Introduction
Chemokines were first described as chemoattractant
cytokines synthesized at sites of inflammation and are the major
regulatory proteins for leukocyte recruitment and trafficking
(1,2). Chemokines are subdivided into four
families, C, CC, CXC and CX3C, based on the number and spacing of
the first two cysteines in a conserved cysteine structural motif
(3). CX3CL1 (also known as
fractalkine), the sole member of the CX3C class of chemokines, is
unique, as it can exist in both soluble and membrane-anchored forms
(4–6). The cognate receptor of CX3CL1 is a
G-protein coupled receptor, named CX3CR1 (7). Along with strong expression on
certain leukocyte populations, such as macrophages, lymphocytes,
and natural killer cells, CX3CR1 is abundant on glial cells and
astrocytes (8,9). Recently, CX3CR1 expression in tumors
was confirmed (10–19). In addition, metastasis of cancer
cells to the skeleton mediated by CX3CL1-CX3CR1 binding was also
reported in a variety of tumors, including prostate cancer
(10,12,15).
Prostate cancer is the second-leading cause of
cancer death in western males with an estimated 240,890 new cases
and 33,720 deaths in the USA in 2011 (20). Although early stage prostate
cancers are not life threatening, development of metastatic
prostate cancers is responsible for the majority of cancer-related
death. Clinical observations provide evidence that despite current
therapies, about one third of prostate cancers invade surrounding
tissue, metastasize to distant organs, and consequently cause death
(21,22). Survival of a patient with prostate
cancer is directly related to the spread of the tumor (23).
Hypoxia, a common phenomenon in solid tumors, is
associated with malignant progression and resistant to radiotherapy
and chemotherapy (24–26). Clinical studies also demonstrated
that low oxygen tension in neoplasm is an independent prognostic
indicator of poor outcome and correlates with an increased risk to
develop distant metastasis with prostate cancer, independent of
therapeutic treatment (27).
The pathophysiological responses of tumor cells to
hypoxia arise from specific alterations in the expression of a
large number of genes, which are regulated directly or indirectly
by transcription factors. The activity of several transcription
factors, including hypoxia-inducible factor-1 (HIF-1), nuclear
factor-κB (NF-κB), AP-1, p53 and early growth response-1 (Egr-1)
are upregulated in hypoxic cancer cells, which subsequently
activate target genes expression to allow the tumor to adapt to
decreased oxygen levels (28,29).
Lack of oxygen causes the cells of tumor to spread
to new locations (30). Chemokines
and their receptors have been demonstrated to play major roles in
the process of metastasis. Multiple cancers are found to express
chemokine receptors, while the corresponding ligands of the
receptors are expressed at sites of tumor metastases (10,12,15,31,32).
Numerous studies showed that hypoxia can promote cell migration and
invasion of several different types of tumor by upregulating the
specific chemokine receptors, such as CCR2, CXCR1, CXCR2, CXCR4 and
CXCR6 (33–37). However, whether hypoxia mediates
the metastatic property of cancer cells through regulating the
CX3CR1 expression has not been examined previously. The regulation
pattern of CX3CR1 expression in hypoxic cancer cells remains to be
elucidated.
The current study was performed to determine whether
hypoxia regulates CX3CR1 expression and promotes metastasis of
prostate cancer cells by CX3CL1/CX3CR1-axis, as well as the
mechanism involved in the process. Our data showed that the
expression of CX3CR1 was upregulated by hypoxia, which resulted in
an increasing sensitivity to CX3CL1-stimulated migration and
invasion in prostate cancer cells.
Materials and methods
Cell culture and hypoxic treatment
Human prostate cancer cell lines DU145 and PC-3 were
purchased from American Type Culture Collection (Manassas, VA, USA)
and PC-3M was obtained from the Peking University Health Science
Center (Beijing, China). Cells were maintained in RPMI-1640 medium
containing 10% fetal bovine serum (FBS) (Gibco-BRL, Grand Island,
NY, USA) and 1x penicillin/streptomycin (Invitrogen, Carlsbad, CA,
USA) at 37°C in a humidified atmosphere (5% CO2/95%
air). For experiments involving hypoxia, cells were incubated in a
hypoxic chamber (Thermo Scientific) maintained at 1% O2,
5% CO2 and 94% N2 at 37°C for 24–48 h.
CoCl2 (100 μM) (Sigma, St. Louis, MO, USA) or 500 μM DFX
(Sigma) was supplemented in the medium to mimic the hypoxic
environment under standard culture conditions.
RNA isolation and semi-quantitative
real-time PCR
Total-RNA from cells in normoxic or hypoxic
conditions was extracted using TRIzol reagent (Invitrogen). RNA was
converted to cDNA with Superscript II reverse transcriptase
(Invitrogen) by following the manufacturer’s instructions.
Subsequently PCR was performed using ABI 7500 real-time PCR system
(Applied Biosystems). Primers used in this study were: β-actin,
sense: 5′-TACCTCATGAAGATCCTCACC-3′; antisense: 5′-TTT
CGTGGATGCCACAGGAC-3′; CX3CR1, sense: 5′-CTTCTG GTGGTCATCGTG TT-3′;
antisense: 5′-GGTATCTTCTGAAC TTCTCCC-3′; CXCR4, sense:
AATCTTCCTGCCCACCA TCT; antisense: GACGCCAACATAGACCACCT; Bcl-2,
sense: AAAGGACCTGATCATTGGGG; antisense: CAACTCTTTT CCTCCCACCA. All
the primers were purchased from Sangon Biotech Co. Ltd. (Shanghai,
China). Annealing temperature was ∼58.5–60.5°C for CX3CR1 and 55°C
for CXCR4, Bcl-2 and β-actin. Amplification cycles were 35 for
CX3CR1, 28 for CXCR4 and Bcl-2, and 23 for β-actin. Each sample was
tested in triplicate for analysis of relative gene expression and
transcript levels of β-actin were monitored as internal
control.
