Introduction
Tumors are characterized by a change of the
transcriptome, which occurs to a large extent due to alterations in
the chromatin structure. A key element of chromatin regulation is
the post-translational modification of histones, including their
acetylation and methylation on lysine residues. While histone
acetylation is normally correlated with increased gene
transcription, histone methylation is able to induce silencing or
activation of genes depending on which histone lysine residue is
modified and whether this lysine becomes mono-, di- or
trimethylated (1). A prominent
family of histone lysine-modifying enzymes comprises the Jumonji C
domain (JMJD) demethylases, whose dysregulation has been suggested
in cancer development (2,3).
One subfamily of JMJD proteins consists of JMJD2A,
JMJD2B, JMJD2C and JMJD2D (4).
These four JMJD2 proteins share extensive homology at their
N-termini harboring the catalytic domain, however, JMJD2D is
different from the other three JMJD2 proteins in that it lacks
their extensive C-terminus which contains PHD and TUDOR domains
involved in binding to modified histone residues (5,6). In
addition, whereas JMJD2A, B and C are capable of demethylating
histone 1.4 on lysine 26 as well as histone 3 on lysines 9 and 36,
JMJD2D is unable to demethylate the latter lysine residue (7–13).
Moreover, compared to JMJD2A and JMJD2C, JMJD2B seems to be much
less catalytically active (7,10).
Thus, the four JMJD2 family members exhibit different biochemical
properties and are thought to perform distinct physiological
functions.
The mRNA expression of JMJD2B, but not of JMJD2A or
JMJD2C, is induced by estrogen and JMJD2B is therefore potentially
important in estrogen receptor α (ERα)-positive tumors.
Consistently, JMJD2B is more highly expressed in ERα-positive
compared to ERα-negative human breast tumors and is required for
the maximal proliferation of estrogen-dependent MCF7 breast cancer
cells (14–16). Furthermore, only JMJD2B and JMJD2C
appear to be overexpressed in medulloblastomas and their enzymatic
activities may contribute to the neoplastic transformation of brain
cells (17). In the present study,
we focused on JMJD2A, also known as KDM4A (lysine demethylase 4A),
and assessed its potential role in breast cancer.
Materials and methods
Immunohistochemistry
Tissue microarrays consisting of 12 (AccuMax
A712[14]) or 20 (AccuMax A312) matching samples of human breast
tumors and normal tissues were purchased from ISU Abxis. Staining
with JMJD2A antibodies (Bethyl A300-861A or Abcam ab70786) was
performed as described in a previous study (18) and graded by a board-certified
pathologist.
Coimmunoprecipitation assays
MCF7 cells were grown in the absence or presence of
1 nM estradiol (19). Cells were
lysed in 50 mM Tris (pH 7.4), 50 mM NaF, 150 mM NaCl, 0.5% Igepal
CA-630, 0.1 mM DTT, 0.5 mM PMSF, 0.25 mM
Na3VO4, 10 μg/ml leupeptin, 2
μg/ml aprotinin and 1 μg/ml pepstatin A (20). Immunoprecipitations were performed
with ERα antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz,
CA, USA; sc-543), as previously described (21). Coprecipitated JMJD2A was then
detected by western blotting utilizing JMJD2A antibodies (Bethyl
Laboratories, Inc., Montgomery, TX, USA; A300-861A) and enhanced
chemiluminescence (22).
Similarly, coimmunoprecipitation experiments with transiently
transfected 293T cells were conducted (23).
Luciferase assays
CV-1 cells were grown in 12-well plates and
transiently transfected utilizing the calcium phosphate
coprecipitation method (24). The
reporter plasmid ERE-luc (500 ng), pSG5-ERα (2.5 ng), Flag-JMJD2A
(500 ng) or empty vector pEV3S (500 ng), and pBluescript
KS+ (1500 ng) were cotransfected (25). After transfection, cells were
incubated for 36 h in phenol red-free media containing 5%
charcoal-stripped serum with or without 1 nM estradiol. The cells
were then lysed (26) and
luciferase activities determined in a luminometer, as described in
a previous study (27). Averages
with standard errors of triplicate experiments were determined.
RNA interference
Small hairpin RNA (shRNA) directed against JMJD2A
was cloned into pSIREN-RetroQ (Clontech). Sequences targeted within
the human JMJD2A mRNA were 5′-GUUGAGGAUGGUCUUACCU-3′ (shRNA #3) and
5′-GGACUUAGCUUCATAACUA-3′ (shRNA #5). Retrovirus was then produced
in 293T cells, as previously described (28). The resultant retrovirus was
employed to infect T47D cells (29). Infected cells were selected with
2.5 μg/ml puromycin for three days, after which cells were
lysed by boiling in Laemmli buffer (30). Protein extracts were subjected to
SDS polyacrylamide gel electrophoresis, followed by western
blotting (31). The following
antibodies were employed for the detection of proteins: JMJD2A
(Bethyl A300-861A), actin (GenScript A00730), c-Jun (Santa Cruz
Biotechnology sc-45) and cyclin D1 (Cell Signaling #2926).
