Introduction
Every third patient with localized prostate cancer
(PCa) who undergoes radical prostatectomy (RP) will experience
biochemical failure (BcF) within 10 years (1,2) and,
without further treatment, one third will progress to metastatic
disease within 8 years (1).
Postoperative radiotherapy has been delivered to patients with
recurrent PCa for the last 2–3 decades, but not until recently have
data shown that adjuvant radiotherapy (ART) increases disease-free
survival and also provides an appreciable survival benefit in
patients with non-radically excised tumors (3–5).
Definitions of BcF have varied over the years, although they have
all included rising PSA after RP and no evidence of clinical or
radiological residual disease. Furthermore, there is now level 1
evidence that ART plays a role after non-radical RP, but the vast
majority of patients with PSA relapse have not received this
treatment.
Thus, therapy decisions must be made as to whether
these individuals should be offered salvage radiotherapy (SRT).
Several ongoing studies include patients randomized to ART or SRT;
however, data are not to be expected within the next few years,
and, hence, decisions must be based on outcome data from
non-randomized studies. Numerous questions concerning SRT remain to
be answered, although recommendations and guidelines have emerged
(6,7). Common to these is that SRT provides
long-term control of the disease in approximately one third of
patients with BcF, particularly those with relatively low pre-SRT
PSA levels. Patients with poorly differentiated cancer and short
PSA doubling time (PSADT) seem to benefit most from SRT (8). Recently, well-designed matched-pair
analyses were performed to compare ART with early SRT (9,10)
and these investigations, like the three large randomized adjuvant
trials, suggested that ART is superior. However, it is difficult to
draw extensive conclusions from the indicated observations,
because, by definition, the SRT patients suffered from more
advanced disease. Treatment decisions can be based on previously
reported outcome data. However, knowledge is still limited
regarding issues such as long-term side-effects, impact of
treatment on health-related quality of life (HRQoL), influence of
co-morbidity on treatment outcome, effects of neoadjuvant hormone
therapy (NHT).
The aim of the present study was to evaluate the
long-term outcome of SRT, with consideration paid to genitourinary
(GU) and gastrointestinal (GI) toxicity, the impact on HRQoL, and
the influence of co-morbidity. To our knowledge, this is the first
large, single-institution study to use a uniform radiation
treatment protocol combined with short-term NHT.
Patients and methods
Patients
Treatment outcome was analyzed in 184 consecutive
PCa patients with post-RP BcF. The patients had been referred for
SRT to the Radiumhemmet and Södersjukhuset facilities at the
Karolinska University Hospital (KS) in Stockholm, Sweden, between
October 2001 and February 2007. Patients were available for all the
following characteristics: pT stage 2–3, record of digital rectal
examination (DRE), post-RP BcF, NX or N0, Gleason score (GS) of the
post-RP specimen and at least 12 months post-SRT follow-up. Median
age was 64 years (range 39–77), and median follow-up was 48 months
(range 12–92). After SRT, all patients were monitored for BcF with
periodic PSA values. If recurrence/metastases were suspected,
scintigraphy and/or [F18]-FDG or acetate positron
emission tomography were performed.
Treatment
Radical prostatectomy was retropubic in 161 (87%),
laparoscopic in 3 (2%), and robot-assisted in 20 (11%). Three
different clinical target radiotherapy volumes (CTVs) were used
according to individual factors: A) inclusion of the prostatic
fossa (PF) and seminal vesicles (SVs) in the 78 (43%) patients with
SV invasion (SVI) at surgery; B) inclusion of only the PF in 19
(10%) patients whose SVs had been removed completely; C) a
shrinking field in 87 (47%) patients with residual distal parts of
the SVs after RP. The planning target volume (PTV) was defined as
CTV plus 20 mm in all directions except posteriorly, where 15 mm
was used. The daily fraction was 2 Gy delivered 5 days/week. For
CTVs A and B, the prescribed dose was 70 Gy; in C, the PF and the
SVs were irradiated to 54 Gy, and thereafter only the PF to 70 Gy.
At least 95% of the PTV received the prescribed doses. Less than
15% of the outlined rectal volume received doses >70 Gy. All
patients were given 3D conformal therapy with high-energy linear
accelerators equipped with multi-leaf collimators, and they were
treated in the supine position. The external beam radiation was
performed with a four-field at Radiumhemmet, whereas a three-field
technique (one anterior and two lateral fields) was used at
Södersjukhuset; all fields were equally weighted at both
facilities.
A median of 3 months of NHT was given to 165 (90%)
of the patients. Of these, 151 (92%) received antiandrogens
(bicalutamide 50 mg × 1 or flutamide 250 mg × 3) combined with a
gonadotropin-releasing hormone (GnRH) analogue, and 14 (8%) were
given antiandrogens or GnRH analogue as monotherapy.
Data collection
Clinical data were collected from electronic medical
records (KS database). Side-effects were reported according to the
GU-GI toxicity scale of the Radiation Therapy Oncology Group (RTOG)
and the European Organization for Research and Treatment of Cancer
(EORTC) (11). The Charlson
co-morbidity index adjusted for age was analyzed to correlate
co-morbidities and RTOG toxicity (12,13).
DRE was performed during the regular follow-up after RP. A palpable
post-RP recurrence was found in 46 (25%) patients; 35 (76%) of
these underwent transrectal ultrasound guided fine-needle
aspiration cytology. Pathological evidence of local post-RP
recurrence was found in 10 (29%). The patient characteristics are
described in Table I. The
investigation was approved by the local ethics committee (approval
number 2006/620-31/1).
 | Table I.Baseline clinical
characteristics. |
Table I.
