Signal transducer and activator of transcription 3
(STAT3) is a transcription factor which in humans is encoded by the
STAT3 gene. The protein is a latent cytoplasm transcription factor
that relays signals of cytokines and growth factors from the cell
membrane to the nucleus to regulate gene expression critical to
normal cellular processes, including cell development,
differentiation, proliferation, survival, angiogenesis and immune
function (1–5). Constitutive STAT3 activation is
associated with various human cancers and commonly suggests poor
prognosis (6–9). Thus STAT3 has been studied as a
tumour therapeutic target. Very recently a tumour suppressor role
of STAT3 in a few tumours has also been reported (10,11),
but this is not the focus of the present review.
In mammals, the STAT family consists of seven
protein members: STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b and
STAT6, which are mapped to different human chromosomal regions
(12). STAT2, STAT4 and STAT6 are
only activated in normal human function, while STAT1, STAT3 and
STAT5 play an important role in cancer development (STAT1 as tumour
suppressor, STAT3 and STAT5 as oncogenes) (13,14).
STAT3 appears to be a critical regulator and is therefore the focus
of this review. All of the STAT family comprise 750–850 amino acids
and 6 conserved domains: the NH2-terminal, coiled-coil, DNA
binding, linker, SH2, and C-terminal transactivation domains
(15,16). The C-terminal transactivation
domain of STAT3 plays an important role in activation through a
tyrosine residue at position 705 and a serine residue at position
727 (17). The SH2 region, a
well-characterized small protein module of approximately 100 amino
acids (18), is responsible for
the binding of STAT3 to the tyrosine-phosphorylated receptors and
also the homodimerization or heterodimerizaion of two STAT monomers
that is necessary for DNA binding and gene expression (19). SH2 region, NH2-terminal and
DNA-binding domain became the targets for development of STAT3
inhibitors.
This review aimed principally to summarise the
therapeutic potential of STAT3 inhibitors, specifically STAT3
inhibition and preclinical studies, as well as inhibiting STAT3 in
clinical trials.
The STAT3 signalling pathways regulate the gene
expression of proliferation, survival, migration and invasion, as
well as angiogenesis (2,20) in human. Furthermore, STAT3 is
essential at early stages of embryo development (21) and modulates embryonic stem cell
differentiation such as TH17 helper T cells (22). In benign cells, the signalling by
STAT3 is under tight regulation so that the signal is transient in
accordance with physiological responses.
The process of STAT activation commences with the
Janus kinases (JAKs) which bind to and are phosphorylated by
cytokine or growth factor receptors in response to external signals
such as interleukin-6 (IL-6), interferon-α, tumour necrosis factor
(TNF), epidermal growth factor (EGF), platelet-derived growth
factor (PDGF), transforming growth factor-alpha (TGF-α) and TGF-β1
(17,23–25).
Subsequently, phosphorylated JAKs in turn cause multiple
phosphorylations of tyrosine residues within the cytoplasmic domain
of the cytokine receptor (26–28).
Monomeric unphosphorylated STAT is recruited to these activated
receptors through an interaction between the STAT SH2 domain and
phosphotyrosine docking sites on the receptors. JAKs then
phosporylate the critical tyrosine on C-terminal domain of STAT,
which causes dissociation of STAT from the receptor, this then
leads to dimerization of two STAT monomers through reciprocal
interaction (2,29,30).
Dimeric STAT complexes translocate to the nucleus where they bind
to specific DNA response elements in the promoters of target genes
(31).
The non-receptor tyrosine kinases such as Src can
activate the STAT signalling pathway in the absence of receptor
engagement (32–34). In some cases, JAKs as
intermediaries are simultaneously involved in activation of STAT by
non-receptor tyrosine kinases (35).
In a hepatocellular carcinoma cell (HCC) study,
JAK/STAT activated by interferon-α inhibited MAPK signalling by
cross-talking with MEK/ERK pathway (36). Our results in sarcoma suggest this
may be due to IFNα induced alteration of the balance between STAT1
and STAT3/ STAT5 favouring inhibition of proliferation and
subsequent apoptosis (37).
Cross-talking among the signalling pathways is complicated and
requires further investigations. Increased understanding of
cross-talking may provide the rationale for combination therapy
using STAT3 inhibitor and other drugs.
Constitutive activation of STAT3 has been reported
at high frequency in large numbers of malignant cell lines, in
vivo animal experiments and human tumours (35,38,39).
Table I includes a broad range of
pre-clinical studies, which show that (activated)
phosphorylated-STAT3 (pSTAT3) is a common characteristic of many
cancers. Constitutive activation of STAT3 involves multiple
signalling pathways in both a cell- and tissue-specific manner,
making single upstream pathway treatment difficult. So far, there
has been no STAT3 gene mutation detected in any cancer. Persistent
activation of STAT3 has been attributed to dysregulation of
upstream tyrosine kinases and negative regulators in the STAT3
signalling pathway (20,40).
Activated STAT3 regulates many genes whose
expression is required in aspects of cancer initiation, development
and progression, including uncontrollable proliferation,
anti-apoptosis, invasion, angiogenesis and immune surveillance
evasion. STAT3 signalling has been implicated in the up-regulation
of cell proliferation by cylin D2 and c-Myc (20,34,41–43).
In addition, pSTAT3 contributes to malignancy by preventing
apoptosis, allowing accumulation of long-lived tumour cells and
mediating chemoresistance via increased expression of
anti-apoptotic genes such as Bcl-2 family member Bcl-XL
and Mcl-2 as well as survivin (43–49).
Recently, miRNA-21 has been proved to be STAT3 target gene, which
functions as an inhibitor of apoptosis in multiple myeloma and
Sézary cells (50,51). Increased VEGF expression in
cultured cell lines, animal models and patient cancer specimens, as
well as tumour angiogenesis in vivo is also induced via
STAT3 pathway in diverse human cancers from head, neck, breast,
pancreas, cervix as well as melanoma (27,52-54).
Over-expression of matrix metalloproteinase-2 (MMP-2) in melanoma
and MMP-9 in breast cancer is attributed to elevated STAT3 activity
(55,56). STAT3, which may also be activated
in tumour-infiltrating immune cells, can inhibit tumour immune
function by promoting the expression of immune suppressive factors
and inhibiting the product of pro-inflammatory mediators (39,57–60).
