Introduction
Pulmonary carcinoids (PCs) account for ∼2–5% of all
primary lung tumors (1) and on the
basis of the histopathological, biological and clinical features
are distinct in typical and atypical carcinoids (TCs and ACs
respectively) (2). This
distinction is based on neuroendocrine morphology, number of
mitoses, absence or presence of necrosis and size of primary tumor
(3). Most PCs are confined to the
main or lobar bronchi (4),
however, 10–15% of cases present with regional lymph node
metastases, thus they are classified as malignant, albeit
low-grade, neoplasms (1). Distant
metastases involving liver, bone, adrenal gland and brain occur in
15% of cases (5). The 5-year
survival of TCs is 87–100%, whereas ACs have a more aggressive
clinical course, with a 5-year survival of 37–71% (4).
PCs are rare and usually occur sporadically.
Infrequently (5%) they arise in association with multiple endocrine
neoplasia type 1 (MEN1), an autosomal-dominant familial tumor
syndrome characterized by a high frequency of endocrine neoplasms
(6). The MEN1 gene is also
implicated in the pathogenesis of sporadic PCs, and mutations of
this gene have been the first genetic alterations identified in
these tumors (6). Somatic
MEN1 mutations have been detected in 35% of bronchial
carcinoid tumors (7). Overall,
inactivation of the MEN1 gene by mutation is detectable in
∼47% of sporadic TCs and in ∼70% of sporadic ACs (8). Recently, somatic inactivating
mutations in MEN1 have been also reported in 44% of
pancreatic neuroendocrine tumors (9,10).
Menin, the protein encoded by the MEN1 gene, is a component
of histone methyltransferase complexes (11–13)
and is ubiquitously expressed. It is predominantly a nuclear
protein in non-dividing cells, but in dividing cells it is found
mainly in the cytoplasm (7). Menin
regulates gene transcription, cell proliferation, apoptosis and
genomic stability. One of the proteins interacting with menin is
β-catenin, an E-cadherin signaling component, that acts as a
transcription factor and whose dysregulation has been associated
with the development and progression of many solid tumors,
including several types of endocrine tumors (14,15).
The E-cadherin/β-catenin complex localizes at the cell membrane in
essentially all normal and hyperplastic neuroendocrine cells of the
lower respiratory tract, giving rise to a membrane-linear
immunostaining pattern. The expression of the E-cadherin/β-catenin
complex appears conserved in pulmonary neuroendocrine tumors.
However, the subcellular compartmentalization of E-cadherin and
β-catenin is profoundly heterogeneous in diverse tumor types, and
reflects in a differential distribution of the
membrane-linear/disarrayed immunostaining pattern ratio (13). Only a minority of lung
neuroendocrine tumors show a nuclear translocation of β-catenin,
most cases showing a membranous colocalization with E-cadherin. The
β-catenin nuclear accumulation appears to be an exclusive feature
of a subset of high-grade neuroendocrine tumors (14). Consistently, abnormal cytoplasmic
and/or nuclear localization of the E-cadherin/β-catenin complex are
independent predictors of lymph node metastasis in ACs (14,15).
Finally, LOH and point mutations of the TP53 locus on
chromosome 17p13 have also been detected in 10% of TCs and in 45%
of ACs, and were proposed to increase with the severity of the
tumor type (16).
However, a comprehensive scenario of the molecular
alterations associated with PCs and of their interactions is still
missing. Hence, we investigated 38 sporadic PCs for protein
expression/localization (nuclear, cytoplasmic and membranous) of
menin, p53, E-cadherin and β-catenin combined with mutational
analysis of MEN1, TP53, CTNNB1 genes. Our findings show
correlations of specific alterations patterns in different
sub-sets, thus suggesting different molecular mechanisms in tumor
sub-groups. This may reflect in differential molecular taxonomy of
PCs.
Materials and methods
Tissue samples
Archived formalin-fixed paraffin-embedded (FFPE)
blocks of 38 apparently sporadic PCs consecutively diagnosed
between 2001–2008 at the Institute of Pathology, ‘S.S.Annunziata’
Hospital, Chieti, Italy were retrieved. All tumors were reviewed
for diagnosis. Cases were classified as TC (30 cases) or AC (8
cases) carcinoid tumors (WHO classification) (2). For each case both tumor and normal
tissues were available. The study was reviewed and approved by the
ethics committee of the ‘S.S. Annunziata’ Hospital.
