Introduction
Hepatocellular carcinoma (HCC) is a major health
problem worldwide, with an estimated incidence ranging between
500,000 and 1,000,000 new cases annually. It is the fifth most
common cancer in the world and the third most common cause of
cancer-related death (1,2). The prognosis of HCC is generally poor
because of the high recurrence rate (1,3,4).
Surgical resection (SR) remains the best curative treatment, but is
only suitable in 9–27% of patients. The presence of significant
background liver cirrhosis often precludes hepatic resection in
patients with HCC. Recurrence in the liver remnant is also common
in patients who have undergone radical hepatic resection (5).
Transcatheter arterial chemoembolization (TACE) is
one of the available locoregional therapies for HCC. It involves
injection of an embolizing agent into the hepatic artery to deprive
the tumor of its major nutrient source via embolization of the
nutrient artery, resulting in ischemic necrosis of the tumor
(6–8). According to the current guidelines,
TACE is generally performed in intermediate-stage HCC patients
(9). However, TACE has also been
performed as preoperative adjuvant chemotherapy in resectable HCC
patients with the aim of improving survival (3,10,11).
The purpose of preoperative TACE is to reduce tumor
volume, induce tumor necrosis and prevent cancer cell dissemination
during the surgical procedure (3,10,11).
To the best of our knowledge, four randomized controlled trials
have assessed the efficacy of preoperative TACE in terms of
survival (3,10,12,13).
However, the results of these trials are difficult to compare
because of differences in baseline clinical characteristics such as
tumor size, cause of liver disease and chemotherapeutic agents used
when performing TACE. Hence, the postoperative survival benefits of
preoperative TACE for HCC remain a matter of debate.
The aim of the present study was to evaluate the
influence of preoperative TACE on survival after SR for HCC.
Patients and methods
Patients
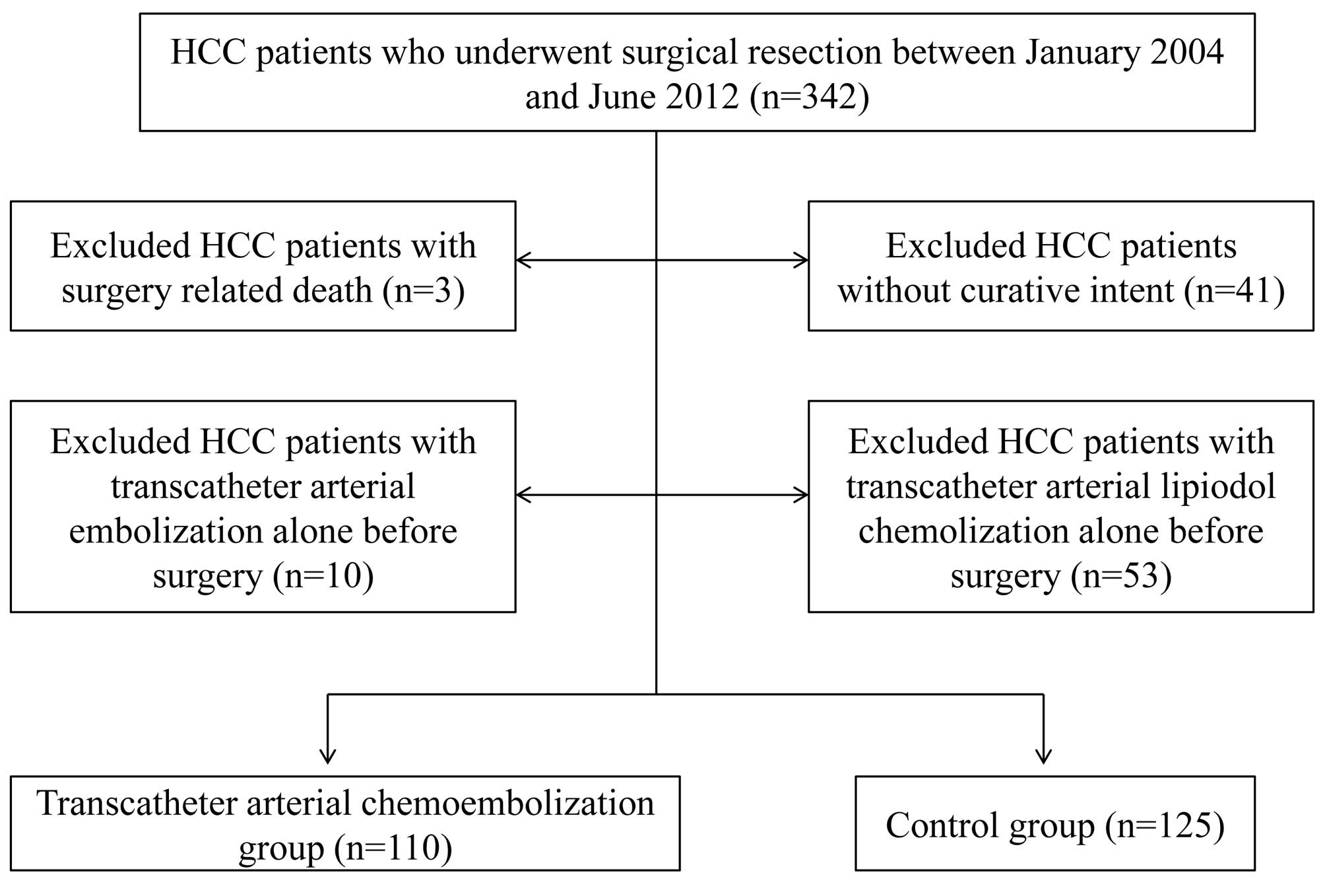
SR was performed in 342 treatment-naïve HCC patients
at the Department of Surgery, Osaka Red Cross Hospital, Japan,
between January 2004 and June 2012. Of these, we excluded patients
operated on without curative intent (n=41), with surgery-related
death (n=3), with TACE alone before surgery (n=10), and with
transcatheter arterial lipiodol chemolization alone before surgery
(n=53). We defined curative surgery as the resection of all tumors
detectable using imaging modalities. A total of 235 HCC patients
who underwent SR were therefore analyzed in the present study
(Fig. 1), including 110 patients
who underwent TACE before surgery (TACE group) and 125 patients who
did not (control group). All patients in the TACE group received
one session of TACE, and patients in the control group received
angiography alone. The decision to perform TACE prior to surgery
was made mainly by the attending physician. Written informed
consent was obtained from all patients prior to TACE and surgery,
and the study protocol complied with all of the provisions of the
Declaration of Helsinki. The present study comprised a
retrospective analysis of patient records, and all treatments were
conducted in an open-label manner.
