Introduction
Cancer/testis (CT) antigens are immunogenic proteins
with expression restricted to the testis and a wide range of human
tumor types, eliciting both humoral and cellular immune responses
in cancer patients (1). They are
considered ideal targets for vaccine-based immunotherapy, and more
than 100 CT antigens, including MAGE, NY-ESO-1, GAGE, BAGE, LAGE,
and SSX2, have been identified (2). CT antigens are divided into those
that are encoded on the X chromosome (CT-X antigens) and those that
are not (non-X CT antigens) (3).
Many CT antigens exhibit heterogeneous expression patterns within
the same tumor tissue (2,4). Therefore, multiple CT antigens are
needed to develop polyvalent cancer vaccines that overcome the
limited frequency and heterogeneity of CT antigen expression. CT
antigens have been identified through various techniques, including
T cell epitope cloning, MHC peptide elution, differential gene
expression analysis, and serologic expression cloning (SEREX)
(5–8). Using DNA microarray analysis, MAA-1A
was identified (9). Recently,
massively parallel signature sequencing (MPSS) was utilized to
compare the mRNA expression profiles of testis, melanoma cell
lines, and other somatic tissues (10). This resulted in the identification
of CT45. In addition to these experimental approaches, in
silico analyses have also identified CT antigens, including
BRDT (11), CT46 (12), PAGE (13), and XAGE1 (14).
Among all these methods, SEREX seems to be effective
for the identification of CT antigens. SEREX screening of various
cancer types was broadened to include screening of cDNA libraries
derived from allogeneic tumors, tumor cell lines, and testis
(15,16). This investigation and similar
studies by other researchers have led to the identification of more
than 2,000 SEREX-defined antigens over several years (http://ludwig-sun5.unil.ch/CancerImmunomeDB/). CT
antigens identified by SEREX include MAGE-A (16), NY-ESO-1 (17), SSX2 (18), SCP1 (19), NY-SAR-35 (15), SLCO6A1 (20), CAGE-1 (21), and BCP-20 (22). In the present study, we performed
SEREX analysis to screen a testicular cDNA library with the aim of
isolating novel CT antigens. In addition to a previously defined
CAGE CT antigen (23), a novel CT
antigen, KP-CoT-23 (CCDC83), was identified.
Materials and methods
Human tissues, sera, and cell lines
Human tumor tissues and sera were obtained from the
Department of Pathology, Pusan National University Hospital after
diagnosis and staging. Tissues were frozen in liquid nitrogen and
stored at −80°C until use. The human colon cancer cell lines
SNU-C1, SNU-C2A, SNU-C4, and SNU-C5; the human ovarian cancer cell
lines SNU-8 and SNU-840; the human lung cancer cell lines SK-LC-5
and SK-LC-14; and the human breast cancer cell line MCF7 were
obtained from the Korean Type Culture Collection (KTCC) and the
American Type Culture Collection (ATCC). All these cell lines were
maintained in RPMI-1640 medium (Gibco-BRL Life Technologies Inc.,
Grand Island, NY, USA) supplemented with 10% fetal bovine serum, 2
mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml
streptomycin. The study was conducted under an approved protocol
from Ethical Committeee in this institution.
Total RNA extraction from tissues and
cell lines
Total RNA was isolated from human tissue samples and
human tumor cell lines using the standard TRIzol reagent (Life
Technologies, Gaithersburg, MD, USA) and RNA isolation kit (RNeasy
Maxi Kit, Qiagen, Hilden, Germany) following the manufacturer's
instructions. The amount of RNA isolated was measured at 260 nm by
a spectrophotometer (Ultrospec 2000, Pharmacia Biotech, Cambridge,
UK). Normal tissue total RNA was purchased from Clontech
Laboratories, Inc. (Palo Alto, CA, USA) and Ambion, Inc. (Austin,
Texas, USA). Total RNA from several cancer cell lines other than
the ones used in this experiment was obtained from the Ludwig
Institute for Cancer Research (LICR), New York Branch at the
Memorial Sloan-Kettering Cancer Center.
Preparation of cDNA library and sera
Poly(A)+ RNA from normal testis was purchased from
Clontech Laboratories Inc. mRNA (5 μg) was used to construct
a cDNA library in the ZAP Express vector (Stratagene, La Jolla, CA,
USA), following the manufacturer's instructions. The library
contained approximately 1 million recombinants and was used for
immunoscreening without prior amplification. Serum was prepared
from a colon cancer patient and was diluted 1:200 for SEREX
analysis. The colon cancer patient was immunized frequently (5
times) with a new dendritic cell vaccine (DC-Vac) into which
autologous tumor lysate was loaded by electroporation and pulse.
Serum from a human colon cancer patient was kindly provided by Dr
Chi-Dug Kang (Pusan National University, Pusan City, Korea). The
patient is a 56-year-old male with stage IV colon cancer. All sera
from colon cancer patients and healthy individuals used in this
study were diluted 1:200. To remove serum antibodies that react
with Escherichia coli/bacteriophage-related antigens, sera
were absorbed with E. coli/bacteriophage lysates as
described by Lee et al (15).