Tissue microarray (TMA) construction and
IHC
TMA was constructed by extracting 2-mm diameter
cores of histologically confirmed neoplastic area and re-embedding
the cores into gridded paraffin blocks, using a precision
instrument (Beecher Instruments, Sun Prairie, WI). TMA sections
were stained using the anti-Menin polyclonal rabbit antibody (1:350
dilution, 30 min, Bethyl Laboratories Inc., Montgomery, TX). For
β-catenin, in order to validate the results of the
immunohistochemistry analysis, we used two commercially available
mouse monoclonal antibodies raised against the C-terminal domain of
β-catenin, clone 17C2 (1:100 dilution, 60 min, Novocastra,
Laboratories Ltd., Newcastle, UK) and 14/β-catenin (1:150 dilution,
60 min, BD Transduction Laboratories, San Jose, CA). The
anti-E-cadherin (1:50 dilution, 30 min, HECD-1, Zymed Laboratories
Inc., San Francisco, CA) and the anti-p53 (1:50 dilution, 30 min,
DO7, Novocastra) mouse monoclonal antibodies were also used.
Antigen retrieval was performed by microwave treatment at 750 W for
10 min in 10 mM sodium citrate buffer pH 6.0 (S2031, Dako,
Glostrup, Denmark), except for sections stained with the anti-p53
antibody that were treated in thermostatic bath at 96°C for 40 min
in sodium citrate buffer (Dako) The anti-mouse and the anti-rabbit
EnVision kits (Dako) were used for signal amplification, as
appropriate. In control sections the specific primary antibody was
omitted or replaced with non-immune serum or isotype-matched
immunoglobulins.
Immunohistochemical results were evaluated by two
patho-logists (M. Piantelli and R. Lattanzio) by consensus without
knowledge of the clinicopathologic information. Menin and p53
status were considered positive when ≥1% of the tumor cells were
stained. The immunostaining pattern of tumor cells for β-catenin
and E-cadherin was defined as arrayed or disarrayed according to
the immunohistochemical criteria proposed by Pelosi et
al(14). Arrayed staining was
defined as a membrane-associated, linear pattern of
immunoreactivity for β-catenin and E-cadherin, which decorated
entire the cell membrane. Disarrayed staining was defined as a
membrane staining observed along with variable cytoplasmic
accumulation or if a prevalent cytoplasmic staining with only
minimal or absent membrane labeling.
DNA extraction
Representative areas of tumor and normal tissues
were identified within hematoxylin-counterstained deparaffinized
sections and separated by manual microdissection into 1.5 ml
polypropilene vials. For DNA extraction we cut FFPE unsectioned
core samples from the interior of the paraffin blocks selecting
tumor and surrounding normal lung tissue areas. Tumor and non-tumor
DNAs were extracted using the RecoverAll™ Total Nucleic Acid
Isolation kit according to the manufacturer’s instructions (Applied
Biosystems, Forster City, CA). Purified DNA was easily amplifiable
and suitable for denaturing high performance liquid chromatography
(DHPLC) analysis.
Mutational analysis
DHPLC and direct sequence techniques were used to
analyze the entire coding sequence of MEN1 gene, exons 5–8
of TP53 gene and exon 3 of the CTNNB1 gene for
somatic mutations. DHPLC was performed using the Wave®
Nucleic Acid Fragment Analysis system (Transgenomic Inc., San Jose,
CA) and sequencing analysis using an ABI PRISM 3100 Genetic
Analyzer (Applied Biosystems). Each sample was analysed for somatic
nucleotide variants, by sequence comparison of tumor and non-tumor
DNA. We analyzed PCR amplicons of DNA extracted from FFPE tissues
by DHPLC. Tumor DNAs were analyzed for the entire MEN1
coding sequence, including intron-exon boundaries, using 13 PCR
primer sets for exons 1–10, as previously described (17). We designed also a set of primers to
amplify the last part of MEN1 exon 10 (forward:
AACTCGAGCGCCATCAAGC; reverse: GGGCTCAGAGTTGGGGGACTA).
For exons 5–8 of the TP53 gene nested PCR
amplifications were performed using primers previously described
(18,19). Direct PCR for exon 3 of the
CTNNB1 gene was carried out using the forward primer
designed in our laboratory (forward: TGATTTGATGGAGTTGGAC) and the
reverse primer previously reported (15). Tolerability prediction of amino
acid changes was tested by SIFT version 2 (available at http://blocks.fhcrc.org/sift/SIFT.html)
(20). The fruitfly software
(www.fruitfly.org) was used to assess in silico
predicted effects on splicing of intron nucleotide variants.
Statistical data analysis
Comparisons between molecular markers were done by
the Spearman’s Rho correlation. The independent samples t-test was
used to compare the expression of molecular markers in PCs
according to MEN1 gene status. The SPSS program (version
15.0, SPSS Inc., Chicago, IL, USA) was used for statistical
analysis. All cited P-values are two-sided; P<0.05 was
considered as statistically significant.
