Introduction
Allyl isothiocyanate (AITC) is a compound of the
natural isothiocyanates found in cruciferous vegetables such as
brussels sprouts, cauliflower, cabbage, kale, horseradish and
wasabi (1–4). AITC is known to have multiple effects
such as antipathogenic bacteria (5), anti-inflammatory (6), anti-fungicidal (7) and anticancer activities (4). Previous studies have demonstrated
that AITC exhibits significant antitumor activities against human
prostate (8), colorectal (9,10),
bladder (11,12), cervical cancer cells (13) and leukemia cells (14). The anticancer activities by AITC
are involved in the induction of cell cycle arrest and apoptosis as
well as inhibition of cell metastasis (15–17).
Our previous study demonstrated that AITC triggers G2/M
phase arrest and apoptosis in human brain malignant glioma GBM 8401
cells (16). However, there is no
report addressing whether or not AITC inhibits cell proliferation,
promotes cell cycle arrest and induces apoptosis in human breast
adenocarcinoma cells.
Breast cancer is one of the leading causes of death
in women worldwide (18).
According to statistical results from GLOBOCAN in 2008 year, about
1.38 million new patients were diagnosed in breast cancer, and
458,400 people died from breast cancer in the worldwide (19). In Taiwan, 14.8 per 100,000 women
die from breast cancer each year according to the Department of
Health in 2010. Breast cancer cells that lack of estrogen receptor
(ER), progesterone receptor (PR), and human EGF receptor 2
(HER-2/neu) expressions are known as triple negative breast
cancer (TNBC) (20,21). TNBC is the most clinically invasive
breast cancer and the characteristics are more invasive, less
responsive to chemotherapy agents and associated with poorer
prognosis (22). The current
therapy for TNBC includes surgery and systemic chemotherapy but the
clinical treatment is still unsatisfactory (23). Discovering TNBC therapeutic agents
from dietary natural products provides a useful application and
chemo-preventive or chemotherapeutic effectiveness on TNBC
(24,25). The goal of this study was to
explore whether the anti-TNBC activity of AITC mediates through the
direct cytotoxic effects and to understand the molecular mechanisms
in human breast adenocarcinoma MDA-MB-468 cells. This study is
focused on the cell cycle arrest and apoptotic cell death-induced
by AITC in the MDA-MB-468 cells. Our data demonstrated that AITC
inhibits cells viability, induces apoptotic death, and
simultaneously causes cell cycle arrest in G2/M phase
through the extracellular signal-regulated kinase (ERK) signaling
pathway in MDA-MB-468 cells.
Materials and methods
Chemicals
AITC, propidium iodide (PI), dimethyl sulfoxide
(DMSO), RNase A, N-acetyl-L-cysteine (NAC), U0126 and Triton
X-100 were purchased from Sigma-Aldrich Corp. (St. Louis, MO, USA).
The fluorescent probes 2′,7′-dichlorofluorescin diacetate
(H2DCF-DA) and 3,3′-dihexyloxacarbocyanine iodide
DiOC6(3), Dulbecco’s
modified Eagle’s medium (DMEM), L-glutamine, fetal bovine serum
(FBS), penicillin-streptomycin and trypsin-EDTA were obtained from
Life Technologies (Carlsbad, CA, USA). Anti-p-ERK, anti-ERK,
anti-Bcl-2, anti-p-Bcl-2 (Ser-70), anti-cytochrome c,
anti-Apaf-1, anti-cyclin B and anti-CDK1 and second antibodies were
purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA).
Anti-p21/WAF-1 (Cat. 05–345) and Immobilon Western Chemiluminescent
HRP substrate (Cat. WBKLS0500) were bought from Merck Millipore
Corp. (Bedford, MA, USA).
Cell culture
Human breast adenocarcinoma MDA-MB-468 cell line was
made by Dr Wei-Chien Huang (Graduate Institute of Cancer Biology,
China Medical University, Taichung, Taiwan). Cells were placed into
75-cm2 tissue culture flasks under a humidified 5%
CO2 and 95% air grown at 37°C and one atmosphere in DMEM
supplemented with 10% FBS, 2 mM L-glutamine, 100 U/ml penicillin
and 100 μg/ml streptomycin. Subconfluent cells (80%) were passaged
with a solution containing 0.25% trypsin and 0.02% EDTA.
Determinations of cell number and cell
viability
Cells at a density of 2×105 were seeded
in 12-well plates and then exposed to 5, 10, and 20 μM AITC or 0.1%
DMSO (as a vehicle control) for 24 and 48 h, then the cells were
harvested and the cell number determined using trypan blue stain by
Countess Automated Cell Counter (Invitrogen/Life Technologies)
(26,27). Cells were incubated with or without
5, 10 and 20 μM of AITC for 24 and 48 h in presence and absence of
NAC (a ROS scavenger) or U0126 (an ERK inhibitor). Cells were
determined for viability utilizing thiazolyl blue tetrazolium
bromide (MTT) assay as previously described (28,29).
Each data point was represented from three independent
experiments.
Cell morphological analysis
Approximately 2×105 cells/well of
MDA-MB-468 cells in 12-well plates were incubated with or without
20 μM AITC and equal amount of DMSO as a control for 24 h at 37°C.
At the end of treatment, cells were examined and photographed under
a phase-contrast microscope at a ×200 magnification for examining
the cell morphological changes (30,31).
Analysis for cell cycle distribution and
sub-G1 population
Approximately 2×105 cells/well of
MDA-MB-468 cells in 12-well plates were incubated in presence and
absence of 5, 10 and 20 μM AITC then were placed in an incubator
for 24 h, and then cells were harvested by centrifugation at 1,000
× g for 5 min, pellet were washed twice with cold PBS then fixed
gently by 70% ethanol at 4°C overnight. Then cells were washed
twice with cold PBS then resuspended in PBS containing 40 μg/ml PI
and 0.1 mg/ml RNase A and 0.1% Triton X-100 in the dark for 30 min
at 37°C then the cells were analyzed with a flow cytometer
(FACSCalibur, BD Biosciences, San Jose, CA, USA) equipped with an
argonion laser at 488 nm wavelength. Then the cell cycle and
sub-G1 (apoptosis) group were determined and analyzed
(32,33).
