Introduction
Personalized cancer treatment is at present one of
the most challenging goals in cancer research in order to improve
health and quality of life of cancer patients (1,2).
Since many tumor cells are distinct on the molecular level
(3), and modern cancer drugs
target selected molecular pathways, the definitive goal is to
identify cancer patients at risk and those who may benefit from a
certain cancer therapy (1,2). For this purpose, the isolation of
circulating tumor cells (CTC) from the peripheral blood of patients
afflicted with cancer becomes increasingly important and thus is in
the focus of cancer research and the pharmaceutical industry
(4). Enrichment and enumeration of
CTC offer the potential as a prognostic cancer biomarker and may
fulfill the criteria for a surrogate biomarker to evaluate the
response of patients to cancer therapy (5,6).
Molecular characterization of CTC is a rapidly developing research
field aiming at revealing novel drug targets and to investigate
mechanisms of tumor metastasis (7).
Over the past decade, several in vitro
methodological approaches to isolate and detect rare CTC in the
peripheral blood of cancer patients have been reported, including
flow cytofluorometry (8),
image-based immunological approaches (9), fluidic microchip technology (10), and PCR methods (11–13).
At present, an antibody-coated magnetic particle isolation system
targeting the epithelial cell surface EpCAM is used in most studies
designed for ex vivo quantification of CTC in the blood of
patients with advanced breast, colon, or prostate cancer (14–16).
Patients with metastasized cancer diseases exhibit detectable
numbers of CTC in their blood (17), however, since all ex vivo
detection systems are limited by the blood volume that can be
obtained from the patients or handled by the detection system
(18), these technologies are of
relatively low sensitivity.
To overcome the limitations of small blood sample
volumes of the ex vivo CTC isolation techniques, new
approaches are needed for screening large blood volumes in
vivo using for example the principles of photoacoustic flow
cytofluorometry (19). We
developed an alternative medical device: a structured and
functionalized medical wire (FSMW) based on a Seldinger guidewire
(20) that offers the opportunity
of capturing CTC from the circulating blood of cancer patients.
Like in other ex vivo CTC-detection technologies,
identification of CTC captured by the FSMW is performed by
phenotyping CTC with antibodies directed to cytokeratins and/or
epithelial cell markers. Occasionally trapped hematologic cells are
identified by antibodies directed to respective hematologic cell
surface markers. Here, we describe a novel in vivo
CTC-catching medical device, the FSMW, its biocompatibility and
first results of its application for in vivo enrichment of
CTC from the peripheral blood of patients presenting with breast
cancer or non-small cell lung cancer (NSCLC).
Materials and methods
Functionalized structured medical
wire
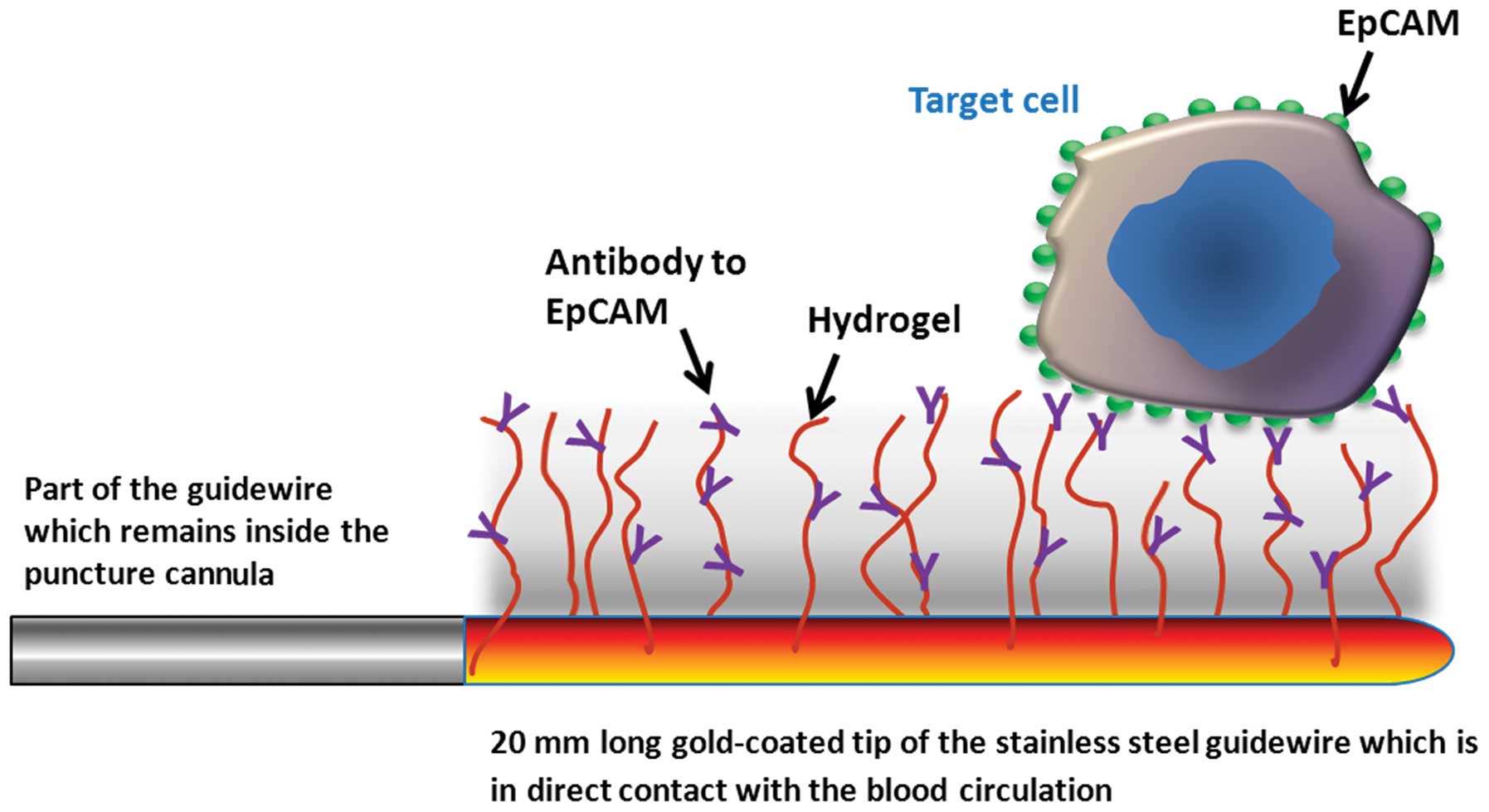
The FSMW (Fig. 1)
is based on a stainless steel medical wire (Seldinger guidewire) of
0.5 mm in diameter and 160 mm in length (EPflex, Dettingen,
Germany). The first 20 mm are plated with a 2 μm thick gold layer
deposited on the device by galvanization (OTEK, Brieselang,
Germany). Subsequently, a hydrogel layer composed of a linear,
synthetic polycarboxylate is attached to the gold layer (Xantec
Bioanalytics, Düsseldorf, Germany). The carboxyl groups present in
the hydrogel are then activated with EDC
(1-ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride) and
NHS (N-hydroxysuccinimide) (Sigma-Aldrich GmbH, Seelze, Germany)
allowing for functionalization via covalent coupling of a chimeric
antibody directed to the epithelial cell adhesion molecule CD326
(EpCAM; HEA 125, kindly provided by Dr G. Moldenhauer, German
Cancer Research Center, DKFZ, Heidelberg, Germany) present on the
surface of most CTC. Functionalization of the FSMW is carried out
under clean room conditions. The FSMW is a sterile medical device
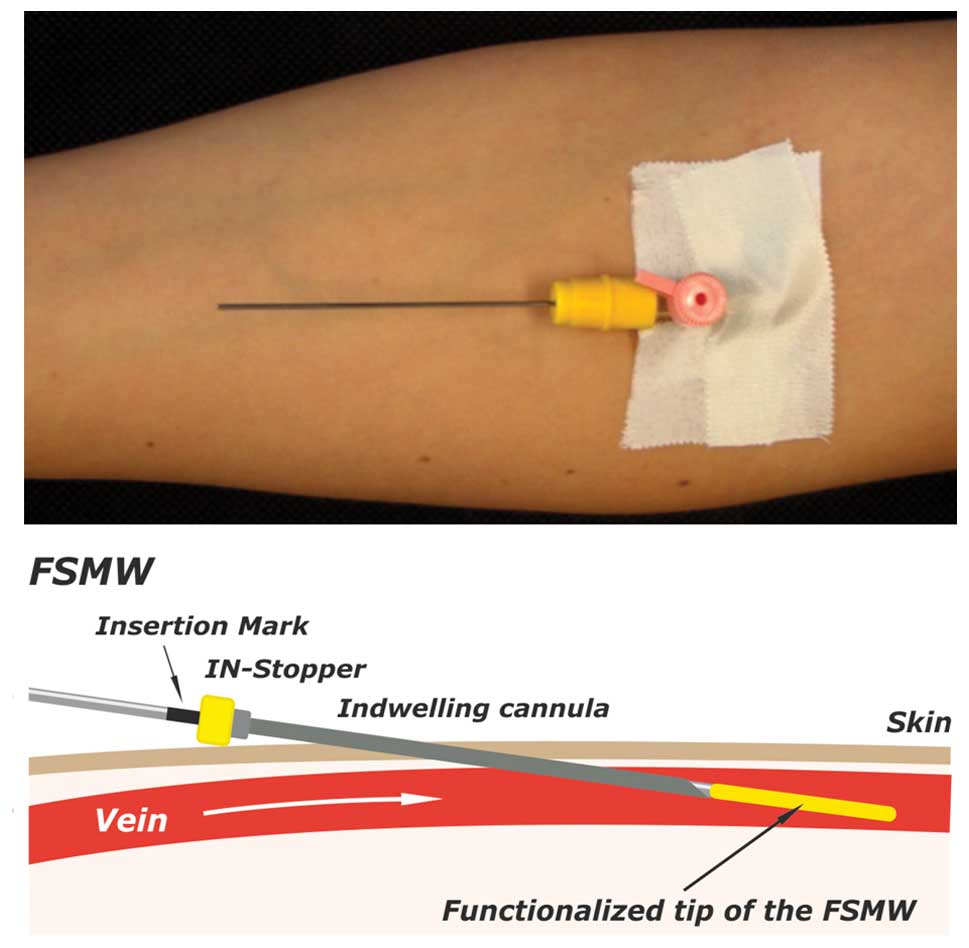
and intended for single in vivo use only. For the in
vivo application, the sterile FSMW is inserted into a standard
20G (pink color code) intravenous cannula (Fig. 2).