Baseline clinical
characteristics.
|
Characteristics | No. (%)a |
|---|
| Total no. of
patients | 184 |
| Pre-prostatectomy
age, median (range), ng/ml | 64 (39–77) |
| Pre-prostatectomy
PSA level, median (range), ng/ml | 9.5(1.8–48) |
| PSA <10 | 101 (55) |
| PSA 10–20 | 68 (37) |
| PSA >20 | 15 (8) |
| Pathological
stage | |
| pT2 | 63 (34) |
| pT3 | 121 (66) |
| Gleason score after
radical prostatectomy | |
| ≤6 | 42 (23) |
| 7 | 112 (61) |
| 8–10 | 30 (16) |
| Digital rectal
examination | |
| Positive | 46 (25) |
| Biopsy | 35 (76) |
| Positive | 10 (29) |
| Pre-radiotherapy
PSA level, median (range), ng/ml | 0.47 (0.1–6.3) |
| PSA doubling time,
median (range), months | 6 (0.4–78) |
| PSA doubling time
<10 months | 134 (73) |
| Positive surgical
margins | 121 (66) |
| Seminal vesicle
invasion | 40 (22) |
| Disease-free
interval between prostatectomy and radiotherapy, median (range),
months | 14 (2–89) |
| Persistent
detectable PSA level after radical prostatectomy | 85 (46) |
| Neoadjuvant
androgen deprivation therapy before salvage radiotherapy | 165 (90) |
| Radiation dose | 70 Gy |
| Entire seminal
vesicles | 78 (43) |
| Shrinking field
after 54 Gy | 87 (47) |
| Only prostate
bed | 19 (10) |
| Follow-up after
radiotherapy, median (range), months | 48 (12–92) |
RTOG score and Charlson co-morbidity
index adjusted for age (CCIAA) (Table
II)
HRQoL EORTC QLQ-C30 and PR-25
(prostate specific) (14,15)
Up to October 2009, 9 of the 184 consecutive
patients had died during follow-up. To each of the remaining 175,
an envelope containing both questionnaires, a letter inviting
participation in the study, and a prepaid return envelope was sent.
In all, 148 (85%) patients returned the questionnaires; four were
blank, which left 144 (83%) for evaluation.
Statistical analysis
Pre-SRT BcF was defined as follows: a rise in PSA
>0.1 ng/dl when nadir was undetectable, or any rise in PSA above
nadir. Clinical characteristics such as pre-RP PSA and pT stage
(extracapsular extension), pre-RT PSADT, post-RP GS, pre-SRT PSA,
were dichotomized. Post-SRT BcF was defined as any detectable PSA
with an undetectable nadir or an elevation of 0.1 ng/ml above
nadir. Undetectable PSA was reported as <0.05 ng/ml, and an
approximation to 0.04 ng/ml was done in the statistical
calculation. The PSADT calculation was done according to Pound
et al(1) The pT stage was
defined according to the 1997 TNM classification system.
No biochemical evidence of disease (bNED), overall
survival (OS), PCa-specific survival, and metastasis-free survival
(MFS) were estimated by using the Kaplan-Meier and log-rank methods
to demonstrate differences. Time to event was calculated from the
date of SRT to the date of the event or death. A univariate
regression was performed in which BcF was evaluated as an outcome
variable. The multivariate regressions were conducted with stepwise
variable elimination, using BcF and bone metastases as outcome
variables. Both univariate and multivariate analyses were performed
using Cox proportional hazards regression and were compared with
the results presented by Stephenson et al(16) as listed in Table III. Logarithmic transformation of
the pre-SRT PSA was performed to run a logistic regression.
 | Table III.Univariate and multivariate
regression analysis. |
Table III.
Univariate and multivariate
regression analysis.
| Univariate | Stephenson et
al(16)
| Present study
|
|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value |
|---|
| Pre-prostatectomy
PSA level, ng/ml >10 | | | 0.04 | 0.90 | 0.55; 1.45 | 0.66 |
| Gleason score | | | <0.001 | 0.39 | 0.23; 0.68 | <0.001 |
| Pre-radiotherapy
PSA level | | | <0.001 | 0.39 | 0.23; 0.67 | <0.001 |
| Surgical
margins | | | 0.002 | 1.13 | 0.69; 1.85 | 0.48 |
| PSA doubling time,
<10 months | | | <0.001 | 0.80 | 0.45; 1.43 | 0.44 |
| Seminal vesicle
invasion | | | <0.001 | 0.41 | 0.25; 0.67 | <0.001 |
| Extracapsular
extension (pathological T stage) | | | 0.07 | 0.56 | 0.32; 0.99 | 0.046 |
| Lymph node
involvement | | | 0.12 | | | - |
| Disease-free
interval, <12 months | | | 0.31 | 1.01 | 0.62; 1.65 | 0.95 |
| Detectable PSA
after radical prostatectomy | | | 0.005 | 0.73 | 0.45; 1.18 | 0.20 |
| NHT prior to
salvage radiotherapy | | | 0.77 | 0.84 | 0.40; 1.78 | 0.60 |
| Radiotherapy
dose | | | 0.24 | | | - |
| Detectable PSA
after SRT | | | - | 4.96 | 1.36; 18.53 | <0.001 |
| Multivariate | Stephenson et
al(16)
| Present study (BcF,
stepwise)
| Present study
(Mets, stepwise)
|
|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value |
|---|
| Positive DRE | | | - | | | NS | | | NS |
| Pre-prostatectomy
PSA level, ng/ml >10 | 1.1 | 0.8; 1.4 | 0.73 | | | NS | | | NS |
| Gleason score
7 | 1.5 | 0.98; 2.2 | 0.06 | | | - | | | - |
| Gleason score
8–10 | 2.6 | 1.7; 4.1 | <0.001 | 2.06 | 1.15; 3.67 | 0.015 | | | NS |
| Pre-radiotherapy
PSA level 1–2 | 1.2 | 0.8; 1.7 | 0.31 | 2.07 | 1.20; 3.56 | 0.009 | | | NS |
| Pre-radiotherapy
PSA level >2 | 2.3 | 1.7; 3.2 | <0.001 | | | - | | | - |
| Surgical
margins | 1.9 | 1.4; 2.5 | <0.001 | | | NS | | | NS |
| PSA doubling time,
<10 months | 1.7 | 1.2; 2.2 | 0.001 | | | NS | | | NS |
| Seminal vesicle
invasion | 1.4 | 1.1; 1.9 | 0.02 | 1.92 | 1.14; 3.23 | 0.015 | 18.20 | 2.21; 149.83 | 0.007 |
| Extracapsular
extension (pathological T stage) | 1.2 | 0.9; 1.6 | 0.21 | | | NS | | | NS |
| Lymph node
involvement | 1.5 | 0.7; 3.0 | 0.32 | | | NS | | | NS |
| Disease-free
interval, <12 months | 1.0 | 0.7; 1.3 | 0.71 | | | NS | | | NS |
| Detectable PSA
after radical prostatectomy | | | - | | | NS | | | NS |
| NHT prior to
salvage radiotherapy | 0.8 | 0.5; 1.1 | 0.16 | | | NS | | | NS |
| Radiotherapy
dose | 1.0 | 0.7; 1.3 | 0.96 | | | - | | | - |
| Detectable PSA
after SRT | | | - | 3.38 | 1.64; 6.96 | 0.001 | | | NS |
The raw scores of the questionnaires were linearly
transformed into a 100-point scale, and missing values were imputed
according to the EORTC scoring manual (17). Mean questionnaire scores for
patients with bNED and with BcF were calculated with 95% confidence
intervals.
