Introduction
Hypoxia is a common condition found within a variety
of solid tumors. Adaptive responses of tumor cells to hypoxia
develop the malignant phenotypes of the tumor cells, which promotes
angiogenesis, invasion, metastasis and resistance to chemotherapy
and radiotherapy (1–3). Hypoxia-inducing factor-1 (HIF-1)
plays a pivotal role in the adaptive responses of tumor cells to
hypoxia. HIF-1, which is a heterodimer with an oxygen-sensitive
HIF-1α subunit and a constitutively expressed HIF-1β subunit, binds
the hypoxia-responsive element and activates the transcription of
target genes (4–6). Under normoxia, HIF-1α is rapidly
degraded by the ubiquitin-proteasome pathway; however, under
hypoxia, HIF-1α is stabilized and accumulates in cells (5,7).
Tumor cells express NKG2D ligands on their cell
surface, which are ligands of an activating receptor, NKG2D, that
is expressed on the cell surface of cytotoxic immune cells, such as
NK, γδ+ T and CD8+ αβ+ T cells
(8,9). In humans, there are two families of
NKG2D ligands, the MHC class I-related chain molecules A and B
(MICA and MICB) and the UL16-binding proteins (ULBP) (8). The binding of an NKG2D receptor to
its ligand activates NK and γδ+ T cells and
co-stimulates tumor-antigen-specific CD8+ αβ+
T cells (8,9). Therefore, the NKG2D ligands on the
surface of tumor cells are important for the cytotoxicity of immune
cells. On the other hand, tumor cells produce soluble forms of
NKG2D ligands by proteolytic cleavage of their extracellular
domains (8,10–13).
Soluble forms of NKG2D ligands interfere with the binding of NKG2D
ligands on the surface of tumor cells to NKG2D receptors on the
surface of cytotoxic immune cells, and the binding of soluble NKG2D
ligands to NKG2D receptors downregulates the NKG2D receptors on the
surface of cytotoxic immune cells (8,13–16).
Therefore, a decrease in the expression of NKG2D ligands on the
surface of tumor cells and an increase in the secretion of soluble
NKG2D ligands attenuate the susceptibility of tumor cells to
cytotoxic immune cells.
Regarding the effects of hypoxia on immune
surveillance, there are many studies on the hypoxia-induced
inhibition of cytotoxic activity of immune cells (17); however, only a few studies have
addressed the effects of hypoxia on immune escape by tumor cells
(18,19). In this study, we examined the
effects of hypoxia on the expression of the cell surface NKG2D
ligands of osteosarcoma cells, the secretion of soluble NKG2D
ligands and the role of HIF-1α in the hypoxia-induced effects.
Several studies have previously shown such hypoxia-induced effects
to be produced by the hypoxia-induced inhibition of the nitric
oxide (NO) signaling pathway (18–22)
and that NO modulates hypoxia-induced cellular events (23,24).
Therefore, we also examined the role of NO in hypoxia-induced
effects.
Materials and methods
Cell culture
U2-OS human osteosarcoma cells and NK-92 human
natural killer cells were purchased from the American Type Culture
Collection (Rockville, MD, USA). HOS and SaOS-2 human osteosarcoma
cells were purchased from the Riken BRC Cell Bank (Tsukuba,
Ibaragi, Japan). All osteosarcoma cells were maintained in
Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, Carlsbad,
CA, USA) containing 10% fetal bovine serum (FBS) (PAA, Pasching,
Oberosterreich, Austria). Human NK cells were cultured in Minimum
Essential Medium Alpha Medium (Invitrogen) containing 12.5% horse
serum (MP Biomedicals, Solon, OH, USA), 12.5% FBS, 0.2 mM inositol
(Sigma, St. Louis, MO, USA), 0.1 mM 2-mercaptoethanol (Wako, Osaka,
Japan), 0.02 mM folic acid (Sigma) and 100 U/ml recombinant human
IL-2 (Peprotech, Rocky Hill, NJ, USA). For the cell cultures under
20% O2 conditions, the cells were cultured in a
humidified atmosphere of 5% CO2 in air at 37°C. For the
cell cultures under 1% O2 conditions, the cells were
cultured in a humidified atmosphere of 1% O2 and 5%
CO2 in N2 at 37°C using a CO2
Multi GAS incubator (Astec, Fukuoka, Japan).
Growth of the osteosarcoma cells
Cells seeded at 1×103 cells per well of
96-well tissue culture plates were cultured in 100 μl of DMEM
containing 10% FBS under either 20 or 1% O2 conditions
for 72 h. The number of viable cells in each well was estimated
using a Cell counting kit-8 (Dojindo, Kumamoto, Japan).