Preparation of total cell protein and
nuclear fractions
Cells cultured in normoxic or hypoxic conditions, or
treated with different chemicals were washed with cold PBS and
lysed in ice-cold lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China) containing protease inhibitor
cocktail (Sigma) and phosphatase inhibitor cocktail (Sigma) for 10
min on ice. The lysate was centrifuged at 12,000 x g at 4°C for 15
min and the supernatant was collected.
The nuclear protein was prepared using Nuclear and
Cytoplasmic Protein Extraction kit according to the manufacturer’s
instructions (Beyotime Institute of Biotechnology). Briefly, cells
were washed in ice-cold PBS and the pellet was resuspended in
cytoplasmic protein extraction agent A supplemented with 1 mM PMSF.
After incubated on ice for 15 min, the cytoplasmic protein
extraction agent B was added and the tubes were incubated on ice
for 1 min. Then the samples were centrifuged for 5 min at 14,000 x
g at 4°C. The pellet was resuspended in nuclear protein extraction
agent supplemented with PMSF. After vortexing the tubes ∼15–20
times for 30 min and centrifuging for 10 min at 14,000 x g, the
supernatants containing the nuclear extracts were obtained. The
protein concentrations were determined using the BCA Protein Assay
Reagent (Beyotime Institute of Biotechnology).
Western blot analysis
Total protein extraction was subjected to western
blot analysis of CX3CR1 and β-actin, and nuclear fraction was
subjected to western blot analysis of HIF-1α, p65 and lamin B.
Equal amounts of protein (60 µg/lane) was loaded, fractionated by
12% SDS-PAGE and transferred onto nitrocellulose membrane by
electro-blotting. After blocked with 5% fat-free milk for 2 h at
room temperature, membranes were blotted with rabbit anti CX3CR1
polyclonal antibody (Abcam, Cambridge, MA, USA), rabbit anti
β-actin polyclonal antibody (Abmart, Omaha, NE, USA), mouse
anti-HIF-1α monoclonal antibody (Santa Cruz Biotechnology, Santa
Cruz, CA, USA), rabbit anti p65 polyclonal antibody and rabbit
anti-lamin B polyclonal antibody (Proteintech group. Inc.) at 4°C
overnight. Bound primary antibody was detected by incubating with
appropriate horse-radish peroxidase-conjugated secondary antibodies
(Proteintech group Inc.) for 2 h. Immunoreactive bands were
visualized using the western blot analysis Super ECL plus Detection
Reagents (Applygen Technologies Inc., Beijing, China). The
expression levels of β-actin or lamin B were monitored as internal
control.
Stable transfection of
pcDNA3.1-CX3CR1
The pcDNA3.1-CX3CR1 was constructed by our
laboratory. cDNA encoding human CX3CR1 was inserted into the
pcDNA3.1-vector. DU145 cells were transfected with 2 μg
pcDNA3.0-CX3CR1 (DU145-CX3CR1) or pcDNA3.1 empty vector
(DU145-Mock) using Lipofectamine 2000 (Invitrogen) according to
manufacturer’s instructions. G418 (800 μg/ml) (Gibco-BRL) was
applied to select G418 resistant cells.
siRNA transfection
For siRNA treatment, oligonucleotides corresponding
to nucleotide sequences of CX3CR1 and HIF-1α were synthesized
commercially by Invitrogen and used at the concentration of 100 nM.
A scrambled oligonucleotide was included in all experiments as
negative control at the same concentration. Sequences are as
follows: CX3CR1-siRNA, 5′-UUUGUUUCCACAUUGCGGAGCACGG-3′;
HIF-1α-siRNA, 5′-AUAUGAUUGUGUCUCCAGCGGCUGG-3′. Signal Silence NF-κB
p65 siRNA kit was purchased from Cell Signaling Technology and
trasfection was performed according to the manufacturer’s
recommendations. The transfected cells were collected at 48 or 72 h
for mRNA isolation and protein extraction, respectively.
Flow cytometry analysis
Cells cultured in normoxia or hypoxia, or treated
with different chemicals such as siRNAs, DMSO (Sigma), YC-1 (Sigma)
and PDTC (Beyotime Institute of Biotechnology) were suspended in
ice-cold PBS and washed two times. Then cells were incubated with
FITC-conjugated anti-human CX3CR1 antibody (Biolegend, San Diego,
CA, USA) or FITC-conjugated rat IgG2b κ isotype control (Biolegend)
at 4°C for 30 min and washed twice with ice-cold PBS. The samples
were finally analyzed by flow cytometer (FACSCalibur, BD
Biosciences, San Jose, CA, USA) using CellQuest software.