Immunoscreening
Immunoscreening of the cDNA library was performed as
previously described (15,22). Briefly, E. coli XL1 blue MRF
cells were transfected with the recombinant phages, and then plated
at a density of approximately 5,000 pfu/150-mm plate (NZCYM-IPTG
agar). The plates were incubated at 37°C for 8 h, and transferred
to nitrocellulose filters (Protran BA 85, 0.45 μm;
Schleicher & Schuell, Keene, NH, USA). The filters were then
incubated with a 1:200 dilution of the patient's sera, which had
been preabsorbed with E. coli-phage lysate. The
serum-reactive clones were detected with an AP-conjugated secondary
antibody and visualized by incubation with
5-bromo-4-chloro-3-indolyl-phosphate/nitroblue tetrazolium
(BCIP/NBT). After screening, the isolated positive clones were
removed from the plate and preserved in suspension medium (SM)
buffer with 25 μl of chloroform. Positive phages were mixed
with a helper phage to co-infect XL-1 Blue MRF, and they were
rescued into pBluescript phagemid forms by in vivo excision.
The excised phagemids were transformed into the host bacteria
(XLOLR) to amplify plasmid DNA for extraction and stock. The size
of the inserted cDNA was determined by restriction enzyme digestion
with EcoRI and XhoI. The cDNA was sequenced
commercially (Macrogen, Seoul, Korea).
RT-PCR
The cDNAs were prepared for use as templates for
RT-PCR using the Superscript first strand synthesis kit (Invitrogen
Life Technologies, Carlsbad, CA, USA) with 1 μg of total
RNA. The specific primers used for KP-CoT-23 were
GCGATGAAGGAAAAATGGAA (forward) and GAGC
CAGTCATTCTCCCAGATIIIIIGATAACTGC (reverse). For blocking extension
of non-specifically primed templates and generating consistently
high PCR specificity, we used a dual priming oligonucleotide (DPO)
which contains two separate priming regions joined by a
polydeoxyinosine linker (Segene Ins. Seoul, Korea). PCR primers
used to verify the CCDC83 variants were GGA TGTTGAAGAAGCGATGAAGGA
(forward) and CCAGGG GGCCCAAGTTTACA (reverse). The cDNA template
concentrations were normalized to the amplification of GAPDH. For
PCR, a 20 μl reaction mixture containing 2 μl of
cDNA, 0.2 mM dNTP, 1.5 mM MgCl2, 0.25 μM each of
the gene specific forward and reverse primers, and 3 units of Taq
DNA polymerase (Solgent, Daejun, Korea) was preheated to 94°C for 5
min, followed by 35 cycles at 94°C for 30 sec, 60°C for 30 sec, and
72°C for 1 min followed by a final elongation step at 72°C for 5
min. Amplified PCR products were analyzed on 1.5% agarose gels
stained with ethidium bromide.
Generation of recombinant KP-CoT-23
fusion proteins
To generate His-tagged KP-CoT-23 proteins, we
selected an open reading frame (ORF) cDNA from codons 514 to 1,748
within the CCDC83 ORF (NM_173556), including the target sequence
for KP-CoT-23 variants. The PCR products contained the NdeI
and XhoI restriction enzyme sites. The primers for the
partial protein were GGAATTCCATATGGAAAACTCAGGG (forward) and
CCGCTCGAGGAGAAAAGACTTCA (reverse). The PCR product was subcloned
into the pET21a expression plasmid. E. coli BL21 cells
containing the KP-CoT-23 plasmid were grown in LB liquid medium,
and IPTG was added to a final concentration of 0.5 mM. Affinity
chromatography using Ni-NTA resin (Qiagen) was performed to purify
KP-CoT-23 recombinant protein. The purity of the recombinant
protein was determined by SDS-PAGE and western blotting using
anti-His antibody (Invitrogen Life Technologies) (Fig. 4C).
Western blot analysis
A 100 ng of purified 6X His-KP-CoT-23 protein was
separated on a 10% SDS-PAGE and transferred to a nitrocellulose
membrane (Hybond-ECL; GE Healthcare, Little Chalfont, UK). After
blocking with TBST (TBS and 0.1% Tween-20) containing 5% skim milk
for 1 h at room temperature, the membrane was incubated in sera
(1:200 dilution) overnight at room temperature. The membranes were
washed and incubated with horseradish peroxidase-conjugated sheep
anti-human IgG antibody (GE Healthcare) (1:3,000 dilution) for 1 h
at room temperature. After washing with TBST and incubating with
chemiluminescence reagent plus (PerkinElmer, Waltham, MA, USA), the
membrane was exposed to Kodak medical X-ray film.
Results
Identification of colon cancer antigens
by SEREX
A testis cDNA expression library containing
approximately 2x105 clones was immunoscreened with serum
from a colon cancer patient immunized with dendritic cells. As
shown in Table I, 64 clones
representing 40 genes were isolated, and the antigens were
designated the names KP-CoT-1 through KP-CoT-40.
 | Table I.Colon cancer antigens by SEREX. |
Table I.