Flow cytometry
The cells were harvested using a brief treatment
with 0.25% trypsin and 0.1 mM EDTA in phosphate-buffered saline
(PBS), pH 7.4, resuspended in an ice-cold FACS buffer (PBS
containing 2% FBS) and incubated with either phycoerythrin
(PE)-labeled mouse anti-human MICA mAb (monoclonal IgG antibody)
(100-fold dilution), mouse anti-human MICB mAb (100-fold dilution),
mouse anti-human ULBP1 mAb (100-fold dilution), mouse anti-human
ULBP2 mAb (100-fold dilution) or mouse anti-human ULBP3 mAb
(100-fold dilution) on ice for 30 min. As a control, cells were
incubated with mouse IgG. When incubated with an unlabelled mAb,
the cells were washed with FACS buffer and further incubated with
the PE-labeled goat anti-mouse IgG (200-fold dilution) on ice for
30 min. The cells were then washed with FACS buffer and analyzed
using a FACSCalibur flow cyto-meter (Becton Dickinson, Mountain
View, CA, USA) and the percentage of positive cells was determined.
Cells (10,000) were used for each flow cytometric analysis. All
antibodies and mouse IgG used for flow cytometry were purchased
from R&D Systems (Minneapolis, MN, USA).
Western blot analysis
The cultured cells were homogenized with lysis
buffer containing 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1% SDS, 1%
Triton X-100, 1% sodium deoxycholate and a cocktail of protease
inhibitors (Complete) (Roche, Penzberg, Bavaria, Germany) using a
sonicator (Sonics & Materials, Newtown, CT, USA). An aliquot of
the cell homogenate containing 25 μg of proteins was boiled in a
SDS sample buffer (New England BioLabs, Ipswich, MA, USA) and
subjected to electrophoresis in a denaturing 5–10%
SDS-polyacrylamide gradient gel (Atto, Tokyo, Japan). The separated
proteins were transferred onto a polyvinylidene difluoride membrane
(Fine Trap) (Nihon Eido, Tokyo, Japan). The membranes were blocked
with 5% non-fat dry milk in PBS containing 0.1% Tween-20 and
incubated with primary mouse anti-human HIF-1α mAb (2,000-fold
dilution) (BD Biosciences, Franklin Lakes, NJ, USA), rabbit
anti-human MICA pAb (polyclonal antibody) (2,000-fold dilution)
(GeneTex, Irvine, CA, USA) and rabbit anti-GAPDH pAb (4,000-fold
dilution) (GeneTex) or rabbit anti-human β-actin pAb (4,000-fold
dilution) (Thermo Fisher Scientific, Fremont, CA, USA) at room
temperature for 1 h. Proteins bound to the primary antibodies were
detected using a horseradish peroxidase (HRP)-conjugated goat
anti-rabbit IgG or HRP-conjugated goat anti-mouse IgG (Santa Cruz
Biotechnology, Santa Cruz, CA, USA) and ECL Western Blotting
Detection reagents (GE Healthcare, Little Chalfont, UK).
Quantitative real-time reverse
transcription-polymerase chain reaction (real-time RT-PCR)
Total-RNA was extracted from the cells using a
TRIzol reagent (Invitrogen) and DNase (1 U/μl) (Wako). The cDNAs
were synthesized and amplified using an RNA-direct™ SYBR-Green
Real-time PCR Master mix (Toyobo, Osaka, Japan) and the following
specific primers: sense, 5′-AGATTTTGGCAGCAACGACA-3′ (1637–1656) and
antisense, 5′-GCGGTGGGTAATGGAGACAT-3′ (1752–1771) for the HIF-1α
cDNA, sense, 5′-ACTGCTTGAGCCGCTGAGA-3′ (2–20)
and antisense, 5′-GAGGTGCAAAAGGGAAGATGC-3′ (74–94) for MICA cDNA,
sense, 5′-GGGGCGCAGGTGACTAAAT-3′ (33–51) and antisense,
5′-CCTACGTCGCCACCTTCTCA-3′ (93–112) for MICB cDNA, and sense,
5′-CGTGGCTAAACAGGTACTGCTG-3′ (88–109) and antisense,
5′-GGAGGTTTGCCAGGTA-3′ (177–197) for ribosomal protein L13a
(RPL13a) cDNA. The real-time PCR was performed under the PCR
conditions: 45 cycles at 95°C for 15 sec, at 62°C for 15 sec and at
74°C for 35 sec using an ABI PRIAM 7900HT (Applied Biosystems,
Foster, CA, USA). The amount of mRNA in each gene was corrected by
the amount of mRNA of RPL13a in a corresponding sample.
Cytotoxicity assay
Osteosarcoma cells (T, target cells;
2×104 cells) suspended in 100 μl of DMEM containing 10%
FBS were mixed with NK cells (E, effector cells) suspended in 100
μl of DMEM containing 10% FBS at various T:E ratios (1/10–1/2.5)
and placed into wells of 96-well plates. The cells were incubated
at 37°C for 4 h in a humidified atmosphere of 5% CO2 in
air. The cytotoxicity assay was performed using a Cytotoxicity
Detection kitplus (Roche).