Cell proliferation assay
Infected, puromycin-selected T47D cells (as
mentioned above) were seeded into 96-well plates in DMEM
supplemented with 10% fetal calf serum. One day after seeding, the
cell number was counted for the first time (defined as day 0) and
two days later, the cell number was counted again. The TACS MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] kit
(Trevigen Inc., Gaithersburg, MD, USA) was employed to count the
cell numbers, according to the manufacturer’s recommendations.
Averages with standard errors of triplicate experiments were
determined.
Results
Overexpression of JMJD2A in breast
tumors
To study the expression pattern of JMJD2 genes in
human breast tumors, publicly available microarray data retrieved
from The Cancer Genome Atlas were analyzed through the Oncomine web
portal (www.oncomine.org). Although JMJD2A and B mRNA
expression was robustly upregulated in invasive lobular and ductal
carcinomas compared to the normal breast tissue, the opposite
behavior was noted for JMJD2C and D (Fig. 1). These data suggest that JMJD2A
and B have different functions in breast tumors compared to JMJD2C
and D and are consistent with the hypothesis that as with JMJD2B,
JMJD2A is a potential breast-relevant oncoprotein.
To substantiate this view, we compared the degree of
JMJD2A protein overexpression between normal and cancer breast
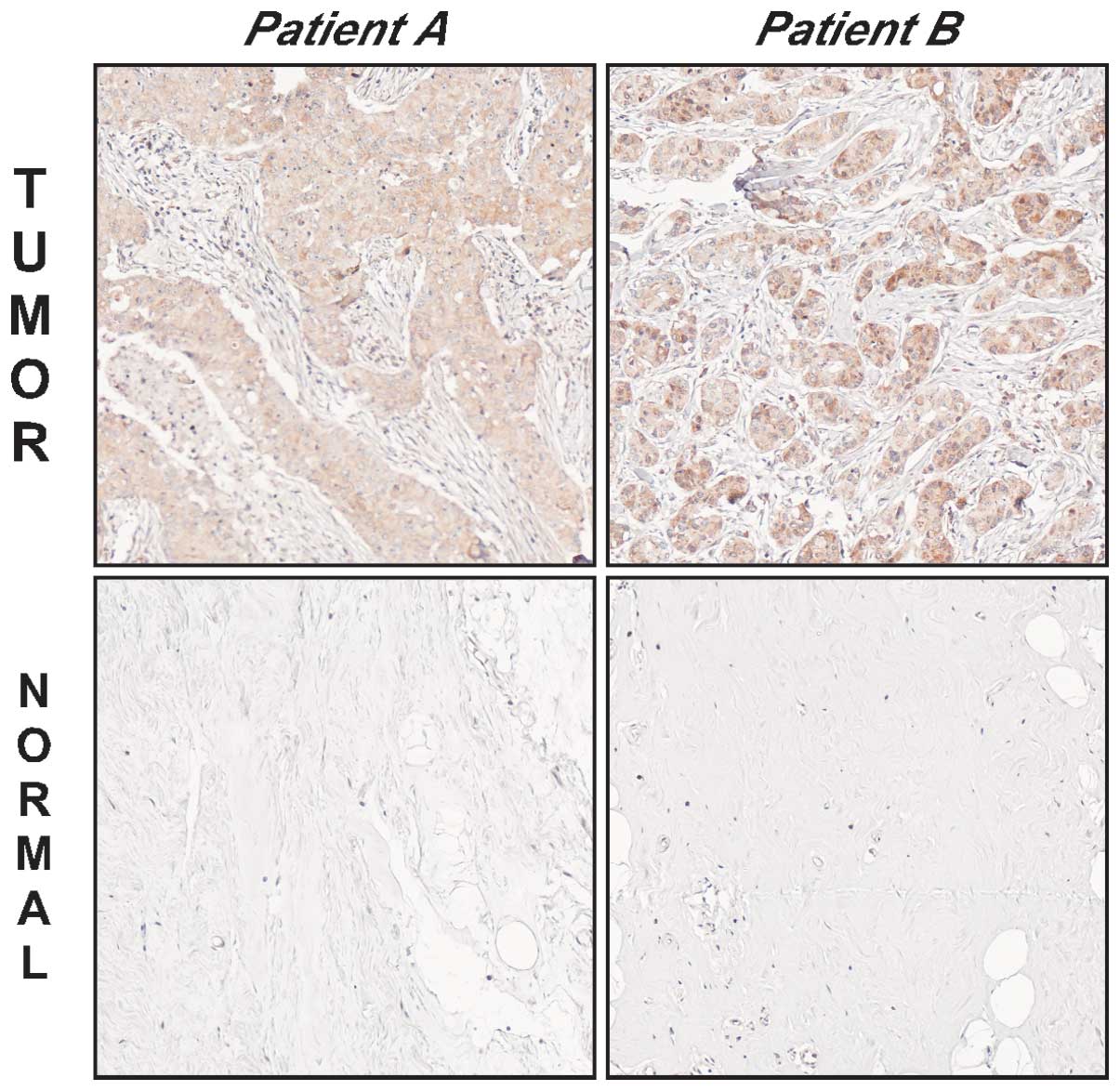
tissue. To this end, we stained a total of 32 pairs of human breast
tumors and matching normal breast tissues with two different JMJD2A
antibodies. The two antibodies exhibited a similar staining pattern
showing JMJD2A overexpression in 19 (Abcam antibody) or 20 (Bethyl
antibody) tumors out of the 32 matching samples (Fig. 2). Thus, approximately 60% of all
human breast tumors overexpress the JMJD2A protein.