In vitro experiments
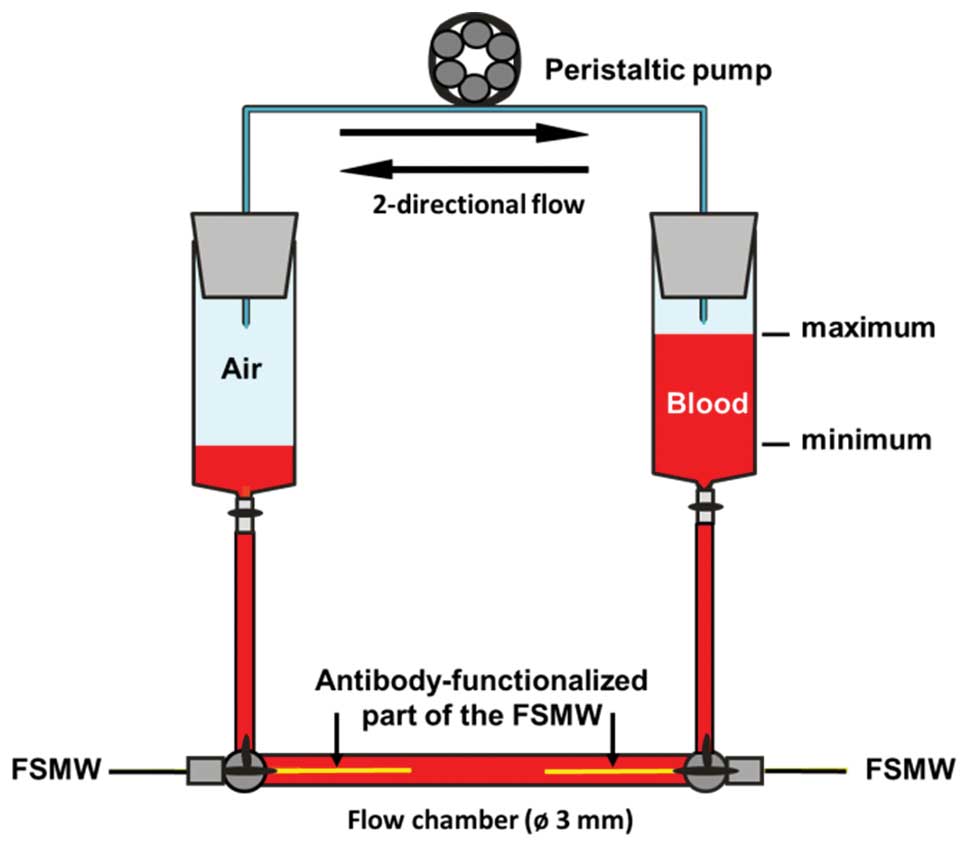
Prior to FSMW application in patients, a hemodynamic
flow system was applied (Fig. 3).
Within this system, blood was routed via flexible tubes through a
flow chamber into which up to two FSMW are inserted. The flow rate,
velocity, and flow direction can be regulated by a peristaltic
pump. A flow rate of 20 ml/min was applied to reflect in
vivo flow conditions within cubital veins (21). In this in vitro flow system
the FSMW was tested for binding of cultured EpCAM-positive SK-BR-3
breast cancer cells (CLS, Eppelheim, Germany), which were grown in
cell culture flasks until cell monolayers reached a confluency of
∼80%. Adherent SK-BR-3 cells were detached with trypsin/EDTA
(ethylene diamine tetraacetate) (Biochrom AG, Berlin, Germany),
centrifuged, and suspended in phosphate-buffered saline (PBS). In
addition, EDTA-anti-coagulated blood samples obtained from healthy
donors were spiked with those cells and tested for binding to the
FSMW. Furthermore, blood samples from breast or lung cancer
patients were also assessed in the in vitro flow system to
test for CTC binding to the FSMW.
Cytotoxicity tests
To examine potential cytotoxic effects of the FSMW,
we performed direct FSMW cell contact and material elution tests
in vitro, based on the requirements for a class IIa medical
device, as outlined in the ISO Guideline 10993-5 (www.iso.org). For the elution test, eluates of one or
three FSMW devices (three to identify any variation in the
production process), and of reference materials known to be toxic
(copper wire, Goodfellow GmbH, Bad Nauheim, Germany) or non-toxic
(Teflon wire, PTFE, Goodfellow GmbH) for normal human dermal
fibroblasts (NHDF, C-12302, PromoCell GmbH, Heidelberg, Germany)
were generated (0.095 m2 FSMW surface area eluted with 5
ml of RPMI-medium, FG 1235, Biochrom AG, at 37°C for 24 h), and
their influence on cell morphology and viability of NHDF examined.
Evaluation of potential effects of these eluates on cell morphology
was done qualitatively by microscopic analysis for changes in cell
morphology, cell adhesive capacity, and cell disintegration.
Viability of cells was tested by use of the colorimetric TTC assay
(triphenyltetrazolium chloride test).
In brief, for the microscopical inspection, NHDF
were exposed to FSMW eluates for 48 h at 37°C. Eluates of reference
materials known to be toxic (copper wire, Goodfellow GmbH) or
non-toxic (Teflon wire, PTFE, Goodfellow GmbH) for NHDF were tested
in parallel. After 48 h of incubation, cells were stained with the
dye CFSE [5-(and-6)-carboxyfluorescein diacetate succinimidyl
ester; Invitrogen, Carlsbad, USA] and inspected under the
fluorescence microscope. Impairment of cell morphology was scored
according to a common reaction index (none versus slight, moderate
or severe).
To test for viability of NHDF, a colorimetric assay
(TTC assay) was performed, which allows for the quantitative
assessment of cell viability in the presence of material-derived
eluates. After 48 h of incubation of NHDF with the eluates, a
yellow tetrazolium compound (EZ4U, Cell Proliferation and
Cytotoxicity Assay, Biomedica Medizinprodukte GmbH & Co. KG,
Vienna, Austria) was added (3 h, 37°C) which was converted due to
metabolic mitochondrial cellular activity to a brick-red formazan.
Change in color was recorded at 450 nm by use of a microtiter plate
reader (SPECTROstar-Omega, BMG Labtech, Ortenberg, Germany). For
direct contact tests, the FSMW device was placed on a layer of
adherent NHDF, followed by a 24 h incubation of this set-up at
37°C. Reference wires made of Teflon or copper were tested in
parallel.
Acute systemic toxicity
To assess potential hazardous effects of the FSMW in
humans, possibly occurring, acute systemic toxicity of the FSMW was
tested. In a parallel clinical study, aiming at the in vivo
enrichment of trophoblasts from pregnant women, potential acute
systemic toxicity of the eluates had already been monitored.
According to these tests, no adverse effects were caused by the
FSMW (22). For evaluation of the
acute systemic toxicity of the FSMW, four test groups of five mice
each [Charles River, Sulzfeld, Germany; NMRI (Han) mice, female
non-pregnant, nulliparous, 19–24 g body weight, 4–5 weeks old) were
injected intravenously or intraperitoneally with a single dose of
four different extracts of the FSMW. The amount of eluates
administered were adjusted to body weight at a volume of 50 ml/kg
for 0.9% (w/v) NaCl, 5% (v/v) ethanol, and cotton seed oil, and at
10 g/kg for polyethylene glycol-400. Four control groups of five
mice each were treated in the same manner with the corresponding
extraction vehicle not previously exposed to the FSMW.
