Introduction
Wedelolactone
(7-methoxy-5,11,12-trihydroxy-coumestan) is a plant-derived natural
product synthesized mainly by members belonging to the Asteraceae
family (1,2). A major source of WDL is the plant
genus Eclipta (or Bhringaraj) which is an acrid, bitter herb
medicine traditionally used extensively for hair and skin health
and for preventing liver damage due to alcohol overdose and
jaundice (1–5). This herb expels intestinal worms,
cures cough, prevents inflammation, reduces symptoms of bronchitis
and asthma, and is used to alleviate uterine pain after delivery.
In addition to its use as folk medicine, it has also been used in
the treatment of infective hepatitis in India (2–4),
snake venom poisoning in Brazil (6–9) and
septic shock in China (10).
Active compounds in Eclipta were observed to inhibit
protease activity as well as the activity of phospholipase A2
(11–14). The coumestan compounds
wedelolactone and demethyl-wedelolactone were tested to show
anti-hepatotoxic effect in liver cells (2,3). WDL
and other compounds from the plant Wedelia sinensis have
also been reported to block androgen receptor function (15), and to inhibit polymerase activity
of hepatitis C virus (16).
Interestingly, the coumestan derivative, wedelolactone, has been
found to be a potent and selective inhibitor of 5-Lox
(IC50 ∼2.5 μM) which inhibits 5-Lox activity by
an oxygen radical scavenging mechanism (17,18).
Thus, WDL has emerged as a candidate drug for prevention as well as
treatment of inflammatory diseases and cancer.
Emerging evidence from several studies has revealed
that prostate cancer cells continuously generate 5-Lox metabolites
and inhibition of 5-Lox by specific inhibitors induces apoptosis
both in androgen-sensitive as well as androgen-independent prostate
cancer cells (19–25). Apoptosis is prevented by
metabolites of 5-Lox, but not by 12-Lox or 15-Lox, suggesting that
5-Lox activity plays an essential role in the viability of prostate
cancer cells (20). Inhibition of
5-Lox activates caspases and blocking caspase activity by specific
inhibitors prevents induction of apoptosis suggesting that this
type of apoptosis is caspase-dependent. It was also observed that
inhibition of 5-Lox triggers rapid activation of c-Jun N-terminal
kinase (JNK) in prostate cancer cells which is detectable within
1–2 h post-treatment (26).
Blocking JNK activity by specific chemical inhibitors prevent 5-Lox
inhibition-induced caspase activation as well as apoptotic
degradation of nuclear DNA to nucleosomal fragments, suggesting
that JNK plays an important role in the apoptosis process. JNK has
already been reported to play an important role in apoptosis in
various types of cells (27–31).
In regard to downstream signaling, recently we found that 5-Lox
metabolites signal via an Akt-independent, PKCε-dependent mechanism
(32,33). Altogether, these findings
demonstrated that 5-Lox activity plays a critical role in the
survival of prostate cancer cells and suggested that 5-Lox may be
used as a molecular target for prevention and treatment of prostate
cancer.
Alongside the use of synthetic inhibitors, screening
and testing of compounds from natural sources are becoming more and
more popular for obtaining improved solubility, potency, and cancer
selectivity. We sought to test natural compound inhibitors of 5-Lox
activity for their effects on induction of apoptosis in prostate
cancer cells with an intention to find novel agents for prostate
cancer therapy. Though the 5-Lox inhibitory effect of WDL is known
for a while, its effect on induction of apoptosis in prostate
cancer cells and the underlying mechanisms have not been addressed
before. Thus, we examined the in vitro effects of WDL on a
range of human prostate cancer cells. Our results show that WDL
strongly affects the viability of both androgen-sensitive (LNCaP)
as well as androgen-independent (PC3, DU145) human prostate cancer
cells with minimal effect on the viability of normal, non-tumor
prostate epithelial cells (PrEC). Moreover, WDL was observed to
induce caspase-dependent apoptosis in prostate cancer cells which
was associated with dramatic inhibition of PKCε but no inhibition
of Akt. Apoptosis was effectively prevented by exogenous
metabolites of 5-Lox. These findings indicate that WDL selectivity
induces caspase-dependent apoptosis in prostate cancer cells via a
novel mechanism involving inhibition of PKCε but without inhibition
of Akt and suggest that WDL should be tested further as a novel
candidate drug for development of an effective therapy against
clinical prostate cancer.
Materials and methods
Cell culture and reagents
Human prostate cancer cells (LNCaP, PC3 and DU145)
were purchased from American Type Culture Collection (Manassas, VA,
USA). Cells were grown in RPMI-1640 medium (Invitrogen, Carlsbad,
CA, USA) as described before (20). Normal prostate epithelial cells
(PrEC) and the growth medium (PrEGM complete) were purchased from
Lonza (Walkersville, MD, USA), polyclonal antibodies against
histone H2A.X, phosphohistone H2A.X, c-JNK, phospho-JNK, Akt and
phospho-Akt were purchased from Cell Signaling (Danvers, MA, USA).
Antibodies against PARP, cyclin D1 and PKCε were purchased from
Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-β-actin
antibody, WDL and ibuprofen were purchased from Sigma Chemical Co.
(St. Louis, MO, USA). 5-Oxoeicosatetraenoid (5-oxoETE) and
15-oxoETE were purchased from Cayman Chemicals (Ann Arbor, MI,
USA).