Interaction of JMJD2A with ERα
The majority of breast tumors are ERα-positive and
therefore endocrine therapy is applicable to most breast cancer
patients (32). Thus, one
mechanism by which JMJD2A is likely to contribute to breast tumor
formation is through the coactivation of ERα. A prerequisite for
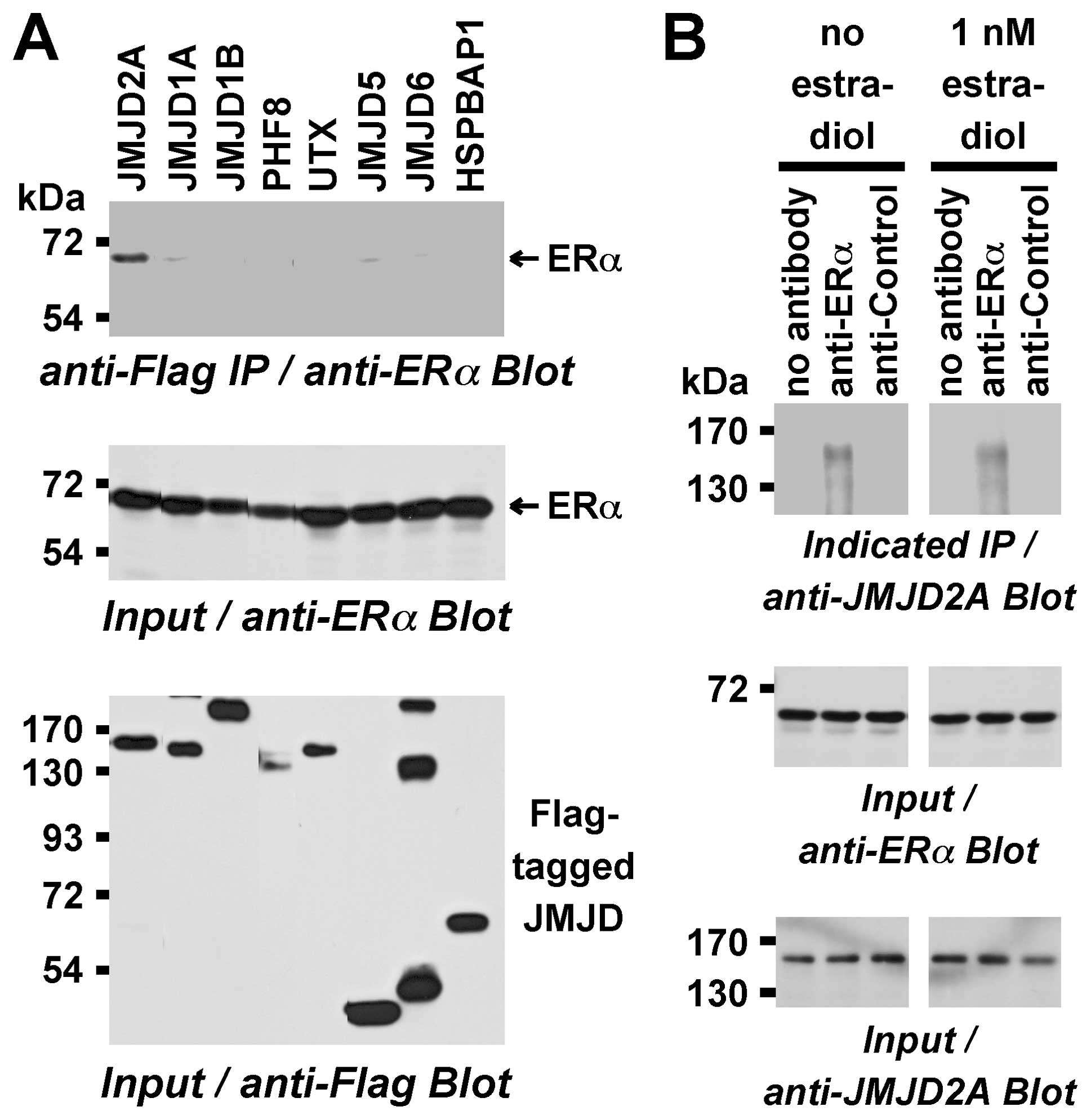
this would be an interaction of the two proteins. To explore this,
Flag-tagged JMJD2A was overexpressed with ERα in human 293T cells,
performed anti-Flag immunoprecipitations and assayed for any
coprecipitated ERα by respective western blotting. ERα was found to
robustly coprecipitate with JMJD2A (Fig. 3A). This is not an unspecific
interaction of JMJD2A with ERα, since seven other JMJD proteins
tested (JMJD1A, JMJD1B, PHF8, UTX, JMJD5, JMJD6, HSPBAP1) did not
significantly form complexes with ERα (Fig. 3A).
