Introduction
Colorectal cancer (CRC) is a malignant neoplasm
arising from the lining of the large intestine (colon and rectum)
(1). CRC is the third most common
cancer and the third leading cause of cancer-related death in the
United States (2). The incidence
of CRC in Korea has increased significantly over the past few
decades. According to the National Cancer Registry of Korea,
age-standardized incidence rates increased from 27.0 to 50.2 per
100,000 for men and from 17.1 to 26.9 per 100,000 for women between
1999 and 2009. The overall incidence of CRC increased by 6.7%
annually in men and 5.1% in women from 1999 to 2009, while the
incidence rates of the most common cancers, such as stomach and
liver cancer, decreased during the same period (3). While more than 50% of CRC patients
with surgical resection is cured, 40–50% of these subjects
eventually experience recurrences and the possibility of
re-operation is very low (4).
Because of incomplete therapeutic options for CRC, there is a need
to develop novel preventive treatment approaches for this
malignancy.
Apoptosis, a controlled and energy-dependent
process, is the best-described form of programmed cell death. There
are two major apoptotic pathways, which are the extrinsic pathway
and the intrinsic pathway (5). The
extrinsic pathway is initiated by the binding of transmembrane
death receptors [Fas, tumor necrosis factor receptor 1 (TNFR1),
TNF-related apoptosis-inducing ligand (TRAIL) receptors] with
cognate extracellular ligands (6).
Ligand receptors recruit adaptor proteins [TNFR-associated death
domain (TRADD) and Fas-associated death domain (FADD)], which
interact with and trigger the activation of caspase-8. Activated
caspase-8 thereby stimulates the effector caspases-3, -6 and -7
which ultimately execute apoptosis (7). TNF-α is known to trigger apoptotic
death via TNFR1 (8). The intrinsic
pathway is dominated by the Bcl-2 family of proteins which govern
the release of cytochrome c from the mitochondria (9,10).
Cytochrome c stimulates the formation of apoptosome (Apaf-1,
dATP, cytochrome c and caspase-9) followed by activation of
caspase-9, which in turn causes the activation of the ‘executioner’
caspases (-3, -6 and -7) (9,11,12).
Psorospermin, a natural product isolated from the
tropical plant Psorospermum febrifugum, consists of a
xanthone scaffold and was reported to exhibit potent anticancer
activity in vitro and in vivo (13,14).
Acronycine, an alkaloid first isolated from Acronychia
baueri Schott (Rutaceae) consists of an acridone
scaffold and showed a broad spectrum of activity against a variety
of solid tumors including sarcoma, myeloma, carcinoma, and melanoma
(15). On the basis of the
chemical structures of psorospermin with a xanthone template and
acronycine derivatives with an acridone template, MHY-449 and
MHY-450 (Fig. 1A) constructed on a
1,2-dihydrobenzofuro [4,5-b][1,8]naphthyridin-6(11H)-one scaffold
were designed and synthesized as potential anticancer agents
(16). Their cytotoxicities were
evaluated against five human cancer cell lines, such as prostate
cancer cell lines (LNCap, DU145 and PC3) and breast cancer cell
lines (MCF-7/ADR and MCF-7). MHY-449 and MHY-450 have shown
cytotoxicity against human prostate and breast cancer cells and
induction of G2/M phase arrest of the cell cycle in MCF-7/ADR
cells, however, the underlying molecular mechanisms of these
cytotoxic effects and G2/M phase arrest were not fully elucidated.
Therefore, the current study was designed to investigate the
effects on HCT116 human colon cancer cells and its mechanisms by
which these novel derivatives induce apoptosis and modulation of
the cell cycle in HCT116 human colon cancer cells.
Materials and methods
Chemicals
The simplified code names and structures of MHY-449
[(±)-(R*)-5-methoxy-11-methyl-2-((R*)-2-methyloxiran-2-yl)-1,2-dihydrobenzofuro[4,5-b][1,8]naphthyridin-6(11H)-one]
and MHY-450
[(±)-(R*)-5-methoxy-11-methyl-2-((S*)-2-methyloxiran-2-yl)-1,2-dihydrobenzofuro[4,5-b][1,8]naphthyridin-6(11H)-one]
used in this study are shown in Fig.
1. Detailed methods for the design and synthesis of these
compounds are described elsewhere (16). They were dissolved in
dimethylsulfoxide (DMSO) and stored at −20°C before the experiments
and dilutions were made in culture medium. The maximal
concentration of DMSO did not exceed 0.1% (v/v) in the treatment
range of 0.125-0.5 μM, where there was no influence on the cell
growth.
Cell culture and growth study
The human colon cancer HCT116 cells were cultured in
RPMI-1640 (Hyclone, Logan, UT, USA) supplemented with 10% fetal
bovine serum (FBS, Hyclone), 2 mM glutamine (Sigma-Aldrich Co., St.
Louis, MO, USA), 100 U/ml penicillin (Hyclone) and 100 μg/ml
streptomycin (Hyclone) at 37°C in a humidified 5% CO2.
