Introduction
Cervical cancer is the second most common cancer in
women worldwide and approximately 529,000 cases are diagnosed each
year (1). In Sweden, about 450
women are diagnosed with invasive cervical cancer annually and 150
die from the disease each year (2). In the last decade, an increase in
incidence of invasive adenocarcinoma, which currently comprises
10–20% of all cervical cancers, has been observed in several
countries where cervical screening programs exist (3–5). In
Stockholm county, Sweden, before screening was introduced the
percentage of adenocarcinomas of all uterine cervical cancers
varied between 2 and 7% (6).
During the screening period, the incidence of adenocarcinoma was
higher and accounted for 25.7% of cases. In the screening cohort, a
marked difference was observed between younger and older women; the
percentage of adenocarcinomas among younger women was about 6 times
higher and in women ≥70 years about 3 times higher than in the
prescreening cohort (6). In
general, clinical screening programs that have led to a substantial
decrease in the incidence of squamous cell carcinoma (SCC) have
only had a limited protective effect on the occurrence of
adenocarcinoma and its precursor lesion, adenocarcinoma in
situ (7). The prognosis for
cervical adenocarcinoma has also been reported to be slightly worse
than for cervical SCC (8). Known
prognostic factors include FIGO stage, tumor lesion size, and lymph
node metastasis status (8).
High-risk human papillomavirus (HPV) is an important
etiological agent in the pathogenesis of cervical adenocarcinoma
(9). HPV DNA is usually detected
in 30–90% of cervical adenocarcinomas, with HPV 18 as the
predominant type (10,11). Although closely linked to cervical
cancer development, HPV infection alone is not sufficient for
oncogenic transformation; additional factors are required for
malignant transformation of normal cervical epithelium into
adenocarcinoma (12).
The human leucine-rich repeats and
immunoglobulin-like domains (LRIG) gene family includes:
LRIG1, LRIG2 and LRIG3. LRIG1 has been shown
to negatively regulate receptor tyrosine kinases, including members
of the epidermal growth factor receptor family (13–15),
MET (16) and RET (17). LRIG1 has been suggested to function
as a tumor suppressor in various tissues (reviewed in ref. 18), and was recently shown to be a bona
fide tumor suppressor in mouse intestine (19). Less is known about the functions of
LRIG2 and LRIG3. High expression of LRIG1 correlates with better
prognosis in breast cancer (20),
early stage invasive squamous cervical cancer (21) and in cutaneous squamous cell
carcinoma (22). In contrast,
LRIG2 expression correlates with poor survival in oligodendroglioma
(23) and in early stage squamous
cervical cancer (24). Both LRIG1
and LRIG2 have been found to correlate with increasing grade of
cervical intraepithelial neoplasia (25). Thus, LRIG proteins seem to play an
important role in both precancerous lesions and invasive squamous
cervical cancer, but the possible prognostic value of the LRIG
proteins in cervical adenocarcinoma is largely unknown since it has
only been studied in a small series of cases (21).
The aim of this study was to evaluate the
immunohistochemical expression of the LRIG proteins in 86 cervical
adenocarcinomas, and to investigate possible correlations between
LRIG expression, patient survival and HPV status.
Materials and methods
Patients and tumor characteristics
This study looked at a cohort of 86 patients with
primary cervical adenocarcinoma treated at Karolinska University
Hospital, Huddinge, between 1992 and 2000. The study was approved
by The Regional Ethics Review Board in Stockholm and all women
provided written informed consent to participate in the study. All
tumor cases were identified from the Swedish National Cancer
Registry and were presented in a previous study (26). The previous study included 101
patients, while the current study conducted immunohistochemical
analyses for the expression of LRIG proteins in 86 patients, after
exclusion of 15 patients due to an insufficient amount of
material.
The histopathological diagnoses were based on WHO
criteria and the FIGO system was used for clinical classification.
All tumors were cervical adenocarcinomas and lacked any squamous
cell component; 79 were invasive, 4 were in situ and
histopathological diagnosis could not be ascertained for the
remaining 3 patients. Table I
presents grade of differentiation. FIGO classification was obtained
for 72 patients and Table I
presents the distribution between stages. All patients were
retrospectively followed up from time of diagnosis until January
2011 and survival data were recorded.
 | Table I.Patient and tumor characteristics. |
Table I.
