Introduction
Inflammatory processes caused by pathogens or by
chemical or physical agents are important components in the
pathogenesis of many human cancers (1). Increasing evidence suggests that also
in the prostate gland inflammation may be implicated in
oncogenesis. A recent study performed on 70,000 North American men
showed that a clinical history of prostatitis significantly
increases the relative risk for prostate cancer (PCa) (1.3; 95% CI:
1.1–1.5), and that a longer duration of the disease may further
increase this risk (2). More
indirect meta-analytic evidence showed that chronic consumption of
aspirin or other NSAIDs can significantly decrease the odds of
prostate cancer (PCa) (0.92; 95% CI: 0.86–0.97) (3).
In recent years, extensive research efforts have
been devoted to investigate the link between inflammation and PCa
and to find which inflammatory lesions of the glandular epithelium
might act as early forerunners of neoplastic transformation. A new
‘injury-and-regeneration’ model, linking the effects of chronic
inflammation to the molecular and cellular modifications underlying
the pathogenesis of prostate cancer has been advanced by De Marzo
and coworkers (4). According to
this model, inflammatory cells infiltrating the prostate in
response to an injury caused by infection or by the action of
endogenous/exogenous irritants or cytotoxins may cause the
initiation of neoplastic transformation by releasing genotoxic
reactive oxygen species (ROS). Tissue injury and cell loss would at
the same time trigger a tumor promotion step with proliferative
regeneration of the damaged epithelium, leading to the appearance,
mainly in the peripheral zone of the gland, of putative cancer
‘risk’ lesions of atrophic appearance, generally referred to as
‘Proliferative Inflammatory Atrophy’ (PIA). Proliferating atrophic
cells, further exposed to ROS-induced oxidative DNA damage, might
subsequently progress to the in situ cancer precursor
prostatic intraepithelial neoplasia (PIN) and, ultimately, to frank
adenocarcinoma (4).
The hypothesis pointing to PIA as a risk-lesion for
prostate cancer has been investigated at the preclinical, clinical,
cellular and molecular levels (reviewed in ref. 5). Very recently, the presence of atrophy
has been linked to advanced prostate cancer (6,7). At
the morphological level, it has been shown that high-grade (HG) PIN
and PCa may merge with PIA lesions, thus suggesting the existence
of a continuum between these entities (8,9).
However, the issue of whether these histological findings are true
signs of an undergoing PIA-cancer transition is still controversial
(10–14), though some experts cautiously
suggest that the great genetic instability of atrophic cells makes
them more vulnerable to lesions possibly leading to neoplastic
transformation (15,16).
At the genetic level, it was shown that a number of
hallmarks of high-grade PIN and PCa are found in PIA cells
(4,17). For example, chromosomal aberrations
commonly found in PCa, like 8p22 loss, 8q24 gain, 8c gain and X
gain, are also detected in PIA, albeit in the form of somatic
aberrations (18–21). At the molecular level, a number of
proto-oncogenes, tumor-suppressors and transducers of
growth/survival signals such as NKX3.1, MSR1, Ki-67, Bcl-2,
p16/CDKN2, p27, p53, GSTP1 and Cox-2, were found to be
upregulated/disregulated in PIA, sometimes to an extent similar or
identical to high-grade PIN or PCa (reviewed in refs. 4 and 5).
The increasing evidence aimed at linking PIA, inflammation and PCa
extends to findings involving the role of corpora amylacea
(22), the expression of the
prostate tumor overexpressed-1 (PTOV1) gene in atrophy (23), the involvement of immune regulatory
cells (e.g., TH17 cells) and cytokines, and the influence of a
number of PCa-associated polymorphisms in the cyclooxygenase-2 gene
(reviewed in refs. 17 and
24).
Despite this mounting host of molecular evidence,
very scant morphological/topographic data are available linking PIA
or PIN to inflammatory findings or to clinical chronic prostatitis
(CP).
To increase knowledge in this area, we investigated
at the morphological level 1367 prostate biopsies from 98 patients
affected by chronic prostatitis (CP) and 32 patients with a history
of CP and a biopsy positive for carcinoma. The aim of this study
was to investigate the topographic and quantitative relationship
between inflammation, proliferative inflammatory atrophy and low-
or high-grade proliferative intraepithelial neoplasia.
Materials and methods
Biopsy material
This study was performed on biopsy specimens from
patients randomly selected from a historical collection of
hematoxylin and eosin-stained, fully anonymized prostate biopsies,
collected in the years 2000–2010.
Patients had been subjected to prostate biopsy to
exclude the presence of malignancy, in the presence of elevated
total serum PSA levels (>4 ng/ml) and suspicious clinical
findings.
The inclusion criterion for this retrospective study
was a recent history (<3 months) of class II chronic bacterial
prostatitis (CBP, 28% of total patients) or class III chronic
prostatitis/chronic pelvic pain syndrome [CP/CPPS, inflammatory
subtype IIIa; NIH-NIDDK classification, (25)] (72% of total patients). At
diagnosis of chronic prostatitis (CP), patients were subjected to a
combined pharmacological treatment protocol of 4 weeks, including
antibacterial agents, α blockers and anti-inflammatory agents
(26,27).