Measurement of cell viability
Prostate cancer cells (4×103 per well)
were plated in 96-well plates overnight in RPMI-1640 medium
supplemented with 10% FBS. PrEC cells were plated in PrEGM complete
medium supplemented with 1% FBS. Then the cells were treated with
varying doses of WDL or solvent vehicle (0.2% DMSO) and the plates
were incubated for 72 h at 37°C in the CO2 incubator.
Cell viability was measured using One Solution Cell Titer AQ Assay
kit following a protocol supplied by the manufacturer (Promega
Corp., Madison, WI, USA).
Microscopy
LNCaP prostate cancer cells (∼3×105) were
plated in RPMI-1640 medium supplemented with 10% FBS overnight onto
60-mm diameter tissue culture plates (Falcon) and allowed to grow
for 48 h. On the day of experiment, the spent culture medium was
replaced with 2 ml fresh RPMI-1640 medium and the cells were
treated with inhibitors. Control cells were treated with solvent
only (0.2% DMSO). Photographs were taken with a Nikon digital
camera attached to a LEICA fluorescence microscope at
magnification, ×400. Image acquisition and data processing were
done with a Dell computer attached to the microscope using
SPOT-Advanced software.
Western blot analysis
LNCaP cells (∼3×105) were plated and
allowed to grow for 48 h. The old medium was then replaced with 2
ml fresh RPMI-1640 medium and the cells were treated with
inhibitors. After treatment, cells were harvested, washed and lysed
in lysis buffer (50 mM HEPES buffer, pH 7.4, 150 mM NaCl, 1 mM
EDTA, 1 mM orthovanadate, 10 mM sodium pyrophosphate, 10 mM sodium
fluoride, 1% NP-40, and a cocktail of protease inhibitors).
Proteins were separated by 12% SDS-PAGE and transferred to
nitrocellulose membranes. Membranes were blocked with 5% non-fat
milk solution and then blotted with appropriate primary antibody
followed by peroxidase-labeled secondary antibody. Bands were
visualized by enhanced chemiluminescence (Amersham, Rockford, IL,
USA).
Annexin V binding
LNCaP cells (∼3×105) were plated in
RPMI-1640 medium and allowed to grow for 48 h. The spent culture
medium was replaced with fresh 2 ml RPMI-1640 medium and the cells
were treated with WDL or ibuprofen for 24 h at 37°C. Then the cells
were treated with FITC-labeled Annexin V and propidium iodide for
15 min in the dark using Annexin V-Binding Detection kit following
a protocol supplied by the manufacturer (BD Biosciences, San Jose,
CA, USA). After washing, cells were photographed with a Nikon
digital camera attached to a LEICA fluorescence microscope at
magnification, ×200. Image acquisition and data processing were
done with a Dell computer attached to the microscope using
SPOT-Advanced software.
Measurement of caspase activity
LNCaP cells (∼3×105 per plate) were
plated in 60-mm diameter plates and treated with inhibitors or
solvent vehicle for varying periods of time. Then the cells were
lysed in lysis buffer containing 0.2% CHAPS as detergent. Enzymatic
activity of caspase-3 in cell lysates was measured colorimetrically
by a commercially available kit following methods supplied by the
manufacturer (Biomol, Plymouth Meeting, PA, USA).
DNA fragmentation
Apoptosis was quantitatively measured by detecting
degradation of nuclear DNA by sandwich-ELISA. LNCaP cells
(∼3×105) were plated in 60-mm diameter tissue culture
plates and allowed to grow for 48 h. Cells were then treated either
with the experimental agents or solvent vehicle for 24 h. At the
end of incubation period, cells were lysed and the degradation of
nuclear DNA to nucleosomal fragments was measured by Cell Death
Detection ELISAplus as described before (20,26),
following instructions supplied by the manufacturer (Roche,
Indianapolis, IN, USA).
Mitochondrial permeability transition
(MPT)
LNCaP cells (∼3×105) were plated in
RPMI-1640 medium and allowed to grow for 48 h. The spent culture
medium was replaced with fresh 2 ml RPMI-1640 medium and the cells
were treated with WDL or ibuprofen for 8 h at 37°C. Permeability
transition of mitochondria was detected using a kit following
manufacturer’s protocol (BD Biosciences) by treating cells with 40
nM Mitotracker red for 30 min at 37°C in the incubator. Hoechst dye
33342 was used to stain the nuclei. After washing, cells were
photographed with a Nikon digital camera attached to a Leica
fluorescence microscope at magnification, ×400. Image acquisition
and data processing were done with a Dell computer attached to the
microscope using SPOT-Advanced software.
Results
WDL reduces viability of prostate cancer
cells in a dose-dependent manner
Since the role of 5-Lox in the survival and growth
of prostate cancer cells has been observed in various laboratories
(19–25), we wanted to examine the effect of
WDL on the viability of prostate cancer cells, because WDL is known
to be a potent inhibitor of 5-Lox activity (17,18).
We observed that WDL dose-dependently reduced the viability of both
androgen-sensitive (LNCaP) as well as androgen-independent (PC3,
DU145) prostate cancer cells with IC50s between 8–12
μM (Fig. 1). The effect of
WDL was observed to be strongly cancer-specific when compared to
its effect on the viability of normal, non-cancerous prostate
epithelial cells (PrEC).