Patient and tumor characteristics.
| Patient and tumor
characteristics |
|---|
| Patients included in
the study | 86 |
| Age at diagnosis
(years) | |
| Median | 48 |
| Mean | 51 |
| FIGO stage
(n=72) | |
| 0 | 4 |
| I | 52 |
| II | 10 |
| III | 6 |
| Histopathological
grade (n=79) | |
| Well
differentiated | 35 |
| Moderately
differentiated | 34 |
| Poorly
differentiated | 10 |
| HPV status
(n=86) | |
| Positive | 57 |
| Negative | 29 |
| Outcome (n=83) | |
| Dead | 27 |
| Alive | 56 |
HPV status
Detailed descriptions of detection of HPV status and
HPV screening analysis results were previously published (26). Briefly, DNA was extracted from a
10-μm thick section of paraffin block and polymerase chain
reaction (PCR) was used to amplify the L1 region of the HPV genome.
Samples that yielded a PCR product of 130–150 base pairs that were
visible after agarose gel electrophoresis and ethidium bromide
staining were considered positive. Among the 86 cases included in
this study, 57 (66%) were HPV-positive and 29 (34%) were
HPV-negative. Of the HPV-positive tumors, 27 were infected with HPV
16, 25 with HPV 18 and 5 with HPV 45.
Immunohistochemistry for the LRIG
proteins
Immunohistochemical staining of LRIG1, LRIG2 and
LRIG3 was carried out using polyclonal rabbit antibodies against
the cytosolic tails of the proteins, as previously described
(27). Tissue sections containing
squamous cervical cancer were used as positive controls, and
appropriate negative controls were always included.
A senior pathologist (L.K.), blinded to all clinical
data, evaluated the immune staining. Immunohistochemistry results
were scored based on both staining intensity and percentage of
immunoreactive epithelial cells. The percentage of positive cells
was based on a 5-grade semi-quantitative scale: 0, no expression;
1, >0 and <24%; 2, 25–49% positive cells; 3, 50–74% positive
cells; 4, >75% positive cells. Intensity of staining was