Results
Immunohistochemistry
The results of the IHC analysis for menin,
β-catenin, E-cadherin and p53 expression performed on 38 PCs are
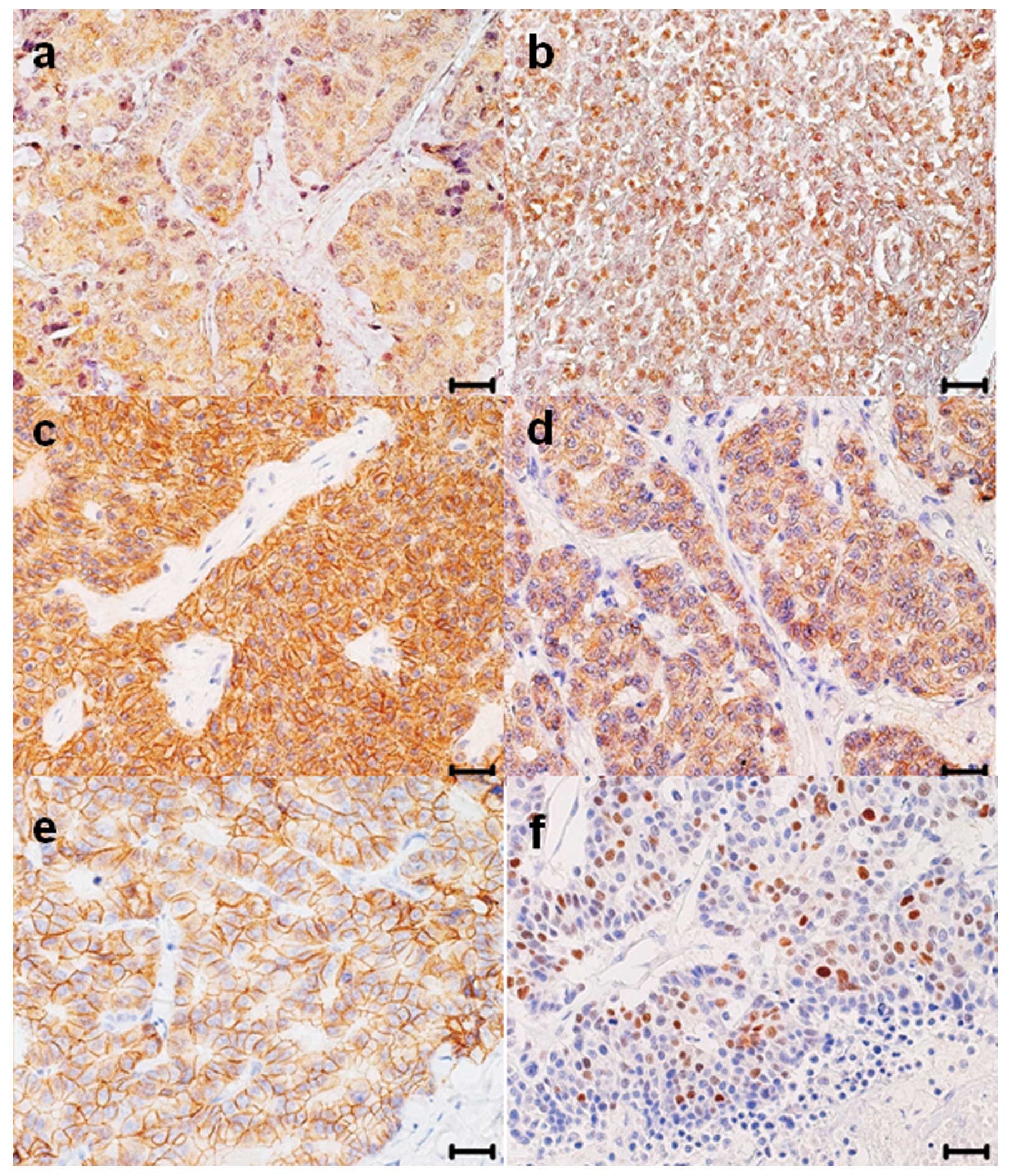
reported in Table I and examples
of specific immunohistochemical stainings are shown in Fig. 1. There were in total 27 menin
positive tumors out of 38 (71.0%), of these, 26 out of 38 (68.4%)
cases expressed menin in the cytoplasm of tumor cells (c-menin)
with a mean value of positive tumor cells of 44.7±6.1 (mean ± SE)
and 3 out of 38 (7.9%) tumors, two of which were also c-menin
positive, showed specific nuclear immunoreactivity for menin
(n-menin) (2.3±2.1).