To compare means, the Student’s t-test was used when
data were normally distributed, and the Mann-Whitney U test when
skewed. Fisher’s exact test was utilized for the count/frequency
analyses. The tests were performed using STATA 9, and p-values
<0.05 were considered statistically significant.
Using the nomogram
The pre-treatment data of patients were used in the
nomogram for radiotherapy as described by Stephenson et
al(7) to create an individual
probability for PSA bNED at 3 years. Thereafter, the means were
calculated.
Results
Nine patients died during follow-up
and one of PCa
This gave a 99% PCa-specific survival and 95% OS.
Seven (4%) developed bone metastases. No statistically significant
differences in OS were found between patients with low/medium and
high CCIAA scores. Using the patients’ pre-treatment data and the
Stephenson nomogram, a mean 3-year bNED probability of 53% (95% CI
49.21; 56.71) was calculated.
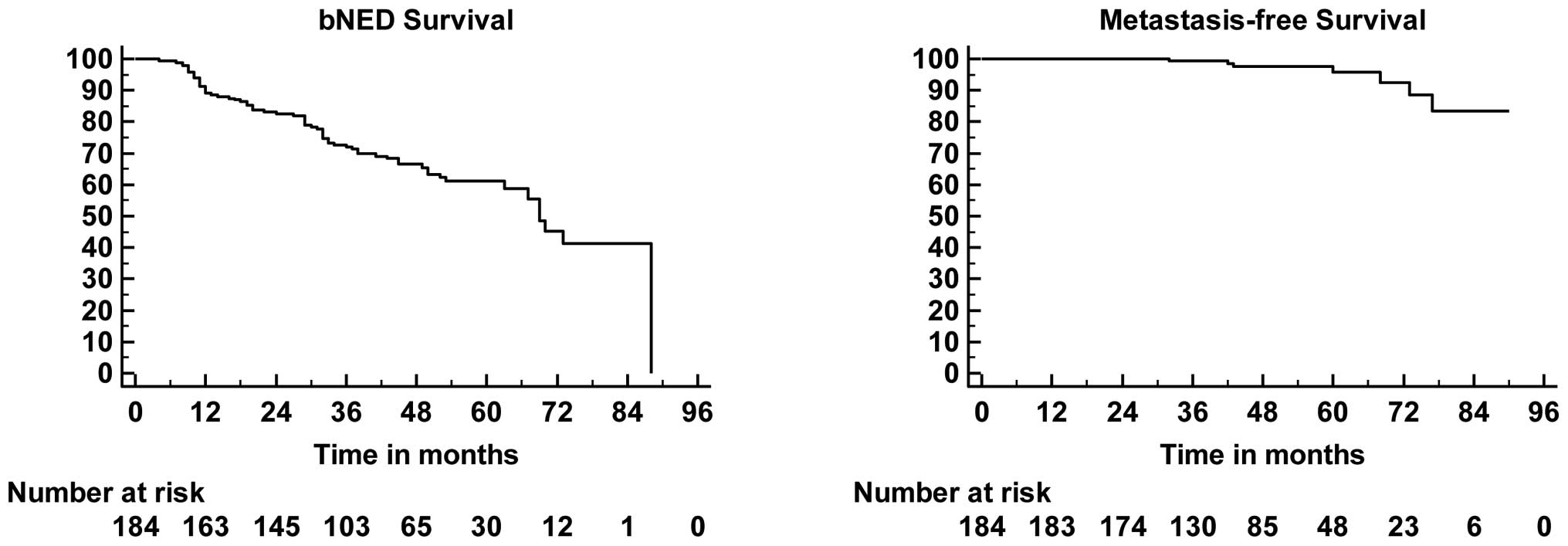
PSA levels after SRT + NHT were undetectable in 171
(93%) patients. BcF developed in 68 (37%). Kaplan-Meier bNED, MFS,
curves are shown in Fig. 1.
In the univariate analyses, post-RP GS, pre-SRT PSA,
pT stage, SVI, and detectable post-SRT PSA were statistically
significant prognostic factors for BcF. In the multivariate
analyses for BcF, statistical significance was found for the same
variables as in the univariate analysis, except for pT stage. In
the multivariate analysis for metastases, only SVI was found to be
significant (Table III). After a
logistic regression, pre-SRT PSA was a positive prognostic factor
for BcF (p<0.001, OR 5.48 95% CI 2.27; 13.23). The area under
the curve was 0.68 (95% CI 0.61; 0.75), and the z-test statistic
4.30 (p<0.001).