Caspase-9 and -3 activity assays
About 5×106 cells of MDA-MB-468 cells in
75-T flasks were treated with or without 5, 10, 15 and 20 μM of
AITC, then incubated for 12 h to detect the activity of caspase-9
and -3 which was assessed according to manufacturer’s instruction
of the Caspase colorimetric kit (R&D Systems Inc., Minneapolis,
MN, USA). Cells were harvested and lysed in 50 μl lysis buffer
containing 2 mM DTT, for 10 min. After centrifugation, the
supernatant containing 200 μg protein were incubated with caspase-9
and -3 substrates in reaction buffer. Then all samples were
incubated in a 96-well flat bottom microplate at 37°C for 1 h.
Levels of released pNA were measured with ELISA reader (Anthos
Labtec Instruments GmbH, Salzburg, Austria) at 405 nm wavelength
(34,35).
Detections of reactive oxygen species
(ROS) and mitochondrial membrane potential (ΔΨm)
MDA-MB-468 cells (2×105 cells/well) in
12-well plates with 0, 5, 10, 15 and 20 μM of AITC were incubated
for 12 h to determine the changes of ROS production and ΔΨm levels.
The cells were harvested by centrifugation and were washed twice by
PBS, then were resuspended in 500 µl of H2DCF-DA (10 μM)
and 500 μl of DiOC6(3)
(1 μmol/l) and incubated at 37°C in the dark for 30 min and were
analyzed immediately by flow cytometry as described previously
(32,33).
Western blot analysis
MDA-MB-468 cells at a density of 5×106
cells/ml seeded into T-75 flasks were treated with 5, 10 and 20 μM
of AITC for 2 and 12 h. Cells were harvested from each treatment
then were washed with cold PBS and then scraped and washed twice by
centrifugation at 1,000 × g for 5 min at 4°C. All pellets were
individually resuspended in the PRO-PREP protein extraction
solution (iNtRON Biotechnology, Seongnam-si, Korea) for 3 h at
−20°C as described previously (36,37).
The lysate from each sample was collected by centrifugation at
12,000 × g for 30 min at 4°C, and the supernatant was stored at
−20°C. Sodium dodecyl sulfate-polyacrylamide electrophoresis
(SDS-PAGE) gels were used to separate proteins before each sample
was incubated with the primary antibodies (Santa Cruz Biotechnology
Inc.) followed by secondary antibodies. These blots were then
detected by Immobilon Western Chemiluminescent HRP substrate (Merck
Millipore Corp.) and autoradiography using X-ray film (GE
Healthcare, Piscataway, NJ, USA) (38,39).
Each Immobilon-P transfer membrane (Cat. IPVH00010, Merck
Millipore) was stripped and reprobed with anti-β-actin antibody as
the loading control for ensuring that equal proteins were loaded
(40,41).
Determination of CDK1 kinase
activity
MDA-MB-468 cells were seeded onto 75-T flask and
then treated with 0, 5, 10 and 20 μM of AITC for 12 h. MDA-MB-468
cells were suspended in a final volume of 0.2 ml buffer containing
20 mM Tris-HCl (pH 8.5), 150 mM NaCl, 0.2% NP-40, 1 mM DTT, 1 mM
EDTA, 1 mM EGTA, 0.2 mM PMSF, 1 μg/ml pepstatin, 0.5 µg/ml
leupeptin, 5 mM β-glycerophosphate, 5 mM NaF, 1 mM
Na3VO4 and 5 mM β-mercaptoethanol. Cell
suspensions were sonicated and centrifuged at 10,000 × g for 30
min. CDK1 kinase activity condition was determined by using MV
Peptide (CycLex Cdc2-Cyclin B kinase assay kit, Medical &
Biological Laboratories Co Ltd, Nagoya, Japan) and measuring
OD492 as described previously (42,43).
Statistical analysis
Our data were performed as means ± SD of at least in