Cell viability was determined by MTT assay and by trypan blue
staining assay. For the MTT assay, HCT116 cells were seeded in a
24-well culture plate at a density of 4×104 cells/well,
cultured for 24 h in the growth media and then treated with or
without various reagents for the indicated concentrations. The
cells were incubated with 0.5 mg/ml MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]
(Sigma-Aldrich) at 37°C for 2 h. The formazan granules generated by
the live cells were dissolved in DMSO and the absorbance at 540 nm
was monitored by using a multi-well reader. For the trypan blue
staining assay, the reagents-treated HCT116 cells were harvested by
trypsinization, suspended in phosphate-buffered saline (PBS),
stained by trypan blue stain (Gibco, Rockville, MD, USA) solution
and the number of viable cells was counted using a
hemacytometer.
Nuclear staining with Hoechst 33342
Cells were washed with PBS and fixed with 3.7%
paraformaldehyde (Sigma-Aldrich) in PBS for 10 min at room
temperature. Fixed cells were washed with PBS and stained with 4
μg/ml Hoechst 33342 for 20 min at room temperature. The cells were
washed two more times with PBS and analyzed by a fluorescent
microscope.
Assessment of DNA degradation
Cells were lysed in a buffer, containing 5 mM
Tris-HCl (pH 7.5), 5 mM EDTA, and 0.5% Triton X-100, for 30 min on
ice. Lysates were vortexed and cleared by centrifugation at 27,000
× g for 20 min. Fragmented DNA in the supernatant was treated with
RNase, followed by proteinase K digestion,
phenol/chloroform/isoamyl alcohol mixture (25:24:1, v/v/v)
extraction and isopropanol precipitation. DNA was separated through
a 1.5% agarose gel, was stained with 0.1 μg/ml ethidium bromide
(EtBr) and was visualized by UV source.
Cell cycle analysis
The DNA content was measured following the staining
of the cells with propidium iodide (PI, Sigma-Aldrich). The cells
were treated under the appropriate conditions for 24 h,
subsequently trypsinized, washed once in cold PBS and then fixed in
70% ethanol at 4°C overnight. The fixed cells were pelleted and
stained in cold PI solution (50 μg/ml in PBS) at room temperature
for 30 min in the dark. Flow cytometry analysis was performed on a
FACScan flow cytometry system (Becton-Dickinson, San Jose, CA,
USA).
Caspase activity assay
The cells were harvested and washed with cold PBS.
Total cells were lysed with the lysis buffer [40 mM Tris (pH 8.0),
120 mM, NaCl, 0.5% NP-40, 0.1 mM sodium orthovanadate, 2 μg/ml
aprotinin, 2 μg/ml leupeptin and 100 μg/ml phenymethylsulfonyl
fluoride (PMSF)] at 4°C for 30 min. The lysed cells were
centrifuged at 14,000 rpm for 10 min and 100 μg protein was
incubated with 100 μl of reaction buffer and 10 μl of colorimetric
tetrapeptides, Z-DEVDpNA for caspase-3, Z-IETD-pNA for caspase-8,
and Ac-LEHD-pNA for caspase-9, respectively. The reaction mixture
was incubated at 37°C for 30 min and liberated p-nitroaniline (pNA)
was measured at 405 nm using a multi-well reader.
Annexin V staining
Annexin V-FITC is used to quantitatively determine
the percentage of cells within a population that are actively
undergoing apoptosis. The cells were treated under the appropriate
conditions for 24 h, subsequently harvested, trypsinized, washed
once in cold PBS, suspended the cells in 1X binding buffer
(Becton-Dickinson, Annexin V-FITC Apoptosis Detection kit). The
counted cells were stained in PI and Annexin V-FITC solution
(Becton-Dickinson, Annexin V-FITC Apoptosis Detection Kit) at room
temperature for 15 min in the dark. The stained cells were analyzed
by flow cytometry within 1 h.
Western blot analysis
The cells were treated under the appropriate
conditions, harvested and washed with cold PBS. Total cells were
lysed in lysis buffer [40 mM Tris (pH 8.0), 120 mM NaCl, 0.5%
NP-40, 0.1 mM sodium orthovanadate, 2 μg/ml aprotinin, 2 μg/ml
leupeptin and 100 μg/ml phenymethylsulfonyl fluoride (PMSF)]. The
supernatant was collected and protein concentrations were then
measured with protein assay reagents (Pierce, Rockford, IL, USA).
Protein extracts were denatured by boiling at 100°C for 5 min in
sample buffer (0.5 M Tris-HCl, pH 6.8, 4% SDS, 20% glycerol, 0.1%
bromophenol blue, 10% β-mercaptoethanol). Equal amount of the total
proteins were subjected to 6–15% SDS-PAGE and transferred to PVDF.
The membranes were blocked with 5% non-fat dry milk in
Tris-buffered saline with Tween-20 buffer (TBS-T) (20 mM Tris, 100
mM NaCl, pH 7.5 and 0.1% Tween-20) for 1 h at room temperature.