evaluated on a four-grade semi-quantitative scale: 0, no staining;
1, weak; 2, moderate; and 3, intense (28,29).
Statistical analysis
Associations between ordinal variables were tested
using a χ2 test or Fisher’s exact test. Clinical
parameters, HPV status and expression of LRIG proteins were
illustrated in a Kaplan-Meier graph to reflect possible prognostic
factors for overall survival, and a log-rank test was used to
compare different groups. The molecular markers found to be
significant in the univariate analyses were considered in a Cox
regression multivariate analysis, including age (young vs. old,
dichotomized based on median age), histology and FIGO stage. All
significance testing was carried out at the 0.05 level, using SPSS
19 software.
Results
Immunohistochemical expression of LRIG
proteins and their correlation to HPV and clinical parameters
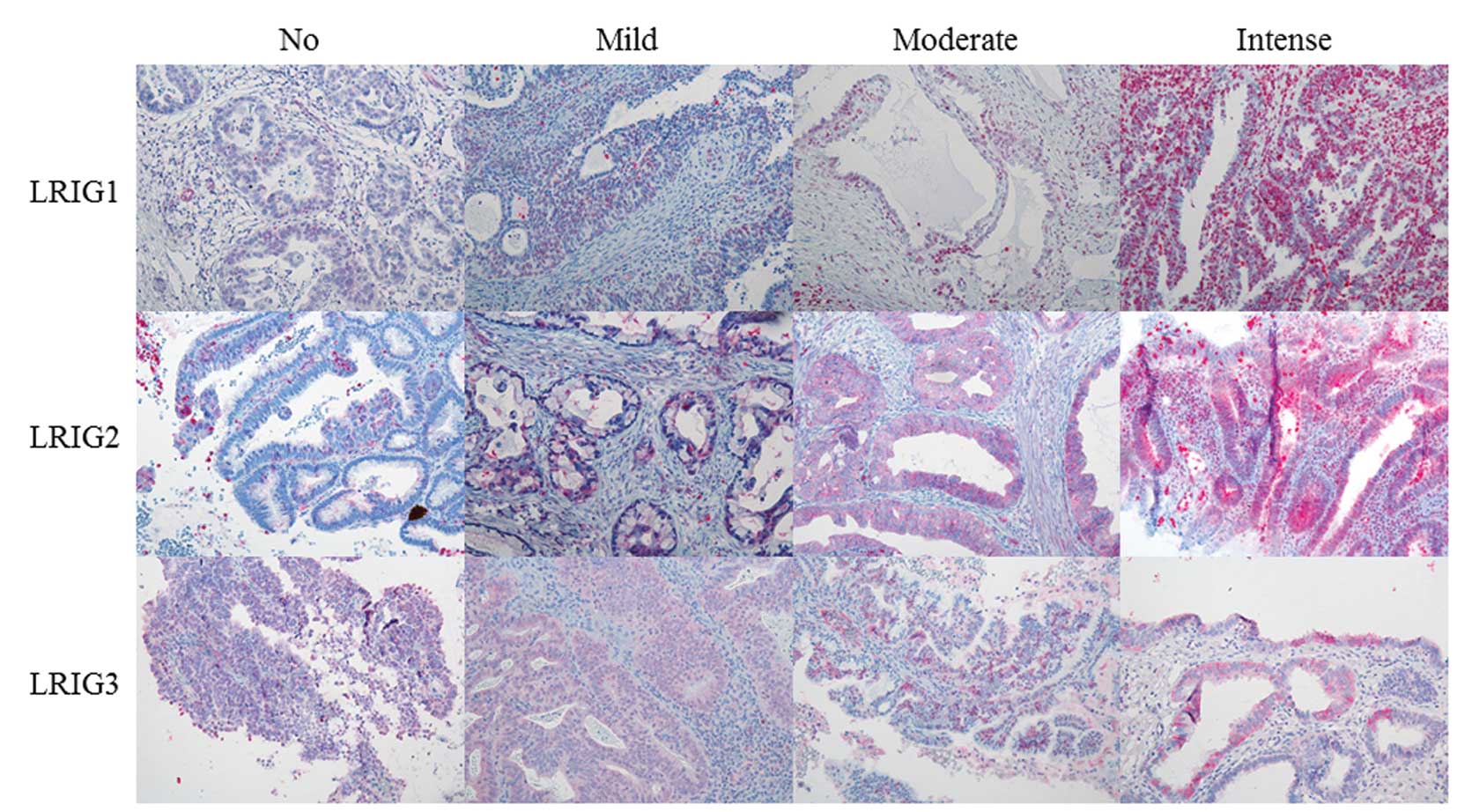
Evaluation of LRIG staining intensity and fraction
of LRIG-positive cells was carried out for all 86 patients, except
in one case each for LRIG1 and LRIG3 staining intensity, and
fraction of LRIG3-positive cells, where technical reasons precluded
evaluation. Table II presents
evaluation of immunohistochemical staining, while Fig. 1 shows examples of the evaluation of
staining intensity. Regarding staining intensity, LRIG1 and LRIG2
were distributed almost equally, in contrast to LRIG3, where most
tumors showed either no staining or weak staining (n=76). When
considering the fraction of positive cells, the majority of cases
demonstrated >50% expressing cells for all three LRIG proteins.
In the majority of cases, immunoreactivity of LRIG1 protein was
seen only in the cytoplasm, LRIG2 immunoreactivity was expressed in
both cytoplasm and the nucleus, while in LRIG3 protein,
immunoreactivity was seen mostly in the nucleus.
 | Table II.Staining intensity (A) and fraction
of positive cells (B) of the LRIG immunohistochemical analysis. |
Table II.