This dosing regime supplied an about 100 times
higher dose of the FSMW than the expected dose in humans. The
animals were followed up immediately after injection, and at 4, 24,
48 and 72 h intervals for body weight and toxic effects. Cage side
observations included spontaneous activity, lethargy, recumbent
position, convulsions, tremors, apnoea, asphyxia, vocalization,
diarrhea, obvious changes in the skin and fur, eyes and mucous
membranes (salivation, discharge). At the end of the observation
period the animals were sacrificed. All animals were subjected to
gross necropsy. Any gross pathological changes were recorded. The
studies were approved by the Ethics Committee of the Medical
University of Poznan, Poland.
Hemocompatibility test
Objectives of the hemocompatibility test were
assessment of the patient’s risk to develop thrombosis,
coagulation, and hemolysis after in vivo exposure to the
FSMW. The test method described in the ISO Guideline 10993-4:2002
(E) and according to Xu and coworkers (23) was employed. To assess any influence
of the FSMW on the blood coagulation cascade, the plasma
recalcification time was determined. The FSMW was incubated for 10
min at 37°C in citrate anti-coagulated platelet-poor plasma, which
was then treated with CaCl2 to induce blood coagulation.
Clotting time of plasma, which had not been exposed to the FSMW,
served as a control. Fibrin formation was determined during a rigid
up and down movement of the FSMW in a plasma sample or in a plasma
sample which was not exposed to the FSMW. Time intervals until
fibrin deposits were formed on the FSMW were recorded. For
examination of any potential hemolytic risk exerted by the FSMW,
erythrocytes were isolated from EDTA anti-coagulated blood of
healthy donors and eluates of the FSMW added. After incubation at
room temperature (RT), the erythrocyte-eluate-suspension was
centrifuged and the hemolysis rate (% increase in cell-free
hemoglobin concentration) determined photo-metrically. Products
that have no hemolytic potential should exhibit a hemolytic rate of
less than 5%.
To assess non-specific blood cell adhesion to the
FSMW device, the antibody-functionalized part of the FSMW was put
into an in vitro hemodynamic flow system (Fig. 3) which simulates the in vivo
blood flow situation, including FSMW contact period, body
temperature, and flow rate. After removal, the FSMW was inspected
microscopically for blood cells deposited on the device. Then, in
order to detect any fibrin deposits on the FSMW, the device was
stained with antibodies directed to fibrin(ogen) [mouse anti-human
fibrin antibody, IgM, 1:100 (2 μg/ml) in PBS; Santa Cruz
Biotechnology Inc., Heidelberg, Germany] for 30 min, followed by
the addition of secondary FITC-conjugated anti-IgM (Santa Cruz
Biotechnology Inc.), 1:200 (2 μg/ml), for 30 min at RT. For
detection of adherent platelets, a fluorescence-labeled antibody
directed to CD41 was applied [anti-CD41a-PE, 1:100 (2 μg/ml) in
PBS; Santa Cruz Biotechnology Inc.].
Study population
Patients were recruited at the Wielkopolska Cancer
Center, Department of Surgical Oncology and General Surgery, and at
the Poznan University of Medical Sciences, both in Poznan, Poland.
The healthy donor population was recruited at the Department of
Obstetrics and Gynecology, Klinikum rechts der Isar, Technische
Universitaet Muenchen, Munich, Germany. The studies were approved
by the Institutional Ethics Committees and written informed
consents of the patients and volunteers were obtained.
For the in vitro functionality test of the
FSMW, we included 17 patients afflicted with breast cancer and 7
patients with NSCLC. For the in vivo functionality test of
the FSMW, we included 12 breast cancer patients and 12 NSCLC
patients (Tables I and II). Patients of both cancer types had
different tumor stages and had not undergone surgery or received
chemotherapy at the time of enrolment. Principal inclusion criteria
for breast cancer and NSCLC patients were >18 years of age,
histopathologically confirmed diagnosis of breast cancer or
potentially resectable NSCLC with eligibility for radical surgery.
Exclusion criteria: history of psychiatric disease, participation
in other clinical trials, history of allergy, anaphylactic
reactions, prior immunological diseases (anti-phospholipid antibody
syndrome, Goodpasture’s syndrome, lupus erythematosus,
polychondritis, rheumatoid arthritis, sarcoidosis, scleroderma,
Sjogren’s syndrome, ANCA positive states), immunodeficiencies,
prior infections with hepatitis viruses, the cytomegalovirus (CMV),
or infectious diseases such as tuberculosis, syphilis, or
toxoplasmosis. Principal exclusion criteria for the 29 healthy
volunteers (premenopausal 24, postmenopausal 5) were >18 years
of age, pregnancy or breastfeeding, any kind of oncological or
allergic disease including asthma and thromboembolic complications.
Median age of the volunteers was 27 (range 22–67).
 | Table IClinical characteristics of breast
cancer patients assessed for CTC enrichment in vivo and
histomorphological features of the primary tumor. |
Table I
Clinical characteristics of breast
cancer patients assessed for CTC enrichment in vivo and
histomorphological features of the primary tumor.
| Classification | No. of
patients | Patients with 1–4
CTC (%) | Patients with ≥5
CTC (%) |
|---|
| All patients | 12 | 3 (25) | 9 (75) |
| ER-/PR-status | | | |
| Positive for
either | 10 | 3 (30) | 7 (70) |
| Negative for
both | 2 | 0 | 2 (100) |
| HER2-status | | | |
| Positive | 3 | 0 | 3 (100) |
| Negative | 9 | 3 (33.3) | 6 (66.7) |
| Histological
classification | | | |
|
Invasive-ductal | 7 | 1 (14.3) | 6 (85.7) |
|
Invasive-lobular | 3 | 1 (33.3) | 2 (66.7) |
|
Invasive-ductal/invasive lobular | 1 | 1 (100) | 0 |
|
Invasive-ductal/bifocal cancer | 1 | 0 | 1 (100) |
| Adjuvant
therapy | | | |
| Chemotherapy | 3 | 1 (33.3) | 2 (66.7) |
| Endocrine
therapy | 1 | 0 | 1 (100) |
| No adjuvant
therapy | 8 | 2 (25) | 6 (75) |
| Tumor stage | | | |
| T1N1M0 | 2 | 1 (50) | 1 (50) |
| T1N3M0 | 1 | 0 | 1 (100) |
| T2N1M0 | 3 | 1 (33.3) | 2 (66.7) |
| T2N3M0 | 1 | 0 | 1 (100) |
| T4N0M0 | 1 | 0 | 1 (100) |
| T4N2M0 | 1 | 0 | 1 (100) |
| T4N+N/A | 1 | 0 | 1 (100) |
| T1N1M1 | 1 | 1 (100) | 0 |
| T3N+N/A | 1 | 0 | 1 (100) |
 | Table IIClinical characteristics of NSCLC
patients assessed for CTC enrichment in vivo and
histomorphological features of the primary tumor. |
Table II
Clinical characteristics of NSCLC
patients assessed for CTC enrichment in vivo and
histomorphological features of the primary tumor.
| Classification | No. of
patients | Patients with 1–4
CTC (%) | Patients with ≥5
CTC (%) |
|---|
| All patients | 12 | 2 (16.7) | 8 (66.7) |
| Histological
classification | | | |
| Squamous cell
lung carcinoma | 8 | 1 (12.5) | 5 (62.5) |
|
Adenocarcinoma | 3 | 1 (33.3) | 2 (66.7) |
| Large cell lung
carcinoma | 1 | 0 | 1 (100) |
| Histological
grade | | | |
| G2 | 9 | 2 (22.2) | 6 (66.7) |
| G3 | 2 | 0 | 1 (50) |
| Unknown | 1 | 0 | 1 (100) |
| Tumor stage | | | |
| T2N0M0 | 4 | 1 (25) | 2 (50) |
| T2N1M0 | 3 | 0 | 3 (100) |
| T2N2M0 | 2 | 1 (50) | 1 (50) |
| T3N0M0 | 1 | 0 | 1 (100) |
| T3N1M0 | 2 | 0 | 1 (50) |
For in vitro studies of the FSMW in an in
vitro flow system, peripheral venous blood from patients
afflicted with breast cancer or NSCLC was harvested into EDTA-tubes
(Sarstedt AG & Co., Nuembrecht, Germany). Blood samples were
kept at RT and processed in the flow system at RT within 72 h after
drawing of the blood.
In vivo application of the FSMW
The FSMW was designed to fit into a standard 20G
intravenous cannula, which is placed into the cubital vein of a
cancer patient or a healthy donor (Fig. 2). An IN-Stopper (Sarstedt AG &
Co.) allows secure fixation to the intravenous cannula. The FSMW is
slowly pushed forward into the cannula until the
EpCAM-antibody-functionalized FSMW surface of 2 cm in length is
exposed to the blood flow within the lumen of the vein. The correct
insertion is indicated by a mark on the distal part of the FSMW,
which is not inserted into the cannula. The FSMW remains in the
cubital vein for 30 min. In the present study, the insertion of the
FSMW was done before the respective patient underwent surgery of
the primary tumor. During the procedure of FSMW application, the
patient remained in a flat or supine position. The total volume of
blood coming into contact with the FSMW during the 30 min
application period is estimated at 1.5–3 liters (24).