In the presence of persisting elevated PSA levels
post-therapy (27), patients were
subjected to transrectal biopsies directed to the peripheral zone
of the gland. A total of 1367 biopsy cores were analyzed for this
study. Cores containing non-prostatic tissue (rectal mucosa and
accessory glands) or gland-free prostatic stroma were discarded.
Biopsy cores, previously screened for cancer by a hospital
pathologist, were blind-analyzed by a histopathologist with
expertise in prostate lesions to detect the presence and extent of
inflammatory infiltrates, focal atrophy and other prostatic
lesions. In case of uncertainty or controversy, an independent
pathologist from a foreign institution was consulted.
Characterization of extent and severity
of inflammatory findings
Chronic inflammation was categorized according to
the consensus classification system proposed by Nickel et
al(28). According to this
system, the localization of inflammatory infiltrates can be
glandular, periglandular or stromal, the extent of prostatic
inflammation is classified as focal, multifocal or diffuse, and the
severity of the inflammatory finding is categorized as mild,
moderate or severe. To classify the severity of inflammation, we
adopted as a visual reference the pictures shown in the Song et
al prostate histologic inflammation study (Fig. 2 in ref. 29). The present study did not focus on
acute inflammation.
Classification and quantitative
estimation of focal prostatic atrophy and other non-neoplastic
glandular lesions
Atrophy detected in biopsy specimens was classified
according to the 2006 International Working Group Classification
System for Focal Prostate Atrophy Lesions (30).
This consensus system recognizes four categories of
focal atrophy: simple atrophy (SA), post-atrophic hyperplasia
(PAH), partial atrophy (PA) and simple atrophy with cyst formation
(SACF). The term: Proliferative Inflammatory Atrophy (PIA) includes
only two lesions: SA and PAH (30). In the present study, PA and SACF
were categorized as ‘NON-PIA’ (Fig.
1).
The extent of prostatic atrophy in each biopsy was
quantitatively expressed as the percentage of extent of atrophic
glands over total glands, according to Billis et al(31). From these values, a ‘PIA index’,
i.e., the total percentage of PIA over total glands per-prostate
(i.e., per patient) was calculated.
When present, high- and low-grade PIN, basal cell
hyperplasia (BCH), adenosis and atypical small acinar proliferation
(ASAP) were identified and categorized according to published
descriptors (32,33).
Clinical findings
The symptoms of clinical chronic prostatitis were
scored using the international, validated questionnaire National
Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI)
(34). Due to the retrospective
nature of this study, the questionnaire was only administered to a
fraction of patients at diagnosis of prostatitis and at the end of
pharmacological therapy (time of collection: 2–4 weeks after
therapy). Questionnaires were fully anonymized. At the time of
questionnaire administration, all patients had given their written
informed consent to handling and publication of their anonymized
clinical data, for scientific purposes.
Statistical analysis
The quantitative relationship between inflammation
and atrophy, inflammation and PIN, atrophy and PIN, inflammation
and BCH, and atrophy and BCH was analyzed calculating the Pearson’s
product-moment correlation coefficient. The XLStatistics 5.71
program (http://www.deakin.edu.au/~rodneyc/XLStatistics/) was
used for analysis of data. Linear regressions, equation-finding and
curve-fitting procedures were performed using the curve-fitting
tool in Apple iWork Numbers ’09, version 2.1.
To analyze contingency tables containing the
proportion of PIA vs. non-PIA lesions in inflammatory vs.
non-inflammatory biopsy cores, we calculated the chi-square and the
probability of a null hypothesis of equal proportions using the
Vassar College (USA) on-line tool (http://vassarstats.net/newcs.html). To correct the
assumption of continuity in the χ2 distribution of
frequencies in 2×2 tables, we adopted the Yates’s modification of
Pearson’s χ2 formula (35).
Results
Equivalent proportions severe/moderate (50.28%) or
mild/absent (49.72%) inflammatory infiltrates were detected in the
biopsy cores analyzed in the present study. Similar numbers of
atrophic lesions, dichotomized as PIA (n=840, 50.79%) or NON-PIA
(n=814, 49.21%), were detected in prostate biopsies. Due to the
equivalent proportions of PIA vs. NON-PIA findings, and of
severe/moderate vs. absent/mild inflammation, normalization was not
deemed as necessary. Low-grade and high-grade PIN were detected in
total 307 and 6 biopsies, respectively.
Relationship between inflammatory
infiltrates and atrophic lesions
In order to investigate the presence and prevalence
of focal atrophy in relationship to the presence and severity of
inflammation, we calculated the number of biopsy cores containing
i) a severe/moderate or ii) a mild or absent inflammatory
infiltrate adjacent to i) a PIA (SA or PAH) or ii) a NON-PIA lesion
(PA or SACF). An example of representative PIA and NON-PIA lesions
is shown in Fig. 1.
PIA was found more frequently in cores containing a
severe/moderate inflammatory finding (n=521 cores), compared to
NON-PIA (n=290 cores, Fig. 2A).