Staining intensity (A) and fraction
of positive cells (B) of the LRIG immunohistochemical analysis.
| A | LRIG staining
intensity, n (%)
|
|---|
| No staining | Weak | Moderate | Intense |
|---|
| LRIG1 | 11 (12.9) | 18 (21.2) | 26 (30.6) | 30 (35.3) |
| LRIG2 | 5 (5.9) | 19 (22.4) | 34 (40.0) | 28 (31.8) |
| LRIG3 | 17 (20.0) | 59 (69.4) | 8 (9.4) | 1 (1.2) |
| B | Fraction of
LRIG-positive cells, n (%)
|
|---|
| 0 | >0 and
<24% | 25–49% | 50–75% | >75% |
|---|
| LRIG1 | 11 (12.8) | 4 (4.7) | 16 (18.6) | 25 (29.1) | 30 (34.9) |
| LRIG2 | 5 (5.8) | 3 (3.5) | 14 (16.3) | 8 (9.3) | 56 (65.1) |
| LRIG3 | 17 (20.0) | 11 (12.9) | 11 (12.9) | 11 (12.9) | 35 (41.1) |
HPV status showed significant correlation with LRIG1
and LRIG3 staining intensity (P=0.001 and 0.004, respectively;
Table III), where high LRIG
staining intensity correlated with HPV positivity. No other
statistically significant correlations were found between LRIG
staining intensity or fraction of positive cells, and HPV status,
FIGO stage or histology, respectively.
 | Table III.Correlation between HPV status and
intensity of staining for LRIG1 (A) and LRIG3 (B), respectively
(P=0.03 and 0.04, respectively). |
Table III.
Correlation between HPV status and
intensity of staining for LRIG1 (A) and LRIG3 (B), respectively
(P=0.03 and 0.04, respectively).
| A | LRIG1 staining
intensity, n (%)
|
|---|
| 0 | 1 | 2 | 3 |
|---|
|
HPV+ | 5 (8.9) | 6 (10.7) | 19 (33.9) | 26 (46.4) |
|
HPV− | 6 (20.7) | 12 (41.4) | 7 (24.1) | 4 (13.8) |
| B | LRIG3 staining
intensity, n (%)
|
|---|
| 0 | 1 | 2 | 3 |
|---|
|
HPV+ | 5 (8.9) | 45 (80.4) | 5 (8.9) | 1 (1.8) |
|
HPV− | 12 (41.4) | 14 (48.3) | 3 (10.3) | 0 (0.0) |
LRIG expression, HPV status, tumor stage
and histology: correlation with patient survival
Reliable follow-up data were available for 83
patients. Median follow-up time was 12 years (range, 0–22 years)
for all women, and 14 years (range, 10–22 years) for those women
who did not die of cervical cancer. The association between
staining intensity and fraction of cells positive for LRIG
proteins, and survival, was estimated using a Kaplan-Meier plot and
the groups were dichotomized using the median as a cut-off to
generate two groups of equal size. Correlations between survival
and HPV status, stage and histology, respectively, were also
evaluated using the Kaplan-Meier method.
Both high staining intensity of LRIG1-positive cells
and high fraction of LRIG3-positive cells correlated with survival
(P=0.03 and 0.04, respectively; Fig.
2). A significant correlation was also found between HPV status
and survival (P<0.001, graph not shown). Survival data from
these patients were previously published (30), but the patient group currently
under study is slightly different, excluding 15 patients as
described above. Moreover, the patient group in the present study
was followed until January 2011, compared with January 2007 in the
previous study (30). As expected,
both stage and histology also correlated with survival (P=0.02 and
<0.001, respectively, graph not shown). All significant
parameters were then included in a Cox regression multivariate
analysis (details presented in Table
IV). The only factors that showed independent statistical
significance in this analysis were age (P=0.002) and fraction of
LRIG3-positive cells (P=0.031).
 | Table IV.Multivariate analysis including LRIG1
staining intensity, fraction of LRIG3-positive cells, HPV status,
age, histology and FIGO stage. |
Table IV.
Multivariate analysis including LRIG1
staining intensity, fraction of LRIG3-positive cells, HPV status,
age, histology and FIGO stage.