Inspection of the FSMW for bound CTC
After removal of the FSMW from the cubital vein, the
FSMW was briefly and gently washed in PBS, followed by incubation
in PBS containing 2% (w/v) bovine serum albumin (BSA, Carl Roth
GmbH, Karlsruhe, Germany, purity grade ≥98%), for 30 min at RT.
Characterization of CTC captured by the FSMW was done by
immunocytochemical staining for EpCAM or cytokeratins 4, 5, 8, 9,
and 18. Cells attached to the FSMW were incubated with an
FITC-conjugated mouse monoclonal antibody directed to EpCAM [1:100
in PBS (10 μg/ml); Acris Antibodies GmbH, Herford, Germany] and a
phycoerythrin (PE)-conjugated rabbit antibody raised against CD45
[1:25 in PBS (2 μg/ml); Life Technologies GmbH, Darmstadt,
Germany]. Cells were counter-stained with the nuclear dye
4,6-diamidino-2-phenylindole (DAPI; 1 μg/ml PBS; Life Technologies
GmbH). Intensity of the immunocytochemical staining of CTC was
evaluated using an Axio Imager.A1m microscope (Zeiss, Jena,
Germany) equipped with an AxioCam digital camera system and the
AxioVision 4.6 software (Zeiss). EpCAM- or cytokeratin-positive
cells included in the count had to disclose additional features
such as a large cell body (diameter 10–50 μm), an irregular cell
shape, a large irregularly shaped nucleus, and a high nuclear to
cytoplasmic ratio (17,25,26).
Cells were counted on each FSMW by an operator blinded to the
clinical background of the patients. Results are given as number of
CTC immobilized on the surface of the EpCAM-antibody-functionalized
FSMW. In some cases, before inspection, cells were fixed with 4%
(w/v) buffered paraformaldehyde.
Results
Isolation and subsequent molecular characterization
of CTC from the blood of cancer patients becomes increasingly
important as it may serve as a ‘liquid biopsy’ with the potential
of monitoring the course of the cancer disease and response to
cancer therapy. For this purpose, but different than currently
employed ex vivo CTC enrichment protocols, we applied a
structured medical Seldinger guidewire (FSMW), functionalized with
a chimeric monoclonal antibody directed to EpCAM, to be used in
vivo to catch and enrich CTC from the peripheral blood pool
(Fig. 1). The FSMW was first
optimized in vitro for its CTC catching ability and then
tested for biocompatibility according to the ISO guidelines for
medical devices. Subsequently, suitability of the FSMW to catch and
enrich CTC in vivo from circulating peripheral blood
(Fig. 2) was tested in breast
cancer and NSCLC patients in the framework of clinical trials, in
comparison to healthy volunteers.
In vitro evaluation of the CTC capture
capability of the FSMW
Functionality of the FSMW in regard to its
antibody-mediated CTC enrichment capability was tested in an in
vitro dynamic flow system (Fig.
3) by capturing SK-BR-3 breast cancer cells simulating human
CTC which had been added to 20 ml of anti-coagulated blood of
healthy volunteers or blood obtained from breast cancer or NSCLC
patients. The intention of these experiments was to test: a)
whether EpCAM-antibody mediated tumor cell immobilization on the
FSMW occurs under conditions similar to venous blood flow and b)
whether non-malignant blood cells are interfering with the capture
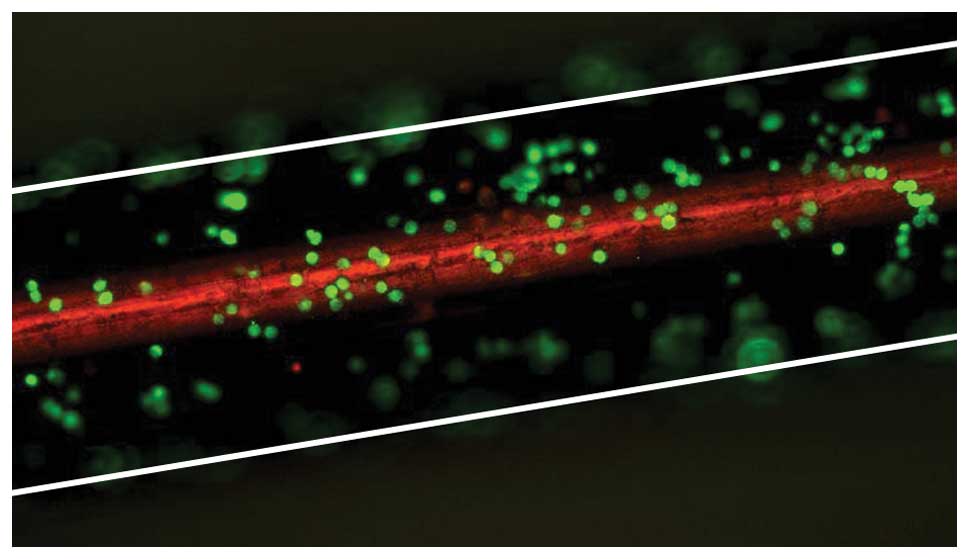
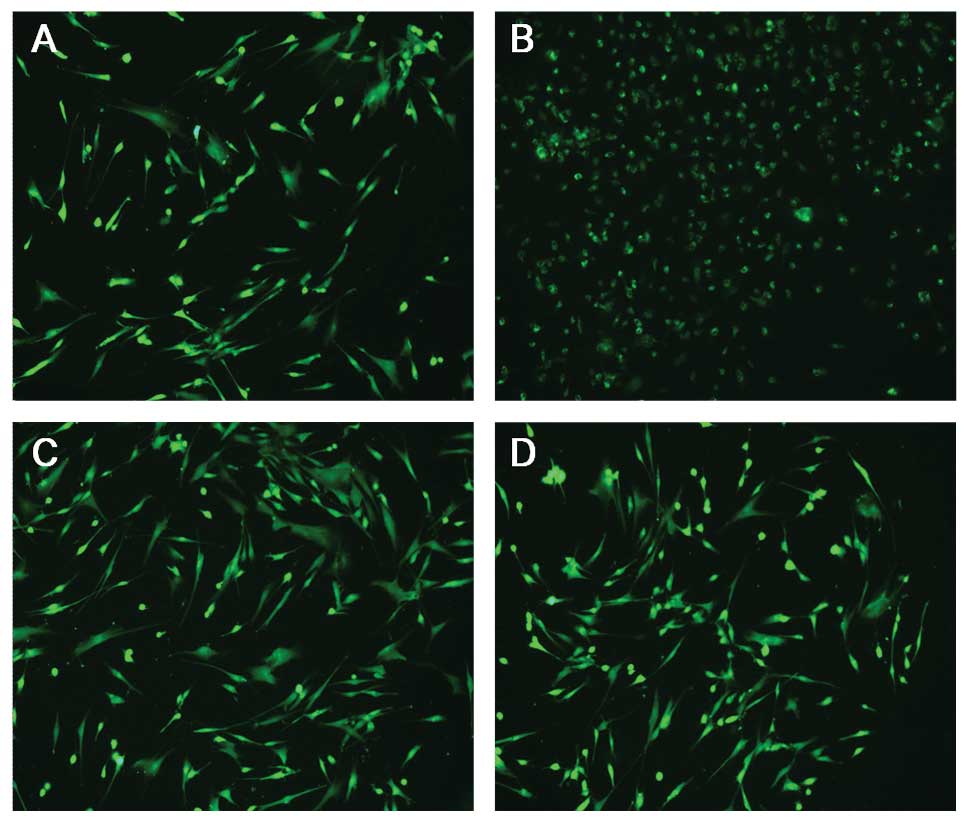
capability of the FSMW. Representative microscopic images of
FSMW-immobilized fluorescence-labeled SK-BR-3 cells from the in
vitro flow system experiments are shown in Fig. 4. The functionality of the FSMW in
the in vitro flow system was also tested with blood samples
from breast cancer and NSCLC patients. Under these conditions, the
FSMW captured CTC in 7 out of 17 (41%) anti-coagulated blood
samples of breast cancer patients (range 1–44, median 5). Also, all
anti-coagulated blood samples of NSCLC patients (n=7) turned out to
be positive for CTC (range 1–8, median 7).
Biocompatibility of the FSMW device
To demonstrate the biocompatibility and safety of
the FSMW for cancer patients or healthy volunteers during its in
vivo application, tests were performed according to ISO
guidelines recommended for class IIa medical devices. Eluates
prepared from the FSMW as described in Materials and methods were
tested for their potential effects on the viability of cultured
NHDF. Microscopic inspection of NHDF after treatment with FSMW or
Teflon wire eluates did not disclose any changes in cell
morphology. In contrast, NHDF subjected to copper wire extract,
detached from the cell culture dish substratum and assumed a
spindle-shaped cell morphology (Fig.