χ2 analysis rejected the null hypothesis of equal
distributions of PIA vs. NON-PIA in cores harboring severe/moderate
vs. absent/mild inflammation (χ2=7.5, P=0.0062).
When PIA and NON-PIA classes were dissected into
single atrophic lesions, simple atrophy was found more frequently
in severe/moderate inflammatory cores (n=372, 62%), than in cores
with absent or mild inflammation (n=224, 38%) (Fig. 2). Conversely, partial atrophy was
found more frequently in non-inflammatory or mildly-inflammatory
cores (n=313, 56%), and to a lesser extent in cores containing a
severe or moderate inflammatory focus (n=242, 44%) (Fig. 2). Interestingly, post-atrophic
hyperplasia, a PIA subtype, was found to be more prevalent in
tissues with mild or absent inflammation (n=241, 62%) than in
inflammatory cores (n=149, 38%) (Fig.
2).
The box-and-whisker diagram in Fig. 3 shows the relationship between the
severity of inflammation and the extent of focal atrophy (PIA or
NON-PIA) in each biopsy core. Compared to non-inflammatory cores,
PIA lesions were found to be more extensive in the presence of
severe or moderate inflammation, whereas NON-PIA lesions were found
to be equally distributed in affected prostates, irrespective of
the severity of inflammation.
Besides being studied at the level of single biopsy
cores, the relationship between atrophy and the severity of
inflammation was investigated at the level of the whole prostate
gland by calculating the percentage of glandular atrophic
epithelium per-patient. If focal lesions were SA or PAH, this
entity was called ‘PIA index’, whereas it was denominated ‘NON-PIA
index’ when atrophy subtypes were SACF or PA.
High correlation was found between the PIA index and
the percentage of biopsy cores per-patient containing at least one
severe or moderate inflammatory focus (Pearson’s r= 0.78;
Fig. 4A). Conversely, poor
correlation was found when the PIA-index was compared to the
percentage of biopsy cores per patient devoid of inflammation, or
containing at least one mild inflammatory focus (Fig. 4B). No correlation was found between
the NON-PIA index and the percentage of biopsy cores per patient
containing at least one inflammatory focus of any severity
(Fig. 4C and D).
Relationship between low-grade PIN,
inflammation and atrophy
To investigate at the morphological level the
linkage between low-grade PIN and inflammation, we calculated the
number of biopsy cores containing i) a severe/moderate or ii) a
mild or absent inflammatory infiltrate adjacent to at least one
low-grade PIN lesion. Low-grade PIN was found more frequently in
cores devoid of inflammation, or with evidence of mild inflammation
(n=205, 63%), compared to cores harboring a moderate or severe
inflammatory focus (n=125, 37%, Fig.
5A).
Low-grade PIN often appears as a tufting growth of
proliferating cells stemming from the lumen of prostate secretory