Colon cancer antigens by SEREX.
| No. of Antigens | Gene names/UniGene
clustera | No. of
redundancies | Previously identified
by SEREXb |
|---|
| KP-CoT-1 |
C6orf204/Hs.656359 | 1 | Y |
| KP-CoT-2 | GKAP1/Hs.522255 | 15 | N |
| KP-CoT-3 | MRPS33/Hs.416207 | 1 | Y |
| KP-CoT-4 |
ANKRD50/Hs.480694 | 1 | N |
| KP-CoT-5 | CCDC19/Hs.647705 | 1 | N |
| KP-CoT-6 | BNC1/Hs.459153 | 1 | N |
| KP-CoT-7 | NACA /Hs.505735 | 1 | Y |
| KP-CoT-8 |
C16orf48/Hs.729159 | 1 | Y |
| KP-CoT-9 | ZC3H15/Hs.731458 | 1 | N |
| KP-CoT-10 | COPB2/Hs.731508 | 2 | N |
| KP-CoT-11 | SPACA7/Hs.97592 | 2 | N |
| KP-CoT-12 |
CEP290/Hs.150444 | 1 | Y |
| KP-CoT-13 |
RGPD5/Hs.469630 | 1 | Y |
| KP-CoT-14 |
IFT81/Hs.528382 | 1 | N |
| KP-CoT-15 | CIR1/Hs.632531 | 1 | N |
| KP-CoT-16 |
TTC29/Hs.378893 | 1 | N |
| KP-CoT-17 |
PLGLB1/Hs.652174 | 1 | Y |
| KP-CoT-18 | BRAP/Hs.530940 | 6 | Y |
| KP-CoT-19 |
NAP1L3/Hs.21365 | 1 | Y |
| KP-CoT-20 |
PALLD/Hs.151220 | 1 | Y |
| KP-CoT-21 | ETFB/Hs.348531 | 1 | N |
| KP-CoT-22 |
LOC220115/Hs.528448 | 1 | Y |
| KP-CoT-23 |
CCDC83/Hs.567774 | 1 | N |
| KP-CoT-24 |
NUPL1/Hs.732281 | 3 | Y |
| KP-CoT-25 |
TTC25/Hs.201134 | 1 | N |
| KP-CoT-26 |
LRRC6/Hs.591865 | 2 | N |
| KP-CoT-27 | CAGE/Hs.434416 | 1 | N |
| KP-CoT-28 |
KIAA0586/Hs.232532 | 1 | Y |
| KP-CoT-29 |
SUDS3/Hs.416630 | 1 | Y |
| KP-CoT-30 |
CMTM2/Hs.195685 | 1 | N |
| KP-CoT-31 |
PIBF1/Hs.441926 | 1 | N |
| KP-CoT-32 | HSF2/Hs.158195 | 1 | Y |
| KP-CoT-33 |
C1orf55/Hs.520192 | 1 | N |
| KP-CoT-34 | CKM/Hs.334347 | 1 | N |
| KP-CoT-35 |
EDDM3A/Hs.304757 | 1 | N |
| KP-CoT-36 |
ATP6V1G1/Hs.388654 | 1 | N |
| KP-CoT-37 |
DYNLRB2/Hs.98849 | 1 | Y |
| KP-CoT-38 |
RPL23A/Hs.419463 | 1 | Y |
| KP-CoT-39 |
H3F3B/Hs.180877 | 1 | N |
| KP-CoT-40 |
POLR2J/Hs.654952 | 1 | N |
When the cDNA sequences encoding the 40 colon cancer
antigens were compared to those in the Cancer Immunome Database, 17
of the 40 antigens (43%) had been previously identified by SEREX
analysis with any cDNA/serum combination, while the remaining 23
(57%) had not been previously reported (Table I). These included 3 genes with
testis-specific expression in the UniGene database (KP-CoT-11,
KP-CoT-23, and KP-CoT-27). Among the 3 genes with testis-specific
profiles, KP-CoT-11, corresponding to sperm acrosome-associated
protein (SPACA7), which was represented by 2 overlapping clones,
was isolated. KP-CoT-27 was previously reported as a CT antigen,
CAGE (23). KP-CoT-23 was
identical to coiled-coil domain containing 83 (CCDC83). KP-CoT-2
(GKAP1), which was represented by 15 clones, was the most
frequently isolated gene. Although GKAP1 mRNA expression was
observed in some normal tissues, the highest expression was
observed in the testis as previously reported (24).