Migration and invasion assay
Cell invasion assay was performed using
Matrigel-coated 24-well Transwell inserts containing poly-carbonate
filters with 8 μm pores (BD Biosciences) according to
manufacturer’s instructions. Briefly, the inserts were prehydrated
with 500 μl/well serum-free RPMI-1640 medium for 2 h at 37°C in a
humidified atmosphere (5% CO2/95% air). Test cells
1x105 in 200 μl serum-free RPMI-1640 medium were seeded
onto the upper chambers, whereas 600 μl complete medium containing
∼0–400 ng/ml recombinant human CX3CL1 (Proteintech group Inc.) was
added to the lower wells. Cells were allowed to invade for 24 h in
hypoxic or normoxic environment. In some experiments, cells were
preincubated 1 h with 3 μg/ml neutralizing antibody against human
CX3CR1 (Abcam), rabbit IgG-matched polyclonal antibody (Abcam), 100
ng/ml PTX (Sigma), 50 µM DMSO, 50 µM YC-1 or 100 µM PDTC before
seeding, respectively. In siRNA transfection experiments, cells
were transfected with scrambled-siRNA, CX3CR1-siRNA, HIF-1α-siRNA
or p65-siRNA for 72 h, then collected and plated onto the upper
chamber. The non-invasive cells on the upper surface of the
membrane were removed by a cotton swab, and the invaded cells
attached to the lower membrane surface were fixed in 4%
paraformaldehyde methanol for ∼10–15 min, subsequently stained with
0.1% crystal violet for 30 min. The number of invading cells was
then counted for a given well under a light microscope (Olympus
IX51, Japan) at a magnification of x200. Five random fields were
numerically averaged and counted for each assay. The invasion index
was calculated by dividing the number of invading cells with the
number of cells that invaded in control group and was expressed as
percentages of control values, as previously described (38). Cell migration assay was performed
through a similar approach without Matrigel coating, and cells in
migration chamber were allowed to migrate for 16 h.
Statistical analysis
Statistical analyses were performed using the SAS
system, version 9.1.3. Quantification of the bands from western
blot analysis were determined by MetaView Image Analyzing System
(Version 4.50; Universal Imaging Corp., Downingtown, PA, USA) and
each band was normalized by its corresponding control. Each
experiment was replicated in triplicate. Results were expressed as
mean ± SEM. p<0.05 was considered as statistically
significant.
Results
Expression of CX3CR1 in prostate cancer
cells
CX3CR1 expression was firstly examined in six
prostate cancer cell lines, which were derived from metastases
localized to different tissues in vivo. Human monocytic
THP-1 cells, which constitutively express high level of CX3CR1,
were used as positive control (Fig.
1). We found that all the cell lines tested were positive for
CX3CR1, indicating that CX3CR1 expression is a common character for
prostate cancer cells. However, the expression level of the protein
was much lower in DU145 cells than that of the other cell lines.
Therefore, DU145 cells, together with PC-3 and PC-3M were selected
for hypoxia treatment because of their relatively low endogenous
CX3CR1 protein expression.
Hypoxia enhances CX3CR1 expression in
prostate cancer cells
We exposed DU145, PC-3 and PC-3M cells to 1%
O2 for the indicated times, and performed a dynamic
analysis to examine the changes in CX3CR1 expression. As shown in
Fig. 2A, the expression of CX3CR1
mRNA was strongly increased with culture time in hypoxia-exposed
cells. The expression remained elevated until at least 24 h.
Because of the hypoxia-mimicking effect, cobalt chloride
(CoCl2) and iron chelator Deferoxamine (DFX) have been
used extensively to study the hypoxic signaling pathway. We also
incubated prostate cancer cells with either 100 μM CoCl2
or 500 μM DFX under normoxic conditions for 24 h. The results
showed, that both CoCl2-treatment and DFX-treatment
increased CX3CR1 protein expression in DU145, PC-3 and PC-3M cells
similarly to 1% O2 (Fig. 2B
and C). Increased surface expression levels of CX3CR1 in the
three cell types after exposure to 1% O2 were also
confirmed by flow cytometry (Fig.
2D).
Hypoxia activates HIF-1 and NF-κB and
induces their target gene expression in DU145 cells
HIF-1 is one of the major regulators of the cellular
response to hypoxia. Western blot analysis revealed a rapid
increase in HIF-1α protein accumulation in nuclear extracts from
hypoxia-exposed DU145 cells. HIF-1α protein level remained elevated
following exposure to hypoxia for 12 h (Fig. 3A). CXCR4, the best characterized
chemokines, has been shown to play critical roles in tumor
progression and metastasis. Studies demonstrated that hypoxia
markedly enhanced CXCR4 expression through HIF-1 in solid tumors.
Therefore, to confirm HIF-1 transcriptional activity in hypoxic
DU145 cells, we performed real-time PCR to analyze the mRNA
expression levels of CXCR4. We found that hypoxia increased CXCR4
mRNA transcript levels in a time-dependent manner (Fig. 3B). Then, we employed siRNA strategy
to deplete HIF-1α expression in DU145 cells and re-examined CXCR4
mRNA expression levels under hypoxic condition. The hypoxia-induced
HIF-1α protein accumulation in nuclear extracts and CXCR4 mRNA
expression were greatly inhibited in the presence of HIF-1α-siRNA
compared with scrambled-siRNA (Fig. 3C
and D). The results confirmed that HIF-1 is active and could
regulate its target gene expression in hypoxic DU145 cells.
NF-κB is another transcription factor that can be
activated by hypoxia. As shown in Fig.
4A and B, hypoxia increased p65 protein accumulation in nuclear
extracts and induced the mRNA expression of known NF-κB target gene
Bcl-2 in DU145 cells. Thereafter, we established p65-siRNA for
inhibition of NF-κB activity. Suppression of p65 expression using
siRNA blocked hypoxia-induced p65 nuclear translocation and
decreased Bcl-2 mRNA transcription (Fig. 4C and D). Taken together, the
results confirmed that the transcription factors HIF-1 and NF-κB
are all active in DU145 cells under hypoxic conditions.
Hypoxia increases CX3CR1 expression by
HIF-1 and NF-κB
Next, we investigated the roles of HIF-1 and NF-κB
in the hypoxia-induced CX3CR1 upregulation in DU145 cells. As
demonstrated in Fig. 5A and B,
CX3CR1 mRNA and protein expression levels were reduced greatly in
HIF-1α-siRNA transfected cells, compared with scrambled-siRNA.