| HR | (95% CI) | P-value |
|---|
| LRIG1
intensity | 0.964 | (0.407–2.285) | 0.934 |
| Fraction of LRIG3
positive cells | 0.401 | (0.175–0.919) | 0.031 |
| HPV status | 0.447 | (0.181–1.104) | 0.081 |
| Age | 5.606 | (1.847–17.015) | 0.002 |
| Histology | 1.804 | (0.994–3.274) | 0.052 |
| FIGO stage | 1.448 | (0.902–2.322) | 0.125 |
Discussion
In this study, LRIG protein immunoreactivity was
evaluated in 86 cervical adenocarcinomas. Both high staining
intensity of LRIG1-positive cells and a high fraction of
LRIG3-positive cells were significantly associated with patient
survival, and positive correlations were found between staining
intensity of both LRIG1 and LRIG3 and HPV status. High expression
of LRIG1 has previously been reported to correlate with better
prognosis in breast cancer (20),
early stage invasive squamous cervical cancer (21) and cutaneous squamous cell carcinoma
(22). LRIG1 expression also
correlates with increasing grade of cervical intraepithelial
neoplasia. Thus, expression of LRIG1 seems to play an important
role in both precancerous lesions and invasive squamous cervical
cancer, and here we showed that LRIG1 also functions as a
prognostic marker in cervical adenocarcinoma. One previous study
investigated the immunoreactivity of LRIG1 in cervical
adenocarcinoma. In this study, no correlation with survival was
found, possibly due to the limited number of cases analyzed (n=36)
(21). The prognostic value of
LRIG1 expression reported for cervical adenocarcinoma suggests its
importance in this disease, as well as in other types of cancer. By
negatively regulating receptor tyrosine kinases, it is possible
that LRIG1 functions as a tumor suppressor in the uterine
cervix.
Less is known about the functions of LRIG2 and
LRIG3. LRIG2 expression has been reported to correlate with worse
survival in cervical SCC (24);
however, in the present study no correlation between LRIG2
expression and clinical parameters in cervical adenocarcinoma was
found. Thus, LRIG2 might be more important as a possible marker of
prognosis in cervical SCC and other cancer types.
Immunohistochemical expression of LRIG3 has been evaluated in
oligodendroglioma and in astrocytic tumors. Perinuclear staining of
LRIG3 in astrocytic tumors correlated with better survival, which
is consistent with the findings of the present study (27), suggesting that LRIG3 might have a
tumor suppressive function similar to that of LRIG1.
The positive correlation between HPV status and
LRIG1 and LRIG3 staining intensity is intriguing. The number of HPV
positive cases (66%) and distribution of HPV types are in line with
that previously reported (31–33).
We previously showed that HPV infection correlated with better
outcome in the present study cohort (30), and this finding still holds after
an additional four years of follow-up. The presence of two groups
of cervical adenocarcinomas, one with positive and one with
negative HPV status, is similar to the situation in tonsillar and
base of tongue cancer, where there is increasing evidence for two
different tumor types, depending on HPV status (34,35).
Whether this also holds true for cervical adenocarcinoma remains to
be investigated, but our results suggest differences between
HPV-positive and -negative cervical adenocarcinomas, at least in
regard to LRIG expression and patient survival.
Since low expression of LRIG1 correlates with worse
survival in cervical adenocarcinoma (Fig. 2A) and other human cancers (20–22),
LRIG1 might be of interest as a potential target for treatment. It
has been reported that LRIG1 ectodomains can be shed and suppress
growth factor signaling in neighboring cells, suggesting that LRIG1
ectodomains can suppress cell proliferation in a paracrine manner.
This non-cell autonomous inhibition of growth factor receptor
signaling could be an interesting strategy for treatment of
cervical adenocarcinoma and other cancers. However, whether
cervical cancers depend on growth factor signaling for their growth
and whether LRIG1 can inhibit this growth remain to be
determined.
Cervical cancer screening programs have decreased
mortality from cervical SCC in many developed countries. However,
due to significant subjectivity and low sensitivity when
interpreting Pap smears and cervical biopsy specimens, an active
search for better biomarkers that may prove useful in clinical
practice for early diagnosis of cervical adenocarcinomas and
neoplastic glandular lesions is underway. Staging of adenocarcinoma
based on morphology and histology is also difficult due to the lack
of a clearly defined preinvasive phase. Thus, sensitivity for
detecting precursor lesions of adenocarcinoma is much lower than
that for SCC (36). A better
understanding of the role of different molecular markers in the
genesis of cervical adenocarcinoma is therefore important to
optimize screening intervention. Molecular markers, such as the
LRIG proteins studied here, may help us to develop more efficient
screening strategies for adenocarcinoma of the cervix. Thus,
analysis of LRIG expression in precursor lesions (i.e.,
adenocarcinoma in situ and glandular dysplasia) will be
important to determine the potential of LRIG proteins as molecular
markers for early detection and progression to invasive
disease.