glands or ducts. In some cases, the lesion extends to an entire
gland. To investigate whether a link existed between atrophy and
low-grade PIN, we analyzed the phenotype of the glandular
epithelium from which PIN lesions were stemming. Three hundred and
thirty-eight biopsy cores contained at least one low-grade PIN
lesion. In 309 cases low-grade PIN was found to stem from a
glandular epithelium of normal appearance, whereas in only 29 cases
PIN emerged from, or merged with, an atrophic gland (Fig. 5B).
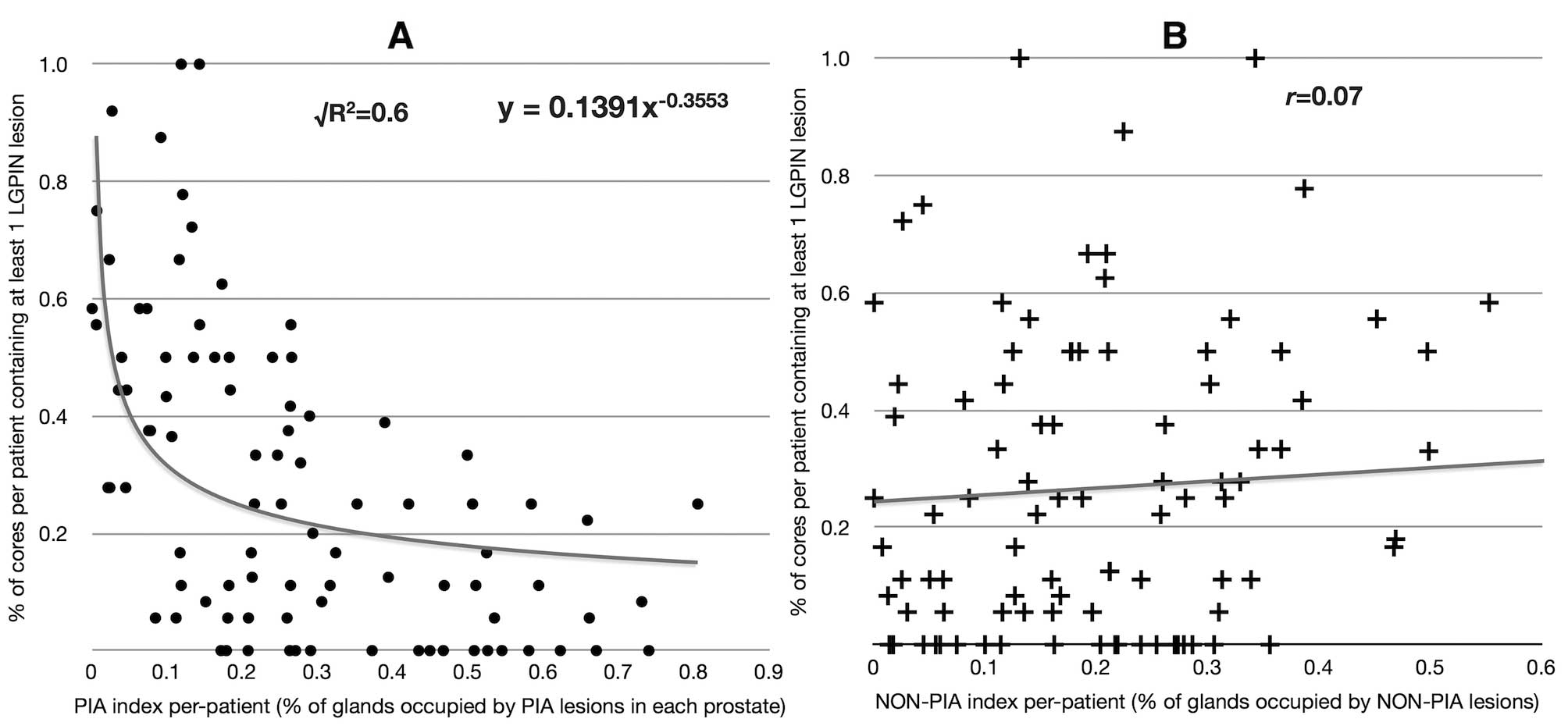
When the PIA index was plotted against the
percentage of biopsy cores per patient showing at least one
low-grade PIN lesion, an inverse relationship was found. This
correlation was best fitted by a power decay curve
(√R2=0.6) (Fig. 6A).
Interestingly, no correlation was found between the NON-PIA index
and the prevalence of low-grade PIN (Fig. 6B).
Relationship between basal cell
hyperplasia, inflammation and atrophy
Poor correlation was found between the extent of
basal cell hyperplasia and inflammatory findings of any degree of
severity (% cores per patient with BCH vs. % cores with
severe/moderate inflammation: r=0.22; vs. % cores with
absent/mild inflammation: r=0.12; graphs not shown).
Poor correlation was found between the extent of
basal cell hyperplasia and the extent of focal atrophy of any kind
(PIA or NON-PIA) (% cores per patient with BCH vs. PIA index:
r=0.21; BCH vs. NON-PIA index: r=0.21; graphs not
shown).
Although additional prostate lesions like high-grade
PIN and ASAP were identified, they were not further investigated
because of the small number of cases. It is important to mention
that we did not observe high-grade PIN stemming from PIA
lesions.
Relationship between atrophy and prostate
cancer
In 32 patients, prostate cancer was diagnosed in
biopsy specimens. The same specimens were subsequently screened for
focal atrophy, low- and high-grade PIN, BCH and ASAP. In these
biopsies, we investigated the relationship between the extent of
PCa and the extent of PIA, NON-PIA and low-grade PIN.
The NON-PIA index was found to be inversely related
to the percent of biopsies per patient showing evidence of
carcinoma. The correlation was best fitted by a power decay curve
(√R2= 0.56; Fig. 7). In
contrast, little correlation was found between the percent of
biopsies showing evidence of prostate cancer and the PIA index per
patient (r=0.13; Fig.
7).
Little correlation was found between the prostate
cancer burden per patient and the percent of biopsies showing at
least one low-grade PIN lesion (r=0.34, graph not
shown).
Relationship between atrophy,
inflammatory findings and clinical symptoms of chronic
prostatitis
A cohort of 34 CP patients included in the present
study filled the NIH-CPSI symptom questionnaire both at diagnosis
of prostatitis and at the end of pharmacological therapy. Prostate
biopsies were taken 3–6 weeks after the end of therapy.