 | Table IExpression of immunohistochemical
markers in PCs. |
Table I
Expression of immunohistochemical
markers in PCs.
| Positive cases
| |
|---|
| Marker | n | (%) | Positive tumor
cellsa |
|---|
| c-Menin | 26 | (68.4) | 44.7±6.1 |
| n-Menin | 3 | (7.9) | 2.3±2.1 |
| m-β-catenin | 14 | (36.8) | 8.0±3.1 |
| c-β-catenin | 12 | (31.6) | 16.7±5.0 |
| n-β-catenin | 0 | | |
| E-cadherin | 32 | (84.2) | 58.6±6.7 |
| n-p53 | 2 | (5.3) | 2.1±1.9 |
β-catenin-positive cases were 26 out 38 (68.4%). No
differences in the β-catenin immunostaining were observed using the
two different monoclonal antibodies. Membranous β-catenin
(m-β-catenin) expression was observed in 14 out of 38 (36.8%)
cases, whereas cytoplasmic β-catenin (c-β-catenin) was detected in
12 out of 38 (31.6%) cases. Coexpression of m- and c-β-catenin was
detected in 6 cases. The mean values of β-catenin-positive tumor
cells were 8.0±3.1 for m-β-catenin and 16.7±5.0 for c-β-catenin.
β-catenin was not expressed in the nucleus of tumor cells.
E-cadherin immunoreactivity was observed exclusively in cell
membrane and detected in 32 out 38 (84.2%) cases with a mean ± SE
of positive tumor cells of 58.6±6.7. The nuclear expression of p53
(n-p53) was observed in 2 out of 38 (5.3%) cases, with a mean ± SE
of tumor positive cells of 2.1±1.9.
Correlations between markers, were analyzed using
the Spearman’s coefficient correlation test. A significant positive
correlation was found between c-menin and c-β-catenin expression
(rho=0.439, P=0.008) (Table IIA).
Furthermore, m-β-catenin showed a positive correlation with both
c-β-catenin and E-cadherin expression (rho= 0.380, P= 0.022 and
rho= 0.360, P=0.040, respectively). With regard to the protein
status of the E-cadherin/β-catenin complex, following the criteria
suggested by Pelosi et al(14) we found a significant positive
correlation between c-menin and β-catenin disarrayed expression
(rho=0.481, P=0.007) (Table
IIB).
 | Table IISpearman’s correlations between
markers in PCs. |
Table II
Spearman’s correlations between
markers in PCs.
A,
|
|---|
| c-Menin | n-Menin | m-β-catenin | c-β-catenin | E-cadherin |
|---|
| c-Menin | | | | | |
| Rhoa | 1 | −0.093 | 0.196 | 0.439 | −0.014 |
| P | | 0.597 | 0.259 | 0.008 | 0.936 |
| n-Menin | | | | | |
| Rho | −0.093 | 1 | −0.077 | 0.007 | 0.004 |
| P | 0.597 | | 0.661 | 0.960 | 0.983 |
| m-β-catenin | | | | | |
| Rho | 0.196 | −0.077 | 1 | 0.380 | 0.360 |
| P | 0.259 | 0.661 | | 0.022 | 0.040 |
| c-β-catenin | | | | | |
| Rho | 0.439 | 0.007 | 0.380 | 1 | 0.172 |
| P | 0.008 | 0.960 | 0.022 | | 0.340 |
| E-cadherin | | | | | |
| Rho | −0.014 | 0.004 | 0.360 | 0.172 | 1 |
| P | 0.936 | 0.983 | 0.040 | 0.340 | |
B,
|
|---|
| c-Menin | β-catenin (arrayed
) | β-catenin
(disarrayed) | E-cadherin
(arrayed) | E-cadherin
(disarrayed) |
|---|
| c-Menin | | | | | |
| Rhoa | 1 | −0.479 | 0.481 | 0.213 | 0.054 |
| P | | 0.230 | 0.007 | 0.318 | 0.856 |
| β-catenin
arrayed | | | | | |
| Rho | −0.479 | 1 | −0.049 | −0.127 | |
| P | 0.230 | | | 0.765 | |
| β-catenin
disarrayed | | | | | |
| Rho | 0.481 | | 1 | 0.081 | 0.029 |
| P | 0.007 | | | 0.765 | 0.922 |
| E-cadherin
arrayed | | | | | |
| Rho | 0.213 | −0.127 | 0.081 | 1 | |
| P | 0.318 | 0.765 | 0.765 | | |
| E-cadherin
disarrayed | | | | | |
| Rho | 0.054 | | 0.029 | | 1 |
| P | 0.856 | | 0.922 | | |
Mutational analysis
DHPLC and direct sequencing analyses were utilized
to detect somatic mutations in MEN1 (entire coding
sequence), TP53 (exons 5–8) and CTNNB1 (exon 3)
genes. MEN1 gene variants (ENST00000312049) were identified
in 13/38 (34%) cases, of which 9/30 TPCs (30%) and 4/8 APCs (50%)
(Table III). Variants included the
frameshift mutation c.427delC, which introduces a stop signal at
codon 184 (p.L143fsX184, case no. 23) and 4 missense variants
(p.T541A, case no. 12; p.G99S, case no. 24; p.A216T, case no. 27;
p.L89R, case no. 33), whose tollerance of amino acid changes was