Accordingly, preincubation of DU145 cells with HIF-1α inhibitor
YC-1 drastically decreased hypoxia-induced CX3CR1 expression both
at mRNA and protein levels. The inhibitory effects of HIF-1α-siRNA
and YC-1 were also confirmed on CX3CR1 surface expression level
(Fig. 5C). Similar responses in
attenuating CX3CR1 mRNA, protein and surface expression levels were
obtained following pretreatment of DU145 cells with p65-siRNA or
NF-κB inhibitor PDTC, respectively (Fig. 5D–F). Taken together, these data
suggest that HIF-1 and NF-κB are involved in CX3CR1 upregulation
induced by hypoxia.
HIF-1 and NF-κB regulate CX3CL1-induced
metastasis of DU145 cells under hypoxic conditions
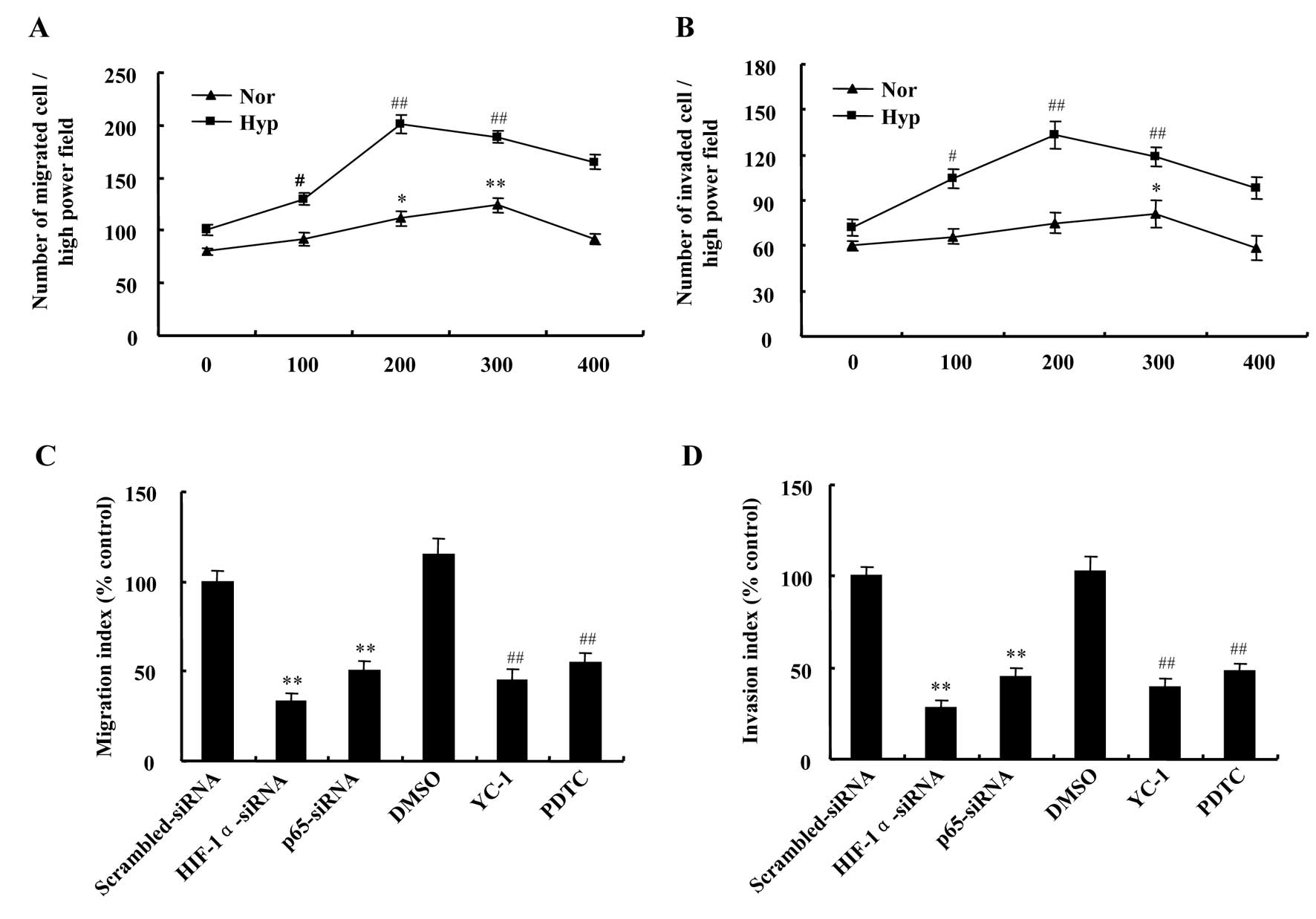
Migration and invasion assays were performed to test
the effect of hypoxia on metastasis of DU145 cells in response to
CX3CL1. The data demonstrated chemotaxis toward CX3CL1 was greatly
changed in a dose-dependent manner and was increased under hypoxic
conditions compared with normoxic. The number of migratory cells
was the highest at the concentration of 300 ng/ml CX3CL1 in
normoxia, but in hypoxia at the concentration of 200 ng/ml
(Fig. 6A). In accordance with
migration assay, number of invaded cells was the highest at the
concentration of 300 ng/ml and 200 ng/ml CX3CL1 in normoxic and
hypoxic conditions, respectively (Fig.
6B).
As mentioned above, we have verified the roles of
HIF-1 and NF-κB in regulation of CX3CR1 expression induced by
hypoxia in DU145 cells. Based on these results, we continued to
explore the contribution of HIF-1 and NF-κB to the chemotactic
response of DU145 cells towards CX3CL1 in hypoxia. The results
showed that hypoxia-induced migration and invasion of DU145 cells
at 200 ng/ml CX3CL1 were attenuated by downregulating HIF-1α or p65
expression, which was more significant in HIF-1α knockdown cells
than in those of p65 knockdown (Fig.