WDL induces severe morphological
alteration in prostate cancer cells
Earlier, we reported that prostate cancer cells show
pronounced alteration in their morphology forming numerous membrane
blebs when treated with synthetic inhibitors of 5-Lox (20). We examined whether WDL also exerts
similar effects on prostate cancer cells. We observed that prostate
cancer cells treated with WDL show a dramatic alteration in their
membrane morphology in a dose-dependent manner. Well-spread
adherent cells gradually withdraw their processes, become round and
eventually detach and float in the growth medium (Fig. 2). Similar effects of WDL were also
observed in PC3 and DU145 cells (not shown). Ibuprofen, an
inhibitor of cyclooxygenase, did not show any appreciable effect on
the morphology of prostate cancer cells in the same experimental
conditions, suggesting a selective action of WDL on these cells.
Morphological change of cells with WDL treatment was reminiscent of
cells undergoing apoptosis.
WDL induces apoptosis in prostate cancer
cells
WDL-induced morphological alteration in prostate
cancer cells prompted us to investigate whether these cells are
undergoing death via induction of apoptosis. Apoptosis-associated
formation of membrane blebs is characterized by cleavage of
cortical cytoskeleton and externalization of phosphatidylserine to
the outer leaflet of plasma membrane. Externalization of
phosphatidylserine can be assessed by its high-affinity binding
with dye-labeled Annexin V. We observed that cells with altered
morphology upon WDL treatment bind with fluorescein
isothiocyanate-labeled Annexin V (Annexin V-FITC), confirming
externalization of phosphatidylserine in these cells after
treatment (Fig. 3A).
Ibuprofen did not induce any appreciable effect on
externalization of phosphatidylserine in the same experimental
conditions. We observed that WDL dose-dependently induced
phosphorylation of the DNA damage-indicator histone H2A.X at
Serine139 (Fig. 3B),
suggesting occurrence of DNA strand breaks. Cleavage of poly-ADP
ribose polymerase (PARP) is an indicator of advanced stage of
apoptosis. PARP is a protein substrate which is cleaved to generate
particular peptide fragments by activated caspases. We observed
that when prostate cancer cells are treated with WDL, the intact
form of PARP protein (molecular weight 116 kDa) is cleaved to
generate a characteristic smaller species of ∼89 kDa which was
detectable at doses 10 μM and above (Fig. 3C). Degradation of DNA to
nucleosomal fragments is an indicator and a well characterized late
event in apoptotic cell death. We observed that treatment with WDL
induces fragmentation of chromatin DNA to nucleosomes in prostate
cancer cells in a dose-dependent manner (Fig. 3D). Ibuprofen did not show any
appreciable effect on phosphorylation of H2A.X, cleavage of PARP or
degradation of DNA.
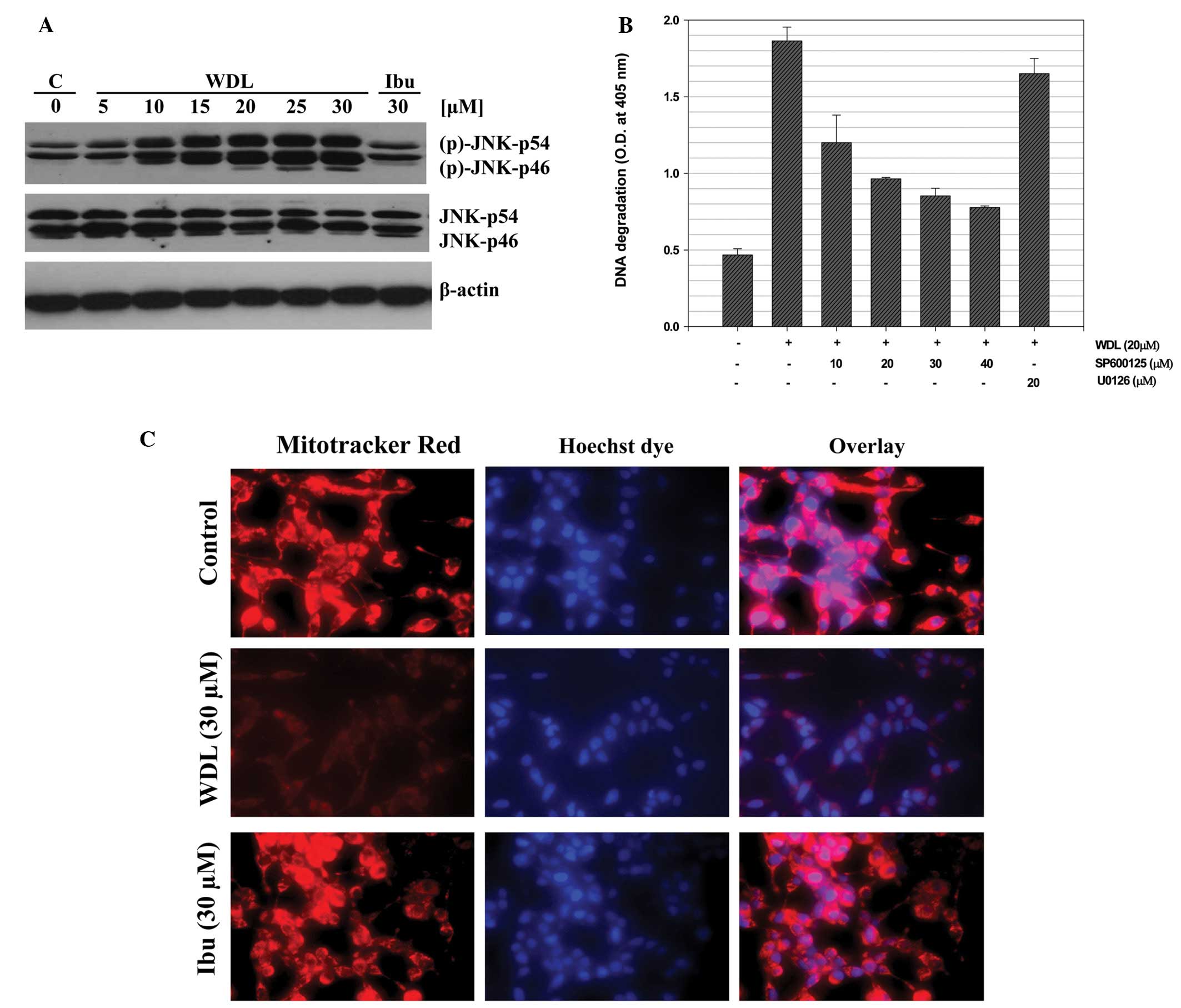
WDL-induced apoptosis in prostate cancer
cells is dependent on activation of c-Jun N-terminal kinase
(JNK)
We previously reported that 5-Lox inhibition induces
apoptosis in prostate cancer cells via rapid activation of c-Jun
N-terminal kinase (26). We
examined whether WDL induces apoptosis in prostate cancer cells via
activation of JNK. We observed that when prostate cancer cells are
treated with WDL a rapid and strong activation of JNK occurs and
that inhibition of JNK blocks induction of apoptosis, suggesting
that WDL-induced apoptosis in prostate cancer cells is dependent on
JNK activity (Fig. 4A and B). We
also observed that WDL damages mitochondrial integrity by inducing
permeability transition and loss of membrane potential-sensitive
dye (Fig. 4C).