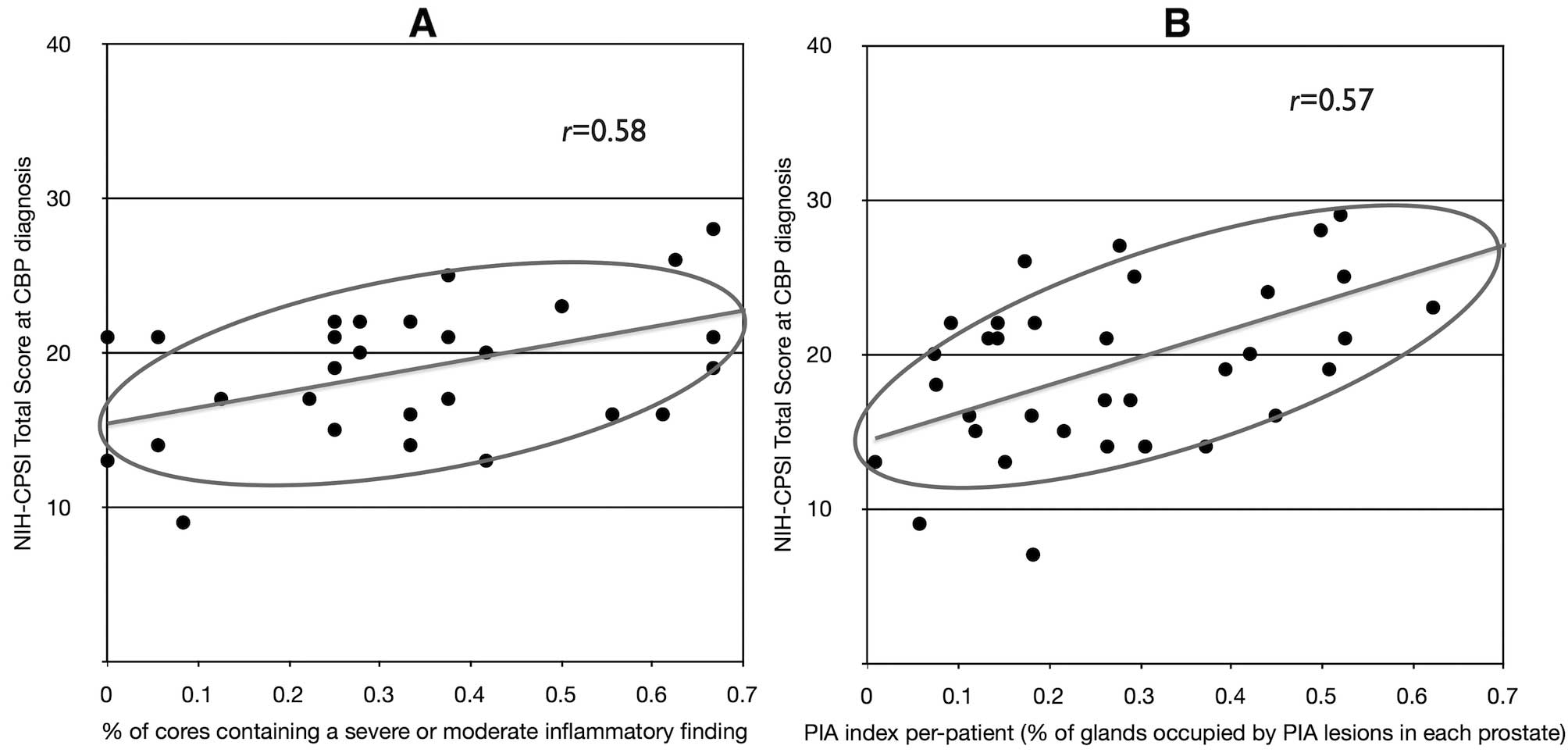
When the total score of the NIH-CPSI test was
plotted against the percent of biopsies per patient showing at
least one moderate or severe inflammatory focus, a positive
correlation (r= 0.58) between clinical symptoms and
histologic inflam mation was found at diagnosis of chronic
prostatitis (Fig. 8A), but not at
the end of therapy (r= 0.05, graph not shown). No
correlation was found between NIH-CPSI scores and mildly- or
non-inflammatory findings lesions either before (r= 0.08) or
after the end of therapy (r= 0.03; graphs not shown).
Similarly, When the PIA burden per patient was
compared with clinical symptoms, a positive correlation (r=
0.57) between the total score of the NIH-CPSI test and the PIA
index was found at diagnosis of chronic prostatitis (i.e., before
therapy, Fig. 8B), but not at the
end of therapy (r= 0.11, graph not shown). No correlation
was found between the NIH-CPSI score and the NON-PIA index either
before (r=0.16) or after the end of therapy (r=0.10;
graphs not shown).
Discussion
Since its first definition, PIA has been considered
as a lesion associated with, or caused by, inflammation. However,
to the best of our knowledge, very few quantitative
histological-clinical studies have been conducted to support this
established empirical observation. The Billis and Magna and the
Ruska et al studies are among the few published reports
investigating the relationship between focal atrophy and prostate
inflammation in autopsy or biopsy material (36,37).
However, both studies focused exclusively on PIA and did not
include NON-PIA lesions. Billis and Magna found that PIA was
associated with inflammation in 66% of analyzed prostates, whereas
in 22% of cases it was not (36).
In our biopsy series simple atrophy, a PIA lesion, was associated
with severe/moderate inflammation in 62.4% of biopsy cores (372/596
cores, Fig. 2), whereas in the
remaining 37.6% of cases contiguity with inflammatory focuses was
not evident. It must be stressed that we cannot exclude that in
these latter cases an inflammatory focus might have been present in
adjacent tissues, not included in the biopsy core. This is an
important limitation of a biopsy study.