Characterization of the KP-CoT-23
clone
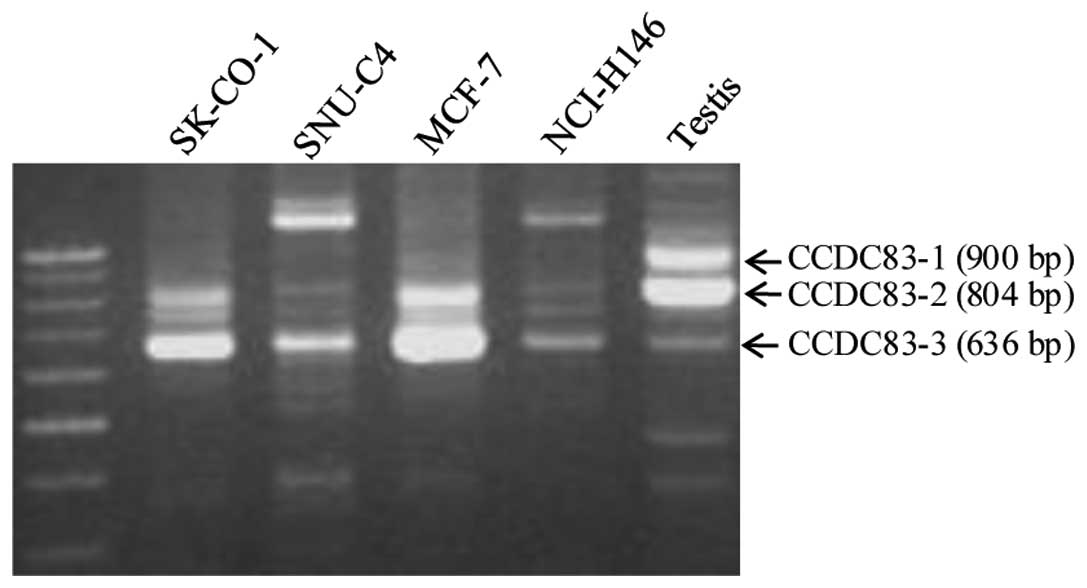
The sequence of the isolated 860-base KP-CoT-23
clone completely matched bases 370 to 1,239 in the 2,345-bp
sequence of CCDC83. Comparison with genome databases showed that
CCDC83 has 3 splice variants of 2,345 bp (CCDC83-1, NM_173556),
2,035 bp (CCDC83-2, BC040208), and 1,030 bp (CCDC83-3, AY251167),
which encode for proteins of 444, 413, and 314 amino acids,
respectively. The CCDC83 gene is approximately 52 kb and contains
11 exons, whereas CCDC83-2 has a deletion in exon 8 and CCDC83-3
has deletions in exons 4 and 8. To verify these 3 variants, we
performed RT-PCR using specific primer sets that included the
deletion regions of testis and several cancer cell lines. As shown
in Fig. 1, RT-PCR analysis yielded
the expected 3 bands, including a major 800-bp band (CCDC83-2), in
testis, whereas only one band (CCDC83-3) or two bands (CCDC83-2 and
-3) were detected in the colon cancer cell line SK-CO-1, the breast
cancer cell line MCF7, and the small lung cancer cell line
NCI-H146. No CCDC83-1 mRNA expression was observed in the cancer
cell lines tested. Each band was cloned and sequenced. We confirmed
that CCDC83-2 has a deletion in exon 8 and CCDC83-3 has deletions
in exons 4 and 8 (data not shown). The KP-CoT-23 clone isolated
matched exons 1–5 of the 11 exons in the CCDC83 gene. In this
region, 2 KP-CoT-23 variants were named KP-CoT-23a, which included
5 exons, and KP-CoT-23b, which had 4 exons (deletion of exon 4 in
KP-CoT-23a).
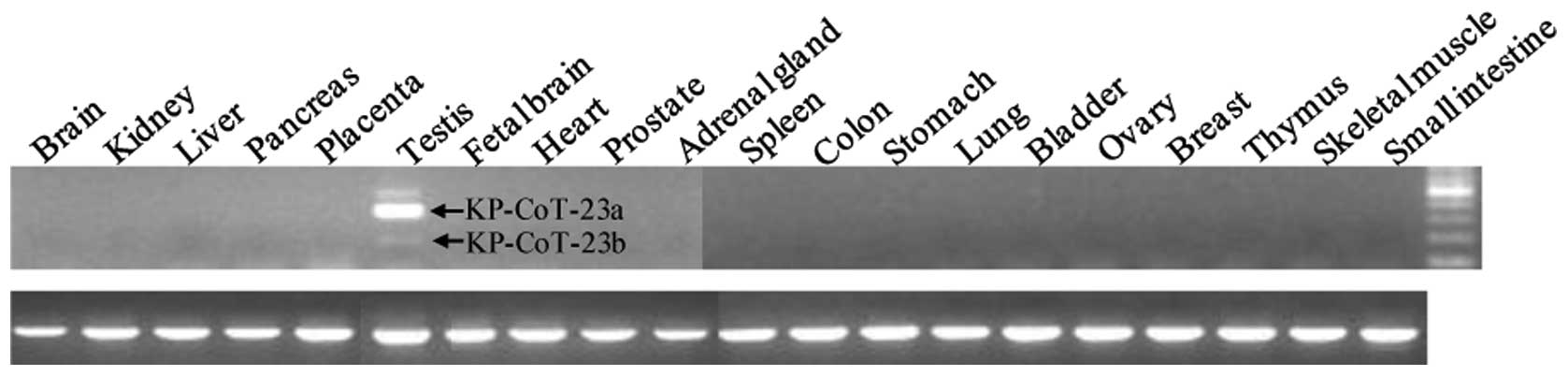