To confirm that endogenous JMJD2A interacts with
endogenous ERα, human MCF7 breast cancer cells that express both
proteins were examined. Furthermore, we tested whether or not such
an interaction would be dependent on estradiol. To this end, ERα
complexes were immunoprecipitated from MCF7 cells treated either
with or without estradiol. Estradiol was added to all buffers
utilized to immunoprecipitate ERα complexes from estradiol-treated
cells. JMJD2A was found to coimmunoprecipitate with ERα both in the
absence and presence of estradiol (Fig. 3B), suggesting that JMJD2A forms
complexes with the hormone-bound and hormone-free receptor. As a
validation for specificity, JMJD2A was not precipitated with no or
control antibodies (Fig. 3B).
Therefore, JMJD2A forms complexes with ERa in vivo and thus
potentially functions as a transcriptional cofactor of ERα.
Stimulation of ERα-dependent gene
transcription by JMJD2A
Whether or not the interaction between JMJD2A and
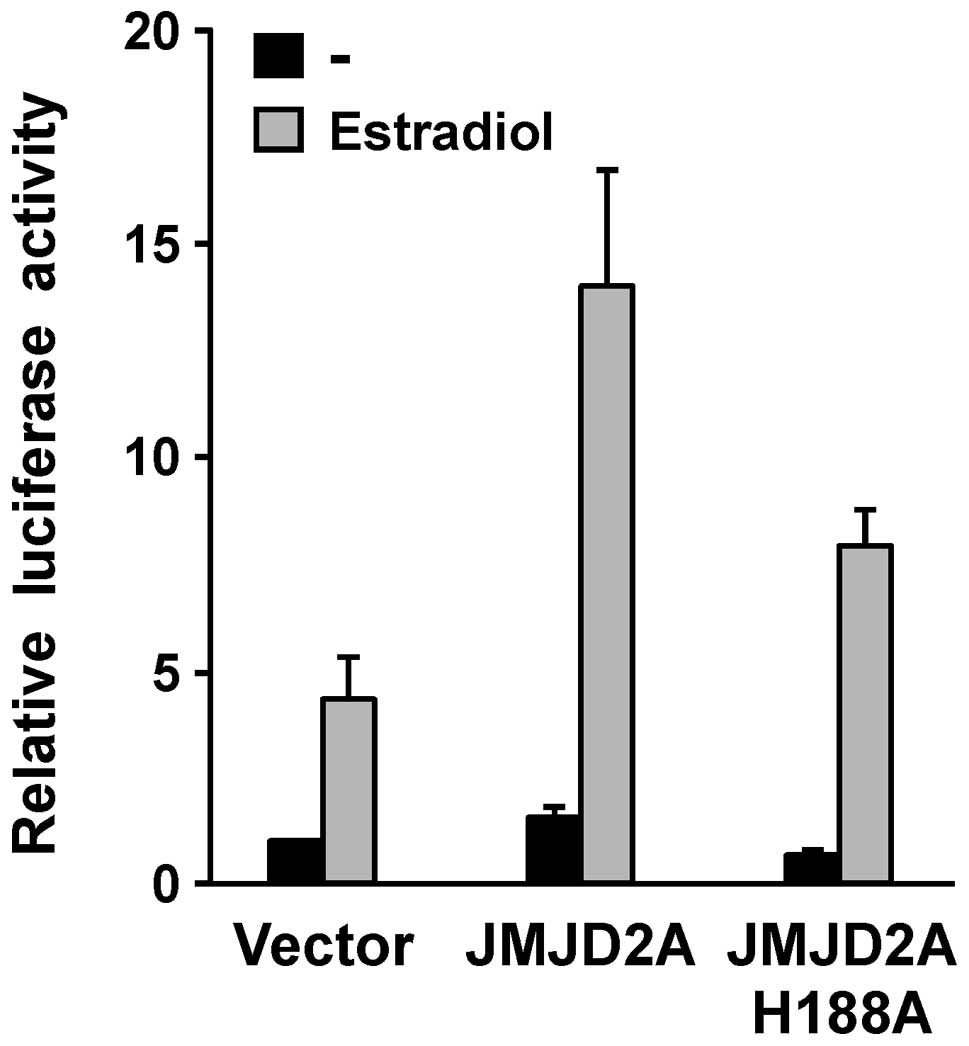
ERα is functionally relevant was assessed. To this end, we employed
a luciferase reporter construct (ERE-luc) that is inducible by
hormone-stimulated ERα (33). When
ERE-luc and ERα were transfected together into CV-1 cells,
stimulation with estradiol led to a ∼5-fold induction of luciferase
activity, as expected (Fig. 4). In
the absence of estradiol, JMJD2A cotransfection had only a slight
impact on luciferase activity, whereas estradiol-dependent
luciferase activity was strongly induced by JMJD2A.
We then determined whether this coactivation of ERα
by JMJD2A is dependent on the catalytic activity of JMJD2A. To this
end, we employed a mutant of JMJD2A (H188A), in which a critical
histidine residue within its catalytic center was mutated to
alanine (7). Replacing wild-type
JMJD2A with this H188A mutant led to a reduction of the
coactivation potential of JMJD2A (Fig.
4), clearly demonstrating that the catalytic activity of JMJD2A
is required for its ability to maximally stimulate
estrogen-dependent gene transcription.