6C and D). In addition, HIF-1α inhibitor YC-1 and NF-κB
inhibitor PDTC were also included in this experiment. As expected,
treatment with YC-1 or PDTC prevented the migratory and invasive
responses to CX3CL1 of DU145 cells under hypoxic conditions
(Fig. 6C and D). The results
showed that HIF-1 and NF-κB play important roles in metastasis of
hypoxic DU145 cells.
The involvement of CX3CR1 in
hypoxia-induced migration and invasion of DU145 cells
In order to clarify the role of CX3CR1 in prostate
cancer cells metastasis, siRNA transfection of DU145 cells for
downregulating CX3CR1 was performed, and the transfection
efficiency was tested by western blot and flow cytometry analysis.
Results in Fig. 7A and B show that
both protein and cell surface levels of CX3CR1 in CX3CR1-siRNA
cells were downregulated significantly compared with
scrambled-siRNA cells. Migration and invasion assays demonstrated
that chemotaxis toward CX3CL1 was greatly decreased in CX3CR1-siRNA
cells compared to scrambled-siRNA cells under hypoxic environment
(Fig. 7C and D).
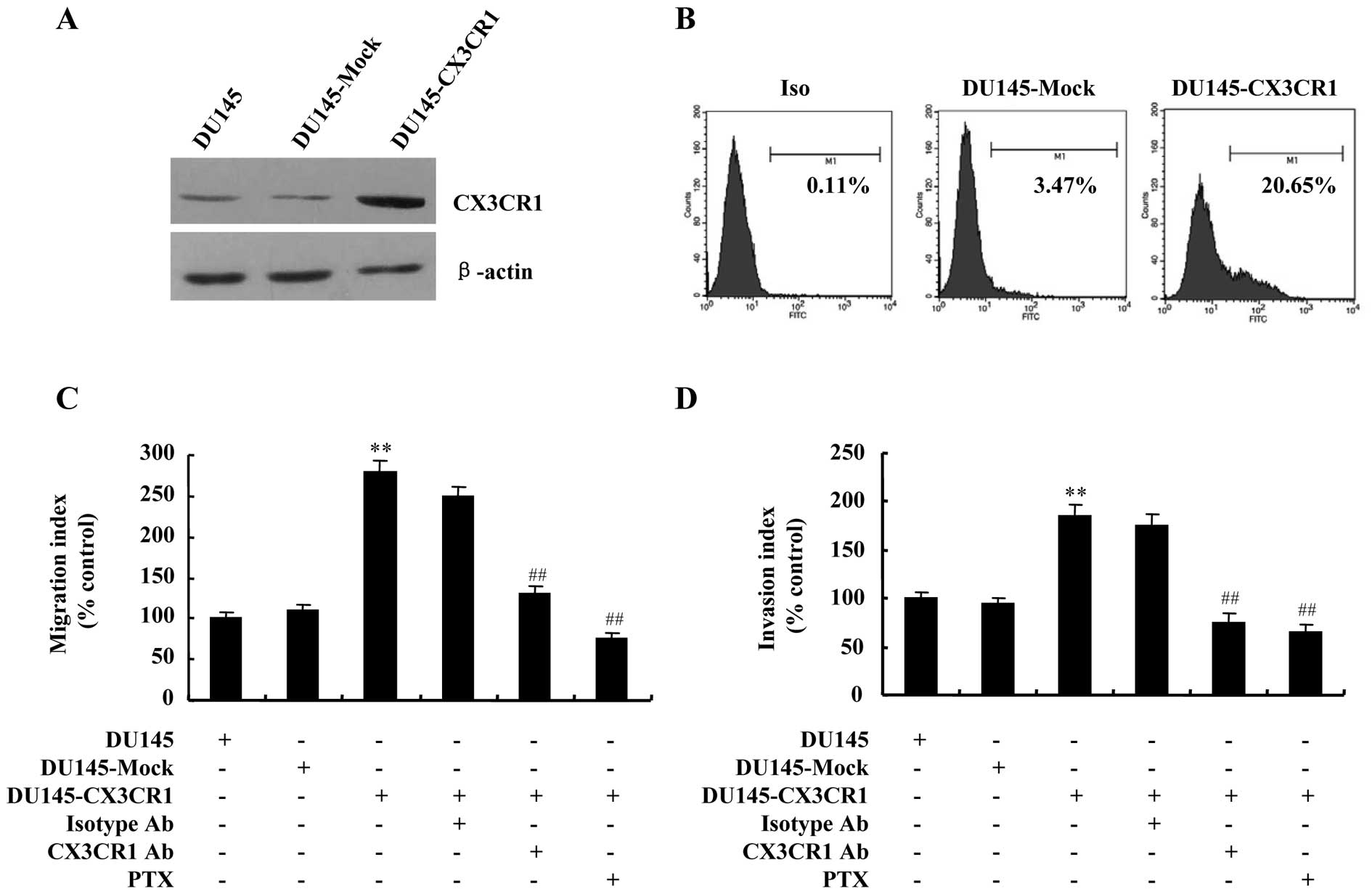
To further verify the contribution of CX3CR1 to
DU145 cells invasiveness, stable transfection of the cells with a
plasmid for overexpression of human CX3CR1 was also employed in
this study to establish high levels of CX3CR1. As shown in Fig. 8A and B, stable CX3CR1 transfectant,
namely DU145-CX3CR1, was detected to have increased protein and
cell surface levels of CX3CR1 compared with control vector
transfectant, the DU145-Mock. Migration and invasion assays were
also performed to determine if CX3CR1 upregulation increased the
migratory and invasive ability of DU145 cells. The results showed
that the number of migrated and invaded cells increased
significantly in DU145-CX3CR1 cells compared to that of DU145-Mock
(Fig. 8C and D).