Then, the membranes were incubated for overnight at 4°C with the
primary antibodies (Table I). The
membranes were washed once for 10 min with 4X TBS-T buffer and
incubated for 1 h with horseradish peroxidase-conjugated
anti-rabbit or anti-mouse immunoglobin (Santa Cruz Biotechnology
Inc., Santa Cruz, CA, USA). The membranes were washed again for 10
min with 4X TBS-T buffer. Antigen-antibody complexes were detected
by the enhanced chemiluminescence (ECL) detection system (Amersham
Biosciences Co., Little Chalfont, UK).
 | Table IPrimary antibodies used in western
blot analysis. |
Table I
Primary antibodies used in western
blot analysis.
| Antibody | 2nd antibody | Dilution ratio | Company |
|---|
| Fas | Rabbit | 1:1,000 | Santa Cruz |
| Fas-L | Rabbit | 1:500 | Santa Cruz |
| TRAIL | Rabbit | 1:1,000 | Santa Cruz |
| DR4 | Rabbit | 1:1,000 | Santa Cruz |
| DR5 | Mouse | 1:1,000 | Santa Cruz |
| Bax | Rabbit | 1:1,000 | Santa Cruz |
| Bcl-2 | Mouse | 1:500 | Santa Cruz |
| Cdc2 | Rabbit | 1:1,000 | Santa Cruz |
| Cdc25c | Rabbit | 1:1,000 | Santa Cruz |
| Cyclin B1 | Mouse | 1:1,000 | Santa Cruz |
| Wee1 | Mouse | 1:500 | Santa Cruz |
| p21 | Mouse | 1:1,000 | Santa Cruz |
| p27 | Mouse | 1:1,000 | Santa Cruz |
| p53 | Mouse | 1:1,000 | Santa Cruz |
| PARP | Mouse | 1:1,000 | Santa Cruz |
| Procaspase-3 | Mouse | 1:1,000 | Santa Cruz |
| Procaspase-8 | Rabbit | 1:500 | Santa Cruz |
| Procaspase-9 | Rabbit | 1:500 | Santa Cruz |
| β-actin | Mouse | 1:10,000 | Sigma-Aldrich |
Statistical analysis
Results were expressed as the mean ± SD of three
separate experiments and analyzed by Student’s t-test. Means were
considered significantly different at *p<0.05 or
**p<0.01.
Results
MHY-449 exhibits more potent cytotoxicity
than MHY-450 against HCT116 cells
To investigate the effects of MHY-449 and MHY-450
(Fig. 1A), the diastereoisomeric
compounds, on the viability of HCT116 cells, the MTT assay was
performed. As shown in Fig. 1B,
both MHY-449 and MHY-450 showed concentration-dependent
cytotoxicity on HCT116 cells. MHY-449, however, exhibited more
potent cytotoxicity than MHY-450. Therefore, further experiments
were performed only with MHY-449.
MHY-449 inhibits the growth of HCT116
cells
MHY449 showed concentration-dependent cytotoxicity
on HCT116 cells. The IC50 value of MHY-449 on HCT116
cells was approximately 0.5 μM (Fig.
2A). Following experiment was performed in order to investigate
whether MHY-449 has an anti-proliferation effect on HCT116 cells by
using the trypan blue staining assay. As shown in Fig. 2B, MHY-449 showed time-dependent
anti-proliferation effect on HCT116 cells.
MHY-449 induces apoptosis in HCT116
cells
To investigate whether the growth inhibitory effects
of MHY-449 were due to the induction of apoptosis in HCT116 cells,
microphotographs were observed. HCT116 cells treated with MHY-449
showed distinct morphological changes compared with control
(Fig. 3A upper panel). They were
rounded and more dispersed with aggregation in a
concentration-dependent manner. Further morphological changes of
cellular structures were assessed with Hoechst 33342 staining. As
shown in Fig. 3A lower panel,
nuclei with chromatin condensation and formation of apoptotic
bodies, which are characteristics of apoptosis, were seen in cells
cultured with MHY-449 in a concentration-dependent manner, whereas
the control cells maintained nuclear structure intact. In addition
to this, to confirm the apoptosis of MHY-449 in the HCT116 cells,
flow cytometry analysis and DNA fragmentation assay were performed.
As shown in Fig. 3B, increases of
early apoptosis (lower right quadrant) and late apoptosis/death
(upper right quadrant) were clearly observed in
concentration-dependent manner. We also analyzed whether DNA
fragmentation, another hallmark of apoptosis, was induced by
MHY-449 treatment on HCT116 cells. Following agarose gel
electrophoresis of HCT116 cells treated with MHY-449 for 24 h, a
typical ladder pattern of internucleosomal fragmentation was
observed in a concentration-dependent manner (Fig. 3C).
