Introduction
Colon cancer is one of the leading causes of cancer
related death worldwide with over 500,000 deaths every year and its
incidence continues to rise in the eastern world (1). There are many possible causes of
colon cancer including advanced age, smoking, diet, environmental
factors, exogenous hormones like estrogens, polyps of the colons,
family history (genetic predisposition), familial adenomatous
polyposis (FAP), hereditary nonpolyposis colorectal cancer (HNPCC),
Lynch syndrome, long-standing ulcerative colitis, and Crohn’s
disease of the colon (2). In its
early stage, surgery can be an effective primary treatment, but
surgical resection is unsatisfactory in cases of metastasis and
reoccurrence (3). Thus other
treatment options such as chemotherapeutic agents have been
introduced, and 5-flurouracil (5-FU) remains a widely used
first-line colon cancer treatment in patients over 50 years of age
(4). However there are
inter-individual differences in response, survival and toxicity in
patients treated with 5-FU (5).
Developing new anticancer agents for use in treatment against colon
cancer are needed in order to provide reliable treatment for
patients.
Several pyrazole derivatives have been reported to
have a wide range of biological activities such as antitumor,
antibacterial, anti-inflammatory, analgesic, and fungistatic
activity (6–9). So, they are increasingly gaining
interest as important compounds in the pharmaceutical industry and
medicinal chemistry. Of these, anticancer activities have been the
most studied. Especially, pyrazole derivatives induce apoptosis and
inhibition of angiogenesis in cancers (10,11).
In addition, pyridine derivatives have been reported to possess
anticancer effect such as inhibition of cell growth, induction of
cell cycle arrest, and apoptosis without cytotoxicity in various
cancer cells (12,13). On the basis of previous finding, we
have synthesized a new active pyrazole and pyridine derivatives,
culminating in the discovery of 2-(4-(2-(4-(dimethylamino) phenyl)
pyridin-4-yl)-5-(3-methoxy-5-methylphenyl)-1H-pyrazol-1-yl)
acetonitrile)•3.5HCl) (Fig. 1,
designated KI-10F).
One of the hallmarks of cancer cells including colon
cancer is uncontrolled cell proliferation and escape of apoptosis
by cancer cells. Especially, angiogenesis plays an important role
in the progression of cancer and correlates with higher incidences
of metastasis and poor prognosis in human colon cancers (14). The presence of microvessels and the
vascular endothelial growth factor (VEGF) are significantly
involved in endothelial cell proliferation, migration, invasion
and, tumor recurrence (15). Also,
apoptosis and proliferation imbalance leads to malignant
transformation and tumorigenesis of normal tissues (16). Therefore, induction of apoptosis
along with inhibiting angiogenesis by non-toxic compounds is a
promising strategy for checking the uncontrolled colon cancer cell
proliferation and survival.
In this study, therefore, we synthesized a new
pyrazole derivative KI-10F and assessed its possibility as a
chemotherapeutic agent against colon cancer. Our results showed
that KI-10F induced apoptosis and inhibited angiogenesis in
vitro and in vivo.
Materials and methods
Cells and materials
The human colon cancer cell lines HT-29, LoVo, RKO
and human umbilical vein endothelial cells (HUVECs) were purchased
from ATCC (Manassas, VA). The HT-29 cells were cultured in Roswell
Park Memorial Institute Media 1640 (RPMI-1640) and the LoVo and RKO
cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM),
supplemented with 10% fetal bovine serum (FBS) and 1%
penicillin/streptomycin. The HUVECs were grown in a gelatin coated
flask in M199 medium containing 20 ng/ml of basic fibroblast growth
factor (bFGF), 100 U/ml heparin and 20% FBS. The cell cultures were
maintained at 37°C in a CO2 incubator with a controlled
humidified atmosphere composed of 95% air and 5% CO2.
The FBS, cell culture media, penicillin-streptomycin, and all other
agents used in the cell culture studies were purchased from
Invitrogen.
Synthesis of KI-10F
2-(4-(2-(4-(Dimethylamino)phenyl)
pyridin-4-yl)-5-(3-methoxy-5-methylphenyl)-1H-pyrazol-1-yl)
acetonitrile (700 mg, 1.65 mM) in hydrogen chloride solution (5 ml,
5 mM, 1 M/diethyl ether) was vigorously stirred for 30 min, and
then filtered to collect the solid which was washed with hexane to
give the pure KI-10F as a yellow solid. Yield: 750 mg; mp
125–128°C; 1H NMR (300 MHz, D2O) δ (ppm) 2.19
(s, 3H), 2,98 (sec, 6H), 3.64 (s, 3H), 5.07 (s, 2H), 6.53 (s, 1H),
6.62 (s, 1H), 7.01 (s, 1H), 7.07 (d, J=8.7 Hz, 2H), 7.24 (d, J=8.7
Hz, 2H), 7.31 (s, 1H), 7.42 (d, J=6.3 Hz, 1H), 8.14 (d, J=4.8 Hz,
2H); elemental analyses
(C26H28.5Cl3.5N5O)
calcd: C, 56.66; H, 5.21; N, 12.71. Found: C, 56.64; H, 5.63; N,
12.60.