As a class the targeted therapies that inhibit
specific biologic pathways represent a novel therapeutic strategy
either as single agents or in combination with conventional
chemotherapeutics in treating a variety of malignancies. However,
patient response has been less than expected and early development
of resistance has been a major issue (65). The identification of novel
alternative signalling pathways represents one resistance
mechanism. For example one recent study demonstrated that EGFR and
IGFR signal through JAK/STAT pathway, in addition to the two
classical pathways of ras-raf-MEK-ERK and PI3K-Akt (66). Indeed STAT3 is a common alternative
pathway for many growth promoting factors effectively bypassing a
single tyrosine kinase inhibitor acting at an early point in the
receptor activation pathway. For example, owing to the high level
of IL-6 in NSCLC, EGFR inhibitor was ineffective due to ongoing
STAT3 activation (13). STAT3 is a
more downstream point of convergence in many ligand/receptor
pathways (such as growth factor and cytokine receptors) and
non-receptor tyrosine kinase pathways (such as Src) and
consequently cross-talk among these signalling pathways may
contribute to resistance to EGFR inhibitors. In addition STAT3 acts
as a nuclear transcription factor (41) upregulating genes of cell survival
and proliferation. Its inhibition represents a promising target for
improving targeted cancer treatment in a number of preclinical
studies. Furthermore, considering its late position in activation
pathways, its inhibition may be less likely to be overcome through
an alternative pathway.
Since STAT3 is involved in regulating fundamental
biological processes and pSTAT3 contributes to malignant
transformation and progression, targeting STAT3 signalling appears
to be a novel approach to preventing and treating cancers. Several
strategies are being developed to target the STAT3 signalling
pathway.
Inhibiting the upstream signals or enhancing
negative regulators of STAT3 signalling pathway is one possible
strategy.
i) Inhibition of STAT3 signalling via targeting the
activation of cytokine and growth factor receptors with monoclonal
antibodies or receptor antagonists: for example, the use of IL-6
receptor super-antagonists, such as Sant7, inhibited IL-6-dependent
human myeloma cell growth (IC50 = 0.16 nM), as well as
induced cell death as a pro-apoptotic factor via STAT3 signalling
(67,68).
ii) Inhibition of upstream tyrosine kinases JAK or
Src with small molecule inhibitors such as AG490, INCB20, PD180970
and Dasatinib: for example AG490, a JAK2-specific inhibitor,
blocked constitutive activation of STAT3 causing a significant
reduction of Bcl-XL mRNA expression and induced cell
apoptosis in certain human myeloma and prostate cancer cell lines
(69). Pan-JAK inhibitor INCB20
was demonstrated to block STAT3 phosphorylation, induce apoptosis
and inhibit human multiple myeloma cell growth with an
IC50 of less than 1 μM, as well as dramatically
delay tumour growth in subcutaneous xenograft model in mice
(26). Dasatinib (BMS-354825),
which inhibits Src tyrosine kinase activity, has shown an
anti-tumour effect on head and neck squamous cell carcinoma and
non-small cell lung cancer cells with low IC50 values
in vitro (70).
iii) Enhancement of negative regulators: a number of
pathways that negatively regulate STAT3 have been identified. These
include suppressors of cytokine signalling (SOCS) family proteins
which are transcriptionally regulated by activated STAT and form a
negative feedback loop to suppress STAT signal by binding to or
inhibiting JAKs or by targeting bound proteins to the proteasome
degradation pathway. Other negative regulators include: protein
inhibitor of activated STAT (PIAS), various tyrosine phosphatises
(SHP1, SHP2, CD45), phosphatase and tensin homolog (PTEN), GRIM-19
(gene associated with retinoid IFN induced mortality-19) as well as
the ubiquitin-proteasome degradation pathway involved in negative
regulation of STAT signalling (71–81).
Recently, neurofibromatosis 2 (NF2) tumour suppressor, schwannomin
was also demonstrated to inhibit STAT3 phosphorylation (82).
However, although these are all theoretically
attractive, any attempt to activate these pathways as a means of
downregulating activated STAT3 is problematic owing to redundancy
of upstream proteins, meaning that the STAT3 pathway might not be
effectively blocked by a single compound. Furthermore, these
compounds might inhibit other downstream targets, which the STAT3
signalling are cross-talking with (39,83)
and consequently cause undesirable side effects.
Another approach for STAT3 inhibition is to affect
translation of STAT3 mRNA by coding RNA interference, such as
domain-negative (DN) STAT3 mutants (STAT3β, STAT3D or STAT3F),
anti-sense STAT3 oligonucleotides or small interfering RNA (siRNA).
Several groups reported that this strategy can inhibit cellular
growth and induce apoptosis in multiple human cancer cells in
vitro and in vivo, accompanied with down-regulation of
STAT3 target genes. Table III
lists some RNAs used in preclinical studies, which target STAT3
mRNA.
Recently, microRNAs, small non-coding RNA molecules,
which act as post-transcriptional regulators have also become the
focus of research on regulation of gene activity. MicroRNA binds to
the 3′ untranslated regions (3′ UTRs) of target mRNA in RNA induced
silencing complexes (RISC), and negatively regulates gene
expression through transcript destabilization and translational
attenuation (84). A variety of
microRNAs are linked with cancer initiation and development as
tumour suppressors or oncogenes (also called oncomirs) (85–90).
Several microRNAs can regulate STAT3 protein expression in
embryonic development and cellular differentiation (91,92).