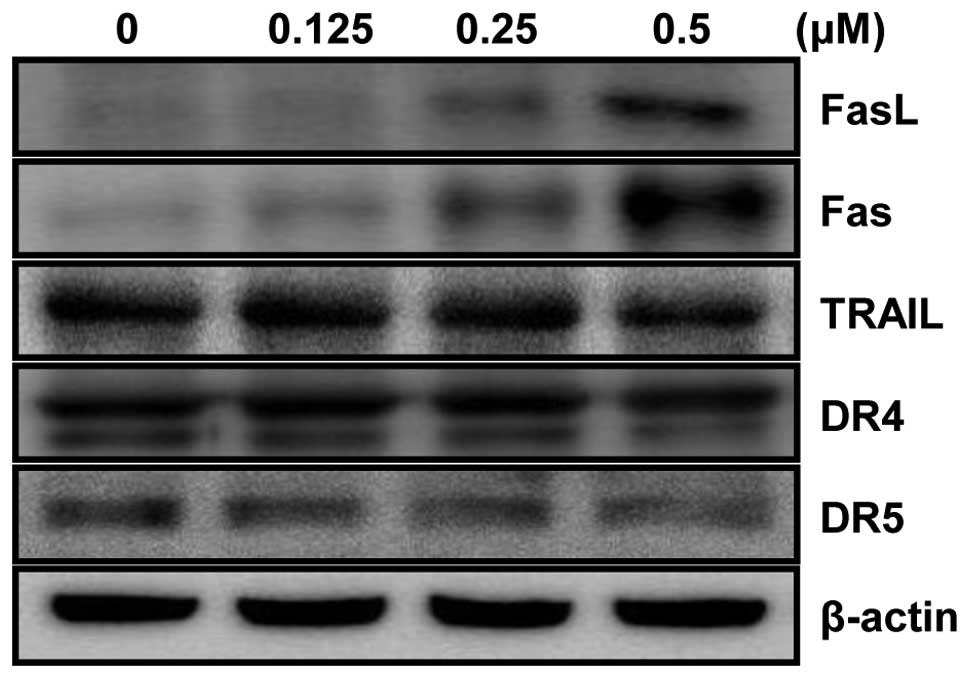
MHY-449 upregulates the expression of
apoptosis-related proteins in the extrinsic pathway
The extrinsic pathway of apoptosis in which Fas/FasL
system plays a key signaling transduction has been proposed as a
therapeutic target in cancer. Thus, involvement of the Fas/FasL
system in HCT116 cells treated with MHY-449 was examined. As shown
in Fig. 4, the levels of Fas as
well as FasL expression were significantly up-reregulated in a
concentration-dependent manner. However, the levels of tumor
necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL),
DR4, and DR5 were not changed after MHY-449 treatment.
MHY-449 increases caspase activity in
HCT116 cells
Significant activation of pro-caspase-3 and -9 were
observed and there was little change in pro-caspase-8 (Fig. 5A). Polypeptide degradation,
including poly(ADP-ribose) polymerase (PARP), was examined to see
the possible involvement of apoptosis-associated protease during
the growth inhibition of the colon cancer cells. PARP cleavage was
evident by the appearance of the p85 PARP cleavage fragment and
clearly observed in the 0.25 μM and 0.5 μM of MHY-449 treatment. In
an attempt to further characterize the mechanisms of apoptosis
induced by MHY-449, the activities of caspase -3, -8 and -9 were
determined by colorimetric assay. The activities of caspase-3, -8
and -9 were increased with the treatment of MHY-449 in
concentration-dependent manner (Fig.
5B). Therefore, these results suggested that MHY-449 induce
caspase-dependent apoptosis in HCT116 cells.
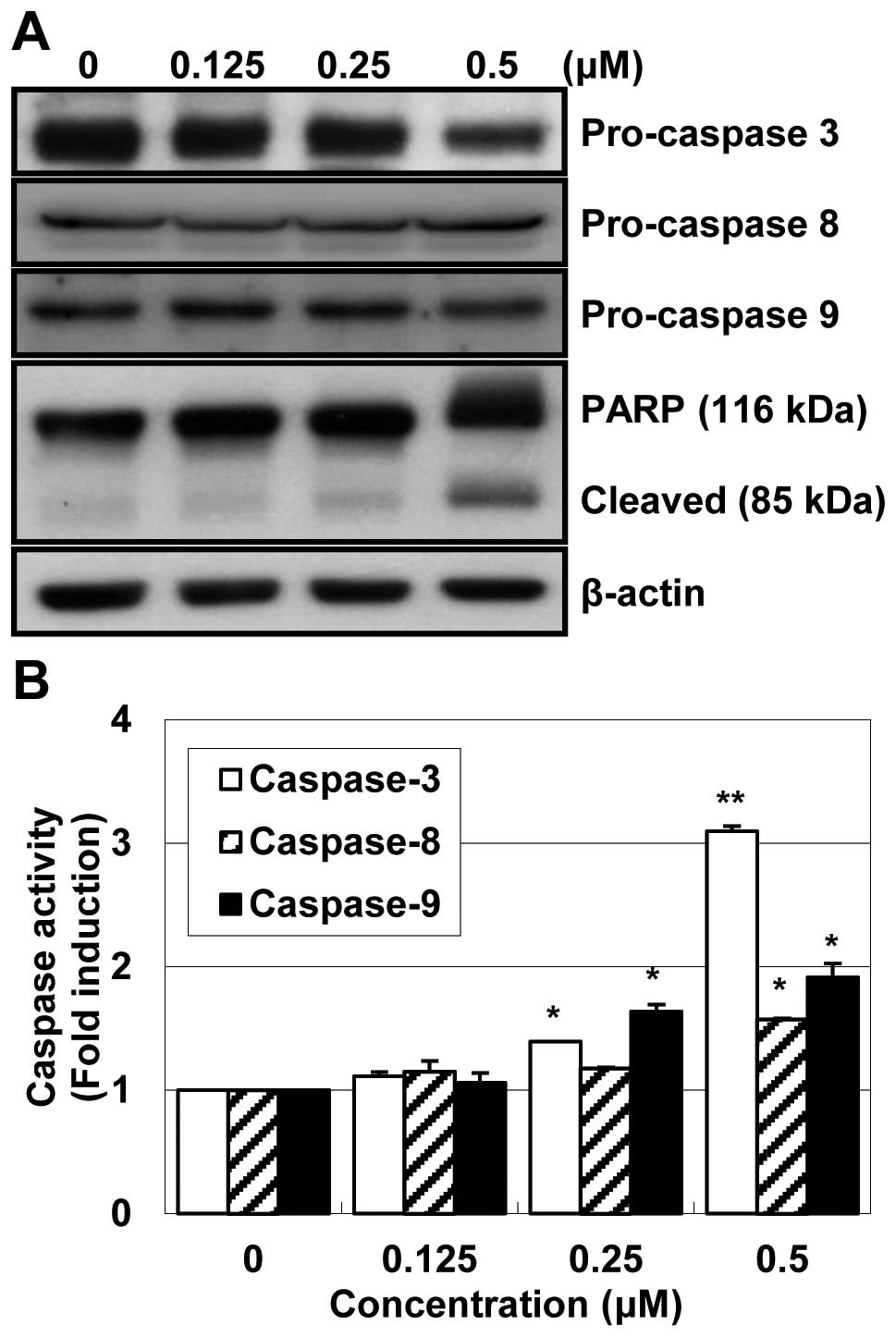 | Figure 5Effect of MHY-449 on activation of
caspase-3, -8, and -9 and degradation of PARP in HCT116 cells. (A)
HCT116 cells were treated with indicated concentrations of MHY-449
for 24 h, collected, lysed and then cellular proteins were
separated and immunoblotted. The membranes were probed with
procaspase-3, pro-caspase-8, procaspase-9 and PARP (116 kDa).
Proteins were visualized using the ECL detection system.
Representative results from three independent experiments are
shown. Actin was used as a loading control. (B) To detect caspase