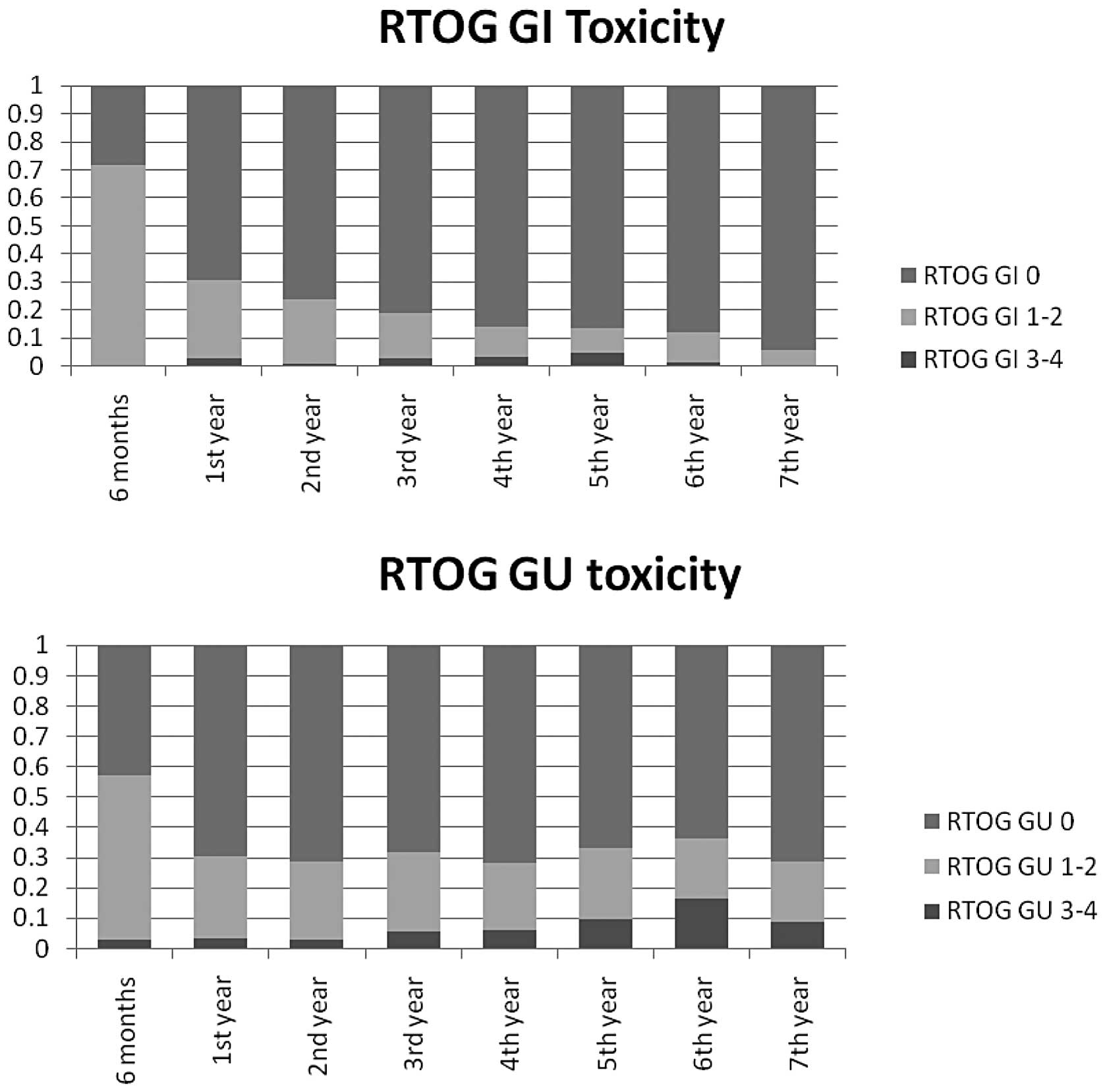
Grade 1–2 acute toxicities were observed in the GU
and the GI region in 100 (54%) and 132 (72%) patients,
respectively, and the former proportion, tended to diminish over
time (Fig. 2). Corresponding
proportions showing RTOG acute toxicity grade 3–4 were observed 5
(3%) and 0 (0%) patients, respectively. An increment in the
frequency of grade over time was observed for both GU and GI
toxicity, although it was more evident for the former. At the 2-
and 5-year follow-ups, respectively, the numbers of patients with
grade 3–4 toxicities were 1 (1%) and 5 (5%) as regards the GI
tract, and 5 (3%) and 11 (9%) as regards the GU region.
The CCIAA score was low/medium for 115 (63%)
patients and high for 69 (38%). No statistically significant
association was found between RTOG toxicity and CCIAA scores
(Table II).
In Table IV, the
results for each domain of the two questionnaires are shown
together with predicted values for the general population in Sweden
and values obtained in a Swedish long-term follow-up of men with
localized prostate cancer ≥5 years after brachytherapy (18,19).
 | Table IV.Total, predicted values (PV), bNED,
and BcF: patients’ mean scores on the various items of the quality
of life and prostate-specific questionnaires vs reference
values. |
Table IV.