Assay of soluble MICA and MICB
The levels of soluble MICA and MICB in culture
medium were determined using DuoSet ELISA Development kits for MICA
and MICB (R&D Systems).
Assay of nitrite and nitrate
Osteosarcoma cells seeded at 5×105 cells
per well in 6-well tissue culture plates were cultured in 5 ml of
medium for 24 h. Then, the culture medium was changed to a medium
with or without 100 μM NOC18
[1-Hydroxy-2-oxo-3,3-bis(2-aminoethyl)-1-triazene] (Dojindo) and
cultured for 24 h under either 20 or 1% O2 conditions.
After the culture was completed, the medium was collected and the
amount of nitrite and nitrate in the culture medium was measured
using a NO2/NO3 Assay kit CII (Dojindo).
RNA interference
Small interfering RNA (siRNA) for human HIF-1α
[siHIF-1 (siRNA ID No. 6539) or siHIF-2 (siRNA ID No. 6541)] and
control siRNA (Catalog No. 4390843) were purchased from Invitrogen.
The transfection of siRNA into the osteosarcoma cells was performed
using a Lipofectamine RNAiMAX (Invitrogen) according to the
manufacturer’s instructions. Briefly, HIF-1α siRNA or control siRNA
and a Lipofectamine RNAiMAX were mixed in 100 μl of Opti-MEM medium
(Invitrogen) in wells of 24-well tissue culture plates. Thereafter,
2×104 osteosarcoma cells suspended in 500 μl of Opti-MEM
medium were added into each well, which resulted in a final siRNA
concentration of 10 nM, and the cells were cultured for 24 h. Then,
the medium was changed to 1 ml of DMEM containing 10% FBS and the
cells were cultured for another 24 h under either 20 or 1%
O2 conditions. After the culture was completed, the
medium and the cells were collected for assay of soluble MICA and
soluble MICB in the medium and analyses of the amounts of HIF-1α
mRNA, HIF-1α protein and MICA.
Statistical analysis
The data of two groups were analyzed using Student’s
t-test, and the data of three or more groups were analyzed using
the Bonferroni multiple comparison test. A P-value of <0.05 was
considered to be significant.
Results
Effects of hypoxia on the growth of
osteosarcoma cell lines
HOS, U2-OS and SaOS-2 cells were cultured in
normoxic (20% CO2) and hypoxic (1% O2)
conditions for 72 h. When the average viable cell number in the
normoxic conditions was expressed as 1, the viable cell numbers
(means ± SE, n=12) in the hypoxic conditions were 1.02±0.06 for HOS
cells, 0.99±0.05 for U2-OS cells and 0.98±0.04 for SaOS-2 cells,
respectively, and hypoxia did not affect the growth of the
osteosarcoma cells for at least 72 h.
Effects of hypoxia on cell surface NKG2D
ligands and their soluble forms
Osteosarcoma cells were cultured for 72 h in
normoxic and hypoxic conditions, and the expression of cell surface
NKG2D ligands and the amounts of their soluble forms in the medium
were examined. As shown in Fig. 1,
three osteosarcoma cell lines expressed cell surface MICA and ULBP2
in high percentages, whereas cell surface MICB, ULBP1 and ULBP3
were expressed in very low percentages. Hypoxia decreased the
expression of cell surface MICA significantly; however, the
expression of ULBP2 did not decrease. Hypoxia increased the
expression of cell surface MICB and ULBP1 in U2-OS cells a little;
however, it did not affect the expression of cell surface MICB or
ULBP1 in HOS and SaOS-2 cells. Hypoxia did not affect the
expression of ULBP3 in any of the three osteosarcoma cell
lines.
The amount of soluble MICA detected in the medium
was much lower than that of soluble MICB. Hypoxia did not affect
the amounts of either soluble MICA or soluble MICB in the medium
(Fig. 2).