In summary, LRIG immunoreactivity is of prognostic
importance in cervical adenocarcinoma and correlate with HPV
status. Therefore, LRIG proteins may be important determinants of
cervical adenocarcinoma progression, which justifies further study
of their diagnostic and prognostic potential in this disease.
Acknowledgements
This study was supported by the
Swedish Cancer Foundation (070623, CAN 2007/1044, 11 0544 CAN
2011/471), KI Cancer Strategic Grants (5888/05-722), Lion’s Cancer
Research Foundation, University of Umeå, Swedish Research Council
(521-2008-2899), Medical Research Council and Cancer Society in
Stockholm, Stockholm County Council.
References
|
1.
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Socialstyrelsen Ec: Cancer incidence in
Sweden 2008. 2008
|
|
3.
|
Vizcaino AP, Moreno V, Bosch FX, Munoz N,
Barros-Dios XM and Parkin DM: International trends in the incidence
of cervical cancer: I. Adenocarcinoma and adenosquamous cell
carcinomas. Int J Cancer. 75:536–545. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Smith HO, Tiffany MF, Qualls CR and Key
CR: The rising incidence of adenocarcinoma relative to squamous
cell carcinoma of the uterine cervix in the United States - a
24-year population-based study. Gynecol Oncol. 78:97–105. 2000.
|
|
5.
|
Bray F, Carstensen B, Moller H, et al:
Incidence trends of adenocarcinoma of the cervix in 13 European
countries. Cancer Epidemiol Biomarkers Prev. 14:2191–2199. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Pettersson BF, Andersson S, Hellman K and
Hellstrom AC: Invasive carcinoma of the uterine cervix associated
with pregnancy: 90 years of experience. Cancer. 116:2343–2349.
2010.PubMed/NCBI
|
|
7.
|
Etherington IJ and Luesley DM:
Adenocarcinoma in situ of the cervix-controversies in diagnosis and
treatment. J Low Genit Tract Dis. 5:94–98. 2001.PubMed/NCBI
|
|
8.
|
Gien LT, Beauchemin MC and Thomas G:
Adenocarcinoma: a unique cervical cancer. Gynecol Oncol.
116:140–146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Bosch FX and Munoz N: The viral etiology
of cervical cancer. Virus Res. 89:183–190. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Andersson S, Larson B, Hjerpe A, et al:
Adenocarcinoma of the uterine cervix: the presence of human
papillomavirus and the method of detection. Acta Obstet Gynecol
Scand. 82:960–965. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Castellsague X, Diaz M, de Sanjose S, et
al: Worldwide human papillomavirus etiology of cervical
adenocarcinoma and its cofactors: implications for screening and
prevention. J Natl Cancer Inst. 98:303–315. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Andersson S, Wallin KL, Hellstrom AC, et
al: Frequent gain of the human telomerase gene TERC at 3q26 in
cervical adenocarcinomas. Br J Cancer. 95:331–338. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Gur G, Rubin C, Katz M, et al: LRIG1
restricts growth factor signaling by enhancing receptor
ubiquitylation and degradation. EMBO J. 23:3270–3281. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Laederich MB, Funes-Duran M, Yen L, et al:
The leucine-rich repeat protein LRIG1 is a negative regulator of
ErbB family receptor tyrosine kinases. J Biol Chem.
279:47050–47056. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Miller JK, Shattuck DL, Ingalla EQ, et al:
Suppression of the negative regulator LRIG1 contributes to ErbB2
overexpression in breast cancer. Cancer Res. 68:8286–8294. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Shattuck DL, Miller JK, Laederich M, et
al: LRIG1 is a novel negative regulator of the Met receptor and
opposes Met and Her2 synergy. Mol Cell Biol. 27:1934–1946. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Ledda F, Bieraugel O, Fard SS, Vilar M and
Paratcha G: Lrig1 is an endogenous inhibitor of Ret receptor
tyrosine kinase activation, downstream signaling, and biological
responses to GDNF. J Neurosci. 28:39–49. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Hedman H and Henriksson R: LRIG inhibitors
of growth factor signalling - double-edged swords in human cancer?