Induction of apoptosis in prostate cancer
cells by WDL treatment is caspase-dependent
Both caspase-dependent and caspase-independent
apoptosis are known to occur depending on cell types and apoptotic
trigger (27). Though we observed
cleavage of PARP (a caspase substrate) after WDL treatment, we
wanted to examine the status and role of caspase-3 activation in
WDL-induced apoptosis in prostate cancer cells. We observed that
treatment with WDL induces activation of caspase-3 in a
dose-dependent manner (Fig. 5A).
Moreover, we observed that inhibition of caspase-3 by specific
inhibitor (DEVD-FMK) significantly prevents apoptotic DNA
degradation, suggesting that WDL-induced apoptosis in prostate
cancer cells is caspase-dependent (Fig. 5B).
WDL-induced apoptosis in prostate cancer
cells occurs via downregulation of PKCε without inhibiting Akt
We recently reported that 5-Lox inhibition-induced
apoptosis in prostate cancer cells occurs via inhibition of PKCε
without inhibition of Akt (32,33).
Thus, we wanted to test whether WDL-induced apoptosis is also
independent of Akt inhibition. We observed that treatment with WDL
downregulates PKCε in a dose-dependent manner, but does not
decrease phosphorylation of Akt in the same experimental conditions
(Fig. 6A and B) which suggests
that WDL induces apoptosis in prostate cancer cells via
downregulation of PKCε but not via inhibition of Akt.
Though it is known that inhibition of 5-Lox induces
apoptosis in prostate cancer cells (20–25),
and that WDL inhibits the activity of 5-Lox (17), WDL is not a specific inhibitor of
5-Lox because at higher doses it inhibits IKKα, topoisomerase IIα,
trypsin and PLA2 (8–13,34).
Thus, we wanted to verify whether the apoptosis-inducing effect of
WDL in prostate cancer cells occurs via inhibition of 5-Lox
activity. Results are depicted in Fig.
6C showing that WDL-induced apoptosis in prostate cancer cells
is effectively prevented by 5-oxoETE, a metabolic product of 5-Lox,
whereas 15-oxoETE, a product of 15-lipoxygenase, was without
effect. These findings suggest that the apoptosis-inducing effect
of WDL in prostate cancer cells is mediated (at least partially)
via inhibition of 5-Lox activity.
Discussion
We observed that the natural compound WDL reduces
viability of both androgen-sensitive (LNCaP) as well as
androgen-independent (PC3, DU145) human prostate cancer cells,
whereas it exerts only marginal effect on normal, non-cancer
prostate epithelial cells (PrEC) in the same culture conditions
(Fig. 1). These observations
document for the first time that WDL possesses significant
cancer-selective action, and suggest that WDL may be effective as a
small molecule agent against prostate cancer. Our observation is of
particular significance because it shows that WDL affects the
viability of both androgen receptor-positive LNCaP (35), and androgen receptor-negative PC3
and DU145 (36,37) human prostate cancer cells with
similar potency (IC50s of ∼8–12 μM), suggesting
that this effect of WDL is independent of androgen receptor status
of these cancer cells. Herbal formulations of the source plants
Eclipta alba and Eclipta prostrata have been used in
India for centuries against liver damage caused by various
hepatotoxins, for hair re-growth, for bronchitis and asthma, and
for general well being as a rejuvenator (1–5).
Crude extracts of plants contain numerous compounds, and the
composition varies from sample to sample and on growth conditions
of plants. However, WDL and demethylwedelolactone were identified
as major components after fractionation of crude plant extracts,
and are now available in pure forms for testing and mechanistic
understanding (1–5,12,18).