activities, cell lysates from cells treated with MHY-449 for 24 h
were assayed for in vitro caspase-3, -8 and -9 activity
using Z-DEVD-pNA, Z-IETD-pNA and Ac-LEHD-pNA, respectively, as
substrates at 37°C for 1 h. The released fluorescent products were
measured. The data represent the mean ± SD values of three
independent experiments. The significance was determined by
Student’s t-test (*p<0.05 and **p<0.01
vs. vehicle-treated control cells). |
MHY-449 modulates the expression levels
of apopotosis-related proteins in HCT116 cells
To determine whether the expression levels of
apoptosis-related proteins were modulated by MHY-449, western blot
analysis was performed. The expression level of Bcl-2 protein was
markedly downregulated, while Bax was upregulated in a
concentration-dependent manner (Fig.
6A). The ratio between Bcl-2 and Bax has been suggested as a
primary event in determining the susceptibility to apoptosis
(17). These data suggest that
MHY-449 induces apoptosis by the alteration in expression ratio of
Bax/Bcl-2 protein (Fig. 6B).
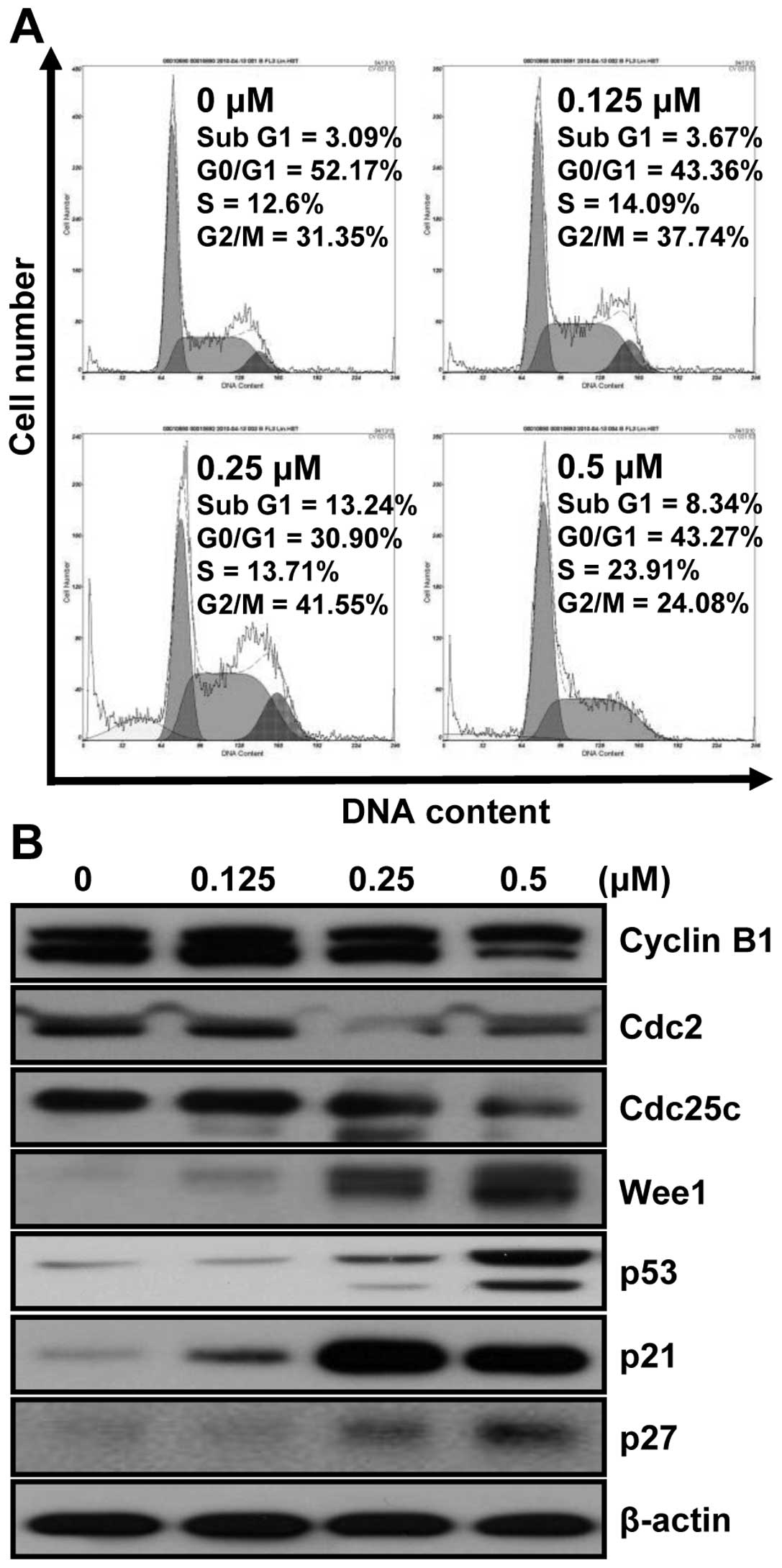
MHY-449 modulates cell cycle in HCT116
cells
To investigate whether MHY-449 has an effect on the
cell cycle, HCT116 cells were treated with various concentrations
of MHY-449 for 24 h and analyzed with flow cytometry. As shown in
Fig. 7A, the cells treated with
MHY-449 were remarkably arrested in the G2/M phase and increased
the proportions of sub-G1 phase cells, both in a
concentration-dependent manner. A total 41.55% of cells cultured
with 0.25 μM MHY-449 were in G2/M phase, compared to 31.35% of
control in G2/M phase. In addition, sub-G1 populations were
increased from 3.09% in control to 13.24% in cells treated with
0.25 μM MHY-449. These results suggest that MHY-449 retard the
growth of HCT116 cells by arresting cell-cycle progression and
apoptosis induction.
MHY-449 modulated cell cycle regulatory
proteins in HCT116 cells
The western blot analysis was conducted to further
characterize the molecular mechanisms whereby MHY-449 inhibit cell
growth and modulate the expression of cell cycle regulating