In contrast to the Billis and Magna study, in which
PAH was exclusively associated with inflammation, we found that
this atrophic lesion is more prevalent in biopsy cores with mild or
no inflammation (63% of cases), and less frequently present in
frank inflammatory specimens (37% of cores with severe/moderate
inflammation) (Fig. 2). This
latter percentage is strikingly similar to the fraction of PAH
associated with moderate/severe chronic inflammation (34%) reported
in the Ruska et al biopsy study (37). Thus, despite its inclusion into the
‘PIA’ category, PAH may appear to be in most cases a
non-inflammatory lesion. Indeed, as stated by McNeal (38) and according to our experience, PAH
may represent a ‘post-inflammatory’ atrophic lesion. This
definition is supported by the morphological evidence that in PAH
the glandular lumen often comprises cell-free areas [defined by
Srigley as the ‘atrophic’ component of PAH, (39)], likely constituting the remnants of
a previous inflammatory injury. Proliferative regeneration of
secretory cells following disruption of the epithelium, may
generate small, hyperplastic glands arranged in a lobular
configuration, the typical feature of PAH. In our opinion, the fact
that in over 60% of cases PAH was not associ ated with a moderate
or severe inflammatory focus, is not sufficient to rule out the
essential inflammatory nature of this lesion.
In the present study, partial atrophy, the most
representative NON-PIA lesion, was found to be in a slight majority
of cases associated with mild or no inflammation (56% of cores;
Fig. 2). In our opinion, the fact
that PA was found to be adjacent to moderate/severe inflammatory
foci in 44% of analyzed cores should not be overlooked. Recently,
Billis and coworkers have demonstrated that a mergence between PA
and SA occurred in 27% of prostatic biopsies, with transitions
often taking place within the same gland (40). The authors hypothesized that PA may
be part of an evolving ‘continuum’ in focal prostatic atrophy, with
SA arising from PA in this morphologic sequence. However, in no
case did the authors find inflammatory cells in biopsy cores
containing PA. Because of this finding, Billis and coworkers
hypothesized that inflammation might represent a secondary
phenomenon in complete focal atrophy. In contrast with these
findings, in our study PA could be found adjacent to moderate or
severe inflammation in more than 40% of cases, although in 217
cases out of 242 inflammation was classified as ‘moderate’ and in
the remaining cases as ‘severe’. Thus, our data may in part support
the hypothesis that PA could precede SA, as the result of a
phenotypic sequential evolution, triggered by inflammation. This is
supported by the fact that, like Billis and coworkers, we observed
occasional PA-SA transitions within the same gland (data not
shown). However, in contrast to Billis and coworkers, we maintain
the view that inflammation is more likely to be a primum
movens in the development of PIA, rather than a mere secondary
phenomenon. A study is in progress to verify the ‘continuum’
hypothesis in patients subjected to multiple biopsies and/or
radical prostatectomy.
An interesting finding in our study was the dramatic
decline of the burden of low-grade PIN lesions, concomitant with
the increase of the extent of PIA in the same gland. This inverse
relation was best fitted by a power function decay curve (Fig. 6). This finding suggests that LG-PIN
and PIA may be mutually exclusive lesions within the same tissue or
tissue area, and it is supported by our finding that in about 95%
of cases LG-PIN arises from a normal rather than from an atrophic
gland (Fig. 5). In 2009, De Marzo
and coworkers promoted their ‘injury-and-regeneration’ model for
prostate carcinogenesis in the frame of a comprehensive review
article (4). In that context, the
authors included low-grade PIN as a possible intermediate lesion,
developing from PIA, and representing a putative - although
non-obligate - forerunner of high-grade PIN or cancer (Fig. 3 in ref. 4). The results of the present study seem
to exclude this hypothesis, also given that low-grade PIN is more
frequently found in non-inflammatory tissues (Fig. 5), which also seems incompatible
with the higher prevalence of PIA adjacent to focuses of severe or
moderate inflammation.
In the present study we failed to demonstrate a
positive correlation between the extent of PIA lesions and the per
patient burden of prostate cancer (Fig. 7). Intriguingly, prostate cancer and
NON-PIA lesions tend to be mutually exclusive in the same tissue or
tissue area (Fig. 7). This
finding, to be confirmed in a larger sample of patients, weakens
the hypothesis of a possible linkage between cancer and NON-PIA
lesions. Notwithstanding this evidence, if a ‘continuum’ has to be
hypothesized between partial atrophy, PIA and cancer, the former
and the latter lesions are most likely to occur far apart in
time.
Another interesting finding of the present study is
the good correlation [Portney and Watkins criteria, (41)] between the total NIH-CPSI symptom
score and the extent of both inflammation and PIA at diagnosis of
chronic prostatitis (Fig. 8). This
is to our knowledge the first study attempting to link the clinical
symptoms of CP to the extent and severity of histological prostate
inflammation, as well as to the extent of focal atrophy. The
absence of a correlation between these entities at the end of
therapy (few days before biopsy) is probably due to the attenuation
of clinical symptoms of CP achieved by aggressive pharmacological
therapy, and may indirectly support the link between inflammation
and symptom severity during the active (inflammatory) phase of CP.