tested through SIFT Version 2 program. In addition, we found 3
synonymous variants (p.S145S, cases no. 15 and 23; p.A49A, case
nos. 29; p.N418N, case nos. 11, 12, 16 and 37 in heterozygosity,
cases no. 2, 28 and 33 in homozygosity). Finally, we characterized
also a novel intronic variant (c.446-5C>T, IVS2, case nos. 9 and
12) that, according to the in silico evaluation (www.fruitfly.org), was not predicted to affect
splicing. In Table III the
expression of c- and n-menin, n-p53, m- and c-β-catenin and
E-cadherin are reported for each MEN1 mutated case. As
shown, 11 out of 13 and 12 out of 13 MEN1 mutated cases
expressed c-menin and E-cadherin, respectively. Nine out of 13 and
7 out of 13 MEN1-mutated cases expressed m- and c-β-catenin,
respectively, while the co-expression of m- and c-β-catenin were
detected in 6 cases, c- and n-menin and n-p53 were co-expressed in
only 1 case. Correlating the menin expression levels with the
presence or absence of MEN1 nucleotide variants, we found
that c-menin was significantly more expressed in tumors with
MEN1 variants compared to tumors without MEN1
variants (P=0.023), whereas n-menin does not show a significance
when compared with PCs with and without MEN1 variants
(Table IV). No differences were
found comparing m- and c-β-catenin, m-E-cadherin and n-p53
expression levels with MEN1 gene status, although a positive
trend in the expression of c-β-catenin marker in MEN1
mutated cases was also observed (data not shown). Mutational
analysis of TP53 exons 5–8 allowed to identify 3 nucleotide
variants (p.A129A, case no. 21; pI255F, case no. 27; p.R213R, case
no. 37) in exons 5, 7 and 6 respectively (Table V), i.e., outside TP53
hotspots of mutations (21). These
nucleotide variants are reported in the IARC TP53 database
(http://www-p53.iarc.fr/), where the tolerance of
amino acid changes was tested through SIFT version 2 program
(http://blocks.fhcrc.org/sift/SIFT.html) and AGVGD
(http://agvgd.iarc.fr/). The variant c.763T at
codon 255, reported to be deleterious, resulted associated with a
high nuclear expression of p53 (68% of positive tumor cells).
Finally, the mutational study of exon 3 of the CTNNB1 gene
resulted negative and no nucleotide variants were detected.
 | Table IIIData on allelic variants of
MEN1 gene and correlations with the percentage of positive
tumor cells expressing menin, p53, β-catenin and E-cadherin
immunostaining. |
Table III
Data on allelic variants of
MEN1 gene and correlations with the percentage of positive
tumor cells expressing menin, p53, β-catenin and E-cadherin
immunostaining.
| Case no. | MEN1
variant | Exon | Effect | c-menin | n-menin | n-p53 | m-β-catenin | c-β-catenin | E-cadherin |
|---|
| TC | | | | | | | | | |
| 2 | c.1254T | 9 | p.N418N | 72 | 0 | 0 | 2 | 54 | 12 |
| 9 | c.446-5C>T | IVS2 | No effect on
splicing | 100 | 0 | 0 | 6 | 63 | 100 |
| c.1254C>T | 9 | p.N418N | | | | | | |
| 11 | c.1254C>T | 9 | p.N418N | 93 | 0 | 0 | 88 | 92 | 74 |
| 12 | c.446-5C>T | IVS2 | No effect on
splicing | 40 | 0 | 0 | 0 | 0 | 26 |
| c.1254C>T | 9 | p.N418N | | | | | | |
| c.1621A>G | 10 | p.T541A | | | | | | |
| 15 | c.435C>T | 2 | p.S145S | 98 | 0 | 0 | 4 | 82 | 78 |
| 16 | c.1254C>T | 9 | p.N418N | 100 | 0 | 0 | 0 | 31 | 97 |
| 23 | c.427delC | 2 | p.L143fsX184 | 81 | 0 | 0 | 3 | 0 | 95 |
| c.435C>T | 2 | p.S145S | | | | | | |
| 24 | c.296A | 2 | p.G99S | 0 | 0 | 0 | 0 | 0 | 41 |
| 37 | c.1254C>T | 9 | p.N418N | 0 | 0 | 0 | 7 | 8 | 0 |
| AC | | | | | | | | | |
| 27 | c.646G>A | 3 | p.A216T | 42 | 4 | 68 | 0 | 0 | 100 |
| 28 | c.1254T | 9 | p.N418N | 49 | 0 | 0 | 42 | 0 | 98 |
| 29 | c.147T>G | 2 | p.A49A | 82 | 0 | 0 | 1 | 0 | 3 |
| 33 | c.266T>G | 9 | p.L89R | 55 | 0 | 0 | 45 | 53 | 96 |
| c.1254T | 2 | p.N418N | | | | | | |
 | Table IVCorrelations between Menin expression
and MEN1 gene status. |
Table IV
Correlations between Menin expression
and MEN1 gene status.