proteins. As shown in Fig. 7B, the
intracellular protein levels of G2/M phase of cell cycle, such as
cyclin B1, Cdc2 and Cdc25c were decreased and Wee1 was increased in
HCT116 cells by MHY-449 treatment in a concentration-dependent
manner. The induction of p21WAF1/CIP1 causes
subsequent arrest in the G0/G1 or G2/M phase of the cell cycle by
binding of the cyclin-CDK complex. The protein levels of
p21WAF1/CIP1 and p27KIP were
increased in HCT116 cells by MHY-449 treatment in a
concentration-dependent manner, and the protein level of p53 was
also increased. These results suggest that the significant change
in the level of p53 by MHY-449 in HCT116 cells leads to the
increase in p21WAF1/CIP. Therefore, the induction
of p21WAF1/CIP1 in HCT116 cells is assumed to be
p53-dependent.
Discussion
This study was conducted to investigate the effects
of MHY-449 on HCT116 human colon cancer cells. MHY-449 induced
apoptosis and the cell cycle arrest in HCT116 human colon cancer
cells. The diastereoisomeric compound, MHY-449 and MHY-450, showed
concentration-dependent cytotoxicity on HCT116 cells and MHY-449
exhibited more potent cytotoxicity than MHY-450. Treatment of
HCT116 cells with MHY-449 resulted in growth inhibition in a
concentration-dependent manner. MHY-449 also resulted in
anti-proliferation in a time-dependent manner. Moreover, MHY-449
treatment showed modulation of the cell cycle and induction of
apoptosis in HCT116 cells.
The treatment of MHY-449 induced apoptosis as
demonstrated by the formation of apoptotic bodies and DNA
fragmentation. Apoptosis (programmed cell death), is an important
process required for homeostasis (12,18).
Apoptosis occurs through two broad pathways: the intrinsic pathway
(the mitochondrial pathway) and extrinsic pathway (the death
receptor pathway) (19). Death
receptors and caspases are key players in the extrinsic pathway,
whereas the Bcl-2 family which consists of more than 20 members of
pro-apoptotic proteins (including Bax, Bak, Bok, Bad and Bid), and
anti-apoptotic proteins (including Bcl-2, Bcl-XL, Mcl-1 and
Bfl-1/A1) (20,21) are key players in the intrinsic
pathway.