KP-CoT-23 mRNA expression in normal
tissues, tumors, and cancer cell lines
To investigate the restricted expression of
KP-CoT-23 variant mRNAs in normal adult tissues, RT-PCR was
performed using gene specific primer pairs to detect KP-CoT-23a and
KP-CoT-23b. As shown in Fig. 2,
expression of the 2 KP-CoT-23 variant mRNAs was restricted to the
testis. KP-CoT-23a was strongly expressed in testis, while
KP-CoT-23b was weakly expressed. The KP-CoT-23a and KP-CoT-23b
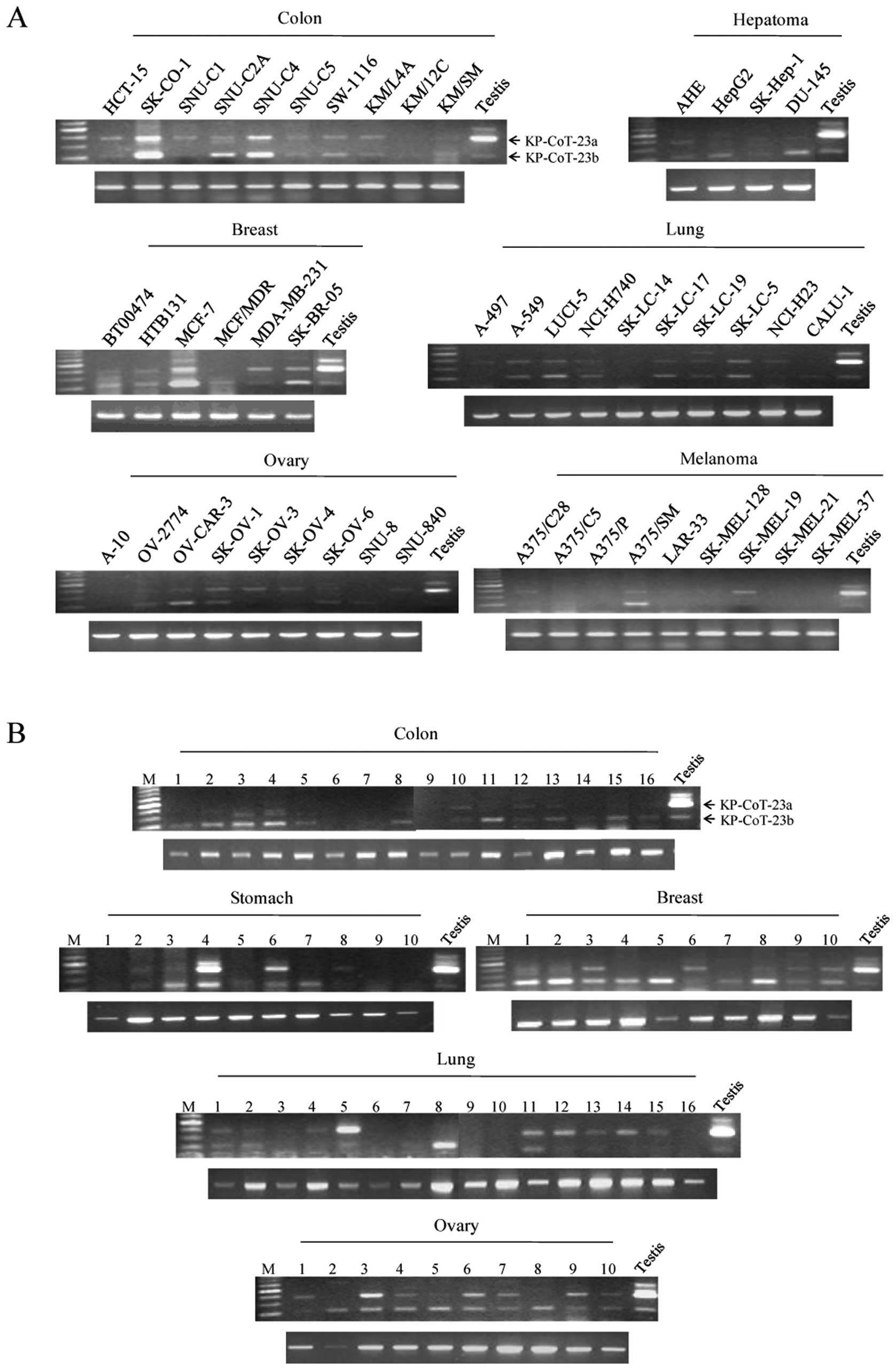
genes were frequently and broadly expressed in a variety of cancer
cell lines, including colon cancer cell lines (9/10 and 10/10,
respectively), hepatoma cell lines (1/3 and 3/3, respectively),
breast cancer cell lines (5/6 and 6/6, respectively), lung cancer
cell lines (8/10 for both), ovary cancer cell lines (6/9 and 5/9,
respectively), melanoma cell lines (3/9 and 2/9, respectively),
renal cancer cell lines (1/2), sarcoma cell lines (3/6 and 6/6,
respectively), and renal cancer cell lines (2/2 for both) (Fig. 3A, Table II). In addition, the KP-CoT-23a and
KP-CoT-23b genes were expressed in various tumors, including colon
cancer (4/16 and 12/16, respectively), stomach tumors (3/10 and
4/10, respectively), breast cancer (4/10 and 9/10, respectively),
lung cancer (9/16 and 6/16, respectively), and ovarian cancer (9/10
and 10/10, respectively) (Fig.
3B). These results indicate that KP-CoT-23 is a novel cancer
testis antigen that is frequently expressed in several types of
cancer, including colon cancer.
 | Table II.Summary of KP-CoT-23 a and b
expression in cancer cell lines. |
Table II.