Measurement of cell proliferation
Cell viability analysis was performed using the MTT
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide)
assay. Briefly, the HT-29, LoVo, and RKO cells were plated at a
density of 1×104 cells/well in 96-well plates and
incubated for 24 h. The medium was removed, and the cells were
treated with either D.W. as a control or various concentrations
(0.01, 0.05, 0.1, 0.5, 1 or 5 μM) of KI-10F or 5-FU. After the
cells were incubated for an additional 48 h, 20 μl of MTT solution
(2 mg/ml) was added to each well and they were incubated for
another 4 h at 37°C. After removing the media, the formed formazan
crystals were dissolved in DMSO (200 μl/well) by constant shaking
for 5 min. The plate was then read on a microplate reader at 540
nm. Three replicate wells were used for each analysis. The median
inhibitory concentration (IC50, defined as the drug
concentration at which cell growth was inhibited by 50%) was
assessed from the dose-response curves.
Western blot analysis
Cells were washed with ice-cold phosphate-buffered
saline (PBS) before lysis. The cells were lysed with buffer
containing 1% Triton X-100, 1% Nonidet P-40, and the following
protease and phosphatase inhibitors: aprotinin (10 mg/ml),
leupeptin (10 mg/ml) (ICN Biomedicals, Asse-Relegem, Belgium),
phenylmethylsulfonyl fluoride (1.72 mM), NaF (100 mM),
NaVO3 (500 mM), and
Na4P2O7 (500 mg/ml)
(Sigma-Aldrich). Protein (50 μg) was separated from the mixture
using 10% sodium dodecyl sulfate-polyacrylamide (SDS) gel
electrophoresis, it was then transferred onto nitrocellulose
membranes and evaluated using Ponceau S solution staining
(Sigma-Aldrich). Immunostaining of the blots was performed using
the primary antibodies followed by the secondary antibody
conjugated to horseradish peroxidase with detection using enhanced
chemiluminescence reagent (ELPS, Seoul, Korea). The primary
antibodies were mouse monoclonal: anticleaved caspase-3, cleaved
caspase-8, cleaved caspase-9 (Cell Signaling Technologies, Danvers,
MA), Bax and Bcl-2 (Santa Cruz Biotechnology, Santa Cruz, CA),
anti-HIF-1α, and anti-β-actin (BD Biosciences, San Jose, CA), and
anti-VEGF (Cell Signaling Technologies, Danvers, MA). The secondary
antibodies were purchased from Amersham Biosciences. The bands were
visualized using the ECL plus system (Amersham Pharmacia Biotech
Inc., Piscataway, NJ).
Cell cycle analysis
HT-29 cells were plated in 100-mm diameter culture
dishes and then incubated. The next day, the cells were treated
with various concentrations (0 to 1 μM) of KI-10F for 24 h.
Floating and adherent cells were collected and fixed in 70% ethanol
at 4°C overnight. After washing, the cells were subsequently
stained with 50 μg/ml of PI and 100 μg/ml of RNase A for 1 h in the
dark and then subjected to flow cytometric analysis in order to
determine the percentage of cells at specific cell cycle phase.
Flow cytometric analysis was performed using a FACSCalibur flow
cytometer (Becton Dickinson, San Jose, CA) equipped with a 488-nm
argon laser. Events were evaluated for each sample and the cell
cycle distribution was analyzed using Cell Quest software (Becton
Dickinson). The results were presented as the number of cells
versus the amount of DNA as indicated by fluorescence signal
intensity. All the experiments were conducted three times.
DAPI staining and TUNEL assay
The HT-29 cells were plated onto 18-mm cover glasses
in RPMI-1640 medium and grown to ∼70% confluence for 24 h. The
cells were then treated with KI-10F at a dose of 0.5 μM for 24 h.
The cells were fixed in 2% ice-cold paraformaldehyde (PFA), washed
with PBS and then stained with 2 μg/ml of
4,6-diamidino-2-phenylindole (DAPI) for 20 min at 37°C. The DAPI
stained cells were examined under a fluorescent microscope in order
to evaluate any nuclear fragmentation. Terminal deoxynucleotidyl
transferase-mediated nick end labeling (TUNEL) was performed
following the manufacturer’s protocol for the use of the TUNEL kit
(Chemicon, Temecula, CA).
HUVEC tube formation assay
Matrigel (200 μl) (10 mg/ml) (BD Biosciences, NJ)
was polymerized for 30 min at 37°C. The HUVECs were suspended in
M199 (5% FBS) medium at a density of 2.5×105 cells/ml.
Then 0.2 ml of cell suspension was added to each Matrigel coated
well either with or without the indicated concentrations of KI-10F,
and then they were incubated for 14 h. The morphological changes of
the cells and HUVEC tube formations were observed under a
phase-contrast microscope and photographed at ×200
magnification.
HUVEC migration assay
HUVECs, plated on 60 mm diameter culture dishes at
90% confluence, were wounded with a 2 mm razor blade and marked at
the injury line. After wounding, the peeled off cells were removed
with serum-free medium and further incubated in M199 with 5% serum,
1 mM thymidine (Sigma-Aldrich) and/or KI-10F. The HUVECs were
allowed to migrate for 24 h and were then rinsed with serum-free
medium followed by fixing with absolute methanol and staining with
Giemsa (Sigma-Aldrich). Migration was quantitated by counting the
number of cells that moved beyond the reference line.