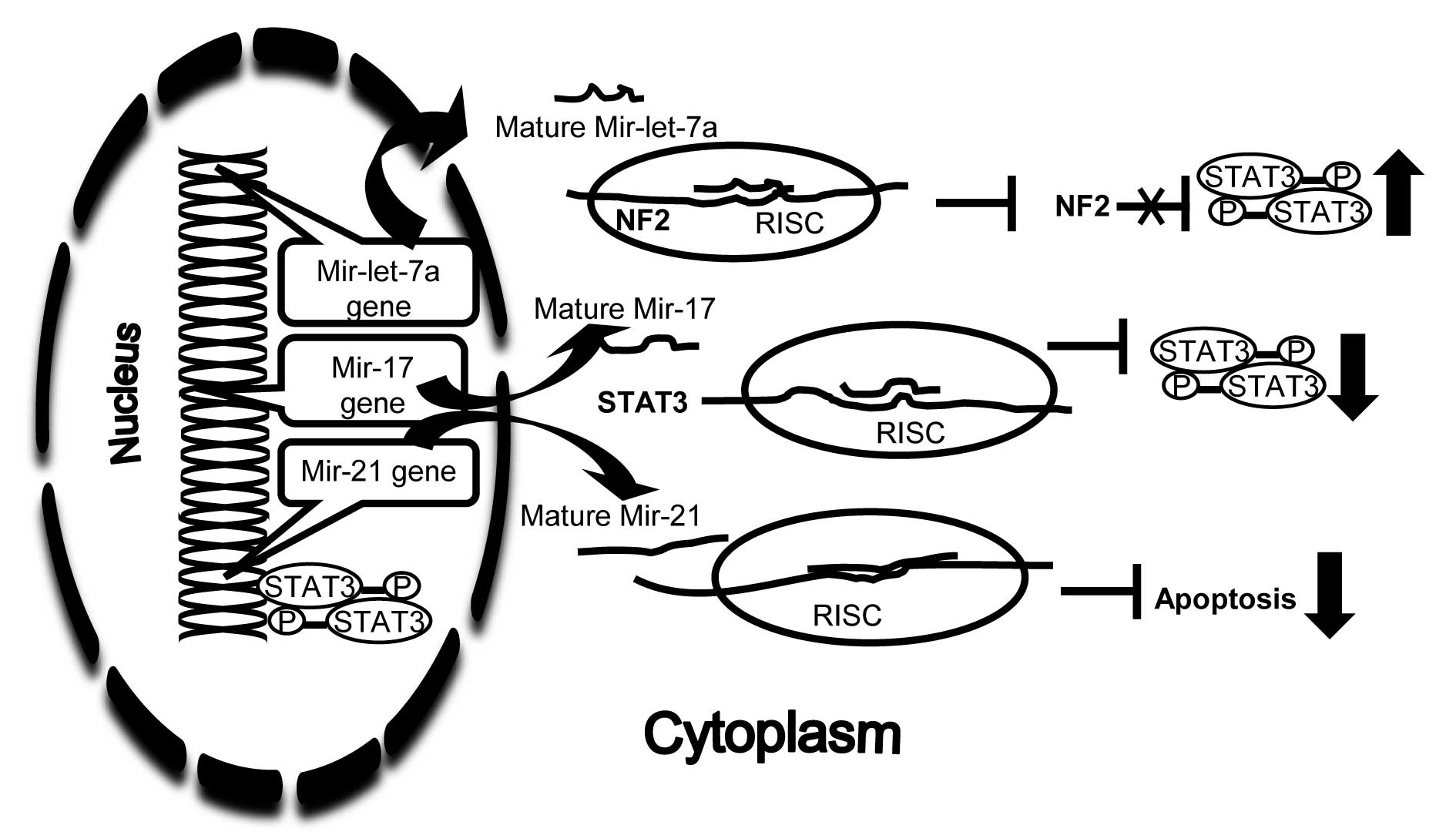
However, in tumour-related studies, only two microRNAs, mir-17 and
mir-let-7a are identified to regulate STAT3 to date (Fig. 1). The Mir-17 family, as a tumour
suppressor, directly regulates STAT3 mRNA expression through
binding to and silencing STAT3 3′ UTRs in a breast cancer cell line
(MDA-MB-231) (91). Mir-let-7a, as
an oncogene, indirectly modulates STAT3 phosphorylation in
malignant human cholangiocytes (93). Neurofibromatosis 2 (NF2), a
negative regulator of STAT3 phosphorylation, is a direct target
gene of mir-let-7a. As such, let-7a microRNA contributes to
elevated STAT3 phosphorylation. Thus, microRNA (anti-let 7a and
Mir-17 mimic) which blocks the oncogene or replace the lost
suppressor function have the potential for an anti-tumour
effect.
Importantly, such gene therapy displayed anti-tumour
bystander effects, in which adjacent tumour cells that did not
receive the gene therapy also underwent apoptosis (94,95).
For example, gene therapy using STAT3β RNA blockade caused the
tumour regression seen with massive apoptosis of both transfected
and bystander tumour cells.
However, more new techniques will be needed to
overcome the small RNA delivery limitations and subsequent immune
response in patients (84,96). Some other major problems of RNA
delivery include rapid excretion by kidney, degradation in
extracellular fluid and permeability to the cell membrane.
Nevertheless, approaches using different delivery agents (liposome,
nanoparticle and LNA oligonucleotide) have entered preclinical and
phase I clinical trials (97,98).
The best approach may be to inhibit STAT3 protein
directly. The three domains of STAT3: NH2-terminal, DNA-binding and
SH2 were identified as selective targets for development of STAT3
inhibitors based on the understanding of the structure and function
of STAT3 (99). The most popular
target is the SH2 domain, since it is necessary for STAT3
recruiting to any activated receptor. In addition, inhibition of
this target can block STAT3 dimerization and consequently inhibit
nuclear translocation and STAT3-dependent gene regulation. So far,
three categories of STAT3 SH2 inhibitors have been developed
including peptides, peptidomimics and small molecule inhibitors.
Several phosphotyrosyl peptides (PpYLKTK), tripeptides (PpYL, ApYL)
and peptidomimetics (ISS610) were demonstrated to inhibit STAT3
dimerization, cell proliferation and gene regulation in
vitro. PpYLKTK, PpYL and ApYL, which are STAT3 SH2
domain-binding peptides, blocked STAT3 DNA-binding activity with an
IC50 of 235, 182 and 217 μM, respectively
(100). The most effective yet
reported ISS610 disrupted STAT3 dimerization, inhibited cell growth
and induced apoptosis, as well as reduced DNA-binding activity with
an IC50 of 42 μM in cells with pSTAT3 (101). However, poor cell permeability
and in vivo stability is the limitation to applying
peptide-based inhibitors to clinical trials (83).
Multiple novel small molecular inhibitors targeting
STAT3 SH2 domain were identified and designed through
structure-based high-throughout virtual screening and have been
demonstrated to inhibit STAT3 dimerization and DNA-binding
activity, as well as inhibit cell proliferation and tumour growth
in cultured cancer cell lines and in animal models respectively.
Table IV summarizes the inhibitors
reported to target SH2 domain, as well as the related mechanism
studies, including STA-21 and analogue, Stattic, S3I-201 (NSC
74859) and analogue, S3I-M2001, 5,15-DPP, LLL12 and STX-0119.
S3I-201 selectively inhibited STAT3 DNA-binding activity, STAT3
dimerization and transcriptional activity, as well as induced
growth inhibition and apoptosis in cells with persistent pSTAT3
(102,103). Moreover, in both human breast and
hepatocellular cancer xenografts in nude mouse model, S3I-201
significantly inhibited the tumour growth with a dose of 5 mg/kg.
OPB-31121, another small molecule inhibitor, inhibits cell
proliferation with low IC50 in a number of hematopoietic
malignancy cell lines through direct blockage of STAT3
phosphorylation in preclinical studies (104). OPB-31121 has been used in
clinical trials and is introduced below in the clinical study
section.
Collectively, small molecule inhibitors targeting
STAT3 directly are the most promising of the possible therapeutic
strategies because: i) modulation of upstream regulators by single
targeted therapy may not completely block the STAT3 pathway due to
multiple regulators and cross-talking among pathways; ii) RNA
inhibition requires yet to be identified improved techniques to
deal with the delivery issues; iii) the preclinical studies
demonstrate that small molecule inhibitors strongly inhibited the
growth both of tumour cells in vitro and xenografts in
animal models with a low IC50 encouraging clinical
development and reducing the risk of development of resistance.