Both PC3 and DU145 cells were isolated from distant metastatic
sites (bone and brain, respectively) and are androgen-independent
(35–38). Thus, our observations open up the
possibility of using WDL against deadly diseases such as
androgen-independent metastatic prostate cancer for which currently
there is no cure available.
A major advancement in our understanding about WDL
as a pure compound is that it severely alters morphology and
induces apoptosis in prostate cancer cells (Figs. 2 and 3). This apoptosis is associated with
externalization of phosphatidylserine, cleavage of PARP,
phosphorylation of H2A.X, and degradation of chromatin DNA to
nucleosomal fragments. Cells undergoing apoptosis externalize
phosphatidylserine which is characterized as a signal from dying
cells for macrophage engulfment and clearance from the system
(39). PARP is a protein substrate
of executioner caspases and its characteristic cleavage is
considered as an indicator of caspase-mediated apoptotic cell death
(40). Degradation of chromatin
DNA to nucleosomal fragments is considered as a hallmark of
advanced stage of programmed cell death (41,42).
Induction of apoptosis in cancer cells has been recognized as an
effective approach to limit cancer growth because cancer cells are
often observed to be endowed with increased capacity to prevent
apoptosis, and pose resistance to chemo- and radiation-therapy
(43–45). This is particularly important for
prostate cancer because clinically prostate cancer is often
characterized as slow-growing where anti-mitogenic therapies are
not much effective. Thus, our observation of the induction of
apoptosis not only adds a new dimension to the pharmacological
properties of WDL but also opens up a possibility of using this
agent to sensitize prostate cancer cells to undergo apoptosis.
Activation of the stress-activated protein kinase
SAPK/JNK is a common, well-characterized cellular process for
induction of apoptosis in various types of cells, and it was
previously reported that 5-Lox inhibition induces apoptosis in
prostate cancer cells via rapid activation of c-Jun N-terminal
kinase (26–31). Thus, we examined whether WDL
induces apoptosis in prostate cancer cells via activation of JNK.
When prostate cancer cells were treated with WDL a rapid and strong
activation of JNK occurred which was inhibited when cells were
treated with inhibitors of JNK which also blocked induction of
apoptosis, suggesting that WDL-induced apoptosis in prostate cancer
cells is dependent on JNK activity (Fig. 4A and B). JNK modulates the function
of mitochondrial apoptosis-regulating proteins and in turn induces
permeability transition to release apoptosis-inducing factors
(46,47). Our observation of WDL-induced
damage of mitochondria which resulted in permeability transition
and loss of membrane potential-sensitive dye suggests that
WDL-induced apoptosis in prostate cancer cells involves JNK
activation as well as loss of mitochondrial function (Fig. 4C).
Caspases are activated by both the mitochondrial and
cell death receptor-mediated apoptosis pathways and play a causal
role in the apoptosis process (48). Caspase-3 is one of the executioner
caspases that is activated by upstream caspases, caspase-8 and -9.
Numerous intracellular peptide substrates of the executioner
caspases have been characterized including PARP, gelsolin,
cytokeratin and endonuclease (49–51).
As a first time report of apoptosis induction by WDL, we wanted to
know whether activation of caspase-3 occurs in this type of
apoptosis process, and whether caspase-3 activation plays any role
in WDL-induced apoptosis in prostate cancer cells. Our analysis
revealed that WDL treatment increases the enzymatic activity of
caspase-3 in a dose-dependent manner (Fig. 5A). Moreover, it was observed that
inhibition of caspase-3 by specific chemical inhibitor
significantly prevents induction of apoptosis, suggesting that
WDL-induced apoptosis in prostate cancer cells is caspase-dependent
(Fig. 5B). This finding is similar
to our previous observations of caspase-dependent apoptosis in
prostate cancer cells induced by other 5-Lox inhibitors (26).
How WDL can induce apoptosis in prostate cancer
cells is an intriguing question. A notable feature of WDL as a pure
compound is that it is a potent inhibitor of 5-Lox
(IC50=2.5 μM) which inhibits 5-Lox activity by an
oxygen radical scavenger mechanism (17,18).
However, WDL is not a specific inhibitor of 5-Lox because it also
inhibits other molecules at various concentrations (8–13,34).
Previous studies have demonstrated an essential role of 5-Lox in
the regulation of survival of both androgen-sensitive as well as
androgen-independent prostate cancer cells (19–25),
because inhibition of 5-Lox induces apoptosis in prostate cancer
cells which is prevented by exogenous metabolites of 5-Lox
(20,26,32,33).
Thus, 5-Lox has emerged as a potential molecular target for
therapeutic development against prostate cancer. However, potency,
solubility, and cancer selectivity of several available 5-Lox
inhibitors have limited their use for prostate cancer therapy.
Based on published reports on the 5-Lox inhibitory effect of WDL,
we expected that WDL, like other 5-Lox inhibitors, will decrease
viability and induce apoptosis in prostate cancer cells via
inhibition of PKCε (33) but
without inhibition of Akt (32).
Indeed we observed that WDL induced-apoptosis in prostate cancer
cells is associated with dramatic inhibition of PKCε, whereas no
inhibition of Akt was observed (Fig.