Tumor xenograft study
Six-week-old male nude mice were obtained from
Central Laboratory Animal Inc. (Seoul, Korea). Animal care and all
experimental procedures were conducted in accordance with the
approval and guidelines of the Inha Institutional Animal Care and
Use Committee of the Medical School of Inha University. The animals
were fed standard rat chow and tap water ad libitum, and
were maintained under a 12 h dark/light cycle at 37°C. The mice
were randomly divided into three groups (control, KI-10F at 1 mg/kg
and 5-FU at 10 mg/kg). The HT-29 cells were harvested, mixed with
PBS (200 μl/mouse) and then inoculated into one flank of each nude
mouse (2×106 of HT-29 cells). When the tumor volume
reached ∼50–100 mm3, the mice were orally administered
either KI-10F (1 mg/kg), 5-FU (10 mg/kg, positive control group) or
the vehicle (200 μl of 0.7% CMC, control group) three times per
week for 3 weeks. The tumor dimensions were measured twice a week
using a digital caliper and the tumor volume was calculated using
the formula: V = length × width2 × 0.5. At the end of
the experiment, the mice were sacrificed, and the tumors were
excised and weighed. A portion of each tumor was fixed in buffered
formalin and then embedded in paraffin. The remaining tissue was
stored at −70°C for further analysis.
Immunohistochemistry in the tumor
tissue
After deparaffinization, immunostaining was
performed on 8-μm thick sections of tumor tissue. Microwave antigen
retrieval was performed in citrate buffer (pH 6.0) for 10 min prior
to peroxidase quenching with 3% H2O2 in PBS
for 10 min. The sections were then washed in water and preblocked
with normal goat or horse serum for 10 min. Next, the tissue
sections were incubated overnight at 4°C in 1:50 dilutions of mouse
anticleaved caspase-3 (Cell Signaling), anti-PCNA and anti-CD34
antibodies (Santa Cruz Biotechnology). The sections were then
incubated with biotinylated secondary antibodies (1:200) for 1 h.
Following a washing step with PBS, streptavidin-HRP was applied.
Finally, the sections were developed with diaminobenzidine
tetrahydrochloride substrate for 10 min, and counterstained with
hematoxylin. At least three random fields of each section were
examined at a magnification, ×200 and analyzed by a computer image
analysis system (Media Cybernetics, Silver Spring, MD).
Statistical analysis
Data are expressed as mean ± SD. Statistical
analysis was performed using ANOVA and an unpaired Student’s
t-test. A p-value ≤0.05 was considered statistically significant.
Statistical calculations were performed using SPSS software for the
Windows operating system (Version 10.0; SPSS, Chicago, IL).
Results
KI-10F inhibited proliferation of human
colon cancer cells
In order to determine the effectiveness of KI-10F on