triplicate. The difference between the AITC-treated and control
groups were analyzed by Student’s t-test, p<0.05 was considered
significant.
Results
AITC reduces cell viability and affects
cell morphological changes and cell cycle arrest in MDA-MB-468
cells
In order to examine the biological effects of AITC,
MDA-MB-468 cells were treated with varying concentrations of AITC
at 0, 5, 10 and 20 μM for 24 and 48 h. Cells were harvested and the
cell number was determined using trypan blue stain by Countess
Automated Cell Counter, and the other cell viability and cell
morphological changes were assayed by MTT and phase-contrast
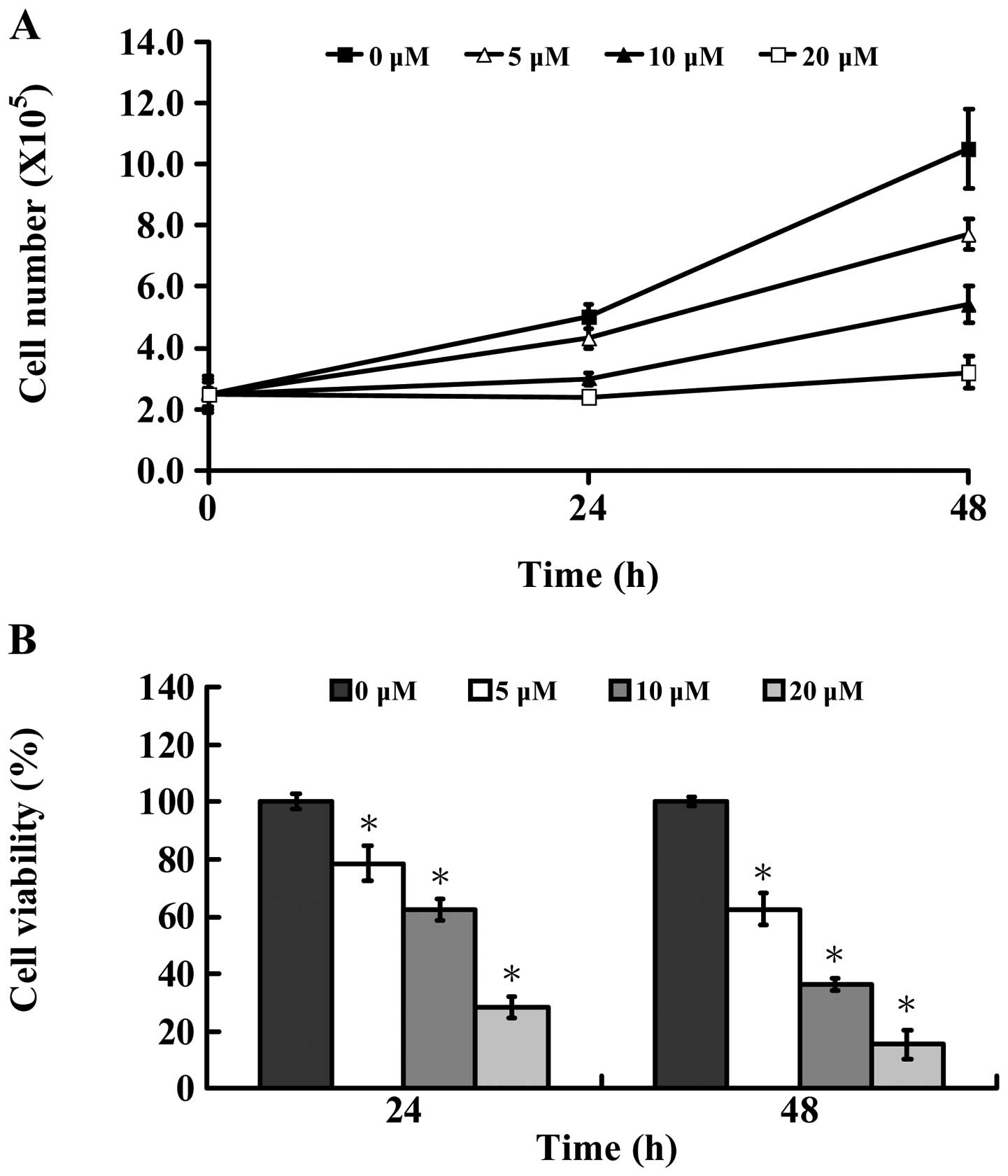
microscope. Fig. 1A shows that
AITC time-and concentration-dependently decreased the number of
MDA-MB-468 cells. Also, the results of these experiments indicated
that AITC caused a decrease of cell viability (Fig. 1B) in a dose- and time-dependent
manner as well as morphological changes (Fig. 2A). Based on these pilot
observations, the treated concentrations were assessed for the
effect of AITC at 5, 10 and 20 μM, which caused strong induction of
viable cells (Fig. 1B) and cell
morphological changes (Fig. 2A)
mostly in a dose- and time-dependent manner in 24 and 48 h. The
half maximal inhibitory concentration (IC50) of AITC was
10.26±1.31 μM after 24-h treatment. According to these results, the
different concentration and cell death effects of AITC are
accompanied by its effect on cell cycle progression and/or
apoptotic cell death. We found that AITC promoted G2/M
phase arrest and sub-G1 population in MDA-MB-468 cells.
Also, these effects were dose- and time-course dependent (Fig. 2B).
AITC stimulates the activities of
caspase-9 and -3 in MDA-MB-468 cells
Cells were exposed to various concentrations of AITC
for 12 h treatment to determine caspase-9 and -3 activities. Our
results indicated that the caspase-9 and -3 activities were
time-and concentration-dependently stimulated in AITC-treated
MDA-MB-468 cells (Fig. 3). Thus,
we suggest that AITC-triggered apoptosis is carried out through
caspase-9 and -3-dependent signaling in MDA-MB-468 cells.
AITC promotes the reactive oxygen species
(ROS) production and loss of ΔΨm levels in MDA-MB-468 cells
The results of flow cytometric analysis for ROS
production and ΔΨm levels are shown in Fig. 4A and B. AITC-treated MDA-MB-468
cells with DCF were observed with increased intracellular ROS
(Fig. 4A). We further explored if
mitochondrial depolarization contributed to AITC-induced apoptosis
of MDA-MB-468 cells. The treated and un-treated cells were exposed
to AITC to investigate the change in ΔΨm in MDA-MB-468 after being
stained with DiOC6(3),
a mitochondria-specific and voltage-dependent dye. Results shown in
Fig. 4B display that AITC
significantly decreased the levels of ΔΨm in MDA-MB-468 cells
(Fig. 4B). Based on these
observations, we found that AITC-provoked cell apoptosis is
involved in ROS production and intrinsic signaling pathway in
MDA-MB-468 cells.
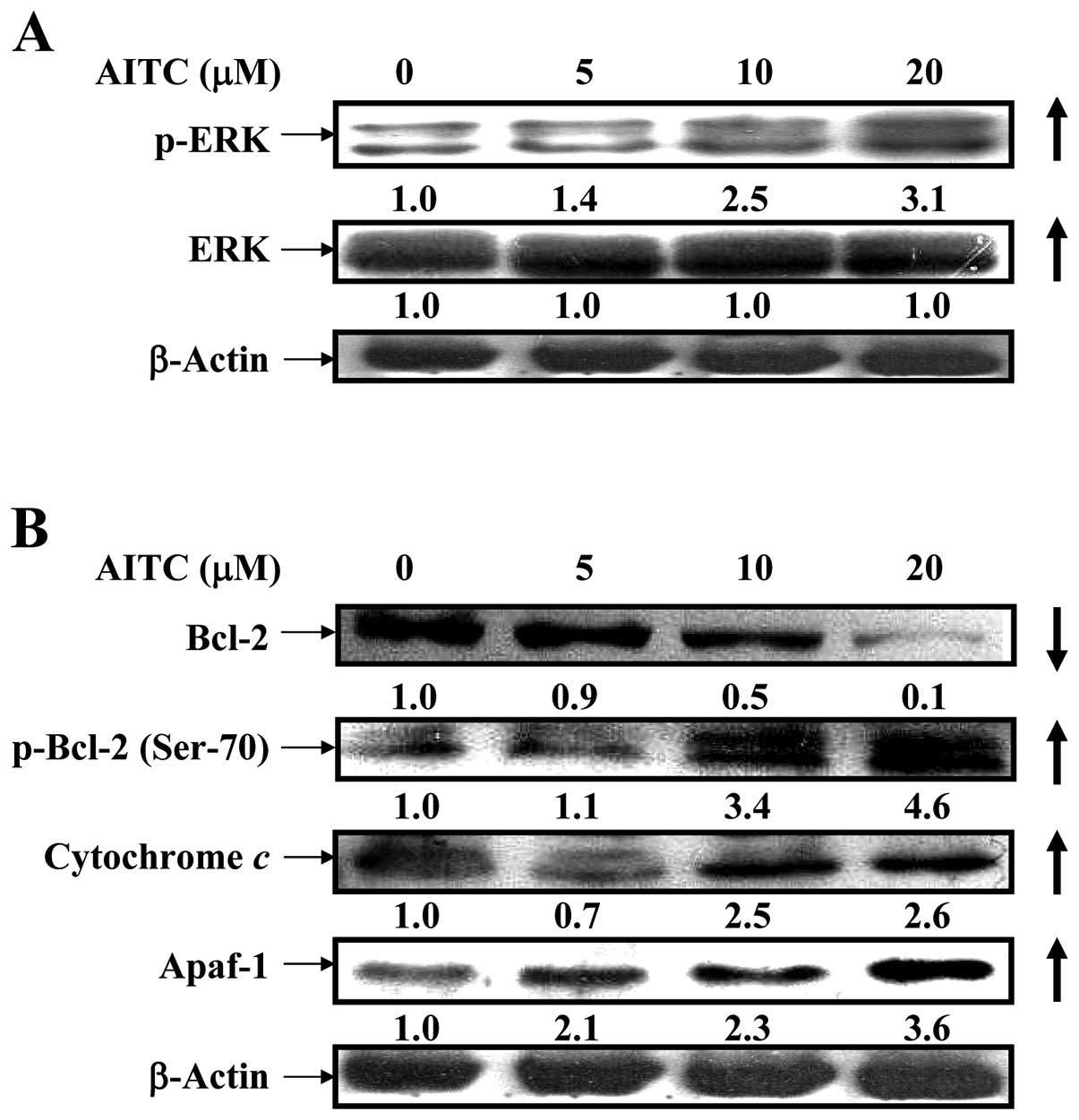
AITC upregulates p-ERK signaling and
alters mitochondria-dependent apoptotic pathway in MDA-MB-468
cells
It is widely reported that ERK/MAPK positively
regulated phosphorylation of Bcl-2 at Ser-70, causing antiapoptotic
function to suppress Bcl-2 expression (44,45).