6A and B). Our observations of the induction of apoptosis in
prostate cancer cells by WDL, and the prevention of apoptosis by
5-oxoETE (a metabolite of 5-Lox), but not by 15-oxoETE (a
metabolite of 15-Lox) are consistent with the idea that the
apoptosis-inducing effect of WDL in prostate cancer cells is
mediated, at least partially, via inhibition of 5-Lox activity
(Fig. 6C). Altogether, these
findings indicate that WDL, a plant-derived coumestan compound,
possesses significant anticancer properties, and suggest that it is
possible to find newer 5-Lox-targeting agents from natural sources
for development of effective therapy against prostate cancer.
Prostate cancer is the most common form of
malignancy and second leading cause of cancer-related deaths in men
in the United States (52). Though
prostate cancer initially responds to anti-androgenic therapy,
androgen-refractory disease almost always develops (53,54).
Development of hormone-independent metastatic prostate cancer
always ends up with a fatal outcome because currently there is no
treatment available for this type of prostate cancer (54). Thus, novel agents and strategies
are urgently needed to improve treatment options for
androgen-independent prostate cancer. Based on the potency,
solubility, and selectivity profile of WDL against metastatic
prostate cancer cells in vitro, it appears that WDL is a
novel, promising candidate drug and should be tested further for
the treatment of both androgen-sensitive as well as
androgen-independent prostate cancers.
Abbreviations:
|
WDL
|
wedelolactone;
|
|
5-Lox
|
5-lipoxygenase;
|
|
PKCε
|
protein kinase C ε;
|
|
5-oxoETE
|
5-oxoeicosatetraenoid;
|
|
PARP
|
poly-ADP ribose polymerase;
|
|
IAP
|
inhibitors of apoptosis;
|
|
ELISA
|
enzyme-linked immunosorbent assay;
|
|
FITC
|
fluorescein isothiocyanate
|
Acknowledgements
Research reported in this publication
was supported by the National Cancer Institute of the National
Institutes of Health under award number RO1 CA 152334, the
Department of Defense Prostate Cancer Research Program
W81-XWH-05-1-0022 and the Henry Ford Health System internal grant
A10203 to JG.
References
|
1.
|
T GovindachariK NagarajanB
PaiWedelolactone from Eclipta albaJ Sci Indust
Res15B6646651956
|
|
2.
|
H WagnerB GeyerY KisoH HikinoGS
RaoCoumestans as the main active principles of the liver drugs
Eclipta alba and Wedelia calendulaceaPlanta
Med52370374198610.1055/s-2007-969188
|
|
3.
|
B SinghAK SaxenaBK ChandanSG AgarwalKK
AnandIn vivo hepatoprotective activity of active fraction from
ethanolic extract of Eclipta alba leavesIndian J Physiol
Pharmacol45435441200111883149
|
|
4.
|
MB PatelVM KadakiaSH MishraSimultaneous
estimation of andrographolide and wedelolactone in herbal
formulationsIndian J Pharma
Sci70689693200810.4103/0250-474X.4542121394279
|
|
5.
|
RK RoyM ThakurVK DixitHair growth
promoting activity of Eclipta alba in male albino ratsArch
Dermatol Res300357364200810.1007/s00403-008-0860-318478241
|
|
6.
|
WB MorsMC do NascimentoJP ParenteMH da
SilvaPA MeloG Suarez-KurtzNeutralization of lethal and myotoxic
activities of South American rattlesnake venom by extracts and
constituents of the plant Eclipta prostrata
(asteraceae)Toxicon2710031009198910.1016/0041-0101(89)90151-72799833
|
|
7.
|
PA MeloMC NascimentoWB MorsG
Suarez-KurtzInhibition of the myotoxic and hemorrhagic activities
of crotalid venoms by Eclipta prostrata (asteraceae)
extracts and
constituentsToxicon32595603199410.1016/0041-0101(94)90207-08079371
|
|
8.
|
PA MeloCL OwnbyAbility of wedelolactone,
heparin, and para-bromophenacyl bromide to antagonize the myotoxic
effects of two crotaline venoms and their PLA2
myotoxinsToxicon37199215199910.1016/S0041-0101(98)00183-49920492
|
|
9.
|
AM SoaresAH JanuarioMV LourencoAM
PereiraPS PereiraNeutralizing effects of Brazilian plants against
snake venomsDrugs
Fut2911051109200410.1358/dof.2004.029.11.851973
|
|
10.
|
M KoboriZ YangD GongV HeissmeyerH ZhuYK
JungM AngelicaM GakidisA RaoT SekineF IkegamiC YuanJ
YuanWedelolactone suppresses LPS-induced caspase-11 expression by
directly inhibiting the IKK complexCell Death
Differ11123130200410.1038/sj.cdd.440132514526390
|
|
11.
|
SD SyedM DeepakS YogishaAP ChandrashekarKA
MuddarachappaP D’SouzaA AgarwalBV VenkataramanTrypsin inhibitory
effect of wedelolactone and demethylwedelolactonePhytother
Res17420421200310.1002/ptr.115312722155
|
|
12.
|
BP SagarR PanwarA GoswamiK KadianK TyagiM
ChughS DalalR ZafarPharmacokinetic interactions of antihepatotoxic
wedelolactone with paracetamol in wistar albino ratsPharmaceutical
Biol44554561200610.1080/13880200600885242
|
|
13.