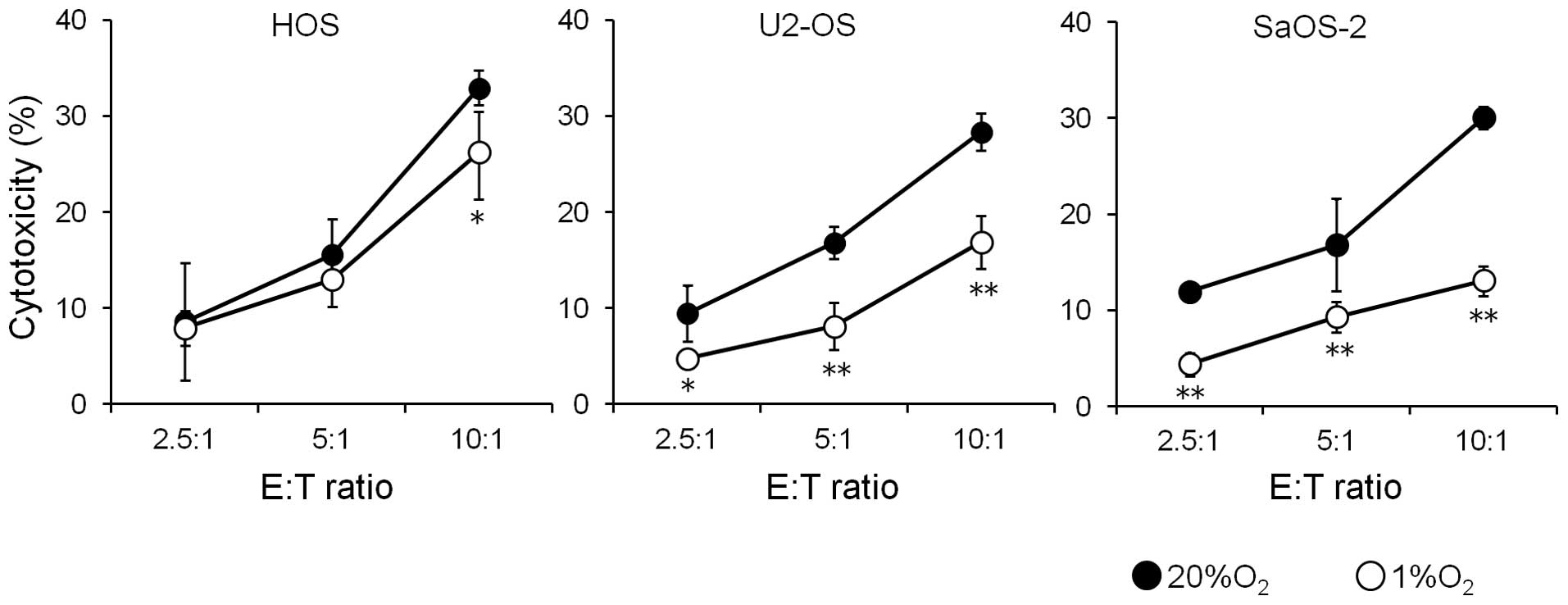
Effects of hypoxia on the susceptibility
of osteosarcoma cell lines to NK cells
Since hypoxia decreased the expression of cell
surface MICA in the osteosarcoma cells, the effect of this decrease
on the susceptibility of osteosarcoma cells to the cytotoxicity of
NK cells was examined (Fig. 3).
Culture of three osteosarcoma cell lines for 72 h under hypoxic
conditions decreased the susceptibility of all three osteosarcoma
cell lines to NK cells.
Role of hypoxia-induced expression of
HIF-1α
It has been reported that hypoxia induces the
accumulation of HIF-1α proteins and that hypoxia-induced cellular
events are mediated by HIF-1α (4,5).
Therefore, the role of HIF-1α in the hypoxia-induced decrease in
the expression of cell surface MICA was investigated.
Hypoxia for 24 h increased the amount of HIF-1α and
decreased the amount of MICA in all three osteosarcoma cell lines
(Fig. 4A). However, hypoxia did
not increase the amount of mRNA of both HIF-1α and MICA (Fig. 4B).