| Positive tumor
cells
|
|---|
| Mean ± SEa | P-valueb |
|---|
| c-Menin | | |
| PCs with
MEN1 mutations (n=13) | 62.5±9.7 | 0.023 |
| PCs without
MEN1 mutations (n=25) | 34.2±7.1 | |
| n-Menin | | |
| PCs with
MEN1 mutations (n=13) | 0.3±0.3 | 0.485 |
| PCs without
MEN1 mutations (n=25) | 3.4±3.2 | |
 | Table VData on nucleotide variants of
TP53, predicted effect on p53 protein and
immunohistochemical expression. |
Table V
Data on nucleotide variants of
TP53, predicted effect on p53 protein and
immunohistochemical expression.
| | | | | | Predicted effect on
the protein
| |
|---|
| Case no. | Histotype | TP53
variant | Exon | Effect | Status
(Reference) | SIFT | AGVGD | IHC |
|---|
| 21 | TC | c.387C>T | 5 | p.A129A | IARC TP53
database | Silent | Silent | 0 |
| 27 | AC | c.763T | 7 | p.I255F | IARC TP53
database | Deleterious | Deleterious | 68a |
| 37 | TC | c.639A>G | 6 | p.R213R | IARC TP53
database | Silent | Silent | NA |
Discussion
In this study we analyzed a series of 38 sporadic
PCs for somatic mutations and for protein expression of genes that
appear to be implicated in the development and progression of the
disease. Combined genetic and IHC findings were used to identify
relevant PC sub-groups and to help defining a combined role of
genes potentially involved in the pathogenesis of PCs. This was
done by evaluating the IHC expression of menin, p53, β-catenin and
E-cadherin at subcellular level and by correlating the expression
of these markers with the mutational spectra of the MEN1,
TP53 and CTNNB1 genes in both TC and AC tumors.
A significant fraction of tumor samples (34%)
harbored MEN1 gene variants. Most samples showed a
cytoplasmic, rather than nuclear, localization of menin.
Cytoplasmic localization of menin was observed in tumors with and
without MEN1 variants. Only two cases with nuclear menin
immunostaining did not display variants of the MEN1
gene.
Notably, tumors carrying MEN1 variants showed
significantly higher cytoplasmic expression of the menin, when
compared with samples negative for MEN1 variants. The
cytoplasmic localization of menin was detected both in TCs and ACs,
the highest fraction being observed in ACs (data not shown), i.e.,
in cases with higher rate of mitosis. Thus, our data on PCs are in
agreement with previous observations in pancreatic endocrine tumors
in which a strong association between MEN1 variants and
cytoplasmic localization of menin were observed (10). This is consistent with a role of
this oncosuppressor gene in the pathogenesis of sporadic PCs. The
analysis of the E-cadherin/β-catenin complex evidenced a
significant correlation in the membranous and/or cytoplasmic
expression of these two proteins. It is known that the complex
plays a crucial role in cell-cell adhesion, and dysregulation of
the E-cadherin/β-catenin-dependent adhesion complex has been
associated with the development and progression of many solid
tumors, including several types of endocrine tumors (14). Thus, we investigated a possible
dysregulation of the complex in PCs. We observed β-catenin
expression in the majority of tumor cases. However, in a fraction
of PCs β-catenin tended to accumulate in the cytoplasm. Notably, we
found also a significant direct relationship of c-β-catenin
expression with c-menin expression and, following the
immunohistochemical criteria suggested by Pelosi et
al(13) who defined the
immunostaining pattern of tumor cells for β-catenin and E-cadherin
as arrayed or disarrayed, we searched for possible correlations
between these patterns of immunostaining with the other markers
analyzed. Intriguingly, we found a significant positive
correspondence between the disarrayed expression of β-catenin with
the c-menin expression. Consistent with the absence of nuclear
β-catenin expression, we did not find mutations of the
CTNNB1 gene (15), further
indicating a role of this gene in PC pathogenesis, but not as a
driver mutation. It is known that menin regulates gene
transcription, cell proliferation, apoptosis and genomic stability
and one of the proteins interacting with menin is β-catenin.