Eur J Cancer. 43:676–682. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Powell AE, Wang Y, Li Y, et al: The
pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker
that functions as a tumor suppressor. Cell. 149:146–158. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Krig SR, Frietze S, Simion C, et al: Lrig1
is an estrogen-regulated growth suppressor and correlates with
longer relapse-free survival in ERalpha-positive breast cancer. Mol
Cancer Res. 9:1406–1417. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Lindstrom AK, Ekman K, Stendahl U, et al:
LRIG1 and squamous epithelial uterine cervical cancer: correlation
to prognosis, other tumor markers, sex steroid hormones, and
smoking. Int J Gynecol Cancer. 18:312–317. 2008. View Article : Google Scholar
|
|
22.
|
Tanemura A, Nagasawa T, Inui S and Itami
S: LRIG-1 provides a novel prognostic predictor in squamous cell
carcinoma of the skin: immunohistochemical analysis for 38 cases.
Dermatol Surg. 31:423–430. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Holmlund C, Haapasalo H, Yi W, et al:
Cytoplasmic LRIG2 expression is associated with poor
oligodendroglioma patient survival. Neuropathology. 29:242–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Hedman H, Lindstrom AK, Tot T, Stendahl U,
Henriksson R and Hellberg D: LRIG2 in contrast to LRIG1 predicts
poor survival in early-stage squamous cell carcinoma of the uterine
cervix. Acta Oncol. 49:812–815. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Lindstrom AK, Asplund A and Hellberg D:
Correlation between LRIG1 and LRIG2 expressions and expression of
11 tumor markers, with special reference to tumor suppressors, in
CIN and normal cervical epithelium. Gynecol Oncol. 122:372–376.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Andersson S, Rylander E, Larson B, et al:
Types of human papillomavirus revealed in cervical adenocarcinomas
after DNA sequencing. Oncol Rep. 10:175–179. 2003.PubMed/NCBI
|
|
27.
|
Guo D, Nilsson J, Haapasalo H, et al:
Perinuclear leucine-rich repeats and immunoglobulin-like domain
proteins (LRIG1-3) as prognostic indicators in astrocytic tumors.
Acta Neuropathol. 111:238–246. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Klussmann JP, Gultekin E, Weissenborn SJ,
et al: Expression of p16 protein identifies a distinct entity of
tonsillar carcinomas associated with human papillomavirus. Am J
Pathol. 162:747–753. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Sano T, Oyama T, Kashiwabara K, Fukuda T
and Nakajima T: Expression status of p16 protein is associated with
human papillomavirus oncogenic potential in cervical and genital
lesions. Am J Pathol. 153:1741–1748. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Muller S, Flores-Staino C, Skyldberg B, et
al: Expression of p16INK4a and MIB-1 in relation to histopathology
and HPV types in cervical adenocarcinoma. Int J Oncol. 32:333–340.
2008.PubMed/NCBI
|
|
31.
|
Seoud M, Tjalma WA and Ronsse V: Cervical
adenocarcinoma: moving towards better prevention. Vaccine.
29:9148–9158. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Tenti P, Romagnoli S, Silini E, et al:
Human papillomavirus types 16 and 18 infection in infiltrating
adenocarcinoma of the cervix: PCR analysis of 138 cases and
correlation with histologic type and grade. Am J Clin Pathol.
106:52–56. 1996.
|
|
33.
|
Andersson S, Rylander E, Larsson B, Strand
A, Silfversvard C and Wilander E: The role of human papillomavirus
in cervical adenocarcinoma carcinogenesis. Eur J Cancer.
37:246–250. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Attner P, Du J, Nasman A, et al: Human
papillomavirus and survival in patients with base of tongue cancer.
Int J Cancer. 128:2892–2897. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Hammarstedt L, Lindquist D, Dahlstrand H,
et al: Human papillomavirus as a risk factor for the increase in
incidence of tonsillar cancer. Int J Cancer. 119:2620–2623. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Krane JF, Granter SR, Trask CE, Hogan CL
and Lee KR: Papanicolaou smear sensitivity for the detection of
adenocarcinoma of the cervix: a study of 49 cases. Cancer. 93:8–15.
2001. View Article : Google Scholar : PubMed/NCBI
|