In addition, synergy may be expected with many other
inhibitors, since STAT3 is a complementary pathway for many growth
factor pathways. The combination therapy of STAT3 and EGFR
inhibitors synergistically and significantly suppressed cancer cell
growth and down-regulated activated STAT3 expression in high-grade
gliomas and pancreatic cancers (41,105). Furthermore, STAT3 inhibition
increased the sensitivity of cancer cells to chemotherapeutic
agents such as taxol and cisplatin (105,106). Therefore, STAT3 is an attractive
target and some new studies on STAT3 mono-therapy and combination
therapy are ongoing.
There are several phase 0/I clinical trials of STAT3
inhibition ongoing or not yet reported (Table V). OPB-31121, which inhibits STAT3
phosphorylation, but did not affect JAK kinase, displayed strong
anti-proliferation effect in cancer cell lines and in mouse model
in vivo studies (104).
Preliminary data are currently only available from one Korean study
reported in the Annual Meeting of the American Society of Clinical
Oncology (107) in 21 patients
with advanced refractory solid tumours treated by OPB-31121. This
showed that toxicities were predominantly grade 1 or 2, with the
dose-limiting toxicities (DLT) of grade 3 vomiting and diarrhoea.
The MTD has not been determined within the designated dose range.
Eight out of 17 patients had the stable disease, with one case more
than 12 months. It seems that this oral STAT3 inhibitor is safe and
tolerable. More data from different clinical trials of different
phases using this kind of drug are required before a clear
conclusion can be made.
STAT3 play a pivotal role in the initiation,
development and progression of cancers, including proliferation,
anti-apoptosis, invasion, angiogenesis and immune surveillance
evasion. Constitutively activated STAT3 is associated with a wide
range of human cancers. As such, STAT3 has been identified as a
novel target to treat and prevent cancers with a broad potential
application. Several STAT3 inhibitors display anti-tumour
effectiveness in preclinical studies. The data for clinical trials
using STAT3 inhibitors are emerging. Targeting STAT3 using a
specific inhibitor may be a useful cancer treatment approach, with
the potential for a broad clinical impact.
|
1
|
Darnell JE: Transcription factors as
targets for cancer therapy. Nat Rev Cancer. 2:740–749. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Darnell JE: STATs and gene regulation.
Science. 277:1630–1635. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bromberg J and Darnell JE: The role of
STATs in transcriptional control and their impact on cellular
function. Oncogene. 19:2468–2473. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ihle JN: STATs: signal transducers and
activators of transcription. Cell. 84:331–334. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Herrington J, Smit LS, Schwartz J and
Carter-Su C: The role of STAT proteins in growth hormone signaling.
Oncogene. 19:2585–2597. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alvarez JV, Greulich H, Sellers WR,
Meyerson M and Frank DA: Signal transducer and activator of
transcription 3 is required for the oncogenic effects of
non-small-cell lung cancer-associated mutations of the epidermal
growth factor receptor. Cancer Res. 66:3162–3168. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yin W, Cheepala S, Roberts JN, Syson-Chan
K, DiGiovanni J and Clifford JL: Active Stat3 is required for
survival of human squamous cell carcimoma cells in serum-free
conditions. Mol Cancer. 5:152006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kusaba T, Nakayama T, Yamazumi K, et al:
Activation of STAT3 is a marker of poor prognosis in human
colorectal cancer. Oncol Rep. 15:1445–1451. 2006.PubMed/NCBI
|
|
9
|
Morikawa T, Baba Y, Yamauchi M, et al:
STAT3 expression, molecular features, inflammation patterns, and
prognosis in a database of 724 colorectal cancers. Clin Cancer Res.
17:1452–1462. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
de la Iglesia N, Konopka G, Lim KL, et al:
Deregulation of a STAT3-interleukin 8 signaling pathway promotes
human glioblastoma cell proliferation and invasiveness. J Neurosci.
28:5870–5878. 2008.PubMed/NCBI
|
|
11
|
de la Iglesia N, Konopka G, Puram SV, et
al: Identification of a PTEN-regulated STAT3 brain tumor suppressor
pathway. Genes Dev. 22:449–462. 2008.PubMed/NCBI
|
|
12
|
Copeland NG, Gilbert DJ, Schindler C, et
al: Distribution of the mammalian Stat gene family in mouse
chromosomes. Genomics. 29:225–228. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lai SY and Johnson FM: Defining the role
of the JAK-STAT pathway in head and neck and thoracic malignancies:
implications for future therapeutic approaches. Drug Resist Updat.
13:67–78. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Quesnelle KM, Boehm AL and Grandis JR:
STAT-mediated EGFR signaling in cancer. J Cell Biochem.
102:311–319. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao L, Zhang L, Hu J, et al:
Down-regulation of signal transducer and activator of transcription
3 expression using vector-based small interfering RNAs suppresses
growth of human prostate tumor in vivo. Clin Cancer Res.
11:6333–6341. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kisseleva T, Bhattacharya S, Braunstein J
and Schindler CW: Sigaling through the JAK-STAT pathway, recent
advances and future challenges. Gene. 285:1–24. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aggarwal BB, Kunnumakkara AB, Harikumar
KB, et al: Signal tansducer and activator of transcription-3,
inflammation, and cancer. Ann NY Acad Sci. 1171:59–76. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pawson T, Gish GD and Nash P: SH2 domains,
interaction modules and cellular wiring. Trends Cell Biol.
11:504–511. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhong Z, Wen Z and Darnell JE: Stat3, a
STAT family member activated by tyrosine phosphorylation in
response to epidermal growth factor and interleukin-6. Science.
264:95–98. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Leeman R, Lui VWY and Grandis JR: STAT3 as
a therapeutic target in head and neck cancer. Expert Opin Biol
Ther. 6:231–241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Takeda K, Noguchi K, Shi W, et al:
Targeted disruption of the mouse Stat3 gene leads to early
embryonic lethality. Proc Natl Acad Sci USA. 94:3801–3804. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang XO, Panopoulos AD, Nurieva R, et al:
STAT3 regulates cytokine-mediated generation of inflammatory helper
T cells. J Biol Chem. 282:9358–9363. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miyoshi K, Takaishi M, Nakajima K, et al:
Stat3 as a therapeutic target for the treatment of psoriasis: a
clinical feasibility study with STA-21, a Stat3 inhibitor. J Invest
Dermatol. 131:108–117. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang HY, Zhang Q, Zhang X, et al:
Cancer-related inflammation and Barrett’s carcinogenesis:
interleukin-6 and STAT3 mediate apoptotic resistance in transformed
Barrett’s cells. Am J Physiol Gastrointest Liver Physiol.