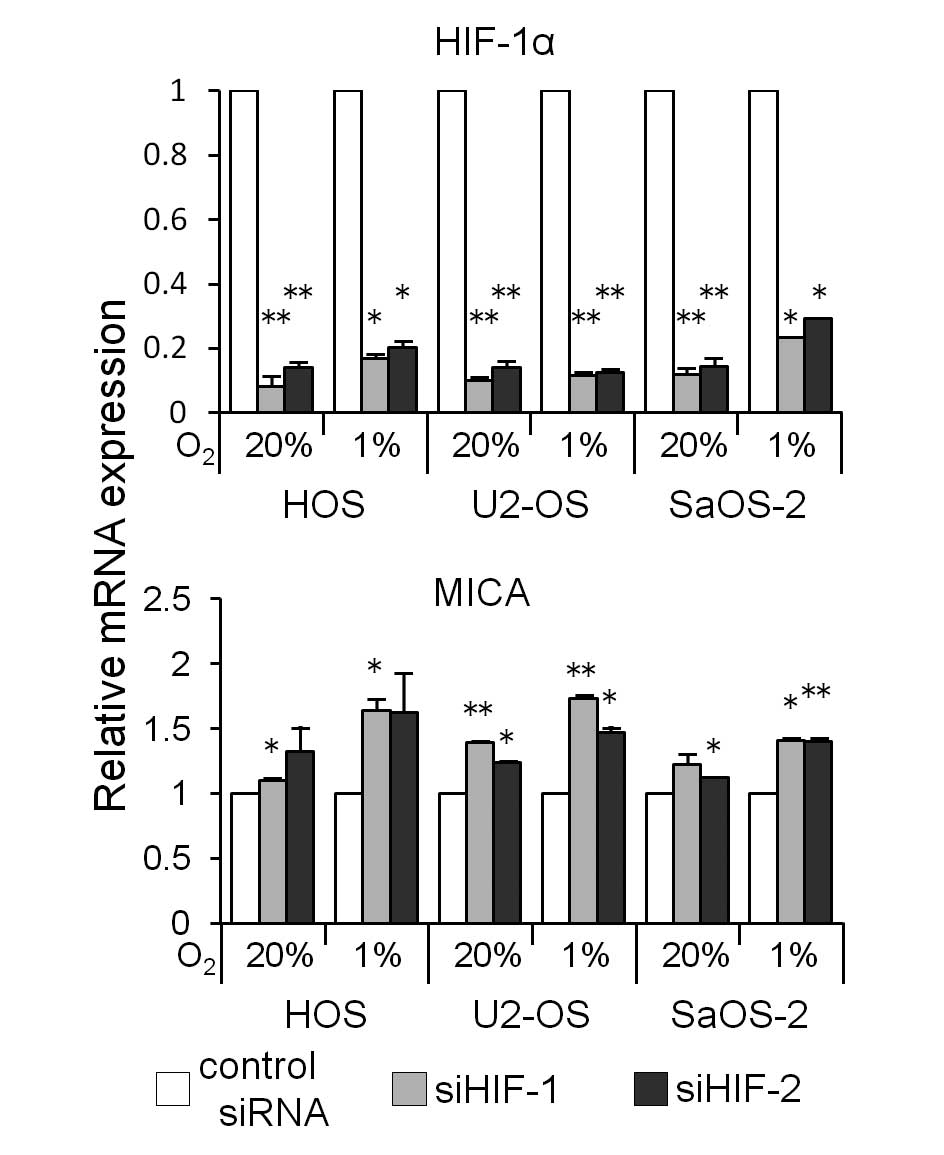
To determine whether the hypoxia-induced decrease in
the amount of MICA is mediated by HIF-1α, we knocked down HIF-1α
mRNA using siRNA (siHIF-1 or siHIF-2). The HIF-1α siRNA
transfection decreased the HIF-1α mRNA expression (Fig. 5) and increased the MICA mRNA
expression in the three osteosarcoma cell lines cultured under both
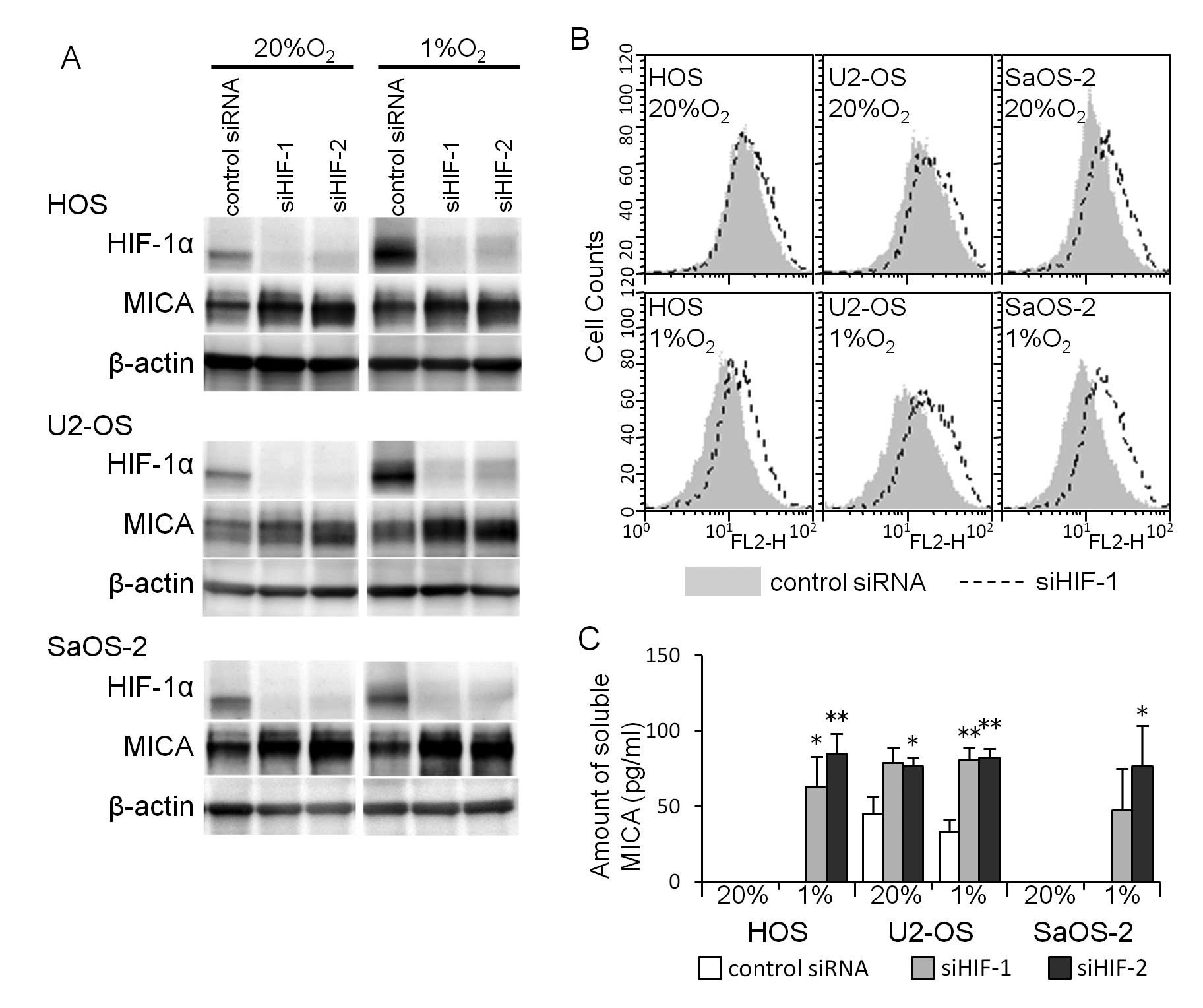
normoxic and hypoxic conditions for 24 h (Fig. 5). A western blot analysis showed
that the HIF-1α siRNA transfection decreased the amount of HIF-1α
proteins and increased the amount of MICA proteins in osteosarcoma
cells cultured under both normoxic and hypoxic conditions (Fig. 6A). A flow cytometric analysis
showed that the increase in the amount of MICA proteins in the
osteosarcoma cell lines reflected the increase in cell surface MICA
(Fig. 6B). The HIF-1α siRNA
transfection increased the amount of soluble MICA in the medium of
all three osteosarcoma cell lines cultured under hypoxic conditions