Total, predicted values (PV), bNED,
and BcF: patients’ mean scores on the various items of the quality
of life and prostate-specific questionnaires vs reference
values.
| QLQ-C30 | Total n=144
| PV | bNED n=91
| BcF n=53
| p-value | Reference values
|
|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Wahlgren et
al(19) | Michelson et
al(18) |
|---|
| QL2 | 77.9 | (74.7–81.1) | 77.2 | 78.0 | (74.1–81.9) | 77.7 | (71.8–83.6) | 0.71 | 75.9
(72.4–79.3) | 76.6
(76.6–76.7) |
| PF2 | 91.6 | (89.4–93.9) | 87.8 | 92.3 | (90.6–95.2) | 89.4 | (84.7–94.2) | 0.54 | 90.4
(87.8–93.0) | 83.7
(83.2–84.2) |
| RF2 | 91.4 | (88.1–94.8) | 86.1 | 94.0 | (90.8–97.1) | 87.1 | (79.7–94.5) | 0.32 | 89.8
(86.5–93.1) | 83.9
(83.6–84.2) |
| EF | 86.9 | (83.8–90.1) | 86.0 | 89.6 | (86.5–92.7) | 82.3 | (75.7–89.0) | 0.12 | 87.0
(83.9–90.1) | 87.5
(97.3–87.7) |
| SF | 81.8 | (77.8–85.9) | 90.3 | 84.6 | (80.4–88.8) | 77.0 | (68.5–85.6) | 0.44 | 84.3
(80.7–87.9) | 89.5
(89.4–89.6) |
| FA | 16.8 | (13.5–20.1) | 19.3 | 14.8 | (11.1–18.4) | 20.3 | (13.7–27.0) | 0.30 | 19.1
(15.7–22.5) | 20.8
(20.6–21.0) |
| PA | 9.4 | (6.0–12.8) | 18.2 | 8.6 | (4.5–12.7) | 10.7 | (4.4–16.9) | 0.63 | 10.2
(7.1–13.3) | 18.8
(18.7–18.9) |
| SL | 16.2 | (12.3–20.1) | 15.2 | 15.4 | 10.8–19.9) | 17.6 | (10.1–25.2) | 0.98 | 21.6
(17.5–25.7) | 13.1
(12.8–13.5) |
| CO | 7.4 | (4.6–10.2) | 4.1 | 7.0 | (3.2–10.8) | 8.2 | (3.8–12.6) | 0.28 | 7.7 (4.8–10.7) | 5.8 (5.6–6.0) |
| DI | 7.9 | (5.0–10.7) | 4.7 | 5.5 | (2.7–8.3) | 11.9 | (5.7–18.2) | 0.08 | 10.8
(7.5–14.1) | 4.4 (4.3–4.4) |
| PR-25 | Total n | Mean | 95% CI | bNED n | Mean | 95% CI | BcF n | Mean | 95% CI | p-value | Reference values
Van Andel et al(15) |
|---|
| URI | 140 | 24.7 | (21.2–28.8) | 91 | 24.0 | (19.8–28.2) | 49 | 25.9 | (19.3–32.5) | 0.51 | 22.7 |
| AID | 42 | 29.4 | (19.6–39.1) | 22 | 24.2 | (11.2–37.3) | 20 | 35.0 | (18.6–51.4) | 0.31 | 22.6 |
| BOW | 142 | 9.4 | (7.2–11.7) | 89 | 9.1 | (6.8–11.4) | 53 | 10.1 | (5.4–14.8) | 0.55 | 5.4 |
| HTS | 144 | 16.3 | (14.3–18.4) | 91 | 15.2 | (13.0–17.3) | 53 | 18.3 | (13.9–22.8) | 0.65 | 11.9 |
| SAC | 144 | 31.6 | (27.6–35.6) | 91 | 31.1 | (26.0–36.2) | 53 | 32.4 | (25.4–39.3) | 0.72 | 27.8 |
| SFU | 89 | 50.4 | (46.0–54.8) | 56 | 52.1 | (46.3–58.0) | 33 | 47.5 | (40.4–54.5) | 0.42 | 53.6 |
Discussion
One of the aims of the present study was to identify
prognostic variables for patients who may derive long-term benefit
from SRT at a dose of 70 Gy combined with short-term NHT.
Stephenson et al(16)
analyzed retrospectively the outcome of 501 patients who were
treated with SRT at five different academic centres with a median
follow-up of 45 months. These patients had received a heterogeneous
pelvic radiation dose (PRD) with a mean absorbed dose of 64.8 Gy.
In that study, GS 8–10, pre-SRT PSA >2.0 ng/ml, positive
surgical margins, PSADT ≤10 months, and SVI were all significant
predictors of disease progression. In the present study, 184
consecutive patients were included in the study and treated with
SRT at a single institution with a homogeneous PRD and NHT (median
3 months). The median follow-up time was 48 months. None of the
patients were lost to follow-up. Our results show that the
significant factors for treatment failure were post-RP GS, pre-SRT
PSA ≥1.0 ng/ml, SVI, and detectable post-SRT PSA, the latter in
accordance with data presented by Wiegel et al(5). We interpret these findings as
indicating that the importance of some of the poor prognostic
factors identified by Stephenson and colleagues can be diminished
by using higher SRT doses combined with short-term NHT.
Once post-RP BcF has been diagnosed, SRT remains the
only potentially curative therapy (16). For SRT, the American Society for
Therapeutic Radiology and Oncology consensus guidelines recommend
the use of the highest dose of radiation that can be delivered with
acceptable morbidity, and suggest a minimum of 64 Gy with
conventional fractionation (20).
However, doses >66.6 Gy have been described to result in
decreased risk of BcF (21). With
the advent of conformal radiotherapy techniques, the maximally
tolerated and effective PRD for recurrent PCa is still unclear
(22) and late toxicity,
especially rectal, is the dose-limiting factor.
MacDonald et al(23) and Choo et al(24) have reported that PCa patients with
palpable recurrence after RP respond poorly to SRT. In our study,
this was not statistically significant as an independent prognostic
factor and no difference between the patients with positive and
negative pathological disease was detected as regards the risk of
BcF or metastasis. However, this is difficult to compare, since the
PRD used in previous studies have been lower and, in many
instances, heterogeneous in terms of dosing and fractionation.