cell growth inhibition, we tested the MTT assay on three colon
cancer cell lines (HT-29, LoVo and RKO). The human colon cancer
cells were exposed to various concentrations of KI-10F and 5-FU,
ranging from 0 to 5 μM, for 48 h. Our results showed that the cell
growth was inhibited strongly by KI-10F in a dose-dependent manner
as compared with 5-FU, a commercially available drug (Fig. 1). The IC50 values of
KI-10F administration in the three colon cancer cells were very low
(HT-29; 0.30 μM, LoVo; 0.59 μM, RKO; 0.33 μM) whereas 5-FU was
unable to inhibit 50% cell proliferation at the highest dose (5
μM). The HT-29 cells were the most sensitive to KI-10F and had the
lowest IC50 value to KI-10F mediated inhibition of
cancer cell growth and proliferation, and were thus chosen for
further experiments.
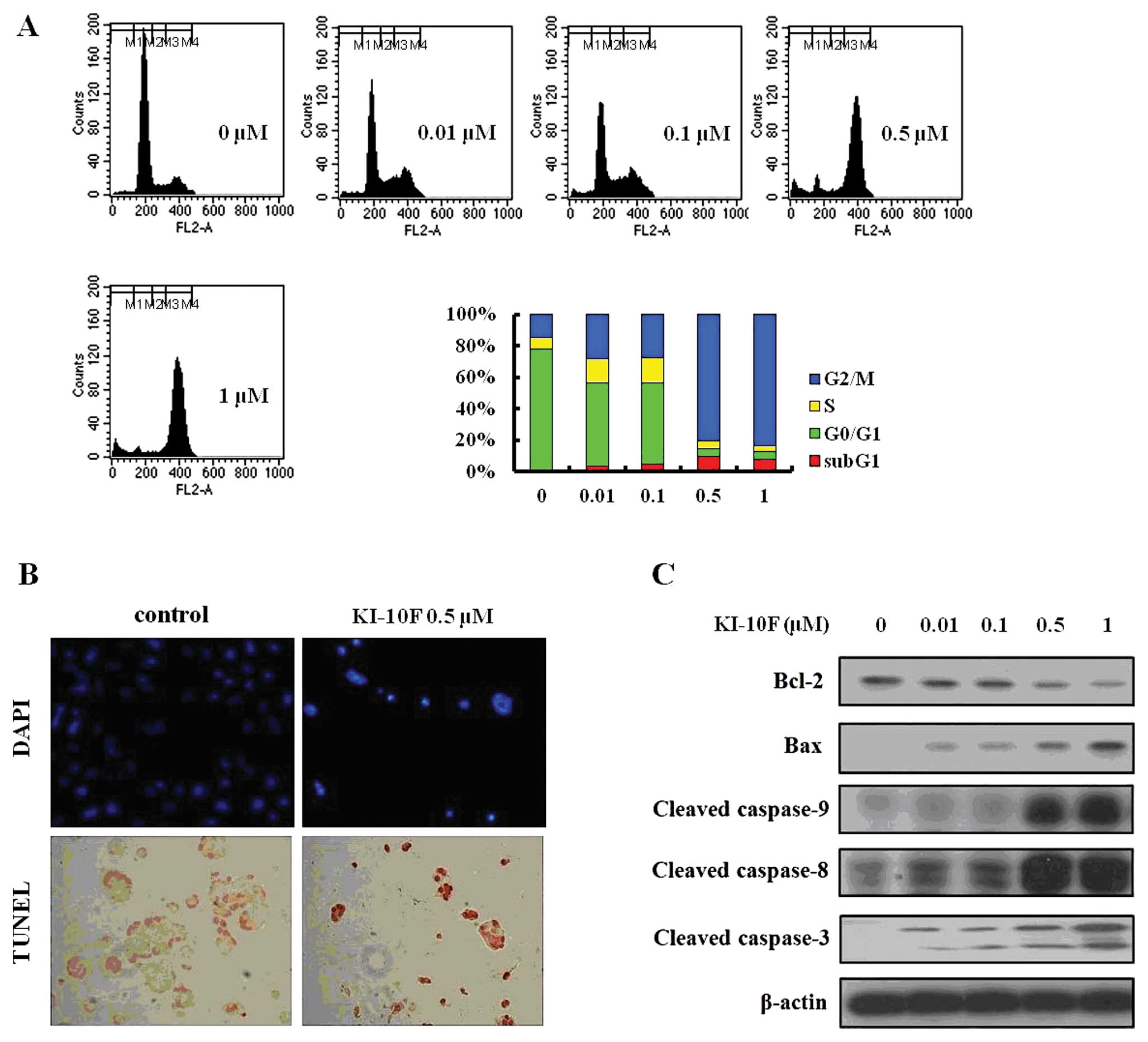
The effects of KI-10F on cell cycle and
apoptosis in HT-29 cells
The cell cycle profile induced by KI-10F was
measured using flow cytometry. The cells were harvested 48 h after
treatment of KI-10F at various concentrations and analyzed for
their cell cycle distributions (sub-G1, G0/G1, S and G2/M).
Treatment with KI-10F increased the occurrence of the sub-G1 phase
and significantly blocked the G2/M phase. At a dose of 0.5 μM,
KI-10F rapidly increased the occurrence of the sub-G1 phase by
7.8-fold and G2/M phase by 5.5-fold as compared to the control (0
μM) (Fig. 2A). In order to
identify the apoptotic effect of KI-10F in the HT-29 cells, we
performed analysis using DAPI and TUNEL staining. When the HT-29
cells were treated with KI-10F (0.5 μM), the cells presented
apoptotic morphological features such as bright nuclear
condensation, DNA fragmentation and perinuclear apoptotic bodies
visible by DAPI and TUNEL staining (Fig. 2B). In results of western blot
analysis, KI-10F dosing seemed to induce apoptosis in the HT-29
cells, whereas it decreased the expression of Bcl-2 and increased
the expression of Bax, induced extrinsic apoptosis by activation of
the caspase-3, caspase-8 and caspase-9 cascades (Fig. 2C).
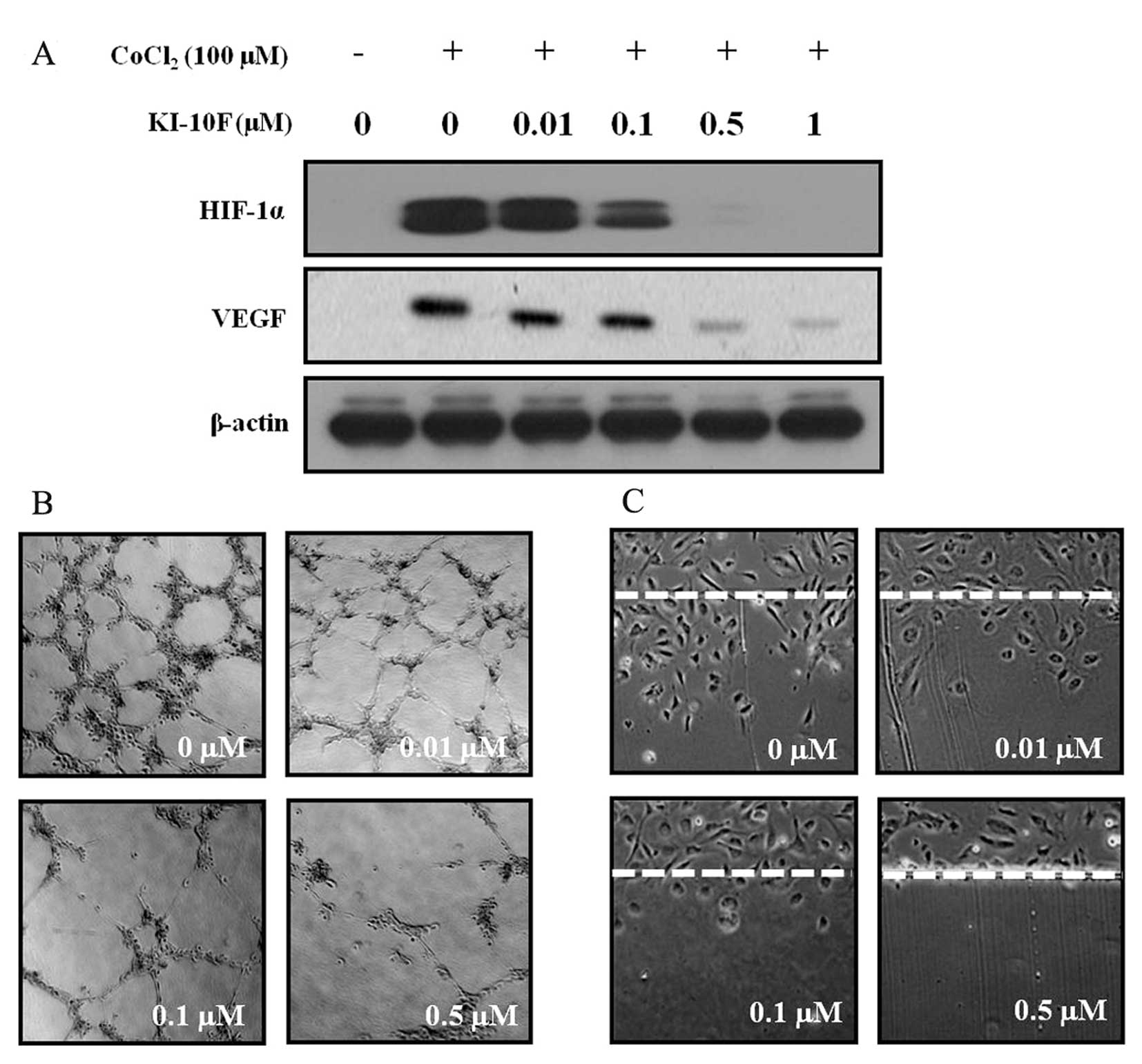
The effects of KI-10F dosing on
angiogenesis
HIF-1α is a major transcriptional modulator of
angiogenic factors such as VEGF. In order to identify the effect of
KI-10F on angio-genesis, HT-29 cells were treated with various
concentration of KI-10F for 6 h under hypoxia mimic conditions
induced by CoCl2 (100 µM), after which we examined the
cellular expression of HIF-1α and VEGF using western blot analysis.
As shown in Fig. 3A, HIF-1α was
highly induced under the hypoxia mimic conditions. However, the
expression of HIF-1α and VEGF decreased in a dose-dependent manner.
Next, we performed an in vitro tube formation assay in order