To clarify whether AITC influences intrinsic apoptotic signaling
through ERK pathway, the results from western blotting are shown in
Fig. 5A and B indicating that the
protein levels of p-ERK and ERK (Fig.
5A), p-Bcl-2 (Ser-70), cytochrome c and Apaf-1 (Fig. 5B) and p21/WAF-1 (Fig. 7B) were upregulated in MDA-MB-468
cells after treatment with AITC, but that of Bcl-2 was
downregulated. Many reports have shown that apoptosis is associated
with the loss of ΔΨm which is an endpoint of apop- which is an
endpoint of apoptosis (46). Our
findings indicated that antiapoptosis signaling involving Bcl-2
phosphorylation is involved in ERK/MAPK pathway in AITC-treated
MDA-MB-468 cells.
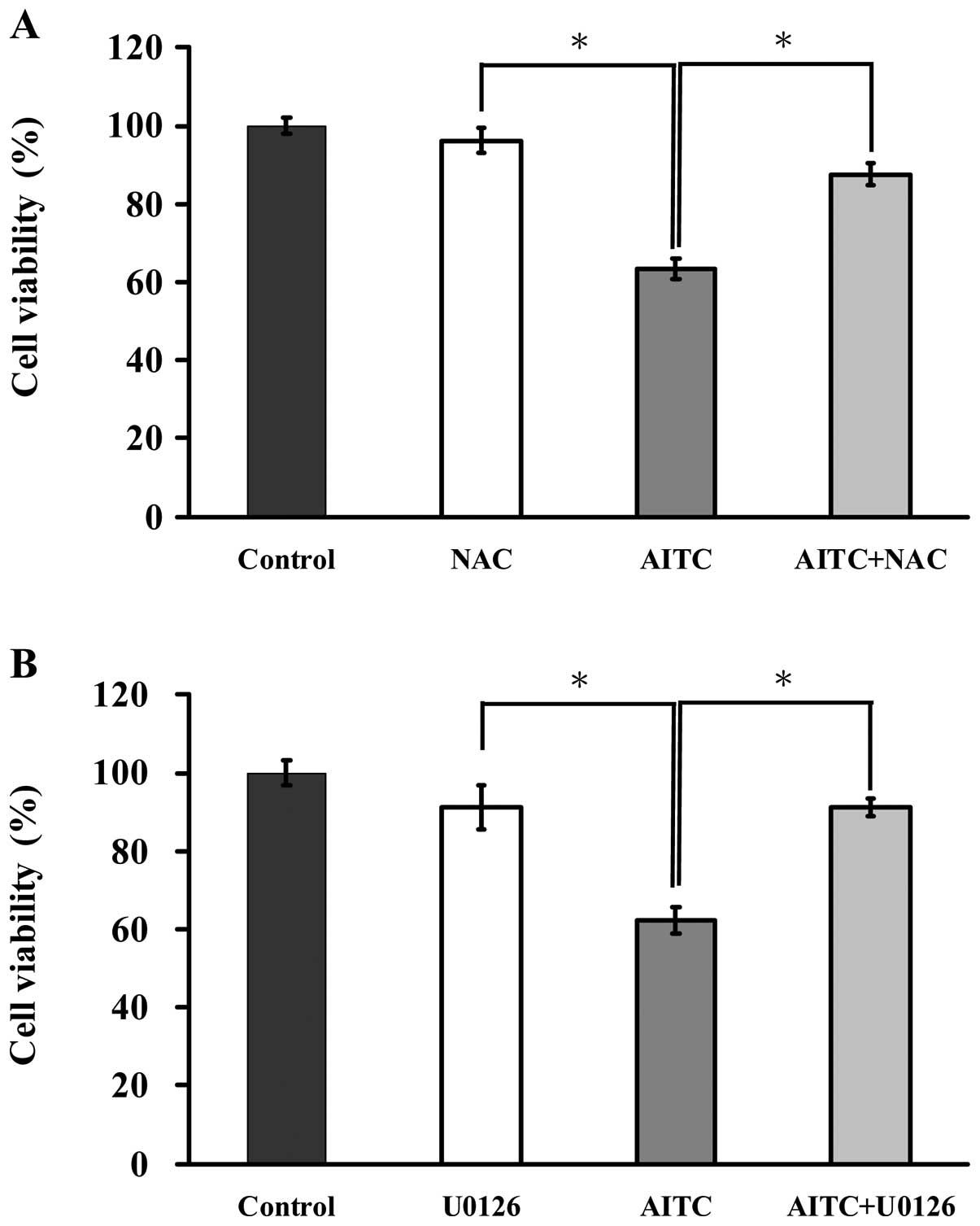
ROS and ERK are associated with the
induction of apoptosis in AITC-treated MDA-MB-468 cells
To elucidate the possible signaling pathways of
AITC-reduced viability of MDA-MB-468 cells, we determined whether
ROS and ERK mediated AITC-regulated apoptotic signaling. Cells were
pretreated with or without NAC (a ROS scavenger) and U0126 (an ERK
inhibitor) and then exposed to AITC (10 μM) for 24 h. Cells were
then determined for measuring cell viability by MTT assay. As shown
in Fig. 6A, cells after treatment
with AITC in presence and absence of NAC were observed to protect
reduction of viability of MDA-MB-468 cells when compare with only
AITC treated sample. Fig. 6B
displays that reduction of cell viability in MDA-MB-468 cells by
AITC was dramatically reversed by U0126 in comparison to
AITC-treated only cells. Thus, ROS and ERK play central roles in
AITC-induced apoptosis of MDA-MB-468 cells.