Also, some other previous studies have indicated that routine
biopsies taken at the anastomotic site before treatment are not
advisable, since a negative biopsy does not exclude local
recurrence, and a positive biopsy does not exclude systemic disease
(21,25). Thus, it remains to be determined
whether a negative or positive biopsy, with no evidence of
metastatic disease, would change the treatment approach used in
patients with BcF after RP. Nevertheless, in the present study, the
PRD may have had an impact on DRE as a prognostic factor. Caution
must be taken when interpreting these results due the following:
the numbers of patients with positive and negative, respectively,
DRE differed and both groups showed statistically significant
differences in clinical characteristics (data not shown).
The current results demonstrate that a substantial
proportion of patients in our study achieved a durable response in
terms of bNED, despite having a high GS, advanced pT stage,
positive DRE, positive surgical margins, rapid PSADT, and SVI, all
of which have been considered to be incurable (Table V). In addition, our observations
indicate 63% bNED (99% of those with still undetectable PSA), 99%
PCa-specific survival, 95% OS, and 96% MFS at 4-year (median)
follow-up. These findings are in contrast to the 4-year
progression-free probability of 45% reported by Stephenson et
al. The data concerning MFS in the present study have to be
interpreted with caution since radiologic follow-up was not
performed systematically and due to the fact that some patients
actively asked for hormone therapy although metastatic disease
could not be verified radiologically.
 | Table V.Proportion of patients with poor
prognostic factors who still had undetectable PSA values after
SRT. |
Table V.
Proportion of patients with poor
prognostic factors who still had undetectable PSA values after
SRT.
| DRE + | Biopsy + | SV + | Gleason 8–10 | pre-SRT PSA
>1 | PSADT <10 |
|---|
| Total n | 46 | 10 | 40 | 29 | 31 | 134 |
| Undetectable PSA, n
(%) | 25 (54.3) | 6 (60) | 17 (42.5) | 13 (44.8) | 11 (35.5) | 80 (59.7) |
| Median follow-up
(months) | | | | | | |
| Total (range) | 50 (12–92) | 41 (30–83) | 54 (28–92) | 42 (13–90) | 46 (24–92) | 50 (1–92) |
| Median follow-up
(months) | | | | | | |
| Patients with
undetectable | 44 (12–79) | 38 (30–42) | 55 (17–83) | 42 (13–83) | 44 (41–64) | 49 (13–84) |
| PSA (range) | | | | | | |
In a study by Tzou et al(26), mean radiation doses of 60–70 and
≥70 Gy were found to be associated with bNED in 25–57% and 58–67%
of cases, respectively. The definition of BcF after RP has been a
matter of debate. The American Urological Association and the
European Association of Urology designate it as a PSA level of 0.2
ng/ml or higher, with a second confirmatory value of >0.2 ng/ml
(27–29). The use of a too low PSA threshold
for SRT involves the risk of over-treating patients. Some
investigators have described that ≤50% of patients with a single
PSA elevation <0.4 ng/ml after RP may be regarded as having
stable non-progressive disease. This is based on the assumption
that residual benign prostatic tissue might be responsible for the
rise in PSA (16,30). However, recent data suggest that
residual benign prostatic elements after RP are an unlikely source
of elevated PSA (31), a finding
which has led to a decision to administer early SRT at our
institute.
The nomogram presented by Stephenson et al
was used in the present study to compare the actual and the
expected outcome of SRT. A substantially higher bNED was found in
the present study, 63% at 4 years, compared with 53% at 3 years
when using the Stephenson nomogram. The reasons for the improved
outcome in our series may be explained by the use of a higher SRT
total dose combined with the use of short-term NHT. However, there
may also exist other plausible explanations for the discrepancies
seen.
Stephenson et al(32) stated that the potential for
morbidity associated with RT argues against indiscriminate use of
this treatment in the salvage setting. The incidence of RTOG GI/GU
grade 3–4 acute toxicity has been reported to be <4% (3,33–37),
which is in agreement with the results obtained in the present
series. GI/GU grade 1–2 and grade 3–4 late toxicities have been
reported to occur in 5–20% and <4% of SRT patients, respectively
(33–36,38).
We observed GU/GI late toxicities as grade 1–2 in 23 and 9%,
respectively, and as grade 3–4 in 9 and 5%, respectively. This
slight increase in GU/GI complications is most probably
dose-related. It may, thus, be possible to reduce the frequency of
these toxicities further by the use of modern techniques such as
intensity-modulated radiation therapy (IMRT) (39). Interestingly, the incidence of
toxicity was not more pronounced in patients with a high, as
compared with low/intermediate, CCIAA score. The occurrence of
co-morbidity often influences the choice of treatment and it is
generally agreed that men with severe comorbidities receive less
aggressive treatment (40). To our
knowledge, the present study is the first to describe the
co-morbidity pattern and the influence of this in patients
undergoing treatment with 70 Gy SRT + NHT.
The data presented in this report support the idea
that patients with a rise in PSA after RP should be offered 70 Gy
SRT with a curative intention. Although there are numerous reports
concerning SRT, ours is one of the largest single-institution
reports describing treatment using a high and homogeneous PRD +
NHT, given to consecutive patients for a delimited period of time
during which no major alterations were done in treatment policies.
Androgen deprivation before radiotherapy has shown improvement in
DSS and OS in patients with locally advanced and high risk tumours
(41). The role for NHT in
combination with SRT still unclear although recent data from other
treatment series have, in addition to ours, shown promising results
in patients undergoing SRT (42,43).
The role for NHT in combination with SRT is currently being
addressed in ongoing randomized studies.
The patients in the current investigation had
slightly better scores on almost all items of the HRQoL (EORTC
QLQ-C30) after 4-year follow-up compared with predicted values from
an age-matched healthy population and the results presented by
Wahlgren et al and Michelson et al(18,19).
Surprisingly, sexual functioning was not poorer in patients
evaluated in the present series. No statistical differences were
found between patients with or without BcF, although some
complications, such as diarrhoea, may be clinically relevant. The
PR-25 results in our study show slightly higher negative impact
than those presented by van Andel et al(15), a finding that was also more or less
anticipated. To our knowledge, the present study is one of the
largest that assesses long-term HRQoL in patients treated with
SRT.