to identify the anti-angiogenic effect of KI-10F on HUVECs. The
HUVECs, suspended in complete medium (5% FBS) either with or
without KI-10F, were seeded onto a Matrigel layer and the process
of capillary tube formation was monitored and photographed 14 h
post-incubation. We observed that KI-10F administration inhibited
the formation of vessel-like structures consisting of the
elongation and alignment of the cells (Fig. 3B). Endothelial cell migration
supports the formation of blood vessels during tumor growth and
metastasis. Thus, we carried out a wound migration assay in order
to examine the effect of KI-10F on the migration of HUVECs. When
these endothelial cells were wounded and then incubated in media
containing 5% FBS and 1 mM thymidine in the presence of KI-10F (100
μg/ml) over a 24 h period, the wound was unable to heal (Fig. 3C).
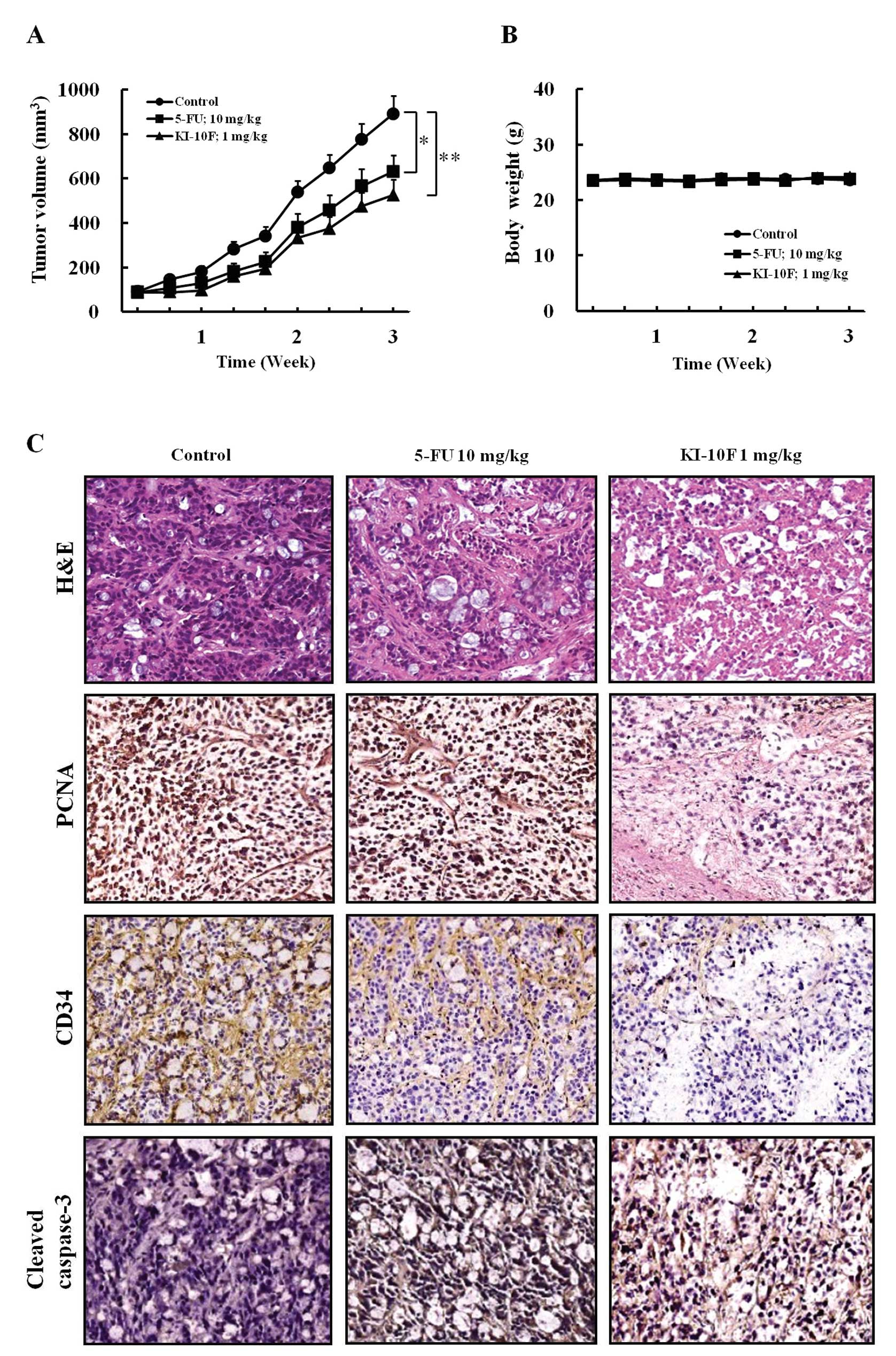
The effects of KI-10F on proliferation,
angiogenesis, and apoptosis in xenograft model
We next examined the in vivo anticancer
activity of KI-10F in an HT-29 cell xenograft model using nude
mice. There was no difference in body weight change in the KI-10F
treated group as compared to the control group (Fig. 4B). This indicated that KI-10F has
low toxicity at the curative dose. As shown in Fig. 4A, 1 mg/kg treatment of KI-10F for 3
weeks inhibited tumor growth as compared to the negative control.
Furthermore, the 1 mg/kg dose of KI-10F was found to suppress tumor
growth more than a 10 mg/kg dose of the commercial available colon
cancer drug, 5-FU (Fig. 4A). Our
results demonstrated the antitumor efficacy of KI-10F against in
vivo colon cancer in a mouse model without any apparent sign of
toxicity.
Using the results of H&E staining and cleaved
caspase-3, we observed that there was a greater degree of tumor
apoptosis and necrosis in the KI-10F treated group than in the
control group (Fig. 4C). In
addition, the IHC results of PCNA showed that administration of
KI-10F decreased the proliferation of tumor tissues. Our in
vitro study showed that KI-10F inhibited angiogenesis and
induced apoptosis. Therefore, in order to evaluate the in
vivo effect of KI-10F on angiogenesis and apoptosis, we
investigated CD34 expression. As observed by microscopic analysis,
the expression of vessel marker CD34 strongly decreased in the
KI-10F treated group as compared to the control group (Fig. 4C). Interestingly, the effects of
KI-10F on proliferation, apoptosis and angiogenesis were more
potent than that of 5-FU as in the in vitro studies.
Discussion
Successfully triggering apoptosis, which causes
irreversible death of cancer cells, is an important hallmark for an
effective chemotherapeutic agent (17). Angiogenesis is essential to the
growth of solid tumors and its inhibition is an effective and