and in the medium of U2-OS cells cultured under normoxic
conditions, while the amount of soluble MICA in the medium of HOS
and SaOS-2 cells cultured under normoxic conditions was
undetectable (Fig. 6C).
Participation of NO in the
hypoxia-induced decrease in the MICA expression
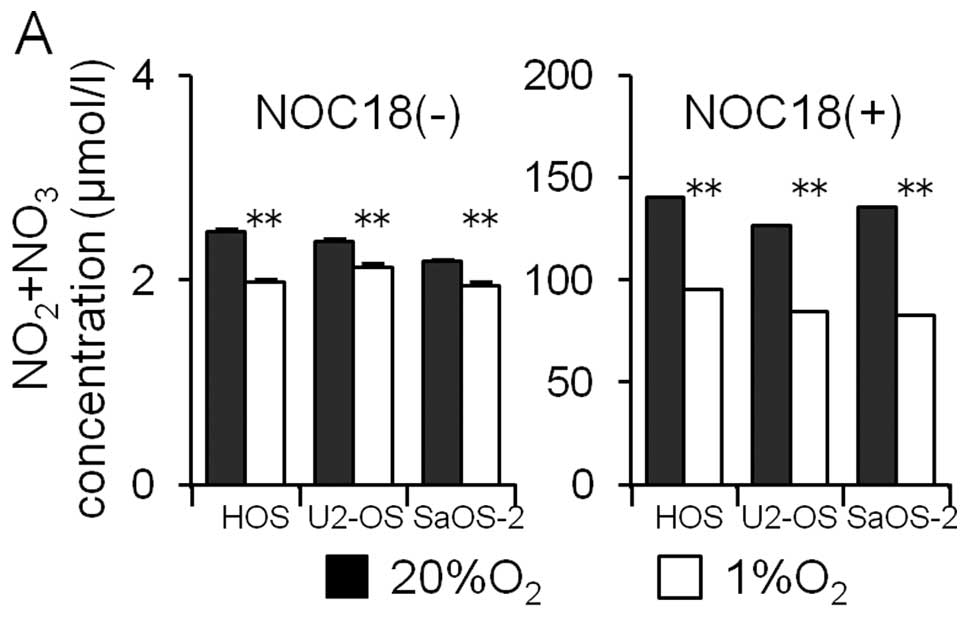
The participation of NO in the hypoxia-induced
decrease in the MICA expression was examined. First, osteosarcoma
cells were cultured for 24 h under normoxic and hypoxic conditions
and the amount of NO metabolites (NO2 and
NO3) in the medium was assayed. Hypoxia decreased the
amount of NO metabolites in the medium (Fig. 7A). Next, the effects of the NO
donor (NOC18) on the amounts of HIF-1α and MICA proteins in the
osteosarcoma cell lines were examined. The NO donor increased the
amount of NO metabolites markedly (Fig. 7A). The NO donor did not affect the
amount of HIF-1α proteins under either normoxic or hypoxic
conditions; however, the amount of MICA under both normoxic and
hypoxic conditions was observed to decrease.