Treatment of HCT116 cells with MHY-449 resulted in
increase in expressions of Fas and FasL in a
concentration-dependent manner. The activation of initiator
caspase-8 and caspase-9 and downstream effector caspase-3, in
response to MHY-449 treatment and cleavage of PARP in HCT116 cells
were also observed. The ratio between Bcl-2 and Bax has been
suggested as a primary event in determining the susceptibility to
apoptosis through maintaining the integrity of the mitochondria and
inhibiting the activation of caspase cascade (17). In this study, MHY-449 treatment
resulted in a significant increase in pro-apoptotic protein Bax and
a decrease in anti-apoptotic protein Bcl-2, resulting in a shift in
Bax/Bcl-2 ratio in favor of apoptosis.
Different classes of cyclins and their
cyclin-dependent kinase (CDK) control cell cycle progression. G2/M
transition provides an effective checkpoint in cell cycle
progression, which is regulated by cyclin B1, Cdc2 and Cdc25C
(22). In this study, flow
cytometric analysis revealed that treatment of HCT116 cells with
MHY-449 resulted in the arrest of cells in G2/M phase and it seems
to be associated with a decrease in the protein levels of cyclin
B1, Cdc2 and Cdc25C.
Uncontrolled cell proliferation is the hallmark of
cancer, and tumor cells have typically acquired damage to gene that
directly regulates their cell cycles. Eukaryotic cell cycle
checkpoints involve the regulation of sequential formation,
activation and subsequent inactivation of CDKs, the activation of
which is dependent upon an association with cyclins (23,24).
The G2/M transition in the cell cycle plays a pivotal role at the
entry into mitosis, the complex of which is formed by the
association of Cdc2 and cyclin B1 (25). Binding to cyclin B and
phosphorylation at threonine 161 by CDK activating kinase (CAK) are
required to activate Cdc2 during G2 and the Cdc2/cyclin B complex
is kept inactive by phosphorylation in tyrosine 15 (Tyr 15) and
threonine 14 of Cdc2 by the kinases Wee1 and Myt1, respectively
(26,27). CDK-cyclin complexes are regulated
by two families of CDK inhibitors (CKIs), the CIP/KIP family
(p21WAF/CIP, p27KIP1, and
p57KIP2) and INK4 family
(p15INK4b, p16INK4a,
p18INK4c, and p19INK4d)
(28). Members of CIP/KIP family,
such as p21WA1F/CIP1, bind to CDK/cyclin
complexes and prevent kinase activation, subsequently regulating
G2/M-phase transition (29,30)
and upregulation in response to the inhibition of cell growth.
p21WAF1/CIP1 is commonly
associated with the G1 check point and G2/M phase, and its
association with inhibiting the expression of the Cdc2/cyclin B1
complex has been also reported (29,31).
p21WAF1/CIP1 transcription can be regulated
through either p53-dependent (32)
or p53-independent pathways (33).
In the present study, p21WAF1/CIP1 was increased
by MHY-449 in HCT116 cells, along with significant increase in the
protein levels of p53. These results suggest that MHY-449
upregulates p21WAF1/CIP1 expression and thereby
leading to G2/M-phase arrest through p53-dependent pathway.
p27KIP1 exerts tumor suppressing
function through inhibitory interactions with the cyclin/CDK
complexes, and downregulation of p27KIP1 protein
is often correlated with poor prognosis in several types of human
cancers. Our result showed that p27KIP1 was
increased by the treatment of MHY-449. However,
p27KIP1 possess oncogenic function as well, and
in many cases it is known to be cyclin/CDK independent and
attributed to its cytoplasmic localization (34).
In summary, MHY-449 suppressed growth and
proliferation of HCT116 cells in a concentration- and
time-dependent manner, respectively, by causing G2/M cell cycle
arrest and induction of apoptosis. Taken together, these results
suggest that novel compound MHY-449 may be useful in the
chemo-prevention and/or treatment of colon cancer.
Acknowledgements
This study was supported by National
Research Foundation of Korea (NRF) grant funded by the Korea
government (MOST) (no. 20090083538). We thank Aging Tissue Bank for
providing research information.
References
|
1.
|
Cooper K, Squires H, Carrol C, Papaioannou
D, Booth A, Logan RF, Maguire C, Hind D and Tappenden P:
Chemoprevention of colorectal cancer: systematic review and
economic evaluation. Health Technol Assess. 14:1–206. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
3.
|
National Cancer Information Center: Cancer
incidence. Goyang. http://www.cancer.go.kr/ncic/cics.
Accessed Dec 29, 2011.
|
|
4.
|
Kelly C and Cassidy J: Chemotherapy in
metastatic colorectal cancer. Surg Oncol. 16:65–70. 2007.
View Article : Google Scholar
|
|
5.
|
Yang SY, Sales KM, Fuller BF, Seifalian AM
and Winslet MC: Apoptosis and colorectal cancer: implications for
therapy. Trends Mol Med. 15:225–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Peter ME: The flip side of FLIP. Biochem
J. 382:E1–E3. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Ashkenazi A: Targeting the extrinsic
apoptosis pathway in cancer. Cytokine Growth Factor Rev.