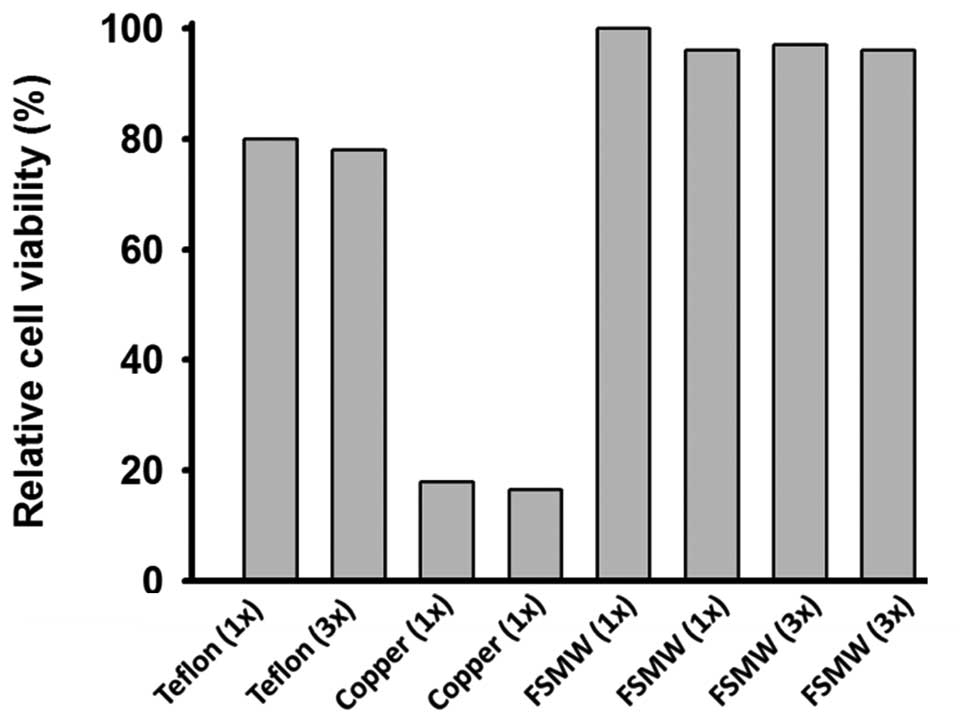
5). Cellular mitochondrial activity (as a surrogate marker for
cell viability) assessed by the TTC test remained unchanged when
NHDF were exposed to the FSMW eluate, compared to that observed in
NHDF treated with copper wire eluate, resulting in a more than 80%
reduction of cell viability (Fig.
6). Direct contact of NHDF with the FSMW gave similar results.
Even after a 24-h incubation period of the FSMW on cultured NHDF,
no perturbation of cell membrane integrity was observed. In
contrast, the copper wire as a positive control had a negative
influence on NHDF since they detached from the substrate and showed
a reduced proliferation rate.
Tests for in vivo biocompatibility of the
FSMW did not reveal any signs of acute toxicity when FSMW eluates
(n=4) were injected intravenously or intraperitoneally into NMRI
mice (n=5; for details see Materials and methods). No FSMW-related
mortalities were recorded. All mice survived the test period of 72
h independent of whether a negative control or a FSMW eluate was
applied and the mice showed normal food intake and unchanged body
weight.
Hemocompatibility tests did not indicate any
hemolytic effects of FSMW eluates. The re-calcification time of
platelet-poor plasma of citrate anti-coagulated blood from healthy
donors in the presence of the FSMW (n=5) was comparable to the
recalcification time of citrate anti-coagulated blood which had not
been exposed to the FSMW (n=5).
In vivo application of the FSMW in
healthy volunteers and in breast cancer and NSCLC patients
The FSMW was inserted into the cubital veins of 29
healthy volunteers, 12 patients afflicted with breast cancer and 12
patients presenting with NSCLC. This invasive procedure was
approved by the local Ethics Committees (Poznan, Munich). All
healthy volunteers and patients tolerated the short-term (30 min)
in vivo exposure to the FSMW without any signs of adverse
events. No CTC or other epithelium-derived cells were detectable on
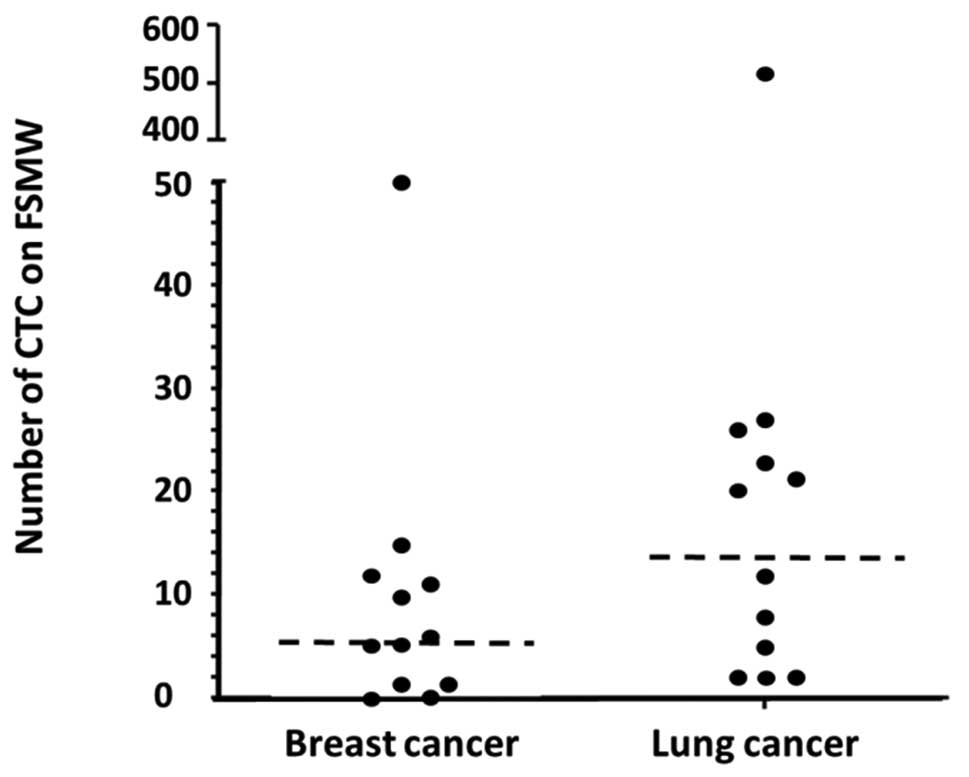
the FSMW applied to healthy volunteers. The FSMW captured CTC in 10
out of 12 patients afflicted with breast cancer (83.3%) with a
median of 5.5 CTC (range 1–50) and a mean of 9.7±14 per FSMW. In
all of the 12 NSCLC patients assessed, CTC were detectable on the
FSMW with a median of 16 CTC (range 2–515) and a mean of 55±145
(Table III). Thus, for the 24
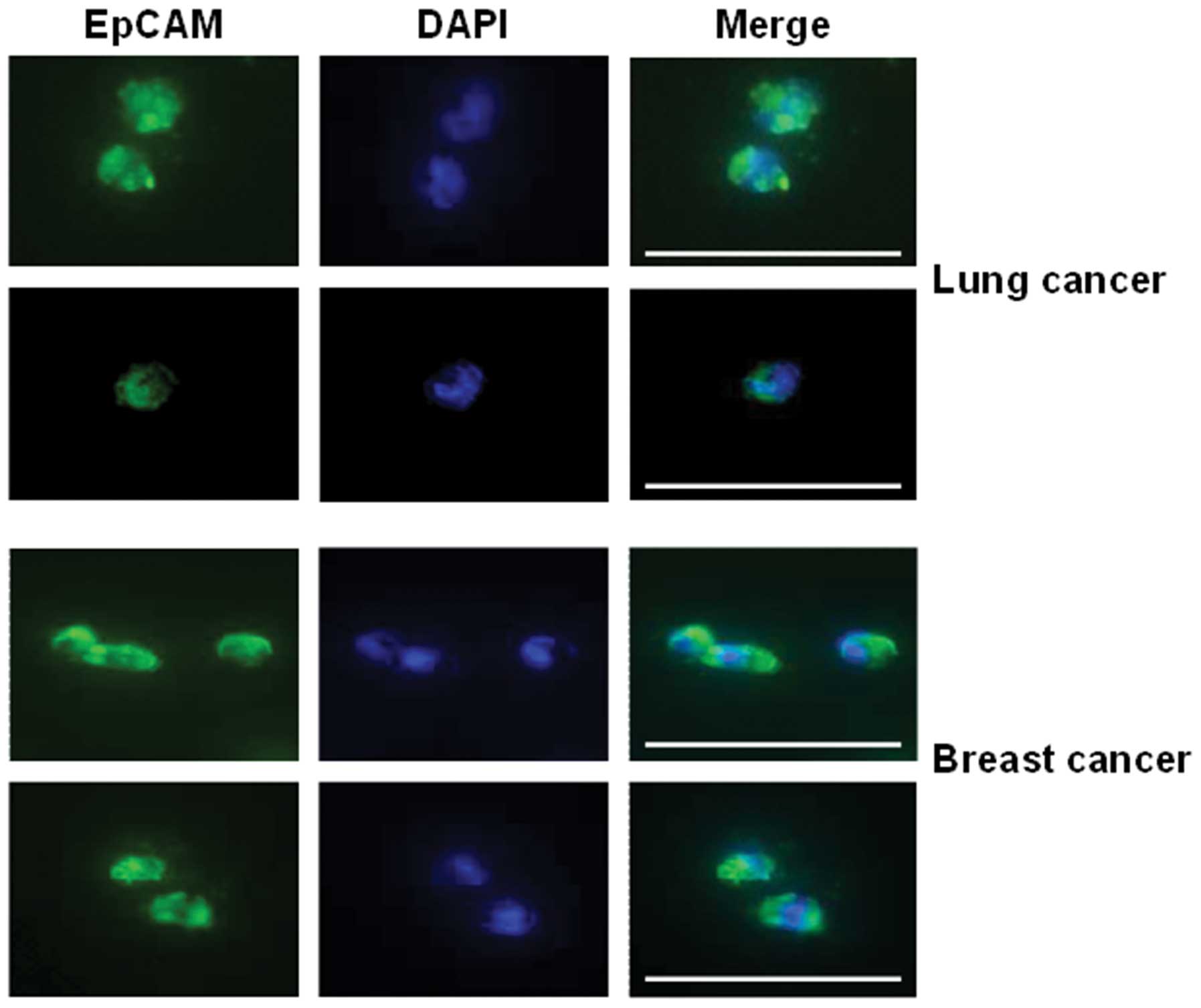
cancer patients tested, the CTC detection rate was 91.6% (Fig. 7). Representative images of
FSMW-captured CTC from breast cancer patients stained for EpCAM
expression are depicted in Fig.