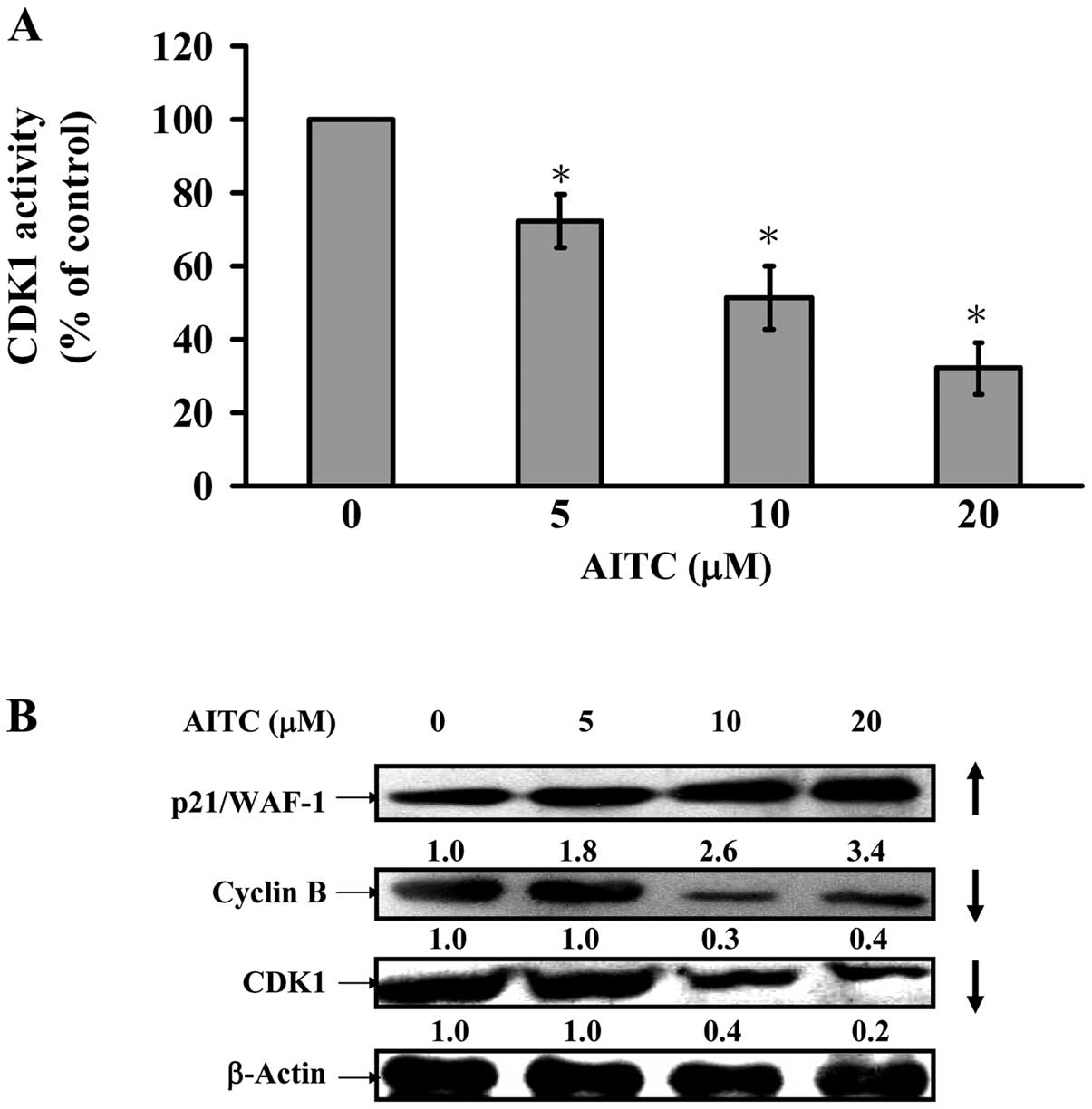
AITC decreases CDK1 activity and alters
G2/M phase-modulated protein levels in MDA-MB-468
cells
We further assessed if G2/M phase arrest
is involved in CDK1 activity, and data showed that AITC at 5–20 μM
dramatically reduced CDK1 activity in MDA-MB-468 cells (Fig. 7A). We also explored the possible
molecular mechanisms in the modulation of G2/M phase
arrest. Fig. 7B indicates that
AITC caused an increase of p21/WAF-1 expression and a decrease of
cyclin B and CDK1 protein levels in MDA-MB-468. Hence, we suggest
that AITC-provoked G2/M phase arrest is mediated through
activating p21/WAF-1 and suppressing CDK1/cyclin B expression in
in vitro cultivation of MDA-MB-468 cells.
Discussion
Many studies in new drug discovery have focused on
breast adenocarcinoma therapeutic agents through the promotion of
cell cycle arrest and induction of apoptotic cell death (47). Previous studies also demonstrated
that AITC arrested HL60 leukemia cells at
G0/G1 phase (14), but caused G2/M arrest in
UMUC-3 cells (48), HeLa cells
(13), HT29 cells (9), SW620 cells (15), GBM 8401 cells (16), PC-3 and LNCaP cells (49). In this study, our results showed
that AITC inhibited the cell growth and proliferation on MCF-7
cells, MDA-MB-231 cells (data not shown), and MDA-MB-468 cells
(Fig. 1) in a time- and
concentration-dependent manner. The IC50 for 24-h
treatment of AITC in MCF-7, MDA-MB-231 and MDA-MB-468 cells were
17.96±2.58, 11.26±1.29 and 10.26±1.31 μM, respectively. One of the
reasons for the differences in sensitivity in IC50 of
different cell lines may be due to the inherently different
doubling time in different cell lines. The doubling time of MCF-7,
MDA-MB-231 and MDA-MB-468 cells were 30.2±0.7, 28.1±1.2 and
29.9±0.5 h (50). The distinct
ability of AITC in growth inhibition of MCF-7, MDA-MB-231 and
MDA-MB-468 cells might be caused by differential gene expression in
different cell types. The MCF-7 cell line is p53 wild-type, but
both of MDA-MB-231 and MDA-MB-468 cells are p53 mutant-type
(51).