Summary of KP-CoT-23 a and b
expression in cancer cell lines.
| Tumor type | KP-CoT-23a
| KP-CoT-23b
|
|---|
Cell lines
| Tissues
| Cell lines
| Tissues
|
|---|
| Positive/total | Positive/total |
|---|
| Breast cancer | 5/6 | 4/10 | 6/6 | 9/10 |
| Colon cancer | 9/10 | 4/16 | 10/10 | 12/16 |
| Hepatoma | 1/3 | ND | 3/3 | ND |
| Lung cancer | 8/10 | 9/16 | 8/10 | 6/16 |
| Melanoma | 3/9 | ND | 2/9 | ND |
| Ovary cancer | 6/9 | 9/10 | 5/9 | 10/10 |
| Stomach cancer | ND | 3/10 | ND | 4/10 |
| Sarcoma | 3/6 | ND | 6/6 | ND |
| Leukemia | 2/4 | ND | 3/4 | ND |
| Prostate
cancer | 1/3 | ND | 3/3 | ND |
| Renal cancer | 2/2 | ND | 2/2 | ND |
| Glioblastoma | 0/2 | ND | 2/2 | ND |
| Bladder cancer | 1/1 | ND | 1/1 | ND |
| Teratoma | 0/1 | ND | 1/1 | ND |
Seroreactivity of KP-CoT-23 by western
blot analysis
To determine whether immune recognition of the
KP-CoT-23 protein is cancer-related, allogeneic sera samples
obtained from 21 healthy blood donors and 37 patients with colon
cancer were tested for KP-CoT-23 reactivity by western blot
analysis. As shown in Fig. 4A and
B, 26/37 (70%) sera from colon cancer patients and 4/21 (19%)
sera from healthy patients were reactive against KP-CoT-23. The
correlation between KP-CoT-23 mRNA expression and positive IgG was
not evaluated due to a lack of paired samples; therefore, this
requires further investigation in a future study. Nonetheless, the
results of KP-CoT-23 recognition by sera from colon cancer patients
and healthy individuals indicate that KP-CoT-23 is an immunogenic
tumor antigen in colon cancer patients.
Discussion
In the current study, 40 distinct antigens were
isolated from colon cancer by SEREX and were designated the names
KP-CoT-1 to KP-CoT-40. Twenty-three of the 40 antigens (57%) had
not been previously identified by SEREX analysis with any
cDNA/serum combination. There were 3 genes with testis-specific
expression in the UniGene database and in the literature, including
KP-CoT-11 (SPACA7), KP-CoT-23 (CCDC83), and KP-CoT-27 (CAGE), a CT
antigen previously identified by SEREX.
KP-CoT-11 (SPACA7) is an uncharacterized sperm
acrosome-associated protein. Conventional RT-PCR demonstrated
strong SPACA7 mRNA expression; however, transcripts encoding SPACA7
were not detected in cancer cell lines and tumor tissues (data not
shown).
KP-CoT-27 (CAGE) was previously reported as a CT
antigen located on chromosome Xp22. CAGE was highly expressed in
several cancer types, including gastric cancer, cervical cancer,
and lung cancer (25). Based on an
ELISA analysis, anti-CAGE antibodies were detected in sera from 12
of 45 endometrial cancer patients, 2 of 20 melanoma patients, and 4
of 33 colon cancer patients. Although CAGE was isolated from
gastric and endometrial cancer, we were the first to find it in
colon cancer by SEREX. Detection of anti-CAGE antibody in 7 of 13
(53.8%) patients with microsatellite instability-positive
endometrial cancer and in 1 of 3 patients with atypical endometrial
hyperplasia (26) suggests that
CAGE may be useful for the prognosis or early diagnosis of patients
with microsatellite instability-positive endometrial cancers. The
expression of CAGE is cell cycle-regulated (25) and confers drug resistance by
regulating expression of p53 through HDAC2 (27).
The KP-CoT-23 gene matched coiled-coil domain
containing 83 (CCDC83). The CCDC83 gene consists of 11 exons and
has 3 variants, CCDC83-1, CCDC83-2, and CCDC-83-3. RT-PCR analysis
revealed that expression of the 3 variants was restricted to the
testis in normal adult tissues. In cancer cell lines, no expression
of CCDC83-1 mRNA was observed, but expression of CCDC83-2 and
CCDC83-3 mRNA was observed in several cancer cell lines (Fig. 1).
The isolated KP-CoT-23 clone matched exons 1-5 of
the 11 exons of the CCDC83 gene, and there were 2 KP-CoT-23
variants, named KP-CoT-23a and KP-CoT-23b. The KP-CoT-23a, and b
genes were frequently expressed in several tumor types and cancer
cell lines, especially in colon cancer tumor (4/16 and 12/16,
respectively) and colon cancer cell lines (9/10 and 10/10,
respectively) (Table II). Since CT
antigen expression rarely exceeds 40% in a given cancer type
(2,28) and is generally heterogeneous
(29), the expression profile of
this CT antigen in cancer cells was extraordinary. SEREX-derived CT
antigens have been shown to induce CD8+ CTLs (30,31),
and a positive correlation was observed between serum positivity
for IgG antibody and induction of CD8+ CTLs against the
cancer testis antigen NY-ESO-1 (31). Western blot analysis of 37 sera
samples from colon cancer patients showed that 23 patients had
reactivity against the recombinant KP-CoT-23. The significant
frequency of IgG antibody responses against KP-CoT-23 suggested
that the strong immunogenicity and CD4 and CD8 T-cell responses
against the antigen should be investigated.