promised target for chemotherapeutics. Therefore, in this study we
investigated anticancer mechanism of the KI-10F in terms of both
apoptosis and anti-angiogenesis. In our previous study, we
synthesized various new pyrazole derivatives and from them we
selected KI-10F which showed high anticancer activity (18). In this regards, we investigated its
possible use as a chemotherapeutic agent against colon cancer cell
proliferation, disrupting the cell cycle, promoting apoptosis and
inhibiting angiogenesis.
Apoptosis is induced by two alternative pathways, an
extrinsic pathway mediated by the death receptor and the intrinsic
pathway mediated by mitochondria (19,20).
In the intrinsic pathway, mitochondrial apoptosis is initiated by
their dysfunction, regulated by the members of the Bcl-2family.
Mitochondria activated by proapoptotic Bcl-2 family members (Bax,
Bak, Bid, etc.) release cytochrome c which binds to
apoptotic protease activating factor 1 (APAF1) to form apoptosome
in the cytosol (21). Apoptosis
through the mitochondrial pathway can be inhibited using
anti-apoptotic proteins of the Bcl-2 family (Bcl-2, Bcl-xl, Bcl-w,
etc.) (22). In the extrinsic
pathway, apoptosis is initiated through the interaction of death
receptors located on the cell surface. When a ligand binds to its
respective death receptor, initiator caspases are recruited
(20,23). Induction of apoptosis leads to
activation of the initiator caspases: caspase-8 for the extrinsic
pathway and caspase-9 for the intrinsic pathway (20). Once the initiator caspases are
activated, they cleave and activate ‘executioner’ caspases:
caspase-3, caspase-6 and caspase-7. Mainly caspase-3 is involved in
the process of apoptosis. As apoptosis plays a pivotal role in the
prevention of cancer, we investigated the apoptotic effect of
KI-10F on HT-29 cells. We observed that KI-10F administration
significantly suppressed the colon cancer cell growth in a
dose-dependent manner. Treatment by KI-10F induced apoptosis in the
HT-29 cells by increasing their expression of cleaved caspase-3, -8
and -9, and Bax. Furthermore, KI-10F dosing decreased the
expression of Bcl-2. In the flow cytometry experiment, DNA
fragmentation and nuclear condensation were confirmed by an
observed increase of cells in the sub-G1 phase. The apoptotic
effects of KI-10F were confirmed by the observed DNA fragmentation,
nuclear condensation, and cell morphology changes as revealed by
TUNEL and DAPI staining. These results implied that the induction
of apoptosis by KI-10F may be a contributing factor in their
suppression of tumor growth. Cell cycle arrest can trigger
proliferation inhibition and apoptosis in cancer cells, and the
G2/M checkpoint is a potential target for a cancer agent (24,25).