300:G454–G460. 2011.
|
|
25
|
Mora LB, Buettner R, Seigne J, et al:
Constitutive activation of Atat3 in human prostate tumors and cell
lines: direct inhibition of Stat3 signaling induces apoptosis of
prostate cancer cells. Cancer Res. 62:6659–6666. 2002.PubMed/NCBI
|
|
26
|
Burger R, Le Gouill S, Tai Y-T, et al:
Janus kinase inhibitor INCB20 has antiproliferative and apoptotic
effects on human myeloma cells in vitro and in vivo. Mol Cancer
Ther. 8:26–35. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wei L-H, Kuo M-L, Chen C-A, et al:
Interleukin-6 promotes cervical tumor growth by VEGF-dependent
angiogenesis via a STAT3 pathway. Oncogene. 22:1517–1527. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lopiccolo J, Blumenthal G, Bernstein WB
and Dennis PA: Targeting the PI3K/Akt/mTOR pathway: effective
combinations and clinical considerations. Drug Resist Updat.
11:32–50. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sasse J, Hemmann U, Schwartz C, et al:
Mutational analysis of acute-phase response factor/Stat3 activation
and dimerization. Mol Cell Biol. 17:4677–4686. 1997.PubMed/NCBI
|
|
30
|
Shuai K, Horvath CM, Huang LHT, Qureshi S,
Cowburn D and Darnell JE: Interferon activation of the
transcription factor Stat91 involves dimerization through
SH2-phosphotyrosyl peptide interactions. Cell. 76:821–828. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fletcher S and Gunning PT: Mild, efficient
and rapid O-debenzylation of ortho-substituted phenols with
trifluoroacetic acid. Tetrahedron Lett. 49:4817–4819. 2008.
View Article : Google Scholar
|
|
32
|
Yu C-L, Meyer DJ, Campbell GS, et al:
Enhanced DNA-binding activity of a Stat3-related protein in cells
transformed by the Src oncoprotein. Science. 269:81–83. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Smithgall TE, Briggs SD, Schreiner S,
Lerner EC, Cheng H and Wilson MB: Control of myeloid
differentiation and survial by Stats. Oncogene. 19:2612–2618. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bowman T, Garcia R, Turkson J and Jove R:
STATs in oncogenesis. Oncogene. 19:2474–2488. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Catlett-Falcone R, Dalton WS and Jove R:
STAT proteins as novel targets for cancer therapy. Curr Opin Oncol.
11:490–496. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Inamura K, Matsuzaki Y, Uematsu N, Hondab
A, Tanaka N and Uchida K: Rapid inhibition of MAPK signaling and
anti-proliferation effect via JAK/STAT signaling by
interferon-alpha in hepatocellular carcinoma cell lines. Biochim
Biophys Acta. 1745:401–410. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang JL, Zahorowska B, Kasim Y, Goldstein
D and Crowe P: Mechanism of the synergistic antiproliferative
effect of gefitinib and interferon-alpha in sarcoma cell lines.
Proc 101st Ann Meet AACR. 398–399:(abs. 1655). 2010.
|
|
38
|
Turkson J and Jove R: STAT proteins: novel
molecular targets for cancer drug discovery. Oncogene.
19:6613–6626. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yu H and Jove R: The STATs of cancer - new
molecular targets come of age. Nat Rev Cancer. 4:97–105. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Aggarwal BB, Sethi G, Ahn KS, et al:
Targeting signal-transducer-and-activator-of-transcription-3 for
prevention and therapy of cancer. Ann NY Acad Sci. 1091:151–169.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jaganathan S, Yue P, Paladino DC,
Bogdanovic J, Huo Q and Turkson J: A functional nuclear epidermal
growth factor receptor, Src and Stat3 heteromeric complex in
pancreatic cancer cells. PLoS One. 6:e196052011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Arany I, Chen S-H, Megyesi JK, et al:
Differentiation-dependent expression of signal transducers and
activators of transcription (STATs) might modify responses to
growth factors in the cancers of the head and neck. Cancer Lett.
199:83–89. 2003. View Article : Google Scholar
|
|
43
|
Masuda M, Suzui M, Yasumatu R, et al:
Constitutive activation of signal transducers and activators of
transcription 3 correlates with cyclin D1 overexpression and may
provide a novel prognostic marker in head and neck squamous cell
carcinoma. Cancer Res. 62:3351–3355. 2002.PubMed/NCBI
|
|
44
|
Chun K-S and Langenbach R: The
prostaglandin E2 receptor, EP2, regulates survivin expression via
an EGFR/STAT3 pathway in UVB-exposed mouse skin. Mol Carcinog.
50:439–448. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Grandis JR, Drenning SD, Zeng Q, et al:
Contitutive activation of Stat3 signaling abrogates apoptosis in
squamous cell carcinogenesis in vivo. Proc Natl Acad Sci USA.
97:4227–4232. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kijima T, Niwa H, Steinman RA, et al:
STAT3 activation abrogates growth factor dependence and contributes
to head and neck squamous cell carcinoma tumor growth in vivo. Cell
Growth Differ. 13:355–362. 2002.PubMed/NCBI
|
|
47
|
Huang M, Page C, Reynolds K and Lin J:
Constitutive activation of Stat 3 oncogene product in human ovarian
carcinoma cells. Gynecol Oncol. 79:67–73. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Niu G, Bowman T, HUang M, et al: Roles of
activated Src and Stat3 signaling in melanoma tumor cell growth.
Oncogene. 21:7001–7010. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen CL, Hsieh FC, Lieblein JC, et al:
Stat3 activation in human endometrial and cervical cancers. Br J
Cancer. 96:591–599. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Van der Fits L, van Kester MS, Qin Y, et
al: MicroRNA-21 expression in CD4+ T cells is regulated
by STAT3 and is pathologically involved in Sézary Syndrome. J
Invest Dermatol. 131:762–768. 2010.PubMed/NCBI
|
|
51
|
Loffler D, Brocke-Heidrich K, Pfeifer G,
et al: Interleukin-6 dependent survival of multiple myeloma cells
involves the Stat3-mediated induction of microRNA-21 through a
highly conserved enhancer. Blood. 110:1330–1333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ahluwalia A, Busse BA, Thiruvengadam SS
and Tarnawski AS: Importins are critical for colorectal cancer
(CRC) growth a nd are novel biomarkers of CRC. Underlying
mechanisms include: increased nuclear transport of P-CREB and
p-STAT3, VEGF gene promoter activation and aberrant VEGF
expression. Gastroenterology. 140:S1842011.
|
|
53
|
Wei D, Le X, Zheng L, et al: Stat3
activation regulates the expression of vascular endothelial growth
factor and human pancreatic cancer angiogenesis and metastasis.