Many reports have shown that AITC-induced
G2/M arrest was associated with a marked decrease in the
protein levels of cyclin B1, CDK1, cdc25B and cdc25C and caused the
disruption of tubulin (9,52,53).
The G2/M phase progression is regulated with CDK1
kinases that are activated in association with cyclin A or cyclin B
(52). The p21/WAF1 is one of the
cyclin-dependent kinase inhibitors (CKI) which inhibits cyclin/CDK
complexes in the G2/M phase. The p21/WAF1 has been found
to be associated with the growth arrest in cells (53). Enhanced p21/WAF1 mRNA expression
occurs through both p53-dependent and -independent mechanisms
(54). Furthermore, ERK MAPK
pathway has recently been reported to cooperate to cause sustained
cell cycle arrest requiring p21/WAF1 expression (55,56).
Our results from cell cycle analysis indicated that AITC induced
G2/M phase arrest (Fig.
2) in MDA-MB-468 cells. The CDK1 and cyclin B proteins were
decreased (Fig. 7B), and
phospho-ERK (Fig. 5A) and
p21/WAF-1 (Fig. 7B) were increased
by AITC treatment in a concentration-dependent manner. AITC also
inhibited the CDK1 activity (Fig.
7A). Our study revealed that the novel molecular mechanism by
which AITC induces G2/M phase arrest and apoptosis in
triple negative breast cancer MDA-MB-468 cells is through
ERK-dependent p21/WAF-1 upregulation.
Two major signaling pathways are involved in
apoptotic cell death (57,58). The extrinsic pathway (also called
death receptor pathway) through the activation of the cell surface
[Fas/Fas ligand (FasL) or TNF-related apoptosis-inducing ligand
(TRAIL)] then promotes caspase-8 activation. The intrinsic pathway
(also called mitochondria pathway) through death signals to
mitochondria result in the release of mitochondrial inter-membrane
proteins such as cytochrome c, which associate with
apoptotic protease-activating factor-1 (Apaf-1) and pro-caspase-9
to form the apoptosome and then active caspase-3. The
caspase-independent pathway is involved in the mitochondria which
led to releases of apoptosis inducing factor (AIF) or endonuclease
G (Endo G) from mitochondria causing cell death (59). Our results showed that AITC induced
apoptotic death (sub-G1 phase) of MDA-MB-468 cells and
this action is concentration-dependent (Fig. 2B). AITC treatment in MDA-MB-468
cells concentration-dependently promoted the activations of
caspase-9 and caspase-3 (Fig. 3).
Cells were pretreated with NAC (a ROS scavenger) and U0126 (an ERK
inhibitor) and exposed to AITC, leading to increase the percentage
of viable cells when compared to the AITC-treated only cells
(Fig. 6). The results in Fig. 5 show that the protein expressions
of p-ERK, p-Bcl-2 (Ser-70), cytochrome c and Apaf-1 were
upregulated in MDA-MB-468 cells after treatment with AITC. Our
results suggested that AITC upregulated ERK signaling and altered
mitochondria-dependent associated apoptotic pathway in MDA-MB-468
cells. Bcl-2 phosphorylation is known to affect antiapoptotic
activity (44,45). Induction of apoptosis associated
with Bcl-2 phosphorylation by anticancer agents has been linked
with altering a variety of cellular signaling pathways, such as
Ras/Raf, protein kinase C, protein kinase A, mitogen-activated
protein kinase, ERK and CDK1 (35). Our results are in agreement with
previous studies (60,61) indicating that that AITC-induced
apoptotic cell death was caused by Bcl-2 phosphorylation and ERK
activation.
The molecular signaling pathways are summarized in
Fig. 8. Our results demonstrate
that the ERK signaling pathway modulated intrinsic signaling and
G2/M phase arrest in AITC-treated MDA-MB-468 cells.
These findings implied that AITC may be used as a novel therapeutic
agent for the treatment of human breast cancer.
Acknowledgements
This study was supported by the grant
CMU-100-ASIA-4 from China Medical University and partly supported
by the Grant-in-Aid from the National Science Council, Taiwan,
R.O.C. (NSC 97-2320-B-039-004-MY3).
References
|
1.
|
Kushad MM, Brown AF, Kurilich AC, et al:
Variation of glucosinolates in vegetable crops of Brassica
oleracea. J Agric Food Chem. 47:1541–1548. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Rungapamestry V, Duncan AJ, Fuller Z and
Ratcliffe B: Changes in glucosinolate concentrations, myrosinase
activity, and production of metabolites of glucosinolates in
cabbage (Brassica oleracea var. capitata) cooked for
different durations. J Agric Food Chem. 54:7628–7634. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Uematsu Y, Hirata K, Suzuki K, Iida K,
Ueta T and Kamata K: Determination of isothiocyanates and related
compounds in mustard extract and horseradish extract used as
natural food additives. Shokuhin Eiseigaku Zasshi. 43:10–17.
2002.(In Japanese).
|
|
4.
|
Zhang Y: Allyl isothiocyanate as a cancer
chemopreventive phytochemical. Mol Nutr Food Res. 54:127–135. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Negri R, Muntoni F and D’Amore R:
Antibiotic effect of the allyl isothiocyanate extracted from
various horticultural forms of the seeds of Raphanus Sativus
L. var. radicula pers towards various bacteria, including two
strains of tubercle bacillus avian type, Cow 18 and Cow 70. Note II
Rend Ist Sup Sanit. 14:186–193. 1951.PubMed/NCBI
|
|
6.
|
Wagner AE, Boesch-Saadatmandi C, Dose J,
Schultheiss G and Rimbach G: Anti-inflammatory potential of
allyl-isothiocyanate - role of Nrf2, NF-(kappa) B and microRNA-155.
J Cell Mol Med. 16:836–843. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Sellam A, Dongo A, Guillemette T, Hudhomme
P and Simoneau P: Transcriptional responses to exposure to the
brassicaceous defence metabolites camalexin and
allyl-isothiocyanate in the necrotrophic fungus Alternaria
brassicicola. Mol Plant Pathol. 8:195–208. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Srivastava SK, Xiao D, Lew KL, et al:
Allyl isothiocyanate, a constituent of cruciferous vegetables,
inhibits growth of PC-3 human prostate cancer xenografts in vivo.
Carcinogenesis. 24:1665–1670. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Smith TK, Lund EK, Parker ML, Clarke RG
and Johnson IT: Allyl-isothiocyanate causes mitotic block, loss of
cell adhesion and disrupted cytoskeletal structure in HT29 cells.