|
LC DiogoRS FernandesS MarcussiDL MenaldoPG
RobertoPV MatranguloPS PereiraSC FrancaS GiuliattiAM SoaresMV
LourencoInhibition of snake venoms and phospholipases A2
by extracts from native and genetically modified Eclipta
alba: isolation of active coumestansBasic Clin Pharmacol
Toxicol104293299200919320636
|
|
14.
|
P PithayanukulB LapettR BavovadaN
PakmaneeR SuttisriInhibition of proteolytic and hemorrhagic
activities by ethyl acetate extract of Eclipta prostrata
against Malayan pit viper venomPharmaceutical
Biol45282288200710.1080/13880200701214805
|
|
15.
|
FM LinLR ChenEH LinFC KeHY ChenMJ TsaiPW
HsiaoCompounds from Wedelia chinensis synergistically
suppress androgen activity and growth in prostate cancer
cellsCarcinogenesis2825212529200717942463
|
|
16.
|
N Kaushik-BasuA Bopda-WaffoTT TaleleA
BasuPR CostaAJ da SilvaSG SarafianosF NoelIdentification and
characterization of coumestans as novel HCV NS5B polymerase
inhibitorsNucleic Acids
Res3614821496200810.1093/nar/gkm117818203743
|
|
17.
|
H WagnerB FesslerIn vitro 5-lipoxygenase
inhibition by Eclipta alba extracts and the coumestan
derivative wedelolactonePlanta Med523743771986(In German).
|
|
18.
|
O WerzInhibition of 5-lipoxygenase product
synthesis by natural compounds of plant originPlanta
Med7313311357200710.1055/s-2007-99024217939102
|
|
19.
|
S GuptaM SrivastavaN AhmadK SakamotoDG
BostwickH MukhtarLipoxygenase-5 is overexpressed in prostate
adenocarcinomaCancer91737743200110.1002/1097-0142(20010215)91:4%3C737::AID-CNCR1059%3E3.0.CO;2-F11241241
|
|
20.
|
J GhoshCE MyersInhibition of arachidonate
5-lipoxygenase triggers massive apoptosis in human prostate cancer
cellsProc Natl Acad Sci
USA951318213187199810.1073/pnas.95.22.13182
|
|
21.
|
J GhoshCE MyersCentral role of
arachidonate 5-lipoxygenase in the regulation of cell growth and
apoptosis in human prostate cancer cellsAdv Exp Med
Biol469577582199910.1007/978-1-4615-4793-8_8410667385
|
|
22.
|
KM AndersonT SeedM VosJ MulshineJ MengW
AlrefaiD OuJH Harris5-lipoxygenase inhibitors reduce PC-3 cell
proliferation and initiate nonnecrotic cell
deathProstate37161173199810.1002/(SICI)1097-0045(19981101)37:3%3C161::AID-PROS5%3E3.0.CO;2-D9792133
|
|
23.
|
RM MorettiMM MontagnaniA SalaM MottaP
LimontaActivation of the orphan nuclear receptor RORalpha
counteracts the proliferative effect of fatty acids on prostate
cancer cells: crucial role of 5-lipoxygenaseInt J
Cancer1128793200410.1002/ijc.20387
|
|
24.
|
P YangP CollinT MaddenD ChanB
Sweeney-GotschD McConkeyRA NewmanInhibition of proliferation of PC3
cells by the branched-chain fatty acid, 12-methyltetradecanoic
acid, is associated with inhibition of
5-lipoxygenaseProstate55281291200310.1002/pros.1024312712407
|
|
25.
|
M MatsuyamaR YoshimuraM MitsuhashiT HaseK
TsuchidaY TakemotoY KawahitoH SanoT NakataniExpression of
lipoxygenase in human prostate cancer and growth reduction by its
inhibitorsInt J Oncol24821827200415010818
|
|
26.
|
J GhoshInhibition of arachidonate
5-lipoxygenase triggers prostate cancer cell death through rapid
activation of c-Jun N-terminal kinaseBiochem Biophys Res
Commun307342349200310.1016/S0006-291X(03)01201-412859962
|
|
27.
|
SP CreganVL DawsonRS SlackRole of AIF in
caspase-dependent and caspase-independent cell
deathOncogene2327852796200410.1038/sj.onc.120751715077142
|
|
28.
|
DN DhanasekaranEP ReddyJNK signaling in
apoptosisOncogene2762456251200810.1038/onc.2008.30118931691
|
|
29.
|
JM KyriakisP BanerjeeE NikolakakiT DaiEA
RubieMF AhmadJ AvruchJR WoodgettThe stress-activated protein kinase
subfamily of c-Jun
kinasesNature369156160199410.1038/369156a08177321
|
|
30.
|
JM KyriakisJ AvruchSounding the alarm:
protein kinase cascades activated by stress and inflammationJ Biol
Chem2712431324316199610.1074/jbc.271.40.243138798679
|
|
31.
|
AC MaroneyJP FinnD Bozyczko-CoyneTM
O’KaneNT NeffAM TolkovskyDS ParkCY YanCM TroyLA GreeneCEP-1347 (KT
7515), an inhibitor of JNK activation, rescues sympathetic neurons
and neuronally differentiated PC12 cells from death evoked by three
distinct insultsJ Neurochem73190119121999
|
|
32.
|
S SarveswaranCE MyersJ GhoshMK591, a
leukotriene biosynthesis inhibitor, induces apoptosis in prostate
cancer cells: synergistic action with LY294002, an inhibitor of
phosphatidylinositol 3′-kinaseCancer Lett291167176201019906484
|
|
33.