Oncogene. 22:319–329. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Xu Q, Briggs J, Park S, et al: Targeting
Stat3 blocks both HIF-1 and VEGF expression induced by multiple
oncogenic growth signaling pathways. Oncogene. 24:5552–5560. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Xie T-X, Wei D, Liu M, et al: Stat3
activation regulates the expression of matrix metalloproteinase-2
and tumor invasion and metastasis. Oncogene. 23:3550–3560. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Dechow TN, Pedranzini L, Leitch A, et al:
Requirement of matrix metalloproteinase-9 for the transformation of
human mammary epithelial cells by Stat3-C. Proc Natl Acad Sci USA.
101:10602–10607. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yu H, Kortylewski M and Pardoll D:
Crosstalk between cancer and immune cells: role of STAT3 in the
tumour microenvironment. Immunology. 7:41–51. 2007.PubMed/NCBI
|
|
58
|
Kortylewski M, Kujawski M, Wang T, et al:
Inhibiting Stat3 signaling in the hematopoietic system elicits
multicomponent antitumor immunity. Nat Med. 11:1314–1321. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kortylewski M and Yu H: Role of Stat3 in
suppressing anti-tumor immunity. Curr Opin Immunol. 20:228–233.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wang T, Niu G, Kortylewski M, et al:
Regulation of the innate and adaptive immune responses by Stat-3
signaling in tumor cells. Nat Med. 10:48–54. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Garcia R, Bowman TL, Niu G, et al:
Constitutive activation of STAT3 by the Src and JAK tyrosine
kinases participates in growth regulation of human breast carcinoma
cells. Oncogene. 20:2499–2513. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Nagpal JK, Mishra R and Das BR: Activation
of Stat-3 as one of the early events in tobacco chewing-mediated
oral carcinogenesis. Cancer. 94:2393–2400. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Deng J-Y, Sun D, Liu X-Y, Pan Y and Liang
H: STAT-3 correlates with lymph node metastasis and cell survival
in gastric cancer. World J Gastroenterol. 16:5380–5387. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Watson CJ and Miller WR: Elevated levels
of members of the STAT family of transcription factors in breast
carcinoma nuclear extracts. Br J Cancer. 71:840–844. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kosaka T, Yamaki E, Mogi A and Kuwano H:
Mechanisms of resistance to EGFR TKIs and development of a new
generation of drugs in non-small-cell lung cancer. J Biomed
Biotechnol. 2011:1–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Himpe E and Kooijman R: Insulin-like
growth factor-I receptor signal transduction and the Janus
kinase/signal transducer and activator of transcription (JAK-STAT)
pathway. Biofactors. 35:76–81. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Sporeno E, Savino R, Ciapponi L, et al:
Human interleukin-6 receptor super-antagonists with high potency
and wide spectrum on multiple myeloma cells. Blood. 87:4510–4519.
1996.PubMed/NCBI
|
|
68
|
Demartis A, Bernassola F, Savino R, Melino
G and Ciliberto G: Interleukin 6 receptor superantagonists are
potent inducers of human multiple. Cancer Res. 56:4213–4218.
1996.PubMed/NCBI
|
|
69
|
Catlett-Falcone R, Landowski TH, Oshiro
MM, et al: Constitutive activation of Stat3 signaling confers
resistance to apoptosis in human U266 myeloma cells. Immunity.
10:105–115. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Johnson FM, Saigal B, Talpaz M and Donato
NJ: Dasatinib (BMS-354825) tyrosine kinase inhibitor suppresses
invasion and induces cell cycle arrest and apoptosis of head and
neck squamous cell carcinoma and non-small cell lung cancer cells.
Clin Cancer Res. 11:6924–6932. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Perry E, Tsruya R, Levitsky P, et al:
TMF/ARA160 is a BC-box-containing protein that mediates the
degradation of Stat3. Oncogene. 23:8908–8919. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Gu Q, Kong Y, Yu Z-B, Bai L and Xiao Y-B:
Hypoxia-induced SOCS3 is limiting STAT3 phosphorylation and NF-κB
activation in congenital heart disease. Biochimie. 93:909–920.
2011.PubMed/NCBI
|
|
73
|
Ulane CM, Kentsis A, Cruz CD, Parisien
J-P, Schneider KL and Horvath CM: Composition and assembly of
STAT-targeting ubiquitin ligase complexes: paramyxovirus V protein
carboxyl terminus is an oligomerization domain. J Virol.
79:10180–10189. 2005. View Article : Google Scholar
|
|
74
|
Lesina M, Kurkowski Magdalena U, Ludes K,
et al: Stat3/Socs3 activation by IL-6 transsignaling promotes
progression of pancreatic intraepithelial neoplasia and development
of pancreatic Cancer. Cancer Cell. 19:456–469. 2011. View Article : Google Scholar
|
|
75
|
Lindemann C, Hackmann O, Delic S, Schmidt
N, Reifenberger G and Riemenschneider MJ: SOCS3 promoter
methylation methylation is mutually exclusive to EGFR amplification
in gliomas and promotes glioma cell invasion through STAT3 and FAK
activation. Acta Neuropathol. 122:241–251. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Kluge A, Dabir S, Vlassenbroeck I,
Eisenberg R and Dowlati A: Protein inhibitor of activated STAT3
expression in lung cancer. Mol Oncol. 5:256–264. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Migone T-S, Cacalano NA, Taylor N, Yi T,
Waldmann TA and Johnston JA: Recruitment of SH2-containing protein
tyrosine phosphatase SHP-1 to the interleukin 2 recepto; loss of
SHP-1 expression in human T-lymphotropic virus type I-transformed T
cells. Proc Natl Acad Sci USA. 95:3845–3850. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Schaper F, Gendo C, ECK M, et al:
Activation of the protein tyrosine phosphatease SHP2 via the
interleukin-6 signal transducing receptor protein gp130 requires
tyrosine Jak1 and limits acute-phase protein expression. Biochem J.
355:557–565. 1998.
|
|
79
|
Irie-Sasaki J, Sasaki T, Matsumoto W, et
al: CD45 is a JAK phosphatase and negatively regulates cytokine
recptor signalling. Nature. 409:349–354. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Sun S and Steinberg BM: PTEN is a negative
regualtor of STAT3 activation in human papillomavirus-infected
cells. J Gen Virol. 83:1651–1658. 2002.PubMed/NCBI
|
|
81
|
Desrivières S, Kunz C, Barash I,
Vafaizadeh V, Borghouts C and Groner B: The biological functions of
the versatile transcription factors STAT3 and STAT5 and new
strategies for their targeted inhibition. J Mammary Gland Biol
Neoplasia. 11:75–87. 2006.PubMed/NCBI
|
|
82
|
Scoles DR, Nguyen VD, Qin Y, et al:
Neurofibromatosis 2 (NF2) tumor suppressor schwannomin and its
interacting protein HRS regulate STAT singling. Hum Mol Genet.