Single or multiple MEN1 sequence variants,
consisting in frameshift, missense and silent variants were
characterized in 13 out of 38 cases, with a prevalence in ACs
compared to TCs. Three of the 4 missense variants characterized in
our study were not reported up to now in association with PCs. The
p.T541A missense variant is a pathogenetic variant affecting the
role of menin in the apoptosis control (22). The missense mutation p.L89R and the
polymorphism p.S145S were previously identified as somatic variants
also in glucagonoma and parathyroid tumors. The p.L89R detected in
case no. 33 was not tolerant using the SIFT Version 2 program and
could interfere with menin protein structure and function.
Cytoplasmic expression of menin and presence of nucleotide variants
of the MEN1 gene may be indicative of a significant
correlation, since cases with MEN1 nucleotide variants are
characterized by a higher number of positive tumor cells expressing
menin in the cytoplasmic compartment. It is noteworthy that high
cytoplasmic expression of menin has been also detected in most of
the cases bearing only the c.1254T polymorphism, suggesting a
hypothetical possibility of this silent variant and c-menin
accumulation in tumor cells. Thus, our data support the hypothesis
that MEN1 gene variants affect the subcellular localization
of the protein causing its accumulation in the cytoplasm. For cases
without MEN1 variants and cytoplasmic menin expression, it
may be possible that other genes, partners of MEN1, are
responsible for the impairment of menin function in PCs. The
results are in agreement with other studies on sporadic lung
carcinoids (6).
Somatic mutation analysis of exons 5–8 of the
TP53 gene, which encode the DNA-binding region where
cancer-associated mutations most frequently occur (23–25),
indicates that genetic alterations of this gene may be implicated
in the development of a limited fraction of PCs. In this contest,
it is relevant to note that the nucleotide variant c.763T at codon
255, detected in an APC with high immunohistochemical expression of
p53, is reported to be deleterious in both the SIFT version 2 and
AGVGD programs, where the tolerance of amino acid changes was
tested. Thus, our results indicate that TP53, rather than
playing a broad role in PCs (16,26),
may operate in specific PC sub-groups, possibly by interacting with
menin. Additional studies are required to test this model.
In conclusion, the present study confirmed the
implication of MEN1 gene in the development of sporadic PC.
Furthermore, the mutational study of MEN1 gene, associated
with the IHC analysis of menin indicated that tumors displaying
MEN1 nucleotide variant were characterized by a higher
accumulation of menin in the cytoplasm and, for our knowledge, this
strong association between MEN1 variants and cytoplasmic
localization of menin has not been previously reported in sporadic
pulmonary carcinoids, thus representing an interesting finding for
this type of tumor.
In addition, this study also indicated that the
subcellular compartmentalization of the E-cadherin/β-catenin
complex was altered in PCs and the disarrayed pattern of the
complex significantly correlated with c-menin accumulation, thus
suggesting a possible cooperative role of menin and
E-cadherin/β-catenin in the development and/or progression of this
endocrine-related tumor.
References
|
1
|
Bini A, Brandolini J, Cassanelli N, Davoli
F, Dolci G, Sellitri F and Stella F: Typical and atypical pulmonary
carcinoids: our institutional experience. Interact Cardiovasc
Thorac Surg. 7:415–418. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Travis WD, Brambilla E, Müller-Hermelink
HK and Harris CC: WHO Classification of Tumors: Pathology and
Genetics of Tumours of the Lung, Pleura,Thymus and Heart. IARC
Press; Lyon: 2004
|
|
3
|
Travis WD, Rush W, Flieder DB, Falk R,
Fleming MV, Gal AA and Koss MN: Survival analysis of 200 pulmonary
neuroendocrine tumors with clarification of criteria for atypical
carcinoid and its separation from typical carcinoid. Am J Surg
Pathol. 22:934–944. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hage R, de la Rivière AB, Seldenrijk CA
and van den Bosch JM: Update in pulmonary carcinoid tumors: a
review article. Ann Surg Oncol. 10:697–704. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jeung MY, Gasser B, Gangi A, et al:
Bronchial carcinoid tumors of the thorax: spectrum of radiologic
findings. Radiographics. 22:351–365. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Debelenko LV, Brambilla E, Agarwal SK, et
al: Identification of MEN1 gene mutations in sporadic carcinoid
tumors of the lung. Hum Mol Genet. 6:2285–2290. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lemos MC and Thakker RV: Multiple
endocrine neoplasia type 1 (MEN1): analysis of 1336 mutations
reported in the first decade following identification of the gene.