|
S SarveswaranV ThamilselvanC BrodieJ
GhoshInhibition of 5-lipoxygenase triggers apoptosis in prostate
cancer cells via down-regulation of protein kinase
C-epslilonBiochim Biophys
Acta181321082117201110.1016/j.bbamcr.2011.07.01521824498
|
|
34.
|
P BenesL KnopfovaF TrckaA NemajerovaD
PinheiroK SoucekM FojtaJ SmardaInhibition of topoisomerase IIα:
novel function of wedelolactoneCancer Lett30329382011
|
|
35.
|
JS HoroszewiczSS LeongE KawinskiJP KarrH
RosenthalTM ChuEA MirandGP MurphyLNCaP model of human prostatic
carcinomaCancer Res43180918181983
|
|
36.
|
ME KaighnKS NarayanY OhnukiJF LechnerLW
JonesEstablishment and characterization of a human prostatic
carcinoma cell line (PC-3)Invest Urol1716231979447482
|
|
37.
|
KR StoneDD MickeyH WunderliGH MickeyDF
PaulsonIsolation of a human prostate carcinoma cell line (DU
145)Int J Cancer21274281197810.1002/ijc.2910210305631930
|
|
38.
|
A van BokhovenM Varella-GarciaC KorchWU
JohannesEE SmithHL MillerSK NordeenGJ MillerMS LuciaMolecular
characterization of human prostate carcinoma cell
linesProstate57205225200314518029
|
|
39.
|
SE LogueM ElgendySJ MartinExpression,
purification and use of recombinant annexin V for the detection of
apoptotic cellsNat
Protoc413831395200910.1038/nprot.2009.14319730422
|
|
40.
|
PJ DuriezGM ShahCleavage of
poly(ADP-ribose) polymerase: a sensitive parameter to study cell
deathBiochem Cell Biol75337349199710.1139/o97-0439493956
|
|
41.
|
PJ HurdAJ BannisterK HallsMA DawsonM
VermeulenJV OlsenH IsmailJ SomersM MannT Owen-HughesI GoutT
KouzaridesPhosphorylation of histone H3 Thr-45 is linked to
apoptosisJ Biol
Chem2841657516583200910.1074/jbc.M109.00542119363025
|
|
42.
|
M RadicT MarionM MonestierNucleosomes are
exposed at the cell surface in apoptosisJ
Immunol17266926700200410.4049/jimmunol.172.11.669215153485
|
|
43.
|
DS ZieglerAL KungTherapeutic targeting of
apoptosis pathways in cancerCurr Opin
Oncol2097103200810.1097/CCO.0b013e3282f310f618043263
|
|
44.
|
FH IgneyPH KrammerDeath and anti-death:
tumour resistance to apoptosisNat Rev
Cancer2277288200210.1038/nrc77612001989
|
|
45.
|
T MashimaT TsuruoDefects of the apoptotic
pathway as therapeutic target against cancerDrug Resist
Updat8339343200510.1016/j.drup.2005.11.00116338161
|
|
46.
|
H AokiPM KangJ HampeK YoshimuraT NomaM
MatsuzakiS IzumoDirect activation of mitochondrial apoptosis
machinery by c-Jun N-terminal kinase in adult cardiac myocytesJ
Biol Chem2771024410250200210.1074/jbc.M11235520011786558
|
|
47.
|
C HorbinskiCT ChuKinase signaling cascades
in the mitochondrion: a matter of life or deathFree Radic Biol
Med38211200510.1016/j.freeradbiomed.2004.09.03015589366
|
|
48.
|
SE LogueSJ MartinCaspase activation
cascades in apoptosisBiochem Soc
Trans3619200810.1042/BST036000118208375
|
|
49.
|
RU JanickeML SprengartMR WatiAG
PorterCaspase-3 is required for DNA fragmentation and morphological
changes associated with apoptosisJ Biol
Chem27393579360199810.1074/jbc.273.16.93579545256
|
|
50.
|
JG WalshSP CullenC SheridanAU LuthiC
GernerSJ MartinExecutioner caspase-3 and caspase-7 are functionally
distinct proteasesProc Natl Acad Sci
USA1051281512819200810.1073/pnas.070771510518723680
|
|
51.
|
S KeithD AbayasiriwardanaD BarboneKU KimC
VivoKK LeeTB DansenAE HuntGI EvanVC BroaddusMalignant mesothelioma
cells are rapidly sensitized to TRAIL-induced apoptosis by low-dose
anisomycin via BimMol Cancer
Ther627662776200710.1158/1535-7163.MCT-07-027817938269
|
|
52.
|
A JemalR SiegelE WardY HaoJ XuMJ
ThunCancer Statistics, 2009CA Cancer J
Clin59225249200910.3322/caac.20006
|
|
53.
|
Z CuligJ HoffmannM ErdelIE EderA HobischA
HittmairG BartschG UtermannMR SchneiderK ParczykH KlockerSwitch
from antagonist to agonist of the androgen receptor: bicalutamide
is associated with prostate tumour progression in a new model
systemBr J Cancer81242251199910.1038/sj.bjc.669068410496349
|
|
54.
|
M NamikiS UenoY KitagawaH KonakaA
MizokamiE KohTY FukagaiHormonal therapyInt J Clin
Oncol12427432200710.1007/s10147-007-0704-8
|