Discussion
Hypoxia downregulated the expression of MICA on the
surface of osteosarcoma cells. Osteosarcoma cells secreted a
relatively small amount of soluble MICA, compared to that of
soluble MICB, in the medium, and hypoxia did not increase the
secretion of soluble MICA. Siemens et al (18) and Barsoum et al (19) have shown that hypoxia decreases the
expression of MICA on the surface of prostatic and breast cancer
cells and ascribes this decrease to the proteolytic cleavage of
cell surface MICA by ADAM10 (a disintegrin and metalloproteinase
10). The tumor cells used for their experiments secreted a much
larger amount of soluble MICA than osteosarcoma cells. Furthermore,
hypoxia for 24 h did not affect the expression of ADAM10 mRNA in
osteosarcoma cells (data not shown). Therefore, the difference
between their results and ours may be due to the use of different
tumor cells.
Hypoxia did not decrease the expression of cell
surface NKG2D ligands, except for that of cell surface MICA. Since
hypoxia did not affect the expression of MICA mRNA in spite of a
decrease in cell surface MICA, the decrease in the expression of
cell surface MICA may be ascribed to an attenuated translation of
MICA mRNA or a rapid degradation of MICA in osteosarcoma cells
under hypoxia. On the other hand, hypoxia showed only slight
effects on the expression of cell surface MICB or the secretion of
soluble MICB. Hypoxia for 3, 6 and 24 h did not affect the
expression of MICB mRNA (data not shown). The mechanisms underlying
the fact that hypoxia exerts different effects on cell surface MICA
and other NKG2D ligands must be further investigated.
Hypoxia increased the expression of HIF-1α without
increasing the amount of HIF-1α mRNA, as a result that is in
agreement with those of previous reports (5,7).
Furthermore, the knockdown of HIF-1α mRNA increased the expression
of MICA mRNA and cell surface MICA and concomitantly increased the
secretion of soluble MICA. These results show that the effects of
hypoxia on the expression of cell surface MICA are mediated by
HIF-1α. It has been reported that HIF-1α upregulates the expression
of MICA in human renal proximal tubular epithelial cells and
cardiomyocytes (25,26). The effects of HIF-1α on the
expression of cell surface MICA may be different between tumor
cells and normal cells.
Several studies have shown that hypoxia-induced
inhibition of NO production is linked to hypoxia-induced tumor
invasion, metastasis, resistance to chemotherapy and shedding of
cell surface MICA (18–22). In this study, hypoxia also induced
the inhibition of NO production in osteosarcoma cells. However,
under hypoxia the NO donor NOC18 decreased the expression of MICA
in osteosarcoma cells. These results indicate that hypoxia-induced
inhibition of NO production is not linked to the hypoxia-induced
decrease in the expression of MICA in osteosarcoma cells.
Under both hypoxia and normoxia, NOC18 showed only
slight effects on the expression of HIF-1α; however, NOC18
decreased the expression of MICA in osteosarcoma cells. Therefore,
it is likely that NO modulates the hypoxia-induced HIF-1α-dependent
decrease in the expression of MICA in osteosarcoma cells.
The present and previous studies (18,19)
show that hypoxia decreases the susceptibility of tumor cells by
reducing the expression of cell surface MICA, although the
mechanisms of the reduction in the expression of cell surface MICA
differ. Hypoxia has been reported to attenuate the antitumor
activity of cytotoxic immune cells (17). Therefore, a hypoxic
microenvironment seems to be more conducive to allowing tumor cells
to escape from immune surveillance.
Acknowledgements
This study was in part supported by
JSPS KAKENHI Grant no. 23590480 and MEXT-Supported Program for the
Strategic Research Foundation at Private Universities. We thank Mr.
Kenta Kobayashi for his technical assistance.
References
|
1.
|
Chouaib S, Messai Y, Couve S, Escudier B,
Hasmim M and Noman MZ: Hypoxia promotes tumor growth in linking
angiogenesis to immune escape. Front Immunol. 3:1–10. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Wilson WR and Hay MP: Targeting hypoxia in
cancer therapy. Nat Rev Cancer. 11:393–410. 2011. View Article : Google Scholar
|
|
3.
|
Höckel M and Vaupel P: Tumor hypoxia:
definitions and current clinical, biologic and molecular aspects. J
Natl Cancer Inst. 93:266–276. 2001.PubMed/NCBI
|
|
4.
|
Melillo G: Inhibiting hypoxia-inducible
factor 1 for cancer therapy. Mol Cancer Res. 4:601–605. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar
|
|
6.