In conclusion, salvage radiotherapy with 70 Gy
delivered in fractions of 2 Gy/day combined with a 3-month NHT
results in long-term undetectable PSA in more than half of patients
presenting with biochemical and/or clinical recurrence after
radical prostatectomy.
The data obtained in the present study show that
non-metastatic patients should not be routinely disqualified from
receiving SRT although per se presenting with poor prognostic
factors at surgery such as SVI and/or high GS or having a pre-SRT
PSA level >1.0 ng/ml and/or a short pre-SRT PSADT. SRT combined
with short-term NHT is associated with acceptable
rectal-genitourinary toxicity. No obvious association was found
between the occurrence of co-morbidity (assessed with CCIAA) and
acute/long-term toxicities. The treatment can be safely delivered
without any major negative effects on long-term QoL.
Acknowledgements
We would like to acknowledge Dr
Hemming Johansson for biostatistical expertise. This study was
supported by the Swedish Cancer Society, the National Council of
Science and Technology (Consejo Nacional de Ciencia y Tecnologia,
CONACyT), UANL, Radiumhemmets Research Funds, and The Af Jochnick
Research Foundation.
References
|
1.
|
Pound CR, Partin AW, Eisenberger MA, Chan
DW, Pearson JD and Walsh PC: Natural history of progression after
PSA elevation following radical prostatectomy. JAMA. 281:1591–1597.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Ward JF, Blute ML, Slezak J, Bergstralh EJ
and Zincke H: The long-term clinical impact of biochemical
recurrence of prostate cancer 5 or more years after radical
prostatectomy. J Urol. 170:1872–1876. 2003.PubMed/NCBI
|
|
3.
|
Bolla M, van Poppel H, Collette L, et al:
Postoperative radiotherapy after radical prostatectomy: a
randomised controlled trial (EORTC trial 22911). Lancet.
366:572–578. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Thompson IM, Tangen CM, Paradelo J, et al:
Adjuvant radiotherapy for pathological T3N0M0 prostate cancer
significantly reduces risk of metastases and improves survival:
long-term follow-up of a randomized clinical trial. J Urol.
181:956–962. 2009. View Article : Google Scholar
|
|
5.
|
Wiegel T, Lohm G, Bottke D, et al:
Achieving an undetectable PSA after radiotherapy for biochemical
progression after radical prostatectomy is an independent predictor
of biochemical outcome - results of a retrospective study. Int J
Radiat Oncol Biol Phys. 73:1009–1016. 2009. View Article : Google Scholar
|
|
6.
|
Buskirk SJ, Pisansky TM, Schild SE, et al:
Salvage radiotherapy for isolated prostate specific antigen
increase after radical prostatectomy: evaluation of prognostic
factors and creation of a prognostic scoring system. J Urol.
176:985–990. 2006. View Article : Google Scholar
|
|
7.
|
Stephenson AJ and Slawin KM: The value of
radiotherapy in treating recurrent prostate cancer after radical
prostatectomy. Nat Clin Pract Urol. 1:90–96. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Moreira DM, Banez LL, Presti JC Jr, et al:
Predictors of secondary treatment following biochemical recurrence
after radical prostatectomy: results from the Shared Equal Access
Regional Cancer Hospital database. BJU Int. 105:28–33. 2010.
View Article : Google Scholar
|
|
9.
|
Ost P, De Troyer B, Fonteyne V,
Oosterlinck W and De Meerleer G: A matched control analysis of
adjuvant and salvage high-dose postoperative intensity-modulated
radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys.
80:1316–1322. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Trabulsi EJ, Valicenti RK, Hanlon AL, et
al: A multi-institutional matched-control analysis of adjuvant and
salvage postoperative radiation therapy for pT3-4N0 prostate
cancer. Urology. 72:1298–1304. 2008. View Article : Google Scholar
|
|
11.
|
Cox JD, Stetz J and Pajak TF: Toxicity
criteria of the Radiation Therapy Oncology Group (RTOG) and the
European Organization for Research and Treatment of Cancer (EORTC).
Int J Radiat Oncol Biol Phys. 31:1341–1346. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Charlson ME, Pompei P, Ales KL and
MacKenzie CR: A new method of classifying prognostic comorbidity in
longitudinal studies: development and validation. J Chronic Dis.
40:373–383. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Extermann M: Measuring comorbidity in
older cancer patients. Eur J Cancer. 36:453–471. 2000. View Article : Google Scholar
|
|
14.
|
Aaronson NK, Ahmedzai S, Bergman B, et al:
The European Organization for Research and Treatment of Cancer
QLQ-C30: a quality-of-life instrument for use in international
clinical trials in oncology. J Natl Cancer Inst. 85:365–376. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
van Andel G, Bottomley A, Fossa SD, et al:
An international field study of the EORTC QLQ-PR25: a questionnaire
for assessing the health-related quality of life of patients with
prostate cancer. Eur J Cancer. 44:2418–2424. 2008.
|
|
16.
|
Stephenson AJ, Shariat SF, Zelefsky MJ, et
al: Salvage radiotherapy for recurrent prostate cancer after
radical prostatectomy. JAMA. 291:1325–1332. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Fayers P, Aaronson N, Bjordal K, Groenvold
M, Curran D and Bottomley A: EORTC QLQ-C30 scoring manual. European
Organization for Research and Treatment of Cancer; 3rd edition.
2001
|
|
18.
|
Michelson H, Bolund C, Nilsson B and
Brandberg Y: Health-related quality of life measured by the EORTC
QLQ-C30-reference values from a large sample of Swedish population.