Since CX3CR1 is a Gi-coupled receptor, and
inhibitory effect of pertussis toxin (PTX), the Gi-protein-specific
inhibitor, was observed in the migration of HUVECs stimulated by
CX3CL1, we preincubated DU145-CX3CR1 cells with PTX for 1 h before
chemotaxis in a further test of specificity. As expected, the
migratory and invasive responses to CX3CL1 were significantly
blocked by treatment with PTX. Similarly, neutralizing anti-CX3CR1
antibody also attenuated CX3CL1-induced migration and invasion of
DU145-CX3CR1 cells (Fig. 8C and
D).
Discussion
Chemokines are secreted chemoattractants that induce
cell migration by activating chemokine receptors. Migrating cells
follow a chemokine gradient that is thought to form through
chemokine diffusion and binding to extra-cellular
glycosaminoglycans of the seven transmembrane G-protein coupled
receptors (39). These signaling
proteins regulate leukocytes accumulation and activation in normal
inflammatory processes, but are also implicated in many disease
states, including HIV-1/AIDS, arthritis, asthma and Crohn’s disease
(40). Furthermore, the broader
impact on tumor growth and metastasis of chemokines has been
appreciated in the last few years. A number of chemokine receptors
have been detected in haematological and solid malignancies. As a
result, chemokines and their receptors are extensively studied as
an area for pharmaceutical intervention. Although the importance of
CX3CR1 has been shown in tumor metastasis, the regulation of CX3CR1
expression in epithelial-derived cancers is poorly understood. In
the present work, we have investigated the effect of hypoxia on
CX3CR1 expression in androgen-independent prostate cancer cells and
the role of CX3CL1/CX3CR1-axis in cancer cells metastasis under
hypoxic environment.
We firstly examined the expression levels of CX3CR1
in six prostate cancer cell lines. We found the expression of
CX3CR1 is a common feature of prostate cancer cells. This is in
accordance with previous research (12). Then we observed that CX3CR1
expression can be regulated by hypoxia in prostate cancer cells.
CX3CR1 mRNA and protein expression were strongly upregulated in
DU145, PC-3 and PC-3M cells treated with 1% O2, 100 μM
CoCl2 or 500 μM DFX for 24 h, as well as CX3CR1 surface
expression level. The results showed that hypoxia could upregulate
CX3CR1 expression in prostate cancer cells.
Many cellular responses to hypoxia are mediated by
HIF-1 and NF-κB. We found hypoxia increased the activity of HIF-1
and NF-κB by analyzing the transcript levels of the known target
genes of HIF-1 and NF-κB, respectively. Previous studies showed
that NF-κB and HIF-1 play important roles in the regulation of
CXCR1 and CXCR2 in response to hypoxia in prostate cancer cells
(41). Therefore, we investigated
whether HIF-1 and NF-κB contribute to the regulation of CX3CR1
expression in prostate cancer cells under hypoxic conditions. Our
studies showed that CX3CR1 protein and surface expression were
greatly reduced when HIF-1 and NF-κB were downregulated by siRNAs
specific for HIF-1α and p65, respectively. In addition, YC-1 and
PDTC, inhibitors for HIF-1 and NF-κB activity, also decreased
hypoxia-induced CX3CR1 expression. The results indicated that
hypoxia regulates CX3CR1 expression via both HIF-1 and NF-κB in
DU145 cells.
Experimental and clinical studies pointed to the
important roles of HIF-1 and NF-κB in tumor invasion and metastasis
(42–44). In the current study we investigated
whether HIF-1 and NF-κB are involved in migration and invasion of
DU145 cells under hypoxic conditions. Previous study showed that
prostate cancer cells migrate toward a medium conditioned by
osteoblasts, which secrete the soluble form of CX3CL1 (12). Therefore, we tested the impact of
HIF-1 and NF-κB activity on the chemotactic response of hypoxic
prostate cancer cells towards CX3CL1. Our results showed hypoxia
increased greatly the migration and invasion of DU145 cell response
to CX3CL1. With the increasing concentration of CX3CL1, the
migration and invasion also increased. When the expression of
HIF-1α and p65 were downregulated by siRNAs, the above phenomena
including migration and invasion were also reduced, respectively.
This chemotaxis was also sensitive to inhibitor YC-1 or PDTC.
Collectively, HIF-1 and NF-κB are essential for CX3CL1-induced
metastasis in DU145 cells under hypoxic environment.
Studies have demonstrated that hypoxia increases
CXCL12-induced metastatic ability of breast cancer cells via
upregulation of CXCR4 (45).
Consistently, hypoxia promotes CCL21-induced metastasis of lung
cancer cells by inducing the expression of CCR7 (46). Based on these results, we presumed
that hypoxia increases migration and invasion of prostate cancer
cells through upregulating CX3CR1 expression. We found CX3CR1 mRNA,
protein, and surface expression were significantly increased in
DU145, PC-3 and PC-3M cells by exposure to hypoxia or reagents that
mimic the response to hypoxia. However, inhibition of CX3CR1
expression by siRNA attenuated the hypoxia-induced migration and
invasion of DU145 cells indicating that hypoxia promotes
CX3CL1-induced metastasis of prostate cancer cells via upregulation
of CX3CR1. To further verify the contribution of CX3CR1 to cell
metastasis, we performed stable transfection of DU145 cells for
CX3CR1 overexpression. The stable CX3CR1 transfectant exhibited
high level of CX3CR1 expression, increased cell migration and
invasion response to CX3CL1 were also detected, indicating that
CX3CR1 expression levels are correlated with invasive ability of
DU145 cells. Thus, our results showed that HIF-1 and NF-κB are
prerequisite for migration and invasion through upregulation of
CX3CR1 in hypoxic prostate cancer cells.