19:325–331. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Inada H, Izawa I, Nishizawa M, Fujita E,
Kiyono T, Takahashi T, Momoi T and Inagaki I: Keratin attenuates
tumor necrosis factor-induced cytotoxicity through association with
TRADD. J Cell Biol. 155:415–426. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Cory S and Adams JM: The Bcl2 family:
regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Kuwana T, Mackey MR, Perkins G, Ellisman
MH, Latterich M, Schneiter R, Green DR and Newmeyer DD: Bid, Bax,
and lipids cooperate to form supramolecular openings in the outer
mitochondrial membrane. Cell. 111:331–342. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Oliver L and Vallette FM: The role of
caspases in cell death and differentiation. Drug Resist Updat.
8:163–170. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Iannolo G, Conticello C, Memeo L and De
Maria R: Apoptosis in normal and cancer stem cells. Crit Rev Oncol
Hematol. 66:42–51. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Kupchan SM, Streelman DR and Sneden AT:
Psorospermin, a new antileukemic xanthone from Psorospermum
febrifugum. J Nat Prod. 43:296–301. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Cassady JM, Baird WM and Chang CJ: Natural
products as a source of potential cancer chemotherapeutic and
chemo-preventive agents. J Nat Prod. 53:23–41. 1990.PubMed/NCBI
|
|
15.
|
Dorr RT, Liddil JD, Von Hoff DD, Soble M
and Osborne CK: Antitumor activity and murine pharmacokinetics of
parenteral acronycine. Cancer Res. 49:340–344. 1989.PubMed/NCBI
|
|
16.
|
Kang JA, Yang Z, Lee JY, De U, Kim TH,
Park JY, Lee HJ, Park YJ, Chu P, Kim KS, Jeong LS and Moon HR:
Design, synthesis and anticancer activity of novel
dihydrobenzofuro[4,5-b][1,8]naphthyridin-6-one derivatives. Bioorg
Med Chem Lett. 21:5730–5734. 2011.PubMed/NCBI
|
|
17.
|
Harris MH and Thompson CB: The role of the
Bcl-2 family in the regulation of outer mitochondrial membrane
permeability. Cell Death Differ. 7:1182–1191. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Call JA, Eckhardt SG and Camidge DR:
Targeted manipulation of apoptosis in cancer treatment. Lancet
Oncol. 9:1002–1011. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Lorenzo HK and Susin SA: Therapeutic
potential of AIF-mediated caspase-independent programmed cell
death. Drug Resist Updat. 10:235–255. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Antonsson B and Martinou JC: The Bcl-2
protein family. Exp Cell Res. 256:50–57. 2000. View Article : Google Scholar
|
|
21.
|
Guo B, Godzik A and Reed JC: Bcl-G, a
novel pro-apoptotic member of the Bcl-2 family. J Biol Chem.
276:2780–2785. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Molinari M: Cell cycle checkpoints and
their inactivation in human cancer. Cell Prolif. 33:261–274. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Sherr CJ: Cancer cell cycles. Science.
274:1672–1677. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Murray AW: Recycling the cell cycle:
cyclins revisited. Cell. 116:221–234. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Sancar A, Lindsey-Boltz LA, Unsal-Kaçmaz K
and Linn S: Molecular mechanisms of mammalian DNA repair and the
DNA damage checkpoints. Annu Rev Biochem. 73:39–85. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Taylor WR and Stark GR: Regulation of the
G2/M transition by p53. Oncogene. 20:1803–1815. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Le Gac G, Estève PO, Ferec C and Pradhan
S: DNA damage-induced down-regulation of human Cdc25C and Cdc2 is
mediated by cooperation between p53 and maintenance DNA
(cytosine-5) methyltransferase 1. J Biol Chem. 281:24161–24170.
2006.PubMed/NCBI
|
|
28.
|
Malumbres M and Barbacid M: Cell cycle,
CDKs and cancer: a changing paradigm. Nat Rev Cancer. 9:153–166.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Baus F, Gire V, Fisher D, Piette J and
Dulić V: Permanent cell cycle exit in G2 phase after DNA damage in
normal human fibroblasts. EMBO J. 22:3992–4002. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Hsu YL, Kuo PL, Lin LT and Lin CC: Asiatic
acid, a triterpene, induces apoptosis and cell cycle arrest through
activation of extracellular signal-regulated kinase and p38
mitogen-activated protein kinase pathways in human breast cancer
cells. J Pharmacol Exp Ther. 313:333–344. 2005. View Article : Google Scholar
|
|
31.
|
Niculescu AB III, Chen X, Smeets M, Hengst
L, Prives C and Reed SI: Effects of p21(Cip/Waf1) at both the G1/S
and the G2/M cell cycle transition: pRb is a critical determinant
in blocking DNA replication and in preventing endoreduplication.
Mol Cell Biol. 18:629–643. 1998.PubMed/NCBI
|
|
32.
|
El-Deiry WS, Tokino T, Velculescu VE, Levy
DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW and
Vogelstein B: WAF1, a potential mediator of p53 tumor suppression.
Cell. 75:817–825. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Gartel AL and Tyner AL: Transcriptional
regulation of the p21(WAF1/CIP1) gene. Exp Cell Res. 246:280–289.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Lee J and Kim SS: The function of p27 KIP1
during tumor development. Exp Mol Med. 41:765–771. 2009. View Article : Google Scholar : PubMed/NCBI
|