Acta Oncol. 39:477–484. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Wahlgren T, Nilsson S, Lennernas B and
Brandberg Y: Promising long-term health-related quality of life
after high-dose-rate brachytherapy boost for localized prostate
cancer. Int J Radiat Oncol Biol Phys. 69:662–670. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Cox JD, Gallagher MJ, Hammond EH, Kaplan
RS and Schellhammer PF: Consensus statements on radiation therapy
of prostate cancer: guidelines for prostate re-biopsy after
radiation and for radiation therapy with rising prostate-specific
antigen levels after radical prostatectomy. American Society for
Therapeutic Radiology and Oncology Consensus. Panel J Clin Oncol.
17:11551999.
|
|
21.
|
Bernard JR Jr, Buskirk SJ, Heckman MG, et
al: Salvage radiotherapy for rising prostate-specific antigen
levels after radical prostatectomy for prostate cancer:
dose-response analysis. Int J Radiat Oncol Biol Phys. 76:735–740.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Kupelian PA, Willoughby TR, Reddy CA,
Klein EA and Mahadevan A: Hypofractionated intensity-modulated
radiotherapy (70 Gy at 2.5 Gy per fraction) for localized prostate
cancer: Cleveland Clinic experience. Int J Radiat Oncol Biol Phys.
68:1424–1430. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
MacDonald OK, Schild SE, Vora S, et al:
Salvage radiotherapy for men with isolated rising PSA or locally
palpable recurrence after radical prostatectomy: do outcomes
differ? Urology. 64:760–764. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Choo R, Morton G, Danjoux C, Hong E,
Szumacher E and DeBoer G: Limited efficacy of salvage radiotherapy
for biopsy confirmed or clinically palpable local recurrence of
prostate carcinoma after surgery. Radiother Oncol. 74:163–167.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Neulander EZ and Soloway MS: Failure after
radical prostatectomy. Urology. 61:30–36. 2003. View Article : Google Scholar
|
|
26.
|
Tzou K, Tan WW and Buskirk S: Treatment of
men with rising prostate-specific antigen levels following radical
prostatectomy. Expert Rev Anticancer Ther. 11:125–136. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Boccon-Gibod L, Djavan WB, Hammerer P, et
al: Management of prostate-specific antigen relapse in prostate
cancer: a European Consensus. Int J Clin Pract. 58:382–390. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Cookson MS, Aus G, Burnett AL, et al:
Variation in the definition of biochemical recurrence in patients
treated for localized prostate cancer: the American Urological
Association Prostate Guidelines for Localized Prostate Cancer
Update Panel report and recommendations for a standard in the
reporting of surgical outcomes. J Urol. 177:540–545. 2007.
|
|
29.
|
Heidenreich A, Aus G, Bolla M, et al: EAU
guidelines on prostate cancer. Eur Urol. 53:68–80. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Amling CL, Bergstralh EJ, Blute ML, Slezak
JM and Zincke H: Defining prostate specific antigen progression
after radical prostatectomy: what is the most appropriate cut
point? J Urol. 165:1146–1151. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Godoy G, Tareen BU and Lepor H: Does
benign prostatic tissue contribute to measurable PSA levels after
radical prostatectomy? Urology. 74:167–170. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Stephenson AJ, Scardino PT, Kattan MW, et
al: Predicting the outcome of salvage radiation therapy for
recurrent prostate cancer after radical prostatectomy. J Clin
Oncol. 25:2035–2041. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Catton C, Gospodarowicz M, Warde P, et al:
Adjuvant and salvage radiation therapy after radical prostatectomy
for adenocarcinoma of the prostate. Radiother Oncol. 59:51–60.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Katz MS, Zelefsky MJ, Venkatraman ES, Fuks
Z, Hummer A and Leibel SA: Predictors of biochemical outcome with
salvage conformal radiotherapy after radical prostatectomy for
prostate cancer. J Clin Oncol. 21:483–489. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Maier J, Forman J, Tekyi-Mensah S, Bolton
S, Patel R and Pontes JE: Salvage radiation for a rising PSA
following radical prostatectomy. Urol Oncol. 22:50–56. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Pacholke HD, Wajsman Z, Algood CB, Morris
CG and Zlotecki RA: Postoperative adjuvant and salvage radiotherapy
for prostate cancer: impact on freedom from biochemical relapse and
survival. Urology. 64:982–986. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Valicenti RK, Gomella LG, Ismail M, et al:
Durable efficacy of early postoperative radiation therapy for
high-risk pT3N0 prostate cancer: the importance of radiation dose.
Urology. 52:1034–1040. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Anscher MS, Clough R and Dodge R:
Radiotherapy for a rising prostate-specific antigen after radical
prostatectomy: the first 10 years. Int J Radiat Oncol Biol Phys.
48:369–375. 2000.PubMed/NCBI
|
|
39.
|
Alongi F, Fiorino C, Cozzarini C, et al:
IMRT significantly reduces acute toxicity of whole-pelvis
irradiation in patients treated with post-operative adjuvant or
salvage radiotherapy after radical prostatectomy. Radiother Oncol.
93:207–212. 2009. View Article : Google Scholar
|
|
40.
|
Berglund A, Garmo H, Tishelman C, Holmberg
L, Stattin P and Lambe M: Comorbidity, treatment and mortality: a
population based cohort study of prostate cancer in PCBaSe Sweden.
J Urol. 185:833–840. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Widmark A, Klepp O, Solberg A, et al:
Endocrine treatment, with or without radiotherapy, in locally
advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase
III trial. Lancet. 373:301–308. 2009. View Article : Google Scholar
|
|
42.
|
Choo R, Danjoux C, Gardner S, et al:
Efficacy of salvage radiotherapy plus 2-year androgen suppression
for postradical prostatectomy patients with PSA relapse. Int J
Radiat Oncol Biol Phys. 75:983–989. 2009.
|
|
43.
|
Pai HH, Eldridge B, Bishop D, et al: Does
neoadjuvanthormone therapy improve outcome in prostate cancer
patients receiving radiotherapy after radical prostatectomy? Can J
Urol. 16:4541–4552. 2009.PubMed/NCBI
|