 48:702–707. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dai C, Whitesell L, Rogers AB and
Lindquist S: Heat shock factor 1 is a powerful multifaceted
modifier of carcinogenesis. Cell. 130:1005–1018. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jego G, Hazoume A, Seigneuric R and
Garrido C: Targeting heat shock proteins in cancer. Cancer Lett.
Nov 13–2010.(available online).
|
|
36
|
Wang RE: Targeting heat shock proteins
70/90 and proteasome for cancer therapy. Curr Med Chem.
18:4250–4264. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Khalil AA, Kabapy NF, Deraz SF and Smith
C: Heat shock proteins in oncology: diagnostic biomarkers or
therapeutic targets? Biochim Biophys Acta. 1816:89–104.
2011.PubMed/NCBI
|
|
38
|
Dalton LW, Page DL and Dupont WD:
Histologic grading of breast carcinoma. A reproducibility study.
Cancer. 73:2765–2770. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tsuda H, Akiyama F, Kurosumi M, Sakamoto G
and Watanabe T: Establishment of histological criteria for
high-risk node-negative breast carcinoma for a multi-institutional
randomized clinical trial of adjuvant therapy. Japan National
Surgical Adjuvant Study of Breast Cancer (NSAS-BC) Pathology
Section. Jpn J Clin Oncol. 28:486–491. 1998. View Article : Google Scholar
|
|
40
|
Mylonas I, Makovitzky J, Jeschke U, Briese
V, Friese K and Gerber B: Expression of Her2/neu, steroid receptors
(ER and PR), Ki67 and p53 in invasive mammary ductal carcinoma
associated with ductal carcinoma in situ (DCIS) versus invasive
breast cancer alone. Anticancer Res. 25:1719–1723. 2005.
|
|
41
|
Lower EE, Glass EL, Bradley DA, Blau R and
Heffelfinger S: Impact of metastatic estrogen receptor and
progesterone receptor status on survival. Breast Cancer Res Treat.
90:65–70. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wolff AC, Hammond ME, Schwartz JN, et al:
American Society of Clinical Oncology/College of American
Pathologists guideline recommendations for human epidermal growth
factor receptor 2 testing in breast cancer. J Clin Oncol.
25:118–145. 2007. View Article : Google Scholar
|
|
43
|
Narod SA, Brunet JS, Ghadirian P, et al:
Tamoxifen and risk of contralateral breast cancer in BRCA1 and
BRCA2 mutation carriers: a case-control study. Hereditary Breast
Cancer Clinical Study Group. Lancet. 356:1876–1881. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Schnitt SJ: Estrogen receptor testing of
breast cancer in current clinical practice: what’s the question? J
Clin Oncol. 24:1797–1799. 2006.
|
|
45
|
Farabegoli F, Champeme MH, Bieche I, et
al: Genetic pathways in the evolution of breast ductal carcinoma in
situ. J Pathol. 196:280–286. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jo BH and Chun YK: Heterogeneity of
invasive ductal carcinoma: proposal for a hypothetical
classification. J Korean Med Sci. 21:460–468. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Steinman S, Wang J, Bourne P, Yang Q and
Tang P: Expression of cytokeratin markers, ER-alpha, PR, HER-2/neu,
and EGFR in pure ductal carcinoma in situ (DCIS) and DCIS with
co-existing invasive ductal carcinoma (IDC) of the breast. Ann Clin
Lab Sci. 37:127–134. 2007.PubMed/NCBI
|
|
48
|
Lauren PA and Nevalainen TJ: Epidemiology
of intestinal and diffuse types of gastric carcinoma. A time-trend
study in Finland with comparison between studies from high- and
low-risk areas. Cancer. 71:2926–2933. 1993. View Article : Google Scholar
|
|
49
|
Walker RA: The complexities of breast
cancer desmoplasia. Breast Cancer Res. 3:143–145. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Haberal AN, Toru S, Ozen O, Arat Z and
Bilezikci B: Diagnostic pitfalls in the evaluation of fine needle
aspiration cytology of the thyroid: correlation with histopathology
in 260 cases. Cytopathology. 20:103–108. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ding L, Ellis MJ, Li S, et al: Genome
remodelling in a basal-like breast cancer metastasis and xenograft.
Nature. 464:999–1005. 2010. View Article : Google Scholar : PubMed/NCBI
|