Impact of JMJD2A on cell growth
To assess the role of JMJD2A in breast cancer cells,
JMJD2A was downregulated with two different shRNAs (#3 and #5) in
human ERα-positive T47D breast cancer cells. The two shRNAs
effectively reduced the expression of endogenous JMJD2A protein
(Fig. 5A). Of note, T47D cell
growth was severely impaired by the two JMJD2A shRNAs compared to
control shRNA (Fig. 5B). Thus,
JMJD2A exerts a pro-proliferative role in T47D breast cancer
cells.
Effects of JMJD2A downregulation on gene
transcription
A target gene of ERα is cyclin D1 (34), which is a crucial cell cycle
regulator and prominent oncogene. Thus, whether or not JMJD2A
downregulation would result in decreased cyclin D1 expression in
T47D cells was analyzed. Cells expressing either of the two
utilized JMJD2A shRNAs exhibited a reduced cyclin D1 expression
(Fig. 6). In addition, we found
that another oncoprotein, c-Jun (35), became downregulated by JMJD2A
ablation (Fig. 6). Thus, JMJD2A
may regulate T47D cell growth through influencing the expression of
at least two oncoproteins, cyclin D1 and c-Jun.
Discussion
In this study, we identified the JMJD2A histone
demethylase as a novel coactivator of ERα, since i) JMJD2A formed a
complex with ERα in vivo, ii) JMJD2A overexpression enhanced
estrogen-dependent transcription, and iii) the downregulation of
JMJD2A reduced transcription of a seminal ERα target gene, cyclin
D1. Moreover, our analyses revealed that JMJD2A mRNA as well as
protein are overexpressed in human breast tumors and that JMJD2A
supports breast cancer cell growth. As such, JMJD2A exhibits
features of an oncoprotein, whose action may be particularly
relevant in ERα-positive breast tumors.
In addition to JMJD2A, our microarray analyses
showed that JMJD2B is overexpressed in invasive breast tumors,
suggesting that JMJD2A and JMJD2B may perform similar functions.
Consistently, it was previously reported that JMJD2B
coimmunoprecipitated with ERα and that JMJD2B downregulation
suppressed growth of the ERα-positive breast cancer cell lines,
MCF7 and T47D (14–16). Similarly, JMJD2A and B (but not
JMJD2C or D) are able to perform comparable roles in the DNA damage
recognition and repair pathway (36). Notably, JMJD2B downregulation had
no effect on the ability of ERα-negative MDA-MB-231 breast cancer
cells to induce tumorigenesis in a nude mouse model (16), and similarly JMJD2A siRNA had a
very modest impact on the proliferation of MDA-MB-231 cells in
vitro (37). Thus, JMJD2A and
B are likely to significantly promote tumorigenesis only in
ERα-positive breast tissue, which probably involves their ability
to coactivate ERα.
However, in contrast to our data showing the
downregulation of JMJD2C in invasive breast tumors at the mRNA
level, another report posits that JMJD2C mRNA is upregulated in
breast tumors (38). One reason
for this discrepancy may be the use of different breast tumor
samples. Regardless, JMJD2C mRNA expression was reportedly higher
in ERα-negative compared to ERα-positive tumors (38), which is opposite to the expression
pattern of JMJD2B (14,15) and is suggestive of JMJD2C not being
a promoter of estrogen-dependent breast tumorigenesis.
Cyclin D1 gene transcription is induced by estradiol
in breast cancer cells, which involves activation of its promoter
by a complex of ERα and the AP-1 transcription factor (39,40).
Notably, cyclin D1 is frequently overexpressed in breast tumors and
a respective transgenic mouse model develops breast cancer
(41). Consistent with JMJD2A
being an ERα coactivator, the downregulation of JMJD2A led to a
reduced cyclin D1 expression in our study, indicating that the
overexpression of JMJD2A likely contributes to breast tumor
formation by inducing this oncogene.
Another oncoprotein, whose expression was reduced
upon JMJD2A downregulation, was c-Jun. This transcription factor is
not known to be directly regulated by ERα, however, it has
important functions in breast tumors. Its overexpression in MCF7
breast cancer cells stimulated their ability to migrate, invade and
form tumors in a xenograft model, while its inactivation caused
cell cycle arrest (42,43). Furthermore, inappropriately
activated c-Jun has been found in invasive human breast tumors and
c-Jun was also critical for cell invasion in ErbB2-induced mouse
mammary tumors (44,45). Therefore, JMJD2A overexpression may
lead to the overexpression of c-Jun that is predicted to cause an
invasive, aggressive phenotype of breast tumors.
Notably, JMJD2A does not only coactivate ERα, but
also the androgen receptor (46).
As with estrogen receptors, the androgen receptor belongs to the
nuclear hormone receptor superfamily and is crucial for the
development of prostate tumors (47,48).
As such, one may predict that JMJD2A also contributes to the
development of prostate cancer. Currently, no data supporting this
hypothesis have been published, and the same holds true for JMJD2B.
In addition, JMJD2A likely contributes to the development of
colorectal tumors, since its downregulation impairs the
proliferation and survival of colon cancer cell lines (49). In contrast to breast and prostate
cancer, nuclear hormone receptors are not prime drivers of
tumorigenesis in colorectal cancer. Thus, it remains to be
elucidated which other pivotal DNA-binding transcription factor(s)
become dysregulated by JMJD2A in colon cancer.