Although the small sample size (n=34) does not allow to draw a
conclusive answer, the correlation between NIH-CPSI scores and
inflammation is indicative of a relationship between the clinical
and histological aspects of chronic prostate inflammation. Whereas
such a relationship can be expected in patients suffering from
inflammatory subtypes of chronic prostatitis (class II CBP and
class IIIa CP/CPPS), the correlation between symptom severity and
the per patient extent of atrophy is more striking, and supports
the hypothesis that an inflammatory injury caused by infection or
other etiological determinants may be the original causative factor
of the atrophic process (4).
In conclusion, the evidence emerging from the
present study i) points to a positive association between tissue
inflammation and PIA, ii) questions the presumed non-inflammatory
nature of partial atrophy and iii) does not seem to support a model
whereby low-grade PIN would arise from PIA lesions.
References
|
1.
|
A MantovaniP AllavenaA SicaF
BalkwillCancer-related
inflammationNature454436444200810.1038/nature07205
|
|
2.
|
I ChengJS WitteSJ JacobsenProstatitis,
sexually transmitted diseases, and prostate cancer: the California
Men’s Health StudyPLoS One5e87362010
|
|
3.
|
S JafariM EtminanK AfsharNonsteroidal
anti-inflammatory drugs and prostate cancer: a systematic review of
the literature and meta-analysisCan Urol Assoc
J3323330200919672448
|
|
4.
|
AM De MarzoEA PlatzS SutcliffeInflammation
in prostate carcinogenesisNat Rev Cancer72562692007
|
|
5.
|
G PerlettiE MontanariA VralG GazzanoE
MarrasS MioneV MagriInflammation, prostatitis, proliferative
inflammatory atrophy: ‘Fertile ground’ for prostate cancer
development?Mol Med Rep33122010
|
|
6.
|
ON KryvenkoM JankowskiDA ChitaleD TangA
RundleS TrudeauBA RybickiInflammation and preneoplastic lesions in
benign prostate as risk factors for prostate cancerMod
Pathol2510231032201210.1038/modpathol.2012.5122460812
|
|
7.
|
S DavidssonM FiorentinoO
AndrénInflammation, focal atrophic lesions, and prostatic
intraepithelial neoplasia with respect to risk of lethal prostate
cancerCancer Epidemiol Biomarkers
Prev2022802287201110.1158/1055-9965.EPI-11-0373
|
|
8.
|
MJ PutziAM De MarzoMorphologic transitions
between proliferative inflammatory atrophy and high-grade prostatic
intraepithelial
neoplasiaUrology56828832200010.1016/S0090-4295(00)00776-7
|
|
9.
|
W WangA BerghJE DamberMorphological
transition of proliferative inflammatory atrophy to high-grade
intraepithelial neoplasia and cancer in human
prostateProstate6913781386200910.1002/pros.2099219507201
|
|
10.
|
A BillisLL FreitasLA MagnaU
FerreiraInflammatory atrophy on prostate needle biopsies: is there
topographic relationship to cancer?Int Braz J
Urol33355360200710.1590/S1677-55382007000300008
|
|
11.
|
AA BrasilWJ FavaroVH CagnonU FerreiraA
BillisAtrophy in specimens of radical prostatectomy: is there
topographic relation to high grade prostatic intraepithelial
neoplasia or cancer?Int Urol
Nephrol43397403201110.1007/s11255-010-9803-y
|
|
12.
|
A BillisProstatic atrophy.
Clinicopathological significanceInt Braz J
Urol36401409201010.1590/S1677-5538201000040000320815946
|
|
13.
|
RC AntonMW KattanS ChakrabortyTM
WheelerPostatrophic hyperplasia of the prostate: lack of
association with prostate cancerAm J Surg
Pathol23932936199910.1097/00000478-199908000-0001110435563
|
|
14.
|
R PostmaFH SchröderTH van der KwastAtrophy
in prostate needle biopsy cores and its relationship to prostate
cancer incidence in screened
menUrology65745749200510.1016/j.urology.2004.10.04615833520
|
|
15.
|
G MikuzF AlgabaAL BeltranR
MontironiProstate carcinoma: atrophy or not atrophy that is the
questionEur
Urol5212931296200710.1016/j.eururo.2007.07.03917761384
|
|
16.
|
R MontironiR MazzucchelliA Lopez-BeltranL
ChengM ScarpelliMechanisms of disease: high-grade prostatic
intraepithelial neoplasia and other proposed preneoplastic lesions
in the prostateNat Clin Pract
Urol4321332200710.1038/ncpuro081517551536
|
|
17.
|
KS SfanosAM De MarzoProstate cancer and
inflammation: the
evidenceHistopathology60199215201210.1111/j.1365-2559.2011.04033.x22212087
|
|
18.
|
S Yildiz-SezerI VerdorferG SchaeferH
RogatschG BartschG MikuzAssessment of aberrations on chromosome 8
in prostatic atrophyBJU
Int98184188200610.1111/j.1464-410X.2006.06233.x16831166
|
|
19.
|
JA MacoskaTM TrybusKJ Wojno8p22 loss
concurrent with 8c gain is associated with poor outcome in prostate
cancerUrology55776782200010.1016/S0090-4295(00)00468-410792100
|
|
20.