In summary, the study presented here demonstrated
that hypoxia increased CX3CL1-induced migration and invasion of
prostate cancer cells by upregulating CX3CR1 expression via HIF-1
and NF-κB. We also showed that CX3CR1, as well as HIF-1 and NF-κB
might represent suitable targets for therapies aiming at metastatic
prostate adenocarcinoma.
Abbreviations:
|
CX3CR1
|
CX3C chemokine receptor 1
|
|
HIF-1α
|
hypoxia-inducible factor-1α
|
|
NF-κB
|
nuclear factor-κB
|
|
Egr-1
|
early growth response-1
|
|
siRNA
|
small interfering RNA
|
|
CoCl2
|
cobalt chloride
|
|
DFX
|
deferoxamine
|
|
PTX
|
pertussis toxin
|
|
YC-1
|
3-(5′-Hydroxymethyl-2′-furyl)-1-benzyl
indazole
|
|
PDTC
|
pyrrolidinecarbodithioc acid ammonium
salt
|
Acknowledgements
This study was supported by grants
from the National Natural Science Foundation of China (no.
30973010), the Natural Science Foundation of Heilongjiang Province
of China (no. QC2009C115), the Foundation of Heilongjiang
Educational Committee (no. 11551227) and the Innovation Fund
Project for Graduate Student of Heilongjiang Province, China (no.
YJSCX2009-218HLJ).
References
|
1.
|
Baggiolini M, Dewald B and Moser B: Human
chemokines: an update. Annu Rev Immunol. 15:675–705. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Rollins BJ: Chemokines. Blood. 90:909–928.
1997.PubMed/NCBI
|
|
3.
|
Goda S, Imai T, Yoshie O, et al:
CX3C-chemokine, fractalkine-enhanced adhesion of THP-1 cells to
endothelial cells through integrin-dependent and -independent
mechanisms. J Immunol. 164:4313–4320. 2000. View Article : Google Scholar
|
|
4.
|
Bazan JF, Bacon KB, Hardiman G, et al: A
new class of membrane-bound chemokine with a CX3C motif. Nature.
385:640–644. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Pan Y, Lloyd C, Zhou H, et al:
Neurotactin, a membrane-anchored chemokine upregulated in brain
inflammation. Nature. 387:611–617. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Fong AM, Robinson LA, Steeber DA, et al:
Fractalkine and CX3CR1 mediate a novel mechanism of leukocyte
capture, firm adhesion, and activation under physiologic flow. J
Exp Med. 188:1413–1419. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Combadiere C, Salzwedel K, Smith ED,
Tiffany HL, Berger EA and Murphy PM: Identification of CX3CR1. A
chemotactic receptor for the human CX3C chemokine fractalkine and a
fusion coreceptor for HIV-1. J Biol Chem. 273:23799–23804. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Imai T, Hieshima K, Haskell C, et al:
Identification and molecular characterization of fractalkine
receptor CX3CR1, which mediates both leukocyte migration and
adhesion. Cell. 91:521–530. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Nishiyori A, Minami M, Ohtani Y, et al:
Localization of fractalkine and CX3CR1 mRNAs in rat brain: does
fractalkine play a role in signaling from neuron to microglia? FEBS
Lett. 429:167–172. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Jamieson-Gladney WL, Zhang Y, Fong AM,
Meucci O and Fatatis A: The chemokine receptor CX3CR1 is directly
involved in the arrest of breast cancer cells to the skeleton.
Breast Cancer Res. 13:R912011. View
Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Valdivia-Silva JE, Franco-Barraza J, Silva
AL, et al: Effect of pro-inflammatory cytokine stimulation on human
breast cancer: implications of chemokine receptor expression in
cancer metastasis. Cancer Lett. 283:176–185. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Shulby SA, Dolloff NG, Stearns ME, Meucci
O and Fatatis A: CX3CR1-fractalkine expression regulates cellular
mechanisms involved in adhesion, migration, and survival of human
prostate cancer cells. Cancer Res. 64:4693–4698. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Jamieson WL, Shimizu S, D’Ambrosio JA,
Meucci O and Fatatis A: CX3CR1 is expressed by prostate epithelial
cells and androgens regulate the levels of CX3CL1/fractalkine in
the bone marrow: potential role in prostate cancer bone tropism.
Cancer Res. 68:1715–1722. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Marchesi F, Piemonti L, Fedele G, et al:
The chemokine receptor CX3CR1 is involved in the neural tropism and
malignant behavior of pancreatic ductal adenocarcinoma. Cancer Res.
68:9060–9069. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Nevo I, Sagi-Assif O, Meshel T, et al: The
involvement of the fractalkine receptor in the transmigration of
neuroblastoma cells through bone-marrow endothelial cells. Cancer
Lett. 273:127–139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Andreasson U, Ek S, Merz H, et al: B cell
lymphomas express CX3CR1 a non-B cell lineage adhesion molecule.
Cancer Lett. 259:138–145. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Erreni M, Solinas G, Brescia P, et al:
Human glioblastoma tumours and neural cancer stem cells express the
chemokine CX3CL1 and its receptor CX3CR1. Eur J Cancer.
46:3383–3392. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Rodero M, Marie Y, Coudert M, et al:
Polymorphism in the microglial cell-mobilizing CX3CR1 gene is
associated with survival in patients with glioblastoma. J Clin
Oncol. 26:5957–5964. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Gaudin F, Nasreddine S, Donnadieu AC, et
al: Identification of the chemokine CX3CL1 as a new regulator of
malignant cell proliferation in epithelial ovarian cancer. PLoS
One. 6:e215462011. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
American Cancer Society: Cancer Facts and
Figures, 2011. American Cancer Society; Atlanta: 2011
|
|
21.
|
Freedland SJ: Screening, risk assessment,
and the approach to therapy in patients with prostate cancer.