In summary, a novel CT antigen, KP-CoT-23 was
expressed in various types of cancer, including colon cancer.
Frequent detection of specific serum IgG antibody in patients with
colon cancer indicated the highly immunogenic nature of KP-CoT-23
in colon cancer. These results suggest that KP-CoT-23 may be useful
not only for the immunotherapy of colon cancer, but also for the
diagnosis of some types of cancer, particularly colon cancer.
Acknowledgements
The study was supported by a grant
from the Basic Research Program of the Korea Science and
Engineering Foundation (R01-2004-000-10224-02006) and a grant from
the Cancer Research Institute, USA.
References
|
1.
|
Scanlan MJ, Gure AO, Jungbluth AA, Old LJ
and Chen YT: Cancer/testis antigens: an expanding family of targets
for cancer immunotherapy. Immunol Rev. 188:22–32. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Caballero OL and Chen YT: Cancer/testis
(CT) antigens: potential targets for immunotherapy. Cancer Sci.
100:2014–2021. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Simpson AJ, Caballero OL, Jungbluth A,
Chen YT and Old J: Cancer/testis antigens, gametogenesis and
cancer. Nat Rev Cancer. 5:615–625. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Kim YD, Park HR, Song MH, Shin DH, Lee CH,
Lee MK and Lee SY: Pattern of cancer/testis antigen expression in
lung cancer patients. Int J Mol Med. 29:656–662. 2012.PubMed/NCBI
|
|
5.
|
Gaugler B, Van den Eynde B, van der
Bruggen P, Romero P, Gaforio JJ, De Plaen E, Lethe B, Brasseur F
and Boon T: Human gene MAGE-3 codes for an antigen recognized on a
melanoma by autologous cytolytic T lymphocytes. J Exp Med.
179:921–930. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
van der Bruggen P, Traversari C, Chomez P,
Lurquin C, De Plaen E, Van den Eynde B, Knuth A and Boon T: A gene
encoding an antigen recognized by cytolytic T lymphocytes on a
human melanoma. Science. 254:1643–1647. 1991.
|
|
7.
|
Pascolo S, Schirle M, Guckel B, Dumrese T,
Stumm S, Kayser S, Moris A, Wallwiener D, Rammensee HG and
Stevanovic S: A MAGE-A1 HLA-A A*0201 epitope identified
by mass spectrometry. Cancer Res. 61:4072–4077. 2001.
|
|
8.
|
Sahin U, Tureci O, Schmitt H, Cochlovius
B, Johannes T, Schmits R, Stenner F, Luo G, Schobert I and
Pfreundschuh M: Human neoplasms elicit multiple specific immune
responses in the autologous host. Proc Natl Acad Sci USA.
92:11810–11813. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
de Wit NJ, Weidle UH, Ruiter DJ and van
Muijen GN: Expression profiling of MMA-1a and splice variant
MMA-1b: new cancer/ testis antigens identified in human melanoma.
Int J Cancer. 98:547–553. 2002.PubMed/NCBI
|
|
10.
|
Chen YT, Scanlan MJ, Venditti CA, Chua R,
Theiler G, Stevenson BJ, Iseli C, Gure AO, Vasicek T, Strausberg
RL, et al: Identification of cancer/testis-antigen genes by
massively parallel signature sequencing. Proc Natl Acad Sci USA.
102:7940–7945. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Scanlan MJ, Altorki NK, Gure AO,
Williamson B, Jungbluth A, Chen YT and Old LJ: Expression of
cancer-testis antigens in lung cancer: definition of bromodomain
testis-specific gene (BRDT) as a new CT gene, CT9. Cancer Lett.
150:155–164. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Chen YT, Venditti CA, Theiler G, Stevenson
BJ, Iseli C, Gure AO, Jongeneel CV, Old LJ and Simpson AJ:
Identification of CT46/ HORMAD1, an immunogenic cancer/testis
antigen encoding a putative meiosis-related protein. Cancer Immun.
5:92005.PubMed/NCBI
|
|
13.
|
Brinkmann U, Vasmatzis G, Lee B and Pastan
I: Novel genes in the PAGE and GAGE family of tumor antigens found
by homology walking in the dbEST database. Cancer Res.
59:1445–1448. 1999.PubMed/NCBI
|
|
14.
|
Sato S, Noguchi Y, Ohara N, Uenaka A,
Shimono M, Nakagawa K, Koizumi F, Ishida T, Yoshino T, Shiratori Y
and Nakayama E: Identification of XAGE-1 isoforms: predominant
expression of XAGE-1b in testis and tumors. Cancer Immun.
7:52007.PubMed/NCBI
|
|
15.
|
Lee SY, Obata Y, Yoshida M, Stockert E,
Williamson B, Jungbluth AA, Chen YT, Old LJ and Scanlan MJ:
Immunomic analysis of human sarcoma. Proc Natl Acad Sci USA.