Hum Mutat. 29:22–32. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Travis WD, Rush W, Flieder DB, Falk R,
Fleming MV, Gal AA and Koss MN: Bronchopulmonary neuroendocrine
tumors. Cancer. 113:5–21. 2008. View Article : Google Scholar
|
|
9
|
Jiao Y, Shi C, Edil BH, et al: DAXX/ATRX,
MEN1, and mTOR pathway genes are frequently altered in pancreatic
neuroendocrine tumors. Science. 331:1199–1203. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Corbo V, Dalai I, Scardoni M, et al: MEN1
in pancreatic endocrine tumors: analysis of gene and protein status
in 169 sporadic neoplasms reveals alterations in the vast majority
of cases. Endocr Relat Cancer. 17:771–783. 2010. View Article : Google Scholar
|
|
11
|
Kim H, Lee JE, Cho EJ, Liu JO and Youn HD:
Menin, a tumor suppressor, represses JunD-mediated transcriptional
activity by association with an mSin3A-histone deacetylase complex.
Cancer Res. 63:6135–6139. 2003.PubMed/NCBI
|
|
12
|
Hughes CM, Rozenblatt-Rosen O, Milne TA,
et al: Menin associates with a trithorax family histone
methyltransferase complex and with the hoxc8 locus. Mol Cell.
13:587–597. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yokoyama A, Wang Z, Wysocka J, et al:
Leukemia protooncoprotein MLL forms a SET1-like histone
methyltransferase complex with menin to regulate Hox gene
expression. Mol Cell Biol. 24:5639–5649. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pelosi G, Scarpa A, Puppa G, et al:
Alteration of the E-cadherin/β-catenin cell adhesion system is
common in pulmonary neuroendocrine tumors and is an independent
predictor of lymph node metastasis in atypical carcinoids. Cancer.
103:1154–1164. 2005.
|
|
15
|
Clavel CE, Nollet F, Berx G, et al:
Expression of the E-cadherin/β-catenin complex in lung
neuroendocrine tumours. J Pathol. 194:20–26. 2001.
|
|
16
|
Leotlela PD, Jauch A, Holtgreve-Grez H and
Thakker RV: Genetics of neuroendocrine and carcinoid tumours.
Endocr Relat Cancer. 10:437–450. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhuang Z, Vortmeyer AO, Pack S, et al:
Somatic mutations of the MEN1 tumor suppressor gene in sporadic
gastrinomas and insulinomas. Cancer Res. 57:4682–4686.
1997.PubMed/NCBI
|
|
18
|
Catalano T, Curia MC, Aceto G, et al:
Mutations in the p53 and Ki-ras genes, microsatellite instability
and site of tumor origin in colorectal cancer. Oncol Rep.
14:625–631. 2005.PubMed/NCBI
|
|
19
|
Mahdavinia M, Bishehsari F, Verginelli F,
et al: P53 mutations in colorectal cancer from northern Iran:
Relationships with site of tumor origin, microsatellite instability
and K-ras mutations. J Cell Physiol. 216:543–550. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ng PC and Henikoff S: Accaounting for
human polymorphisms predicted to affect protein function. Genome
Res. 2:436–446. 2002.PubMed/NCBI
|
|
21
|
Petitjean A, Mathe E, Kato S, Ishioka C,
Tavtigian SV, Hainaut P and Olivier M: Impact of mutant p53
functional properties on TP53 mutation patterns and tumor
phenotype: lessons from recent developments in the IARC TP53
database. Hum Mutat. 28:622–629. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bazzi W, Renon M, Vercherat C, et al: MEN1
missense mutations impair sensitization to apoptosis induced by
wild-type menin in endocrine pancreatic tumor cells.
Gastroenterology. 135:1698–1709. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vogelstein B, Fearon ER, Hamilton SR, et
al: Genetic alterations during colorectal-tumor development. N Engl
J Med. 319:525–532. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cho KR and Vogelstein B: Genetic
alterations in the adenomacarcinoma sequence. Cancer. 70:1727–1731.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Goh HS, Yao J and Smith DR: p53 point
mutation and survival in colorectal cancer patients. Cancer Res.
55:5217–5221. 1995.PubMed/NCBI
|
|
26
|
Sugio K, Osaki T, Oyama T, et al: Genetic
alteration in carcinoid tumors of the lung. Ann Thorac Cardiovasc
Surg. 9:149–154. 2003.PubMed/NCBI
|