Our cell cycle analysis showed that KI-10F administration strongly
induced cell cycle arrest in the G2/M checkpoint.
Inhibition of angiogenesis also can serve as a
potential antitumor therapy. Rapid proliferation of cancer cells
induces intracellular hypoxia conditions, which in turn initiates
the process of the development of new blood vessels (26). In this process, HIF-1α plays an
important role by activating transcription of the promoter of VEGF,
a key factor in tumor angiogenesis (27). Inhibition of tumor angiogenesis can
serve as a potential anti-tumor therapy because angiogenesis is
crucial to tumor growth, invasion and metastasis (28,29).
Supporting this point, our in vitro studies performed under
hypoxia mimic conditions showed that KI-10F inhibited the
expression of HIF-1α and VEGF in HT-29 cells. Thus, KI-10F was
found to inhibit hypoxia induced angiogenesis. Endothelial cells
are the major constituents of new blood vessels whose functioning
is the basis of angiogenesis. In the vasculogenesis process,
endothelial cells migrate on a matrix, such as collagen, and
remodel into tubular structures (30). KI-10F inhibited tube formation and
migration of HUVECs. In addition, the in vivo
anti-angiogenic effect of KI-10F was supported by decreased
expression of CD34, a microvessel endothelial cell.
In this study, we investigated the anticancer
efficacy and associated mechanisms of KI-10F by studying its effect
against colon cancer cells in vitro, and then expanded the
assessment into an in vivo HT-29 xenograft animal model. Our
expanded study revealed that KI-10F inhibited cell and tumor
growth, and induced apoptosis in both HT-29 cells and the xenograft
animal model. In addition, KI-10F suppressed angiogenesis by
decreasing the expression of HIF-1α and VEGF in colon cancer cells,
and inhibited the vasculogenesis process of endothelial cells. In
this regard, it seems that KI-10F has great potential to induce
apoptosis and inhibit angiogenesis. Of note, KI-10F at a dose of 1
mg/kg suppressed greater tumor growth than a 10 mg/kg dose of 5-FU,
which is the current standard care drug used in patients with
advanced colon cancer.
Acknowledgements
This work was supported by the
National R&D Program for Cancer Control (1020250), National
Center of Efficacy Evaluation for the Development of Health
Products Targeting Digestive Disorders (NCEED), Ministry of Health
& Welfare, and National Research Foundation of Korea (NRF)
funded by the Ministry of Education, Science and Technology (NRF
2012-0002988) and Inha University Grant. Also this work was
supported by KIST Program (2E22760).
References
|
1.
|
Shen W, Wang CY, Wang XH and Fu ZX:
Oncolytic adenovirus mediated Survivin knockdown by RNA
interference suppresses human colorectal carcinoma growth in vitro
and in vivo. J Exp Clin Cancer Res. 28:812009. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Potter JD: Colorectal cancer: molecules
and populations. J Natl Cancer Inst. 91:916–932. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Oh BY, Lee RA and Kim KH: siRNA targeting
Livin decreases tumor in a xenograft model for colon cancer. World
J Gastroenterol. 17:2563–2571. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Zhang N, Yin Y, Xu SJ and Chen WS:
5-Fluorouracil: mechanisms of resistance and reversal strategies.
Molecules. 13:1551–1569. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Pullarkat ST, Stoehlmacher J, Ghaderi V,
Xiong YP, Ingles SA, Sherrod A, Warren R, Tsao-Wei D, Groshen S and
Lenz HJ: Thymidylate synthase gene polymorphism determines response
and toxicity of 5-FU chemotherapy. Pharmacogenomics J. 1:65–70.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Manfredini S, Bazzanini R, Baraldi PG,
Bonora M, Marangoni M, Simoni D, Pani A, Scintu F, Pinna E and
Pisano L: Pyrazole-related nucleosides. 4. Synthesis and antitumor
activity of some 1-tetrahydropyranyl-4-substituted pyrazoles.
Anticancer Drug Des. 11:193–204. 1996.PubMed/NCBI
|
|
7.
|
Park HJ, Lee K, Park SJ, Ahn B, Lee JC,
Cho H and Lee KI: Identification of antitumor activity of pyrazole
oxime ethers. Bioorg Med Chem Lett. 15:3307–3312. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Lv PC, Li HQ, Sun J, Zhou Y and Zhu HL:
Synthesis and biological evaluation of pyrazole derivatives
containing thiourea skeleton as anticancer agents. Bioorg Med Chem.
18:4606–4614. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Bandgar BP, Totre JV, Gawande SS,
Khobragade CN, Warangkar SC and Kadam PD: Synthesis of novel
3,5-diaryl pyrazole derivatives using combinatorial chemistry as
inhibitors of tyrosinase as well as potent anticancer,
anti-inflammatory agents. Bioorg Med Chem. 18:6149–6155. 2010.
View Article : Google Scholar
|
|
10.
|
Christodoulou MS, Liekens S, Kasiotis KM
and Haroutounian SA: Novel pyrazole derivatives: synthesis and
evaluation of anti-angiogenic activity. Bioorg Med Chem.