8.
 | Table IIICTC enriched by in vivo use of
the FSMW: summary for lung and breast cancer patients. |
Table III
CTC enriched by in vivo use of
the FSMW: summary for lung and breast cancer patients.
| Breast cancer
(%) | NSCLC (%) |
|---|
| No. of
patients | 12 | 12 |
| Positive | 10 (83.3) | 12 (100) |
| CTC on FSMW | | |
| Range | 0–50 | 2–515 |
| Median | 5.5 | 16 |
| Mean ± SD | 9.7±13.7 | 55.4±145 |
| CTC counts | | |
| 0 | 2 (16.7) | 0 |
| <5 | 2 (16.7) | 3 (25) |
| ≥5 | 8 (66.7) | 9 (75) |
In a series of clinical trials employing the
CellSearch device (Veridex LLC, Warren, NJ) as an immunomagnetic
isolation technique for CTC in metastatic breast cancer patients
(14,27), a disease-specific cut-off was used
to define patient groups with a favorable (<5 CTC per 7.5 ml of
blood) and an unfavorable prognosis (≥5 CTC/7.5 ml of blood). Basic
characteristics of the NSCLC and breast cancer patients enclosed in
the present observatory study and the respective number of CTC
captured in vivo by the FSMW are shown in Tables I and II. Squamous cell lung carcinoma (8 out of
12 cases) was the most frequent NSCLC subtype investigated. In the
peripheral blood of these patients the FSMW captured ≥5 CTC in 5 of
the 8 cases (62.5%). Furthermore, in 66.7% of the 12 NSCLC patients
with a histological grading G2 >5 CTC were captured by the FSMW.
Invasive-ductal carcinoma was the most common subtype of breast
cancer in 58.3% (7 out of 12) of the cases; 85.7% of these cases
(n=6) displayed more than 5 CTC. In breast cancer patients with
positive estrogen and/or progesterone receptor status of their
primary tumors (n=10), the FSMW captured ≥5 CTC in 70% (n=7) of the
cases. We would like to mention that all of the lung and breast
cancer patients covered a variety of stages (Tables I and II), but only one of the 24 patients
presented with distant metastasis (breast cancer, T1N1M1) at the
time of FSMW application.
Discussion
Metastases rather than the primary tumor are the
main cause of death from cancer (28). Although there is still no
comprehensive knowledge of the biology of the metastases that
actually would need treatment, several studies indicate important
molecular differences between primary tumor and metastases at the
gene and protein level (29). The
differential expression of biomarkers between primary tumor and
metastases with proven clinical relevance could imply that
molecular features of metastatic tumor cells do have a superior
predictive value over looking at the primary tumor cells alone
(30). Still, identification of
cancer biomarkers for clinical response in tumor cells and a better
understanding of the mechanisms involved in drug sensitivity would
require repeated biopsies from metastatic lesions.
Yet, only a minority of metastatic lesions is
resected, so most histopathologic studies investigated primary
tumor tissues only (29). This
attitude is based on the fact that taking biopsies is associated
with an increased risk of complications and often painful
discomfort for the cancer patient, and it is only feasible in
patients with easy-to-access lesions. A markedly interesting
alternative to taking biopsies from metastatic lesions is
collecting CTC from the peripheral blood pool of cancer patients.
After cancer cells escape from primary tumor tissues, they
intravasate by lympho-hematogenous dissemination to distant sites
of the body, including the bone marrow and the blood (17,31).
In contrast to CTC disseminated to the bone marrow, CTC can be
easily obtained and enriched from the peripheral blood (28,32).
For this, robust and reproducible laboratory techniques are needed
for blood-borne CTC enrichment and enumeration. In recent years,
antibody-based ex vivo cytometric methods of tumor cell
enrichment and detection have become the standard to identify CTC.
Nonetheless, in the past, also nucleic acid-based detection
approaches were frequently used, with the disadvantage that CTC
enumeration or assessment of cell morphology is not possible by
this technology (28,30,33–37).
Different from these methods, we have developed a
novel, proprietary in vivo CTC detecting technology which
makes use of a structured, FDA-cleared (510k) Seldinger guidewire.
Seldinger guidewires are otherwise used for angiography, insertion
of chest drains, and central venous catheters. This novel
functionalized and structured Seldinger guidewire (FSMW) is coated
at its tip (2 cm of length) with a hydrogel layer to which chimeric
antibodies directed to epithelial cell adhesion molecule (EpCAM)
are covalently attached. The FSMW is placed into the cubital vein
of a cancer patient to allow in vivo binding of rare CTC
from the patient’s entire circulating blood pool of several liters.
This approach is different from any of the other ex vivo CTC
enriching technologies which are detecting CTC in a limited
quantity of blood, usually a few milliliters only (28,33,38).
In this respect, one should keep in mind that CTC in the peripheral
blood of cancer patients exist in extreme rarity and can be as low
as one CTC per 105–107 of other blood cells
in advanced disease stages, with even lower numbers in the blood of
early-stage-disease patients (35,39,40).
While it is understandable that CTC are present in advanced,
metastasized cancer, it is not clear how early in the tumor cell
invasion and dissemination process they will occur in the blood
circulation.
Our novel FSMW may help to clarify if CTC not only
are predictors of patient outcome in the metastatic phase of cancer
of e.g., the breast, ovary, kidney, lung, colon, and head and neck
(27,33,41–47),
but also in earlier stages of the cancer disease (48–52).
This is done in the context of two ongoing cancer therapy trials,
in advanced lung (ISRCTN55277999) and early and advanced stage
breast cancer patients (ISRCTN66203697). Within these clinical
therapy trials, enumeration of CTC is not a primary endpoint; the
FSMW was employed solely to test the feasibility and performance of
the medical device in vivo, but in context of prospective
clinical cancer trials. The FSMW proved to be a non-hazardous, safe
medical in vivo device with no adverse effects on cell
viability in the mandatory in vitro tests. Moreover, no
harmful impact of the FSMW on the blood coagulation system after
the short in vivo exposure time of 30 min was observed,
neither in breast/lung cancer patients nor in healthy
volunteers.
Different from automated ex vivo CTC
enrichment systems, the novel FSMW allows direct microscopic
control of tumor cells bound to the antibody-labeled hydrogel of
the device. Similar to other antibody-based technologies, CTC bound
to the FSMW are subjected to subsequent ex vivo labeling
with antibodies directed to CD45 to identify any non-specifically
attached leukocytes, and with DAPI, to visualize the nucleus of the
cells. Once attached to the FSMW, CTC are fixed to allow
identification with fluorescence-labeled antibodies directed to
cytokeratins and further fluorescent staining for other cellular
biomarkers, such as HER2, estrogen/progesterone receptor, epidermal
growth factor (EGF)-receptor, urokinase-type plasminogen activator
(uPA)/plasminogen activator inhibitor type-1 (PAI-1), and other
cancer biomarkers of interest.
Other ex vivo CTC enrichment methods rely on
the assumption that tumor cells are different in cell density and
dielectric properties or of relative larger size than the majority
of blood cells (28,33,38,53,54),
yet, not allowing an immediate visual microscopic control of the
enriched CTC. Some technologies are using microfluidic filters or
magnetic beads, also coated with antibodies directed to EpCAM, in
some cases combined with magnetic rods, microposts, or herringbones
to catch the antibody-labeled CTC (28,38).
Yet, all these EpCAM-antibody-based technologies assume that tumor
cells of epithelial origin do express the EpCAM antigen. This is
not always so, epithelial cells may lack the EpCAM antigen. In this
case, additional antibodies to other epithelial surface antigens
should be considered for cell trapping, such as CD49f, HER2,
MUC1/2, or carcinoembryonic antigen (CEA) (34,55).