Carcinogenesis. 25:1409–1415. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Smith T, Musk SR and Johnson IT: Allyl
isothiocyanate selectively kills undifferentiated HT29 cells in
vitro and suppresses aberrant crypt foci in the colonic mucosa of
rats. Biochem Soc Trans. 24:381S1996.
|
|
11.
|
Bhattacharya A, Li Y, Geng F, Munday R and
Zhang Y: The principal urinary metabolite of allyl isothiocyanate,
N-acetyl-S-(N-allylthiocarbamoyl)cysteine, inhibits the growth and
muscle invasion of bladder cancer. Carcinogenesis. 33:394–398.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Bhattacharya A, Tang L, Li Y, et al:
Inhibition of bladder cancer development by allyl isothiocyanate.
Carcinogenesis. 31:281–286. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Hasegawa T, Nishino H and Iwashima A:
Isothiocyanates inhibit cell cycle progression of HeLa cells at
G2/M phase. Anticancer Drugs. 4:273–279. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Zhang Y, Tang L and Gonzalez V: Selected
isothiocyanates rapidly induce growth inhibition of cancer cells.
Mol Cancer Ther. 2:1045–1052. 2003.PubMed/NCBI
|
|
15.
|
Lau WS, Chen T and Wong YS: Allyl
isothiocyanate induces G2/M arrest in human colorectal
adenocarcinoma SW620 cells through downregulation of Cdc25B and
Cdc25C. Mol Med Rep. 3:1023–1030. 2010.PubMed/NCBI
|
|
16.
|
Chen NG, Chen KT, Lu CC, et al: Allyl
isothiocyanate triggers G2/M phase arrest and apoptosis
in human brain malignant glioma GBM 8401 cells through a
mitochondria-dependent pathway. Oncol Rep. 24:449–455.
2010.PubMed/NCBI
|
|
17.
|
Hwang ES and Lee HJ: Allyl isothiocyanate
and its N-acetylcysteine conjugate suppress metastasis via
inhibition of invasion, migration, and matrix
metalloproteinase-2/-9 activities in SK-Hep 1 human hepatoma cells.
Exp Biol Med (Maywood). 231:421–430. 2006.
|
|
18.
|
Rodrigues-Ferreira S, Abdelkarim M,
Dillenburg-Pilla P, et al: Angiotensin II facilitates breast cancer
cell migration and metastasis. PLoS One. 7:e356672012. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
20.
|
Ossovskaya V, Wang Y, Budoff A, et al:
Exploring molecular pathways of triple-negative breast cancer.
Genes Cancer. 2:870–879. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Rakha EA, Reis-Filho JS and Ellis IO:
Basal-like breast cancer: a critical review. J Clin Oncol.
26:2568–2581. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Gucalp A and Traina TA: Triple-negative
breast cancer: adjuvant therapeutic options. Chemother Res Pract.
2011:6962082011.PubMed/NCBI
|
|
23.
|
Gelmon K, Dent R, Mackey JR, Laing K,
McLeod D and Verma S: Targeting triple-negative breast cancer:
optimising therapeutic outcomes. Ann Oncol. Apr 19–2012, (Epub
ahead of print).
|
|
24.
|
Li C, Zhao X, Toline EC, et al: Prevention
of carcinogenesis and inhibition of breast cancer tumor burden by
dietary stearate. Carcinogenesis. 32:1251–1258. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Ebert T, Kleine-Gunk B, Altwein JE, Miller
K and Mallmann P: Dietary prevention of carcinomas of the breast
and prostate: fundamental and practical aspects of the Nutritional
Cancer Prevention (NCP) program. Dtsch Med Wochenschr.
127:1392–1396. 2002.(In German).
|
|
26.
|
Chen KT, Hour MJ, Tsai SC, et al: The
novel synthesized
6-fluoro-(3-fluorophenyl)-4-(3-methoxyanilino)quinazoline (LJJ-10)
compound exhibits anti-metastatic effects in human osteosarcoma U-2
OS cells through targeting insulin-like growth factor-I receptor.
Int J Oncol. 39:611–619. 2011.
|
|
27.
|
Liao CL, Lai KC, Huang AC, et al: Gallic
acid inhibits migration and invasion in human osteosarcoma U-2 OS
cells through suppressing the matrix metalloproteinase-2/-9,
protein kinase B (PKB) and PKC signaling pathways. Food Chem
Toxicol. 50:1734–1740. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Yu FS, Wu CC, Chen CT, et al: Diallyl
sulfide inhibits murine WEHI-3 leukemia cells in BALB/c mice in
vitro and in vivo. Hum Exp Toxicol. 28:785–790. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Tsou MF, Peng CT, Shih MC, et al: Benzyl
isothiocyanate inhibits murine WEHI-3 leukemia cells in vitro and
promotes phagocytosis in BALB/c mice in vivo. Leuk Res.
33:1505–1511. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Lu CC, Yang JS, Huang AC, et al:
Chrysophanol induces necrosis through the production of ROS and
alteration of ATP levels in J5 human liver cancer cells. Mol Nutr
Food Res. 54:967–976. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Lu CC, Yang JS, Chiang JH, et al: Novel
quinazolinone MJ-29 triggers endoplasmic reticulum stress and
intrinsic apoptosis in murine leukemia WEHI-3 cells and inhibits
leukemic mice. PLoS One. 7:e368312012. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Lin JP, Yang JS, Chang NW, et al: GADD153
mediates berberine-induced apoptosis in human cervical cancer Ca
ski cells. Anticancer Res. 27:3379–3386. 2007.PubMed/NCBI
|
|
33.
|
Lin YT, Yang JS, Lin HJ, et al: Baicalein
induces apoptosis in SCC-4 human tongue cancer cells via a
Ca2+-dependent mitochondrial pathway. In Vivo.
21:1053–1058. 2007.PubMed/NCBI
|
|
34.
|
Ying WZ and Sanders PW: Cytochrome c
mediates apoptosis in hypertensive nephrosclerosis in Dahl/Rapp
rats. Kidney Int. 59:662–672. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Yang JS, Hour MJ, Huang WW, Lin KL, Kuo SC
and Chung JG: MJ-29 inhibits tubulin polymerization, induces
mitotic arrest, and triggers apoptosis via cyclin-dependent kinase
1-mediated Bcl-2 phosphorylation in human leukemia U937 cells. J
Pharmacol Exp Ther. 334:477–488. 2010. View Article : Google Scholar
|
|
36.
|
Liu KC, Huang AC, Wu PP, et al: Gallic
acid suppresses the migration and invasion of PC-3 human prostate
cancer cells via inhibition of matrix metalloproteinase-2 and -9
signaling pathways. Oncol Rep. 26:177–184. 2011.PubMed/NCBI
|
|
37.
|
Lan YH, Wu YC, Wu KW, et al: Death
receptor 5-mediated TNFR family signaling pathways modulate
γ-humulene-induced apoptosis in human colorectal cancer HT29 cells.