|
Semenza GL: Involvement of
hypoxia-inducible factor 1 in human cancer. Intern Med. 41:79–83.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Giaccia A, Siim BG and Johnson RS: HIF-1
as a target for drug development. Nat Rev Drug Discov. 2:803–811.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Waldhauer I and Steinle A: NK cells and
cancer immunosurveillance. Oncogene. 27:5932–5943. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Nausch N and Cerwenka A: NKG2D ligands in
tumor immunity. Oncogene. 27:5944–5958. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Salih HR, Rammensee H-G and Steinle A:
Cutting edge: down-regulation of MICA on human tumors by
proteolytic shedding. J Immunol. 169:4098–4102. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Salih HR, Goehlsdorf D and Steinle A:
Release of MICB molecules by tumor cells: mechanism and soluble
MICB in sera of cancer patients. Hum Immunol. 67:188–195. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Waldhauer I and Steinle A: Proteolytic
release of soluble UL16-binding protein 2 from tumor cells. Cancer
Res. 66:2520–2526. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Boutet P, Agüera-González S, Atkinson S,
Pennington CJ, Edwards DR, Murphy G, Reyburn HT and Valés-Gómez M:
Cutting edge: the metalloproteinase ADAM17/TNF-α-converting enzyme
regulates proteolytic shedding of the MHC class I-related chain B
protein. J Immunol. 182:49–53. 2009.PubMed/NCBI
|
|
14.
|
Groh V, Wu J, Yee C and Spies T:
Tumour-derived soluble MIC ligands impair expression of NKG2D and
T-cell activation. Nature. 419:734–738. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Raffaghello L, Prigione I, Airoldi I,
Camoriano M, Levreri I, Gambini C, Pende D, Steinle A, Ferrone S
and Pistoia V: Downregulation and/or release of NKG2D ligands as
immune evasion strategy of human neuroblastoma. Neoplasia.
6:558–568. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Märten A, von Lilienfeld-Toal M, Büchler
MW and Schmidt J: Soluble MIC is elevated in the serum of patients
with pancreatic carcinoma diminishing γδT cell cytotoxicity. Int J
Cancer. 119:2359–2365. 2006.PubMed/NCBI
|
|
17.
|
Lee CT, Mace T and Repasky EA:
Hypoxia-driven immunosuppression: a new reason to use thermal
therapy in the treatment of cancer? Int J Hyperthermia. 26:232–246.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Siemens DR, Hu N, Sheikhi AK, Chung E,
Frederiksen LJ, Pross H and Graham CH: Hypoxia increases tumor cell
shedding of MHC class I chain-related molecule: role of nitric
oxide. Cancer Res. 68:4746–4753. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Barsoum IB, Hamilton TK, Li X, Cotechini
T, Miles EA, Siemens DR and Graham CH: Hypoxia induces escape from
innate immunity in cancer cells via increased expression of ADAM10:
role of nitric oxide. Cancer Res. 71:7433–7441. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Postovit LM, Adams MA, Lash GE, Heaton JP
and Graham CH: Oxygen-mediated regulation of tumor cell
invasiveness. Involvement of a nitric oxide signaling pathway. J
Biol Chem. 277:35730–35737. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Postovit LM, Sullivan R, Adams MA and
Graham CH: Nitric oxide signalling and cellular adaptations to
changes in oxygenation. Toxicology. 208:235–248. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Postovit LM, Adams MA, Lash GE, Heaton JP
and Graham CH: Nitric oxide-mediated regulation of hypoxia-induced
B16F10 melanoma metastasis. Int J Cancer. 108:47–53. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Yoon SY, Lee YJ, Seo JH, Sung HJ, Park KH,
Choi IK, Kim SJ, Oh SC, Choi CW, Kim BS, Shin SW, Kim YH and Kim
JS: uPAR expression under hypoxic conditions depends on iNOS
modulated ERK phosphorylation in the MDA-MB-231 breast carcinoma
cell line. Cell Res. 16:75–81. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Quintero M, Brennan PA, Thomas GJ and
Moncada S: Nitric oxide is a factor in the stabilization of
hypoxia-inducible factor-1alpha in cancer: role of free radical
formation. Cancer Res. 66:770–774. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Luo L, Lu J, Wei L, Long D, Guo JY, Shan
J, Li FS, Lu PY, Li PY and Feng L: The role of HIF-1 in
up-regulating MICA expression on human renal proximal tubular
epithelial cells during hypoxia/reoxygenation. BMC Cell Biol.
11:1–13. 2010.PubMed/NCBI
|
|
26.
|
Wei L, Lu J, Feng L, Long D, Shan J, Li S
and Li Y: HIF-1alpha accumulation upregulates MICA and MICB
expression on human cardiomyocytes and enhances NK cell
cytotoxicity during hypoxia-reoxygenation. Life Sci. 87:111–119.
2010. View Article : Google Scholar : PubMed/NCBI
|