Cancer. 117:1123–1135. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Ye XC, Choueiri M, Tu SM and Lin SH:
Biology and clinical management of prostate cancer bone metastasis.
Front Biosci. 12:3273–3286. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Hara T, Miyazaki H, Lee A, Tran CP and
Reiter RE: Androgen receptor and invasion in prostate cancer.
Cancer Res. 68:1128–1135. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Rudolfsson SH and Bergh A: Hypoxia drives
prostate tumour progression and impairs the effectiveness of
therapy, but can also promote cell death and serve as a therapeutic
target. Expert Opin Ther Targets. 13:219–225. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Palayoor ST, Mitchell JB, Cerna D, Degraff
W, John-Aryankalayil M and Coleman CN: PX-478, an inhibitor of
hypoxia-inducible factor-1alpha, enhances radiosensitivity of
prostate carcinoma cells. Int J Cancer. 123:2430–2437. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Babar IA, Czochor J, Steinmetz A, Weidhaas
JB, Glazer PM and Slack FJ: Inhibition of hypoxia-induced miR-155
radiosensitizes hypoxic lung cancer cells. Cancer Biol Ther.
12:908–914. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Pipinikas CP, Carter ND, Corbishley CM and
Fenske CD: HIF-1alpha mRNA gene expression levels in improved
diagnosis of early stages of prostate cancer. Biomarkers.
13:680–691. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Semenza GL and Wang GL: A nuclear factor
induced by hypoxia via de novo protein synthesis binds to the human
erythropoietin gene enhancer at a site required for transcriptional
activation. Mol Cell Biol. 12:5447–5454. 1992.PubMed/NCBI
|
|
29.
|
Koong AC, Chen EY and Giaccia AJ: Hypoxia
causes the activation of nuclear factor kappa B through the
phosphorylation of I kappa B alpha on tyrosine residues. Cancer
Res. 54:1425–1430. 1994.
|
|
30.
|
Wang Y, Li Z, Zhang H, et al: HIF-1alpha
and HIF-2alpha correlate with migration and invasion in gastric
cancer. Cancer Biol Ther. 10:376–382. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Balkwill F: The significance of cancer
cell expression of the chemokine receptor CXCR4. Semin Cancer Biol.
14:171–179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Murphy PM: Chemokines and the molecular
basis of cancer metastasis. N Engl J Med. 345:833–835. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Lu Y, Cai Z, Galson DL, et al: Monocyte
chemotactic protein-1 (MCP-1) acts as a paracrine and autocrine
factor for prostate cancer growth and invasion. Prostate.
66:1311–1318. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Waugh DJ, Wilson C, Seaton A and Maxwell
PJ: Multi-faceted roles for CXC-chemokines in prostate cancer
progression. Front Biosci. 13:4595–4604. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Zhang S, Qi L, Li M, et al: Chemokine
CXCL12 and its receptor CXCR4 expression are associated with
perineural invasion of prostate cancer. J Exp Clin Cancer Res.
27:622008. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Gladson CL and Welch DR: New insights into
the role of CXCR4 in prostate cancer metastasis. Cancer Biol Ther.
7:1849–1851. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Wang J, Lu Y, Koch AE, Zhang J and
Taichman RS: CXCR6 induces prostate cancer progression by the
AKT/mammalian target of rapamycin signaling pathway. Cancer Res.
68:10367–10376. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Xiao LJ, Lin P, Lin F, et al: ADAM17
targets MMP-2 and MMP-9 via EGFR-MEK-ERK pathway activation to
promote prostate cancer cell invasion. Int J Oncol. 40:1714–1724.
2012.PubMed/NCBI
|
|
39.
|
Veldkamp CT, Peterson FC, Hayes PL, et al:
On-column refolding of recombinant chemokines for NMR studies and
biological assays. Protein Expr Purif. 52:202–209. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Baggiolini M: Chemokines in pathology and
medicine. J Intern Med. 250:91–104. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Maxwell PJ, Gallagher R, Seaton A, et al:
HIF-1 and NF-kappaB-mediated upregulation of CXCR1 and CXCR2
expression promotes cell survival in hypoxic prostate cancer cells.
Oncogene. 26:7333–7345. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Wilczynski J, Duechler M and Czyz M:
Targeting NF-kappaB and HIF-1 pathways for the treatment of cancer:
part I. Arch Immunol Ther Exp (Warsz). 59:289–299. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Wilczynski J, Duechler M and Czyz M:
Targeting NF-kappaB and HIF-1 pathways for the treatment of cancer:
part II. Arch Immunol Ther Exp (Warsz). 59:301–307. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Perkins ND: The diverse and complex roles
of NF-kappaB subunits in cancer. Nat Rev Cancer. 12:121–132.
2012.PubMed/NCBI
|
|
45.
|
Liu YL, Yu JM, Song XR, Wang XW, Xing LG
and Gao BB: Regulation of the chemokine receptor CXCR4 and
metastasis by hypoxia-inducible factor in non small cell lung
cancer cell lines. Cancer Biol Ther. 5:1320–1326. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Li Y, Qiu X, Zhang S, Zhang Q and Wang E:
Hypoxia induced CCR7 expression via HIF-1alpha and HIF-2alpha
correlates with migration and invasion in lung cancer cells. Cancer
Biol Ther. 8:322–330. 2009. View Article : Google Scholar : PubMed/NCBI
|