100:2651–2656. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Chen YT, Gure AO, Tsang S, Stockert E,
Jager E, Knuth A and Old LJ: Identification of multiple
cancer/testis antigens by allogeneic antibody screening of a
melanoma cell line library. Proc Natl Acad Sci USA. 95:6919–6923.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Chen YT, Scanlan MJ, Sahin U, Tureci O,
Gure AO, Tsang S, Williamson B, Stockert E, Pfreundschuh M and Old
LJ: A testicular antigen aberrantly expressed in human cancers
detected by autologous antibody screening. Proc Natl Acad Sci USA.
94:1914–1918. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Tureci O, Sahin U, Schobert I, Koslowski
M, Scmitt H, Schild HJ, Stenner F, Seitz G, Rammensee HG and
Pfreundschuh M: The SSX-2 gene, which is involved in the t(X;18)
translocation of synovial sarcomas, codes for the human tumor
antigen HOM-MEL-40. Cancer Res. 56:4766–4772. 1996.PubMed/NCBI
|
|
19.
|
Tureci O, Sahin U, Zwick C, Koslowski M,
Seitz G and Pfreundschuh M: Identification of a meiosis-specific
protein as a member of the class of cancer/testis antigens. Proc
Natl Acad Sci USA. 95:5211–5216. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Lee SY, Williamson B, Caballero OL, Chen
YT, Scanlan MJ, Ritter G, Jongeneel CV, Simpson AJ and Old LJ:
Identification of the gonad-specific anion transporter SLCO6A1 as a
cancer/testis (CT) antigen expressed in human lung cancer. Cancer
Immun. 4:132004.PubMed/NCBI
|
|
21.
|
Park S, Lim Y, Lee D, Cho B, Bang YJ, Sung
S, Kim HY, Kim DK, Lee YS, Song Y and Jeoung DI: Identification and
characterization of a novel cancer/testis antigen gene CAGE-1.
Biochim Biophys Acta. 1625:173–182. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Song MH, Ha JC, Lee SM, Park YM and Lee
SY: Identification of BCP-20 (FBXO39) as a cancer/testis antigen
from colon cancer patients by SEREX. Biochem Biophys Res Commun.
408:195–201. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Cho B, Lim Y, Lee DY, Park SY, Lee H, Kim
WH, Yang H, Bang YJ and Jeoung DI: Identification and
characterization of a novel cancer/testis antigen gene CAGE.
Biochem Biophys Res Commun. 292:715–726. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Domae S, Nakamura Y, Uenaka A, Wada H,
Nakata M, Oka M, Kishimoto K, Tsukamoto G, Yoshihama Y, Matsuoka J,
et al: Identification of CCDC62-2 as a novel cancer/testis antigen
and its immunogenicity. Int J Cancer. 124:2347–2352. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Kim Y and Jeoung D: Role of CAGE, a novel
cancer/testis antigen, in various cellular processes, including
tumorigenesis, cytolytic T lymphocyte induction, and cell motility.
J Microbiol Biotechnol. 18:600–610. 2008.PubMed/NCBI
|
|
26.
|
Iwata T, Fujita T, Hirao N, Matsuzaki Y,
Okada T, Mochimaru H, Susumu N, Matsumoto E, Sugano K, Yamashita N,
et al: Frequent immune responses to a cancer/testis antigen, CAGE,
in patients with microsatellite instability-positive endometrial
cancer. Clin Cancer Res. 11:3949–3957. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Kim Y, Park H, Park D, Lee YS, Choe J,
Hahn JH, Lee H, Kim YM and Jeoung D: Cancer/testis antigen CAGE
exerts negative regulation on p53 expression through HDAC2 and
confers resistance to anti-cancer drugs. J Biol Chem.
285:25957–25968. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Jungbluth AA, Chen YT, Stockert E, Busam
KJ, Kolb D, Iversen K, Coplan K, Williamson B, Altorki N and Old
LJ: Immunohistochemical analysis of NY-ESO-1 antigen expression in
normal and malignant human tissues. Int J Cancer. 92:856–860. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Slingluff CL Jr, Petroni GR,
Chianese-Bullock KA, Smolkin ME, Hibbitts S, Murphy C, Johansen N,
Grosh WW, Yamshchikov GV, Neese PY, et al: Immunologic and clinical
outcomes of a randomized phase II trial of two multipeptide
vaccines for melanoma in the adjuvant setting. Clin Cancer Res.
13:6386–6395. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Coulie PG, Karanikas V, Colau D, Lurquin
C, Landry C, Marchand M, Dorval T, Brichard V and Boon T: A
monoclonal cytolytic T-lymphocyte response observed in a melanoma
patient vaccinated with a tumor-specific antigenic peptide encoded
by gene MAGE-3. Proc Natl Acad Sci USA. 98:10290–10295. 2001.
View Article : Google Scholar
|
|
31.
|
Jager E, Nagata Y, Gnjatic S, Wada H,
Stockert E, Karbach J, Dunbar PR, Lee SY, Jungbluth A, Jager D, et
al: Monitoring CD8 T cell responses to NY-ESO-1: correlation of
humoral and cellular immune responses. Proc Natl Acad Sci USA.
97:4760–4765. 2000. View Article : Google Scholar : PubMed/NCBI
|