11:3179–3189. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Bhasin D, Cisek K, Pandharkar T, et al:
Design, synthesis, and studies of small molecule STAT3 inhibitors.
Bioorg Med Chem Lett. 18:391–395. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Esquela-Kerscher A and Slack FJ: Oncomirs
- microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar
|
|
85
|
Akao Y, Nakagawa Y and Naoe T: let-7
MicroRNA functions as a potential grwoth suppressor in human colon
cancer cells. Biol Pharm Bull. 29:903–906. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Chan JA, Krichevsky AM and Kosik KS:
MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells.
Cancer Res. 65:6029–6033. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Takamizawa J, Konishi H, Yanagisawa K, et
al: Reduced expression of the let-7 microRNAs in human lung cancers
in association with shortened postoperative survival. Cancer Res.
64:3753–3756. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Yanaihara N, Caplen N, Bowman E, et al:
Unique microRNA molecular profiles in lung cancer diagnosis and
prognosis. Cancer Cell. 9:189–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Johnson SM, Grosshans H, Shingara J, et
al: RAS is regulated by the let-7 microRNA family. Cell.
120:635–647. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
He L, Thomson JM, Hemann MT, et al: A
microRNA polycistron as a potential human oncogene. Nature.
435:828–833. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Foshay KM and Gallicano GI: miR-17 family
miRNAs are expressed during early mammalian development and
regulate stem cell differentiation. Dev Biol. 326:431–443. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Krichevsky AM, Sonntag K-C, Isacson O and
Kosik KS: Specific microRNAs modulate embryonic stem cell-derived
neurogenesis. Stem Cells. 24:857–864. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Meng F, Henson R, Wehbe-Janek H, Smith H,
Ueno Y and Patel T: The microRNA let-7a modulates
interleukin-6-dependent STAT-3 survival signaling in malignant
human cholangiocytes. J Biol Chem. 282:8256–8264. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Niu G, Heller R, Catlett-Falcone R, et al:
Gene therapy with dominant-negative Stat3 suprresses growth of the
murine melanoma B16 tumor in vivo. Cancer Res. 59:5059–5063.
1999.PubMed/NCBI
|
|
95
|
Niu G, Shain KH, Huang M, et al:
Overexpression of a dominant-negative signal transducer and
activator of trnascription 3 varian in tumor cells leads to
production of soluble factors that induce apoptosis and cell cycle
arrest. Cancer Res. 61:3276–3280. 2001.PubMed/NCBI
|
|
96
|
Mishra PJ and Merlino G: MicroRNA
reexpression as differentiation therapy in cancer. J Clin Invest.
119:2119–2123. 2009.PubMed/NCBI
|
|
97
|
Liang Z-W, Guo B-F, Li Y, et al:
Plasmid-based Stat3 siRNA delivered by hydroxyapatite nanoparticles
suppresses mouse prostate tumour growth in vivo. Asian J Androl.
13:481–486. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Petrocca F and Lieberman J: Promise and
chanllenge of RNA interference-based therapy for cancer. J Clin
Oncol. 29:747–754. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Fletcher S, Drewry JA, Shahani VM, Page
BDG and Gunning PT: Molecular disruption of oncogenic signal
transducer and activator of transcription 3 (STAT3) protein.
Biochem Cell Biol. 87:825–833. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Turkson J, Ryan D, Kim JS, et al:
Phosphotyrosyl peptides block Stat3-mediated DNA binding activity,
gene regulation, and cell transformation. J Biol Chem.
276:45443–45455. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Turkson J, Kim JS, Zhang S, et al: Novel
peptidomimetic inhibitors of signal transducer and activator of
transcription 3 dimerization and biological activity. Mol Cancer
Ther. 3:261–269. 2004.PubMed/NCBI
|
|
102
|
Siddiquee K, Zhang S, Guida WC, et al:
Selective chemical probe inhibitor of Stat3, identified through
structure-based virtual screening, induces antitumor activity. Proc
Natl Acad Sci USA. 104:7391–7396. 2007. View Article : Google Scholar
|
|
103
|
Lin L, Amin R, Gallicano GI, et al: The
STAT3 inhibitor NSC 74859 is effective in hepatocellular cancers
with disrupted TGF-β signaling. Oncogene. 28:961–972.
2009.PubMed/NCBI
|
|
104
|
Hayakawa F, Sugimoto K, Kurahashi S, et
al: A novel direct STAT3 inhibitor OPB-31121 induces tumor-specific
growth inhibition in a wide4 range of hematopoietic malignacies and
effectively suppresses the chemotherapy resistant quiescent cell in
vivo. 52nd ASH Ann Meet & Expo III-56. 2010
|
|
105
|
Lo H-W, Cao X, Zhu H and Ali-Osman F:
Constitutively activated STAT3 frequently coexpresses with
epidermal growth factor receptor in high-grade gliomas and
targeting STAT3 sensitizes them to Iressa and akylators. Clin
Cancer Res. 14:6042–6054. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Real PJ, Sierra A, De Juan A, Segovia JC,
Lopez-Vega JM and Fernandez-Luna JL: Resistance to chemotherapy via
Stat3-dependent overexpression of Bcl-2 in metastatic breast cancer
cells. Oncogene. 21:7611–7618. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Oh D, Han S, Kim TM, et al: A phase I,
open-label, nonrandomized trial of OPB-31121, a STAT3 inhibitor, in
patients with advanced solid tumors. J Clin Oncol.
28:e130562010.
|
|
108
|
Sartor CI, Dziubinski ML, Yu C-L, Jove R
and Ethier SP: Role of epidermal growth factor receptor and STAT-3
activation in autonomous proliferation of SUM-102PT human breast
cancer cells. Cancer Res. 57:978–987. 1997.PubMed/NCBI
|
|
109
|
Garcia R, Yu C-L, Hudnall A, et al:
Constitutive activation of Stat3 in fibroblasts transformed by
diverse oncoproteins and in breast carcinoma cells. Cell Growth
Differ. 8:1267–1276. 1997.PubMed/NCBI
|
|
110
|
Song L, Turkson J, Karras JG, Jove R and
Haura EB: Activation of Stat3 by receptor tyrosine kinases and
cytokines regulates survival in human non-small cell carcinoma
cells. Oncogene. 22:4150–4165. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Haura EB, Zheng Z, Song L, Cantor A and
Bepler G: Activated epidermal growth factor receptor-Stat-3
signaling promotes tumor survival in vivo in non-small cell lung
cancer. Clin Cancer Res. 11:8288–8294. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Gao SP, Mark KG, Leslie K, et al:
Mutations in the EGFR kinase domain mediate STAT3 activation via
IL-6 production in human lung adenocarcinomas. J Clin Invest.
117:3846–3856. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Pfeiffer M, Hartmann T, Leick M, Catusse
J, Schmitt-Graeff A and Burger M: Alternative implication of CXCR4
in JAK2/STAT3 activation in small cell lung cancer. Br J Cancer.