Originally, JMJD2A was described as a
transcriptional repressor (50,51).
However, published data (46) and
findings of this study clearly indicate that JMJD2A also functions
as a transcriptional coactivator in conjunction with the androgen
receptor or ERα. Thus, the ability of JMJD2A to regulate gene
transcription may depend on which transcription factor recruits
JMJD2A to a particular gene regulatory element. Mechanistically,
JMJD2A particularly demethylates trimethylated lysines 9 and 36 on
histone 3 (2,4). Although the demethylation of histone
3 on lysine 9 has been associated with the activation of gene
transcription (1), the function of
lysine 36 methylation on histone 3 remains to be elucidated
(52). Moreover, JMJD2A is capable
of demethylating histone 1.4 on lysine 26 (12), and this demethylation is predicted
to also induce gene transcription (53,54).
Thus, JMJD2A overexpression should lead to the activation of ERα
target genes, as observed with the ERE-luc reporter construct in
this study. The reduced expression of the ERα target gene cyclin D1
upon JMJD2A downregulation in T47D cells is consistent with the
hypothesis that the function of JMJD2A is to open up chromatin,
thereby facilitating estrogen-dependent transcription. Accordingly,
we found that catalytically inactive JMJD2A was compromised in its
ability to stimulate ERα-mediated transcription. This latter result
strongly suggests that inhibitors of JMJD2A enzymatic activity may
be beneficial for the treatment of ERα-positive breast tumors.
In conclusion, our data have identified JMJD2A as a
novel bona fide ERα coactivator, thus deepening our
understanding of estrogen-dependent transcriptional processes. In
addition, the fact that JMJD2A is overexpressed in human breast
tumors and required for efficient growth of breast cancer cells
suggests JMJD2A as a novel potential drug target.
References
|
1.
|
Kouzarides T: Chromatin modifications and
their function. Cell. 128:693–705. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Cloos PA, Christensen J, Agger K and Helin
K: Erasing the methyl mark: histone demethylases at the center of
cellular differentiation and disease. Genes Dev. 22:1115–1140.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Chi P, Allis CD and Wang GG: Covalent
histone modifications--miswritten, misinterpreted and mis-erased in
human cancers. Nat Rev Cancer. 10:457–469. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Kooistra SM and Helin K: Molecular
mechanisms and potential functions of histone demethylases. Nat Rev
Mol Cell Biol. 13:297–311. 2012.PubMed/NCBI
|
|
5.
|
Klose RJ and Zhang Y: Regulation of
histone methylation by demethylimination and demethylation. Nat Rev
Mol Cell Biol. 8:307–318. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Yap KL and Zhou MM: Keeping it in the
family: diverse histone recognition by conserved structural folds.
Crit Rev Biochem Mol Biol. 45:488–505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Whetstine JR, Nottke A, Lan F, Huarte M,
Smolikov S, Chen Z, Spooner E, Li E, Zhang G, Colaiacovo M and Shi
Y: Reversal of histone lysine trimethylation by the JMJD2 family of
histone demethylases. Cell. 125:467–481. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Cloos PA, Christensen J, Agger K, Maiolica
A, Rappsilber J, Antal T, Hansen KH and Helin K: The putative
oncogene GASC1 demethylates tri- and dimethylated lysine 9 on
histone H3. Nature. 442:307–311. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Klose RJ, Yamane K, Bae Y, Zhang D,
Erdjument-Bromage H, Tempst P, Wong J and Zhang Y: The
transcriptional repressor JHDM3A demethylates trimethyl histone H3
lysine 9 and lysine 36. Nature. 442:312–316. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Fodor BD, Kubicek S, Yonezawa M,
O’Sullivan RJ, Sengupta R, Perez-Burgos L, Opravil S, Mechtler K,
Schotta G and Jenuwein T: Jmjd2b antagonizes H3K9 trimethylation at
pericentric heterochromatin in mammalian cells. Genes Dev.
20:1557–1562. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Shin S and Janknecht R: Diversity within
the JMJD2 histone demethylase family. Biochem Biophys Res Commun.
353:973–977. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Trojer P, Zhang J, Yonezawa M, Schmidt A,
Zheng H, Jenuwein T and Reinberg D: Dynamic histone H1 isotype 4
methylation and demethylation by histone lysine methyltransferase
G9a/KMT1C and the Jumonji domain-containing JMJD2/KDM4 proteins. J
Biol Chem. 284:8395–8405. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Weiss T, Hergeth S, Zeissler U, Izzo A,
Tropberger P, Zee BM, Dundr M, Garcia BA, Daujat S and Schneider R:
Histone H1 variant-specific lysine methylation by G9a/KMT1C and
Glp1/ KMT1D. Epigenetics Chromatin. 3:72010. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Yang J, Jubb AM, Pike L, Buffa FM, Turley
H, Baban D, Leek R, Gatter KC, Ragoussis J and Harris AL: The
histone demethylase JMJD2B is regulated by estrogen receptor alpha
and hypoxia, and is a key mediator of estrogen induced growth.