18:4338–4350. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Fan C, Su H, Zhao J, Zhao B, Zhang S and
Miao J: A novel copper complex of salicylaldehyde pyrazole
hydrazone induces apoptosis through up-regulating integrin beta4 in
H322 lung carcinoma cells. Eur J Med Chem. 45:1438–1446. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Calhelha RC, Ferreira IC, Peixoto D, Abreu
RM, Vale-Silva LA, Pinto E, Lima RT, Alvelos MI, Vasconcelos MH and
Queiroz MJ: Aminodi(hetero)arylamines in the thieno[3,2-b] pyridine
series: synthesis, effects in human tumor cells growth, cell cycle
analysis, apoptosis and evaluation of toxicity using non-tumor
cells. Molecules. 17:3834–3843. 2010.
|
|
13.
|
Pracharova J, Zerzankova L, Stepankova J,
Novakova O, Farrer NJ, Sadler PJ, Brabec V and Kasparkova J:
Interactions of DNA with a new platinum(IV) azide dipyridine
complex activated by UVA and visible light: relationship to
toxicity in tumor cells. Chem Res Toxicol. 25:1099–1111. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Weidner N, Semple JP, Welch WR and Folkman
J: Tumor angiogenesis and metastasis - correlation in invasive
breast carcinoma. N Engl J Med. 324:1–8. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Cristi E, Perrone G, Toscano G, Verzi A,
Nori S, Santini D, Tonini G, Vetrani A, Fabiano A and Rabitti C:
Tumour proliferation, angiogenesis, and ploidy status in human
colon cancer. J Clin Pathol. 58:1170–1174. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Chen JM, Li WH, Wang JD, Feng YD, Wu JH
and Gong JP: Cell balance between apoptosis and proliferation in
colon cancer and its correlation with prognosis. Ai Zheng.
24:554–558. 2005.(In Chinese).
|
|
17.
|
Esposito E and Cuzzocrea S: New
therapeutic strategy for Parkinson’s and Alzheimer’s disease. Curr
Med Chem. 17:2764–2774. 2010.
|
|
18.
|
El-Deeb IM and Lee SH: Design and
synthesis of new potent anticancer pyrazoles with high FLT3 kinase
inhibitory selectivity. Bioorg Med Chem. 18:3961–3973. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Schmitz I, Kirchhoff S and Krammer PH:
Regulation of death receptor-mediated apoptosis pathways. Int J
Biochem Cell Biol. 32:1123–1136. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Igney FH and Krammer PH: Death and
anti-death: tumour resistance to apoptosis. Nat Rev Cancer.
2:277–288. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Soengas MS, Capodieci P, Polsky D, Mora J,
Esteller M, Opitz-Araya X, McCombie R, Herman JG, Gerald WL and
Lazebnik YA: Inactivation of the apoptosis effector Apaf-1 in
malignant melanoma. Nature. 409:207–211. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Nagane M, Levitzki A, Gazit A, Cavenee WK
and Huang HJ: Drug resistance of human glioblastoma cells conferred
by a tumor-specific mutant epidermal growth factor receptor through
modulation of Bcl-XL and caspase-3-like proteases. Proc Natl Acad
Sci USA. 95:5724–5729. 1998. View Article : Google Scholar
|
|
23.
|
Pitti RM, Marsters SA, Lawrence DA, Roy M,
Kischkel FC, Dowd P, Huang A, Donahue CJ, Sherwood SW and Baldwin
DT: Genomic amplification of a decoy receptor for Fas ligand in
lung and colon cancer. Nature. 396:699–703. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Xu X, Zhang Y, Qu D, Jiang T and Li S:
Osthole induces G2/M arrest and apoptosis in lung cancer A549 cells
by modulating PI3K/Akt pathway. J Exp Clin Cancer Res. 30:332011.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Yang CJ, Wang CS, Hung JY, Huang HW, Chia
YC, Wang PH, Weng CF and Huang MS: Pyrogallol induces G2-M arrest
in human lung cancer cells and inhibits tumor growth in an animal
model. Lung Cancer. 66:162–168. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Carmeliet P, Dor Y, Herbert JM, Fukumura
D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R and
Maxwell P: Role of HIF-1alpha in hypoxia-mediated apoptosis, cell
proliferation and tumour angiogenesis. Nature. 394:485–490. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Bolat F, Haberal N, Tunali N, Aslan E, Bal
N and Tuncer I: Expression of vascular endothelial growth factor
(VEGF), hypoxia inducible factor 1 alpha (HIF-1alpha), and
transforming growth factors beta1 (TGFbeta1) and beta3 (TGFbeta3)
in gestational trophoblastic disease. Pathol Res Pract. 206:19–23.
2010. View Article : Google Scholar
|
|
28.
|
Spano D and Zollo M: Tumor
microenvironment: a main actor in the metastasis process. Clin Exp
Metastasis. 29:381–395. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Tao BB, Zhang CC, Liu SY and Zhu YC:
Involvement of HIF-1 in the migration-promoting effects of hydrogen
sulfide in vascular endothelial cells under normoxic conditions.
Sheng Li Xue Bao. 64:129–134. 2012.(In Chinese).
|
|
30.
|
Ergun S, Kilic N, Wurmbach JH,
Ebrahimnejad A, Fernando M, Sevinc S, Kilic E, Chalajour F, Fiedler
W and Lauke H: Endostatin inhibits angiogenesis by stabilization of
newly formed endothelial tubes. Angiogenesis. 4:193–206. 2001.
View Article : Google Scholar : PubMed/NCBI
|