Oncol Rep. 25:419–424. 2011.PubMed/NCBI
|
|
38.
|
Wu SH, Hang LW, Yang JS, et al: Curcumin
induces apoptosis in human non-small cell lung cancer NCI-H460
cells through ER stress and caspase cascade- and
mitochondria-dependent pathways. Anticancer Res. 30:2125–2133.
2010.PubMed/NCBI
|
|
39.
|
Lai WW, Yang JS, Lai KC, et al: Rhein
induced apoptosis through the endoplasmic reticulum stress,
caspase- and mitochondria-dependent pathways in SCC-4 human tongue
squamous cancer cells. In Vivo. 23:309–316. 2009.
|
|
40.
|
Chiang JH, Yang JS, Ma CY, et al:
Danthron, an anthraquinone derivative, induces DNA damage and
caspase cascades-mediated apoptosis in SNU-1 human gastric cancer
cells through mitochondrial permeability transition pores and
Bax-triggered pathways. Chem Res Toxicol. 24:20–29. 2011.
View Article : Google Scholar
|
|
41.
|
Huang WW, Chiu YJ, Fan MJ, et al:
Kaempferol induced apoptosis via endoplasmic reticulum stress and
mitochondria-dependent pathway in human osteosarcoma U-2 OS cells.
Mol Nutr Food Res. 54:1585–1595. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Huang WW, Ko SW, Tsai HY, et al:
Cantharidin induces G2/M phase arrest and apoptosis in
human colorectal cancer colo 205 cells through inhibition of CDK1
activity and caspase-dependent signaling pathways. Int J Oncol.
38:1067–1073. 2011.PubMed/NCBI
|
|
43.
|
Chou LC, Yang JS, Huang LJ, et al: The
synthesized 2-(2-fluorophenyl)-6,7-methylenedioxyquinolin-4-one
(CHM-1) promoted G2/M arrest through inhibition of CDK1 and induced
apoptosis through the mitochondrial-dependent pathway in CT-26
murine colorectal adenocarcinoma cells. J Gastroenterol.
44:1055–1063. 2009. View Article : Google Scholar
|
|
44.
|
Deng X, Kornblau SM, Ruvolo PP and May WS
Jr: Regulation of Bcl2 phosphorylation and potential significance
for leukemic cell chemoresistance. J Natl Cancer Inst Monogr.
30–37. 2001.PubMed/NCBI
|
|
45.
|
Mai H, May WS, Gao F, Jin Z and Deng X: A
functional role for nicotine in Bcl2 phosphorylation and
suppression of apoptosis. J Biol Chem. 278:1886–1891. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Lavrik IN, Golks A and Krammer PH:
Caspases: pharmacological manipulation of cell death. J Clin
Invest. 115:2665–2672. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Choi JA, Kim JY, Lee JY, et al: Induction
of cell cycle arrest and apoptosis in human breast cancer cells by
quercetin. Int J Oncol. 19:837–844. 2001.PubMed/NCBI
|
|
48.
|
Tang L and Zhang Y: Dietary
isothiocyanates inhibit the growth of human bladder carcinoma
cells. J Nutr. 134:2004–2010. 2004.PubMed/NCBI
|
|
49.
|
Xiao D, Srivastava SK, Lew KL, et al:
Allyl isothiocyanate, a constituent of cruciferous vegetables,
inhibits proliferation of human prostate cancer cells by causing
G2/M arrest and inducing apoptosis. Carcinogenesis. 24:891–897.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
50.
|
Watanabe N, Okochi E, Mochizuki M,
Sugimura T and Ushijima T: The presence of single nucleotide
instability in human breast cancer cell lines. Cancer Res.
61:7739–7742. 2001.PubMed/NCBI
|
|
51.
|
Salem SD, Abou-Tarboush FM, Saeed NM, et
al: Involvement of p53 in gemcitabine mediated cytotoxicity and
radiosensitivity in breast cancer cell lines. Gene. 498:300–307.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
52.
|
Lobrich M and Jeggo PA: The impact of a
negligent G2/M checkpoint on genomic instability and cancer
induction. Nat Rev Cancer. 7:861–869. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53.
|
Maeda T, Nagaoka Y, Kawai Y, et al:
Inhibitory effects of cancer cell proliferation by novel histone
deacetylase inhibitors involve p21/WAF1 induction and G2/M arrest.
Biol Pharm Bull. 28:849–853. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
54.
|
Wu L and Levine AJ: Differential
regulation of the p21/WAF-1 and mdm2 genes after high-dose UV
irradiation: p53-dependent and p53-independent regulation of the
mdm2 gene. Mol Med. 3:441–451. 1997.PubMed/NCBI
|
|
55.
|
Ciccarelli C, Marampon F, Scoglio A, et
al: p21WAF1 expression induced by MEK/ERK pathway activation or
inhibition correlates with growth arrest, myogenic differentiation
and onco-phenotype reversal in rhabdomyosarcoma cells. Mol Cancer.
4:412005. View Article : Google Scholar
|
|
56.
|
Lee B and Moon SK: Ras/ERK signaling
pathway mediates activation of the p21WAF1 gene promoter in
vascular smooth muscle cells by platelet-derived growth factor.
Arch Biochem Biophys. 443:113–119. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
57.
|
Kroemer G, Galluzzi L and Brenner C:
Mitochondrial membrane permeabilization in cell death. Physiol Rev.
87:99–163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
58.
|
Lai E, Teodoro T and Volchuk A:
Endoplasmic reticulum stress: signaling the unfolded protein
response. Physiology (Bethesda). 22:193–201. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
59.
|
Park HH: Structural features of
caspase-activating complexes. Int J Mol Sci. 13:4807–4818. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
60.
|
Geng F, Tang L, Li Y, et al: Allyl
isothiocyanate arrests cancer cells in mitosis, and mitotic arrest
in turn leads to apoptosis via Bcl-2 protein phosphorylation. J
Biol Chem. 286:32259–32267. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61.
|
Xu C, Shen G, Yuan X, et al: ERK and JNK
signaling pathways are involved in the regulation of activator
protein 1 and cell death elicited by three isothiocyanates in human
prostate cancer PC-3 cells. Carcinogenesis. 27:437–445. 2006.
View Article : Google Scholar : PubMed/NCBI
|