Cancer Res. 70:6456–6466. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Kawazu M, Saso K, Tong KI, McQuire T, Goto
K, Son DO, Wakeham A, Miyagishi M, Mak TW and Okada H: Histone
demethylase JMJD2B functions as a co-factor of estrogen receptor in
breast cancer proliferation and mammary gland development. PLoS
One. 6:e178302011. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Shi L, Sun L, Li Q, Liang J, Yu W, Yi X,
Yang X, Li Y, Han X, Zhang Y, Xuan C, Yao Z and Shang Y: Histone
demethylase JMJD2B coordinates H3K4/H3K9 methylation and promotes
hormonally responsive breast carcinogenesis. Proc Natl Acad Sci
USA. 108:7541–7546. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Northcott PA, Nakahara Y, Wu X, Feuk L,
Ellison DW, Croul S, Mack S, Kongkham PN, Peacock J, Dubuc A, Ra
YS, Zilberberg K, McLeod J, Scherer SW, Sunil Rao J, Eberhart CG,
Grajkowska W, Gillespie Y, Lach B, Grundy R, Pollack IF, Hamilton
RL, van Meter T, Carlotti CG, Boop F, Bigner D, Gilbertson RJ,
Rutka JT and Taylor MD: Multiple recurrent genetic events converge
on control of histone lysine methylation in medulloblastoma. Nat
Genet. 41:465–472. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Mooney SM, Grande JP, Salisbury JL and
Janknecht R: Sumoylation of p68 and p72 RNA helicases affects
protein stability and transactivation potential. Biochemistry.
49:1–10. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Mooney SM, Goel A, D’Assoro AB, Salisbury
JL and Janknecht R: Pleiotropic effects of p300-mediated
acetylation on p68 and p72 RNA helicase. J Biol Chem.
285:30443–30452. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Shin S and Janknecht R: Concerted
activation of the Mdm2 promoter by p72 RNA helicase and the
coactivators p300 and P/CAF. J Cell Biochem. 101:1252–1265. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Knebel J, De Haro L and Janknecht R:
Repression of transcription by TSGA/Jmjd1a, a novel interaction
partner of the ETS protein ER71. J Cell Biochem. 99:319–329. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Papoutsopoulou S and Janknecht R:
Phosphorylation of ETS transcription factor ER81 in a complex with
its coactivators CREB-binding protein and p300. Mol Cell Biol.
20:7300–7310. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Kim TD, Shin S and Janknecht R: Repression
of Smad3 activity by histone demethylase SMCX/JARID1C. Biochem
Biophys Res Commun. 366:563–567. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Dowdy SC, Mariani A and Janknecht R:
HER2/Neu- and TAK1-mediated up-regulation of the transforming
growth factor beta inhibitor Smad7 via the ETS protein ER81. J Biol
Chem. 278:44377–44384. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
De Haro L and Janknecht R: Cloning of the
murine ER71 gene (Etsrp71) and initial characterization of its
promoter. Genomics. 85:493–502. 2005.PubMed/NCBI
|
|
26.
|
Wu J and Janknecht R: Regulation of the
ETS transcription factor ER81 by the 90-kDa ribosomal S6 kinase 1
and protein kinase A. J Biol Chem. 277:42669–42679. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Goel A and Janknecht R:
Acetylation-mediated transcriptional activation of the ETS protein
ER81 by p300, P/CAF, and HER2/ Neu. Mol Cell Biol. 23:6243–6254.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Shin S, Rossow KL, Grande JP and Janknecht
R: Involvement of RNA helicases p68 and p72 in colon cancer. Cancer
Res. 67:7572–7578. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Kim J, Shin S, Subramaniam M, Bruinsma E,
Kim TD, Hawse JR, Spelsberg TC and Janknecht R: Histone demethylase
JARID1B/ KDM5B is a corepressor of TIEG1/KLF10. Biochem Biophys Res
Commun. 401:412–416. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Kim TD, Oh S, Shin S and Janknecht R:
Regulation of tumor suppressor p53 and HCT116 cell physiology by
histone demethylase JMJD2D/KDM4D. PLoS One. 7:e346182012.
View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Shin S, Kim TD, Jin F, van Deursen JM,
Dehm SM, Tindall DJ, Grande JP, Munz JM, Vasmatzis G and Janknecht
R: Induction of prostatic intraepithelial neoplasia and modulation
of androgen receptor by ETS variant 1/ETS-related protein 81.
Cancer Res. 69:8102–8110. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Normanno N, Di Maio M, De Maio E, De Luca
A, de Matteis A, Giordano A and Perrone F: Mechanisms of endocrine
resistance and novel therapeutic strategies in breast cancer.
Endocr Relat Cancer. 12:721–747. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Paech K, Webb P, Kuiper GG, Nilsson S,
Gustafsson J, Kushner PJ and Scanlan TS: Differential ligand
activation of estrogen receptors ERalpha and ERbeta at AP1 sites.