The novel FSMW is also making use of trapping epithelial-derived
tumor cells with a high-affinity antibody directed to EpCAM but
other cell surface-directed antibodies can be easily covalently
attached to the hydrogel of the FSMW, alone or in combination with
EpCAM-directed antibodies. That way, not only tumor cells of
epithelial origin, but also CTC derived from malignant melanomas,
sarcomas, and other types of cancer can be trapped. Even further,
antibodies can be attached to the FSMW which are targeting
circulating non-tumor cells such as endothelial cells, rare forms
of leukemia cells, or trophoblast cells of pregnant women. Indeed,
in another clinical cell trapping approach employing a FSMW
covalently modified with antibodies directed to HLA-G (a
trophoblast surface antigen), circulating trophoblasts were caught
from the peripheral blood of pregnant women and subjected to
testing of genetic fetal abnormalities.
Recent results by Farace et al point to
important discrepancies between the numbers of CTC enumerated by
different enrichment technologies, also depending on the type of
tumor (56). Specifically, Flores
et al showed for breast and lung cancer patients that even
for one type of CTC enrichment technology, simply by using two
different cell enrichment kits (CellSearch Epithelial Kit versus
CellSearch Profile Kit) on the Veridex CellSearch™ machine, up to
20-fold differences in CTC yield were obtained by using the
CellSearch Profile Kit (57).
Thus, keeping these results in mind, further preclinical studies
are needed to compare performance and yield of the novel in
vivo FSMW CTC enrichment technology with other, established
ex vivo CTC enrichment technologies.
Acknowledgements
This work was supported by the Federal
Ministry of Education and Research (BMBF), grant number 01EZ0863.
The skilled technical assistance of Sandra Hippauf and Rosalinde
Bräuer is highly acknowledged. We also thank Professor Karl-Ludwig
Laugwitz, Technische Universitaet Muenchen, Munich, Germany, for
advise on and assistance with the use of the automated fluorescence
microscope.
References
|
1
|
Doroshow JH and Parchment RE: Oncologic
phase 0 trials incorporating clinical pharmacodynamics: from
concept to patient. Clin Cancer Res. 14:3658–3663. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kuhlmann J and Wensing G: The applications
of biomarkers in early clinical drug development to improve
decision-making processes. Curr Clin Pharmacol. 1:185–191. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kan Z, Jaiswal BS, Stinson J, Janakiraman
V, Bhatt D, Stern HM, Yue P, Haverty PM, Bourgon R, Zheng J,
Moorhead M, Chaudhuri S, Tomsho LP, Peters BA, Pujara K, Cordes S,
Davis DP, Carlton VE, Yuan W, Li L, Wang W, Eigenbrot C, Kaminker
JS, Eberhard DA, Waring P, Schuster SC, Modrusan Z, Zhang Z, Stokoe
D, De Sauvage FJ, Faham M and Seshagiri S: Diverse somatic mutation
patterns and pathway alterations in human cancers. Nature.
466:869–873. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maheswaran S, Sequist LV, Nagrath S, Ulkus
L, Brannigan B, Collura CV, Inserra E, Diederichs S, Lafrate AJ,
Bell DW, Digumarthy S, Muzikansky A, Irimia D, Settleman J,
Tompkins RG, Lynch TJ, Toner M and Haber DA: Detection of mutations
in EGFR in circulating lung-cancer cells. N Engl J Med.
359:366–377. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dotan E, Cohen SJ, Alpaugh KR and Meropol
NJ: Circulating tumor cells: evolving evidence and future
challenges. Oncologist. 14:1070–1082. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Krebs MG, Hou JM, Ward TH, Blackhall FH
and Dive C: Circulating tumour cells: their utility in cancer
management and predicting outcomes. Ther Adv Med Oncol. 2:351–365.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hou JM, Krebs M, Ward T, Sloane R, Priest
L, Hughes A, Clack G, Ranson M, Blackhall F and Dive C: Circulating
tumor cells as a window on metastasis biology in lung cancer. Am J
Pathol. 178:989–996. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Simpson SJ, Vachula M, Kennedy MJ, Kaizer
H, Coon JS, Ghalie R, Williams S and van Epps D: Detection of tumor
cells in the bone marrow, peripheral blood, and apheresis products
of breast cancer patients using flow cytometry. Exp Hematol.
23:1062–1068. 1995.PubMed/NCBI
|
|
9
|
Ring AE, Zabaglo L, Ormerod MG, Smith IE
and Dowsett M: Detection of circulating epithelial cells in the
blood of patients with breast cancer: comparison of three
techniques. Br J Cancer. 92:906–912. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nagrath S, Sequist LV, Maheswaran S, Bell
DW, Irimia D, Ulkus L, Smith MR, Kwak EL, Digumarthy S, Muzikansky
A, Ryan P, Balis UJ, Tompkins RG, Haber DA and Toner M: Isolation
of rare circulating tumour cells in cancer patients by microchip
technology. Nature. 450:1235–1239. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bossolasco P, Ricci C, Farina G, Soligo D,
Pedretti D, Scanni A and Deliliers GL: Detection of micrometastatic
cells in breast cancer by RT-pCR for the mammaglobin gene. Cancer
Detect Prev. 26:60–63. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iakovlev VV, Goswami RS, Vecchiarelli J,
Arneson NC and Done SJ: Quantitative detection of circulating
epithelial cells by Q-RT-PCR. Breast Cancer Res Treat. 107:145–154.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xenidis N, Perraki M, Kafousi M,
Apostolaki S, Bolonaki I, Stathopoulou A, Kalbakis K, Androulakis
N, Kouroussis C, Pallis T, Christophylakis C, Argyraki K, Lianidou
ES, Stathopoulos S, Georgoulias V and Mavroudis D: Predictive and
prognostic value of peripheral blood cytokeratin-19 mRNA-positive
cells detected by real-time polymerase chain reaction in
node-negative breast cancer patients. J Clin Oncol. 24:3756–3762.
2006. View Article : Google Scholar
|
|
14
|
Cristofanilli M, Budd GT, Ellis MJ,
Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ,
Terstappen LW and Hayes DF: Circulating tumor cells, disease
progression, and survival in metastatic breast cancer. N Engl J
Med. 351:781–791. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cohen SJ, Punt CJ, Iannotti N, Saidman BH,
Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC,
Doyle GV, Tissing H, Terstappen LW and Meropol NJ: Relationship of
circulating tumor cells to tumor response, progression-free
survival, and overall survival in patients with metastatic
colorectal cancer. J Clin Oncol. 26:3213–3221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Riethdorf S, Fritsche H, Müller V, Rau T,
Schindlbeck C, Rack B, Janni W, Coith C, Beck K, Jänicke F, Jackson
S, Gornet T, Cristofanilli M and Pantel K: Detection of circulating
tumor cells in peripheral blood of patients with metastatic breast
cancer: a validation study of the CellSearch system. Clin Cancer
Res. 13:920–928. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Allard WJ, Matera J, Miller MC, Repollet
M, Connelly MC, Rao C, Tibbe AG, Uhr JW and Terstappen LW: Tumor
cells circulate in the peripheral blood of all major carcinomas but
not in healthy subjects or patients with nonmalignant diseases.
Clin Cancer Res. 10:6897–6904. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tibbe AG, Miller MC and Terstappen LW:
Statistical considerations for enumeration of circulating tumor
cells. Cytometry A. 71:154–162. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nedosekin DA, Sarimollaoglu M, Ye JH,
Galanzha EI and Zharov VP: In vivo ultra-fast photoacoustic
flow cytometry of circulating human melanoma cells using
near-infrared high-pulse rate lasers. Cytometry A. 79:825–833.
2011. View Article : Google Scholar
|
|
20
|
Barber CJ: Central venous catheter
placement for intravenous digital subtraction angiography: an
assessment of technical problems and success rate. Br J Radiol.
62:599–602. 1989. View Article : Google Scholar
|
|
21
|
Stanton AW, Holroyd B, Northfield JW,
Levick JR and Mortimer PS: Forearm blood flow measured by venous
occlusion plethysmography in healthy subjects and in women with
postmastectomy oedema. Vascular Med. 3:3–8. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Florek E, Bręborowicz GH, Lücke K,
Madejczyk M, Chuchracki M, Dworacki G, Zabel M and Giersig M: The
acute systemic toxicity study for normal catheter and cell-select
catheter (CSC). Arch Perinatal Med. 14:20–31. 2008.
|
|
23
|
Xu FJ, Li YL, Kang ET and Neoh KG:
Heparin-coupled poly(poly(ethylene-glycol)
monomethacrylate)-Si(111)) hybrids and their blood compatible
surfaces. Biomacromolecules. 6:1759–1768. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fortune JB and Feustel P: Effect of
patient position on size and location of the subclavian vein for
percutaneous puncture. Arch Surg. 138:996–1000. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Larson CJ, Moreno JG, Pienta KJ, Gross S,
Repollet M, O’Hara SM, Russell T and Terstappen LW: Apoptosis of
circulating tumor cells in prostate cancer patients. Cytometry A.