|
R ShahNR MucciA AminJA MacoskaMA
RubinPostatrophic hyperplasia of the prostate gland: neoplastic
precursor or innocent bystander?Am J
Pathol15817671773200110.1016/S0002-9440(10)64132-611337374
|
|
21.
|
S Yildiz-SezerI VerdorferG SchaeferH
RogatschG BartschG MikuzGain of chromosome X in prostatic atrophy
detected by CGH and FISH
analysesProstate67433438200710.1002/pros.2053517219381
|
|
22.
|
KS SfanosBA WilsonAM De MarzoWB
IsaacsAcute inflammatory proteins constitute the organic matrix of
prostatic corpora amylacea and calculi in men with prostate
cancerProc Natl Acad Sci
USA10634433448200910.1073/pnas.081047310619202053
|
|
23.
|
M ScarpelliR MazzucchelliF BarbisanA
SantinelliA Lopez-BeltranL ChengR MontironiIs there a role for
prostate tumour overexpressed-1 in the diagnosis of HGPIN and of
prostatic adenocarcinoma? A comparison with alpha-methylacyl CoA
racemaseInt J Immunopathol Pharmacol256774201222507319
|
|
24.
|
V FradetI ChengG CaseyJS WitteDietary
omega-3 fatty acids, cycloxygenase-2 genetic variation, and
aggressive prostate cancer riskClin Cancer
Res1525591566200910.1158/1078-0432.CCR-08-250319318492
|
|
25.
|
JN KriegerL Nyberg JrJC NickelNIH
consensus definition and classification of
prostatitisJAMA282236237199910.1001/jama.282.3.23610422990
|
|
26.
|
V MagriE MontanariV
ŠkerkFluoroquinolone-macrolide combination therapy for chronic
bacterial prostatitis: retrospective analysis of pathogen
eradication rates, inflammatory findings and sexual
dysfunctionAsian J Androl13819827201110.1038/aja.2011.36
|
|
27.
|
V MagriA TrinchieriE MontanariReduction of
PSA values by combination pharmacological therapy in patients with
chronic prostatitis: implications for prostate cancer detectionArch
Ital Urol Androl7984922007
|
|
28.
|
JC NickelLD TrueJN KriegerRE BergerAH
BoagID YoungConsensus development of a histopathological
classification system for chronic prostatic inflammationBJU
Int87797805200110.1046/j.1464-410x.2001.02193.x11412216
|
|
29.
|
L SongY ZhuP HanN ChenD LinJ LaiQ WeiA
retrospective study: correlation of histologic inflammation in
biopsy specimens of Chinese men undergoing surgery for benign
prostatic hyperplasia with serum prostate-specific
antigenUrology77688692201110.1016/j.urology.2010.07.493
|
|
30.
|
AM De MarzoEA PlatzJI EpsteinA working
group classification of focal prostate atrophy lesionsAm J Surg
Pathol3012811291200617001160
|
|
31.
|
A BillisL MeirellesLL FreitasLA MagnaU
FerreiraDoes the type of prostatic atrophy influence the
association of extent of atrophy in needle biopsies and serum
prostate-specific antigen
levels?Urology7411111115200910.1016/j.urology.2009.05.093
|
|
32.
|
R MontironiR MazzucchelliF AlgabaA
Lopez-BeltranMorphological identification of the patterns of
prostatic intraepithelial neoplasia and their importanceJ Clin
Pathol53655665200010.1136/jcp.53.9.65511041054
|
|
33.
|
R MontironiM ScarpelliR MazzucchelliL
ChengA Lopez-BeltranThe spectrum of morphology in non-neoplastic
prostate including cancer
mimicsHistopathology604158201210.1111/j.1365-2559.2011.04000.x22212077
|
|
34.
|
MS LitwinM McNaughton-CollinsFJ Fowler
JrThe National Institutes of Health chronic prostatitis symptom
index: development and validation of a new outcome measureJ
Urol162369375199910.1016/S0022-5347(05)68562-X
|
|
35.
|
F YatesContingency tables involving small
numbers and the χ2 testJ R Stat Soc12172351934
|
|
36.
|
A BillisLA MagnaInflammatory atrophy of
the prostate. Prevalence and significanceArch Pathol Lab
Med127840844200312823038
|
|
37.
|
KM RuskaJ SauvageotJI EpsteinHistology and
cellular kinetics of prostatic atrophyAm J Surg
Pathol2210731077199810.1097/00000478-199809000-000059737239
|
|
38.
|
JE McNealNormal histology of the
prostateAm J Surg
Pathol12619633198810.1097/00000478-198808000-000032456702
|
|
39.
|
JR SrigleyBenign mimickers of prostatic
adenocarcinomaMod Pathol17328348200410.1038/modpathol.3800055
|
|
40.
|
A BillisL MeirellesLL FreitasMergence of
partial and complete atrophy in prostate needle biopsies: a
morphologic and immunohistochemical studyVirchows
Arch456689694201010.1007/s00428-010-0904-x20361207
|
|
41.
|
Foundations of Clinical Research:
Applications and PracticeLG PortneyMP WatkinsAppleton &
LangeNorwalk, Connecticut5095161993
|