Science. 277:1508–1510. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Carroll JS and Brown M: Estrogen receptor
target gene: an evolving concept. Mol Endocrinol. 20:1707–1714.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Shaulian E and Karin M: AP-1 as a
regulator of cell life and death. Nat Cell Biol. 4:E131–E136. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Mallette FA, Mattiroli F, Cui G, Young LC,
Hendzel MJ, Mer G, Sixma TK and Richard S: RNF8- and
RNF168-dependent degradation of KDM4A/JMJD2A triggers 53BP1
recruitment to DNA damage sites. EMBO J. 31:1865–1878. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Li BX, Zhang MC, Luo CL, Yang P, Li H, Xu
HM, Xu HF, Shen YW, Xue AM and Zhao ZQ: Effects of RNA
interference-mediated gene silencing of JMJD2A on human breast
cancer cell line MDA-MB-231 in vitro. J Exp Clin Cancer Res.
30:902011. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Liu G, Bollig-Fischer A, Kreike B, van de
Vijver MJ, Abrams J, Ethier SP and Yang ZQ: Genomic amplification
and oncogenic properties of the GASC1 histone demethylase gene in
breast cancer. Oncogene. 28:4491–4500. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Altucci L, Addeo R, Cicatiello L, Dauvois
S, Parker MG, Truss M, Beato M, Sica V, Bresciani F and Weisz A:
17beta-Estradiol induces cyclin D1 gene transcription,
p36D1-p34cdk4 complex activation and p105Rb phosphorylation during
mitogenic stimulation of G(1)-arrested human breast cancer cells.
Oncogene. 12:2315–2324. 1996.
|
|
40.
|
Cicatiello L, Addeo R, Sasso A, Altucci L,
Petrizzi VB, Borgo R, Cancemi M, Caporali S, Caristi S, Scafoglio
C, Teti D, Bresciani F, Perillo B and Weisz A: Estrogens and
progesterone promote persistent CCND1 gene activation during G1 by
inducing transcriptional derepression via c-Jun/c-Fos/estrogen
receptor (progesterone receptor) complex assembly to a distal
regulatory element and recruitment of cyclin D1 to its own gene
promoter. Mol Cell Biol. 24:7260–7274. 2004.
|
|
41.
|
Velasco-Velazquez MA, Li Z, Casimiro M,
Loro E, Homsi N and Pestell RG: Examining the role of cyclin D1 in
breast cancer. Future Oncol. 7:753–765. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Smith LM, Wise SC, Hendricks DT, Sabichi
AL, Bos T, Reddy P, Brown PH and Birrer MJ: cJun overexpression in
MCF-7 breast cancer cells produces a tumorigenic, invasive and
hormone resistant phenotype. Oncogene. 18:6063–6070. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Liu Y, Lu C, Shen Q, Munoz-Medellin D, Kim
H and Brown PH: AP-1 blockade in breast cancer cells causes cell
cycle arrest by suppressing G1 cyclin expression and reducing
cyclin-dependent kinase activity. Oncogene. 23:8238–8246. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Vleugel MM, Greijer AE, Bos R, van der
Wall E and van Diest PJ: c-Jun activation is associated with
proliferation and angiogenesis in invasive breast cancer. Hum
Pathol. 37:668–674. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Jiao X, Katiyar S, Willmarth NE, Liu M, Ma
X, Flomenberg N, Lisanti MP and Pestell RG: c-Jun induces mammary
epithelial cellular invasion and breast cancer stem cell expansion.
J Biol Chem. 285:8218–8226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Shin S and Janknecht R: Activation of
androgen receptor by histone demethylases JMJD2A and JMJD2D.
Biochem Biophys Res Commun. 359:742–746. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Pienta KJ and Bradley D: Mechanisms
underlying the development of androgen-independent prostate cancer.
Clin Cancer Res. 12:1665–1671. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Attard G, Cooper CS and de Bono JS:
Steroid hormone receptors in prostate cancer: a hard habit to
break? Cancer Cell. 16:458–462. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Kim TD, Shin S, Berry WL, Oh S and
Janknecht R: The JMJD2A demethylase regulates apoptosis and
proliferation in colon cancer cells. J Cell Biochem. 113:1368–1376.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
50.
|
Gray SG, Iglesias AH, Lizcano F,
Villanueva R, Camelo S, Jingu H, Teh BT, Koibuchi N, Chin WW,
Kokkotou E and Dangond F: Functional characterization of JMJD2A, a
histone deacetylase- and retinoblastoma-binding protein. J Biol
Chem. 280:28507–28518. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
51.
|
Zhang D, Yoon HG and Wong J: JMJD2A is a
novel N-CoR-interacting protein and is involved in repression of
the human transcription factor achaete scute-like homologue 2
(ASCL2/ Hash2). Mol Cell Biol. 25:6404–6414. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52.
|
Wagner EJ and Carpenter PB: Understanding
the language of Lys36 methylation at histone H3. Nat Rev Mol Cell
Biol. 13:115–126. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53.
|
Parseghian MH, Newcomb RL, Winokur ST and
Hamkalo BA: The distribution of somatic H1 subtypes is non-random
on active vs. inactive chromatin: distribution in human fetal
fibroblasts. Chromosome Res. 8:405–424. 2000. View Article : Google Scholar
|
|
54.
|
Daujat S, Zeissler U, Waldmann T, Happel N
and Schneider R: HP1 binds specifically to Lys26-methylated histone
H1.4, whereas simultaneous Ser27 phosphorylation blocks HP1
binding. J Biol Chem. 280:38090–38095. 2005. View Article : Google Scholar : PubMed/NCBI
|