100:1949–1956. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Quintanilla-Martinez L, Kremer M, Specht
K, et al: Analysis of signal transducer and activator of
transcription 3 (Stat3) pathway in multiple myeloma, Stat3
activation and cyclin D1 dysregulation are mutually exclusive
events. Am J Pathol. 162:1449–1461. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Chen C-L, Loy A, Cen L, et al: Signal
transducer and activator of transcription 3 is involved in cell
growth and survival of human rhabdomyosarcoma and osteosarcoma
cells. BMC Cancer. 7:1112007. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Grandis JR, Drenning SD, Chakraborty A, et
al: Requirement of Stat3 but not Stat1 activation for epidermal
growth factor receptor-mediated cell growth in vitro. J Clin
Invest. 102:1385–1392. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Grandis JR, Zeng Q and Drenning SD:
Epidermal growth factor receptor-mediated Stat3 singaling blocks
apoptosis in head and neck cancer. Laryngoscope. 110:868–874. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Sriuranpong V, Park JI, Amornphimoltham P,
Patel V, Nelkin BD and Gutkind JS: Epidermal growth factor
receptor-independent constitutive activation of STAT3 in head and
neck squamous cell carcimoma is mediated by the autocrine/paracrine
stimulation of the interleukin 6/gp130. Cancer Res. 63:2948–2956.
2003.
|
|
119
|
Ni Z, Lou W, Leman ES and Gao AC:
Inhibition of constitutively activated Stat3 signaling pathway
suppresses growth of prostate cancer cells. Cancer Res.
60:1225–1228. 2000.PubMed/NCBI
|
|
120
|
Barton BE, Murphy TF, Adem P, Watson RA,
Irwin RJ and Huang HF: IL-6 signaling by STAT3 participates in the
change from hyperplasia to neoplasia in NRP-152 and NRP-154 rat
prostatic epithelial cells. BMC Cancer. 1:192001. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
DeMiguel F, Lee SO, Lou W, et al: Stat3
enhances the growth of LNCaP human prostate cancer cells in intact
and castrated male nude mice. Prostate. 52:123–129. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Barton BE, Karras JG, Murphy TF, Barton A
and Huang HF-S: Signal transducer and activator of transcription 3
(STAT3) activation in prostate cancer: direct STAT3 inhibition
induces apoptosis in prostate cancer lines. Mol Cancer Ther.
3:11–20. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Sanchez A, Nagy P and Thorgeirsson SS:
STAT-3 activity in chemically-induced hepatocellular carcinoma. Eur
J Cancer. 39:2093–2098. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Dhir R, Ni Z, Lou W, De Miguel F, Grandis
JR and Gao AC: Stat3 activation in prostatic carcinomas. Prostate.
51:241–246. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Sachez-Ceja SG, Reyes-Maldonado E,
Vazquez-Manriquez ME, Lopez-Luna JJ, Belmont A and
Gutierrez-Castellanos S: Differential expression of STAT5 and
Bcl-xL, and high expression of Neu and STAT3 in
non-small-cell lung carcinoma. Lung Cancer. 54:163–168.
2006.PubMed/NCBI
|
|
126
|
David D, Rajappan LM, Balachandran K,
Thulaseedharan JV, Nair AS and Pillai RM: Prognostic significance
of STAT3 and phosphorylated STAT3 in human soft tissue tumors - a
clinicopathological analysis. J Exp Clin Cancer Res. 30:562011.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Niu G, Wright KL, Huang M, et al:
Constitutive STAT3 activity up-regulates VEGF expression and tumor
angiogenesis. Oncogene. 21:2000–2008. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Gritsko T, Williams A, Turkson J, et al:
Persistent activation of Stat3 signaling induces survivin gene
expression and confers resistance to apoptosis in human breast
cancer cells. Clin Cancer Res. 12:11–19. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Gao L, Li F, Dong B, et al: Inhibition of
STAT3 and ErbB2 suppresses tumor growth, enhances radiosensitivity,
and induces mitochondria-dependent apoptosis in glioma cells. Intl
J Radiat Oncol Biol Phys. 77:1223–1231. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Song H, Wang R, Wang S and Lin J: A
low-molecular-weight compound discovered through virtual database
screening inhibits Stat3 function in breast cancer cells. Proc Natl
Acad Sci USA. 102:4700–4705. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Fuh B, Sobo M, Cen L, et al: LLL-3
inhibits STAT3 activity, suppresses glioblastoma cell growth and
prolongs survival in a mouse glioblastoma model. Br J Cancer.
100:106–112. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Schust J, Sperl B, Hollis A, Mayer TU and
Berg T: Stattic: a small-molecule inhibitor of STAT3 activation and
dimerization. Chem Biol. 13:1235–1242. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Zhang X, Yue P, Fletcher S, Zhao W,
Gunning PT and Turkson J: A novel small-molecule disrupts Stat3 SH2
domain-phosphotyrosine interactions and Stat3-dependent tumor
processes. Biochem Pharmacol. 79:1398–1409. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Fletcher S, Singh J, Zhang X, et al:
Disruption of transcriptionally active Stat3 dimers with
non-phosphorylated, salicylic acid-based small molecules: potent in
vitro and tumor cell activities. Chem Biol Chem. 10:1959–1964.
2009. View Article : Google Scholar
|
|
135
|
Siddiquee KAZ, Gunning PT, Glenn M, et al:
An oxazole-based small-molecule Stat3 inhibitor modulates Stat3
stability and processing and incuces antitumor cell effects. ACS
Chem Biol. 2:787–798. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Uehara Y, Mochizuki M, Matsuno K, Haino T
and Asai A: Novel high-throughput screening system for identifying
STAT3-SH2 antagonists. Biochem Biophys Res Commun. 380:627–631.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Ball S, Li C, Li P-K and Lin J: The small
molecule, LLL12, inhibits STAT3 phosphorylation and induces
apoptosis in medulloblastoma and glioblastoma cells. PLoS One.
6:e188202011. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Lin L, Hutzen B, Li P-K, et al: A novel
small molecule, LLL12, inhibits STAT3 phosphorylation and activites
and exhibits potent growth-suppressive activity in human cancer
cells. Neoplasia. 12:39–50. 2010.PubMed/NCBI
|
|
139
|
Matsuno K, Masuda Y, Uehara Y, et al:
Identification of a new series of STAT3 inhibitors by virtual
screening. ACS Med Chem Lett. 1:371–375. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Ashizawa T, Miyata H, Ishii H, et al:
Antitumor activity of a novel small molecule STAT3 inhibitor
against a human lymphoma cell line with high STAT3 activation. Int
J Oncol. 38:1245–1252. 2011.PubMed/NCBI
|