62:46–53. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Coumans FA, Doggen CJ, Attard G, De Bono
JS and Terstappen LW: All circulating
EpCAM+CK+CD45− objects predict
overall survival in castration-resistant prostate cancer. Ann
Oncol. 21:1851–1857. 2010.
|
|
27
|
Cristofanilli M, Hayes DF, Budd GT, Ellis
MJ, Stopeck A, Reuben JM, Doyle GV, Matera J, Allard WJ, Miller MC,
Fritsche HA, Hortobagyi GN and Terstappen LW: Circulating tumor
cells: a novel prognostic factor for newly diagnosed metastatic
breast cancer. J Clin Oncol. 23:1420–1430. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhe X, Cher ML and Bonfil RD: Circulating
tumor cells: finding the needle in the haystack. Am J Cancer Res.
1:740–751. 2011.PubMed/NCBI
|
|
29
|
Steeg PS and Theodorescu D: Metastasis: a
therapeutic target for cancer. Nat Clin Pract Oncol. 5:206–219.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Graves H and Czerniecki BJ: Circulating
tumor cells in breast cancer patients: an evolving role in patient
prognosis and disease progression. Patholog Res Int.
2011:6210902011.PubMed/NCBI
|
|
31
|
Molloy TJ, Devriese LA, Helgason HH, Bosma
AJ, Hauptmann M, Voest EE, Schellens JH and van’t Veer LJ: A
multimarker QPCR-based platform for the detection of circulating
tumour cells in patients with early-stage breast cancer. Br J
Cancer. 104:1913–1919. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li Q, Qi H, Zhou HX, Deng CY, Zhu H, Li
JF, Wang XL and Li FR: Detection of micrometastases in peripheral
blood of non-small cell lung cancer with a refined immunomagnetic
nanoparticle enrichment assay. Int J Nanomed. 6:2175–2181. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
O’Flaherty JD, Gray S, Richard D, Fennell
D, O’Leary JJ, Blackhall FH and O’Byrne KJ: Circulating tumour
cells, their role in metastasis, and their clinical utility in lung
cancer. Lung Cancer. 76:19–25. 2012.PubMed/NCBI
|
|
34
|
Sun YF, Yang XR, Zhou J, Qiu SJ, Fan J and
Xu Y: Circulating tumor cells: advances in detection methods,
biological issues, and clinical relevance. J Cancer Res Clin Oncol.
137:1151–1173. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Alunni-Fabbroni M and Sandri MT:
Circulating tumour cells in clinical practice: methods of detection
and possible characterization. Methods. 50:289–297. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ross JS and Slodkowska EA: Circulating and
disseminated tumor cells in the management of breast cancer. Am J
Clin Pathol. 132:237–245. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Allan AL and Keeney M: Circulating tumor
cell analysis: technical and statistical considerations for
application to the clinic. J Oncol. 2010:4262182010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Van de Stolpe A, Pantel K, Sleijfer S,
Terstappen LW and Den Toonder JM: Circulating tumor cell isolation
and diagnostics: toward routine clinical use. Cancer Res.
71:5955–5960. 2011.PubMed/NCBI
|
|
39
|
Sleijfer S, Gratama JW, Sieuwerts AM,
Kraan J, Martens JW and Foekens JA: Circulating tumour cell
detection on its way to routine diagnostic implementation? Eur J
Cancer. 43:2645–2650. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Paterlini-Brechot P and Benali NL:
Circulating tumor cells (CTC) detection: clinical impact and future
directions. Cancer Lett. 253:180–204. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Swaby RF and Cristofanilli M: Circulating
tumor cells in breast cancer: a tool whose time has come of age.
BMC Med. 9:432011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Blümke K, Bilkenroth U, Schmidt U,
Melchior A, Füssel S, Bartel F, Heynemann H, Fornara P, Taubert H,
Wirth MP and Meye A: Detection of circulating tumor cells from
renal carcinoma patients: experiences of a two-center study. Oncol
Rep. 14:895–899. 2005.PubMed/NCBI
|
|
43
|
Nichols AC, Lowes LE, Szeto CC, Basmaji J,
Dhaliwal S, Chapeskie C, Todorovic B, Read N, Venkatesan V, Hammond
A, Palma DA, Winquist E, Ernst S, Fung K, Franklin JH, Yoo J,
Koropatnick J, Mymryk JS, Barrett JW and Allan AL: Detection of
circulating tumor cells in advanced head and neck cancer using the
Cellsearch system. Head Neck. View Article : Google Scholar : [Epub ahead of
print]. 2011.PubMed/NCBI
|
|
44
|
Lecharpentier A, Vielh P, Perez-Moreno P,
Planchard D, Soria JC and Farace F: Detection of circulating tumour
cells with a hybrid (epithelial/mesenchymal) phenotype in patients
with metastatic non-small cell lung cancer. Br J Cancer.
105:1338–1341. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cohen SJ, Punt CJ, Iannotti N, Saidman BH,
Sabbath KD, Gabrail NY, Picus J, Morse MA, Mitchell E, Miller MC,
Doyle GV, Tissing H, Terstappen LW and Meropol NJ: Prognostic
significance of circulating tumor cells in patients with metastatic
colorectal cancer. Ann Oncol. 20:1223–1229. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
De Bono JS, Scher HI, Montgomery RB,
Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ
and Raghavan D: Circulating tumor cells predict survival benefit
from treatment in metastatic castration-resistant prostate cancer.
Clin Cancer Res. 14:6302–6309. 2008.PubMed/NCBI
|
|
47
|
Poveda A, Kaye SB, McCormack R, Wang S,
Parekh T, Ricci D, Lebedinsky CA, Tercero JC, Zintl P and Monk BJ:
Circulating tumor cells predict progression free survival and
overall survival in patients with relapsed/recurrent advanced
ovarian cancer. Gynecol Oncol. 122:567–572. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bear HD: Measuring circulating tumor cells
as a surrogate end point for adjuvant therapy of breast cancer:
what do they mean and what should we do about them? J Clin Oncol.
26:1195–1197. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Pachmann K, Camara O, Kavallaris A,
Krauspe S, Malarski N, Gajda M, Kroll T, Jörke C, Hammer U,
Altendorf-Hofmann A, Rabenstein C, Pachmann U, Runnebaum I and
Höffken K: Monitoring the response of circulating epithelial tumor
cells to adjuvant chemotherapy in breast cancer allows detection of
patients at risk of early relapse. J Clin Oncol. 26:1208–1215.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bidard FC, Mathiot C, Delaloge S, Brain E,
Giachetti S, De Cremoux P, Marty M and Pierga JY: Single
circulating tumor cell detection and overall survival in
nonmetastatic breast cancer. Ann Oncol. 21:729–733. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hofman VJ, Ilie MI, Bonnetaud C, Selva E,
Long E, Molina T, Vignaud JM, Fléjou JF, Lantuejoul S, Piaton E,
Butori C, Mourad N, Poudenx M, Bahadoran P, Sibon S, Guevara N,
Santini J, Vénissac N, Mouroux J, Vielh P and Hofman PM:
Cytopathologic detection of circulating tumor cells using the
isolation by size of epithelial tumor cell method: promises and
pitfalls. Am J Clin Pathol. 135:146–156. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Rolle A, Günzel R, Pachmann U, Willen B,
Höffken K and Pachmann K: Increase in number of circulating
disseminated epithelial cells after surgery for non-small cell lung
cancer monitored by MAINTRAC(R) is a predictor for relapse: a
preliminary report. World J Surg Oncol. 3:182005. View Article : Google Scholar
|
|
53
|
Fehm T, Solomayer EF, Meng S, Tucker T,
Lane N, Wang J and Gebauer G: Methods for isolating circulating
epithelial cells and criteria for their classification as carcinoma
cells. Cytotherapy. 7:171–185. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Gascoyne PR, Noshari J, Anderson TJ and
Becker FF: Isolation of rare cells from cell mixtures by
dielectrophoresis. Electrophoresis. 30:1388–1398. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Mostert B, Kraan J, Sieuwerts AM, van der
Spoel P, Bolt-De Vries J, Prager-van der Smissen WJ, Smid M,
Timmermans AM, Martens JW, Gratama JW, Foekens JA and Sleijfer S:
CD49f-based selection of circulating tumor cells (CTC) improves
detection across breast cancer subtypes. Cancer Lett. 319:49–55.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Farace F, Massard C, Vimond N, Drusch F,
Jacques N, Billiot F, Laplanche A, Chauchereau A, Lacroix L,
Planchard D, Le Moulec S, André F, Fizazi K, Soria JC and Vielh P:
A direct comparison of CellSearch and ISET for circulating
tumour-cell detection in patients with metastatic carcinomas. Br J
Cancer. 105:847–853. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Flores LM, Kindelberger DW, Ligon AH,
Capelletti M, Fiorentino M, Loda M, Cibas ES, Jänne PA and Krop IE:
Improving the yield of circulating tumour cells facilitates
molecular characterisation and recognition of discordant HER2
amplification in breast cancer. Br J Cancer. 102:1495–1502. 2010.
View Article : Google Scholar : PubMed/NCBI
|