HCC diagnosis and stage
HCC was diagnosed using abdominal ultrasound and
dynamic computed tomography (CT) scans (hyperattenuation during the
arterial phase in all or some part of the tumor and hypoattenuation
in the portal-venous phase) and/or magnetic resonance imaging
(MRI), based mainly on the recommendations of the American
Association for the Study of Liver Diseases (9). Arterial and portal phase dynamic CT
images were obtained at ∼30 and 120 sec, respectively, after the
injection of the contrast material. Abdominal angiography combined
with CT assistance was performed on all patients before SR, in line
with the recommendations of Yamasaki et al, who reported
that this technique was useful for detecting small satellite
nodules (14). HCC stage was
determined using the Liver Cancer Study Group of Japan (LCSGJ)
staging system (15). HCC was
confirmed pathologically in resected specimens at surgery, except
for cases with complete necrosis.
TACE procedure
TACE was performed in accordance with Japanese
guidelines (16), and consisted of
catheterization via the femoral artery with super-selective
cannulation to the hepatic artery feeding the target HCC. An
emulsion containing Farmorubicin (epirubicin hydrochloride;
Pfizer), mitomycin C (Kyowa Hakko Kirin Co. Ltd., Tokyo, Japan) and
lipiodol (iodine addition products of ethyl esters of fatty acids
obtained from poppy seed oil; Mitsui, Japan) was infused via the
feeding artery according to tumor size, tumor number and liver
function. Embolization was then achieved by slow injection of
gelatin (Spongel; Yamanouchi, Japan) to prevent reflux into
untreated segments. The injection sites were segmental or
subsegmental in all TACE group patients. The mean doses of
epirubicin, mitomycin and lipiodol in the TACE group were 38.0±12.5
mg (range 10–70 mg), 8.9±3.1 mg (range 2–14 mg) and 5.2±2.7 ml
(range 1–15 ml), respectively.
Treatment efficacy of TACE
The treatment efficacy of TACE was classified into
four grades according to the Response Evaluation Criteria in Cancer
of the Liver proposed by the LCSGJ (17) and based on CT scans performed
within 30 days after TACE: TE4, tumor-necrotizing effect of 100%;
TE3, tumor-necrotizing effect of 50 to <100%; TE2, effects other
than TE3 and TE1; TE1, tumor enlarged by >25% regardless of the
necrotizing effect. In the present study, we defined TACE
responders as TE4 or TE3 and TACE non-responders as TE2 or TE1.
SR procedure
Conventional open hepatectomy was performed in 179
patients (76.2%) and laparoscopic hepatectomy was performed in 56
patients (23.8%). All procedures were performed by one of four
surgeons who had at least 10 years of experience of SR.
Conventional open hepatectomy was carried out under
general anesthesia using a right subcostal incision with a midline
extension. We performed anatomic partial hepatectomy with a
resection margin of at least 1 cm over the tumor, based on
intraoperative ultrasonography (IOUS) guidance. IOUS was performed
routinely to estimate the location, size, number and feeding
vessels of the tumor, as well as to provide a clear vascular map of
the liver anatomy. The Cavitron Ultrasonic Aspiration system (CUSA;
Valley Lab Corp., Boulder, CO, USA) was used to dissect the liver
tissue. Hemostasis was achieved by dipolar electric coagulation and
suturing. The Pringle maneuver was usually used in cases with
cirrhotic liver, with a clamp/unclamp time of 15/5 min policy.
Laparoscopic hepatectomy was performed using the
four-trocar technique. The first trocar was placed by a small
incision below the umbilicus for pneumoperitoneum creation. The
tumor extent and its relationship with the vascular anatomy and
other tumors in the liver were explored using IOUS. The line of the
intended liver parenchymal transection was marked on the surface of
the liver using diathermy. Ultrasonic dissection was performed
using an ultrasonic surgical system. The resected liver was
maneuvered into a plastic bag (18). Patients were discharged when their
liver function returned to normal and any adverse events were
resolved.
Follow-up
Follow-up consisted of periodic blood tests and
monitoring of tumor markers, including α-fetoprotein (AFP) and
des-γ-carboxy prothrombin (DCP), measured using a chemiluminescent
enzyme immunoassay (Lumipulse PIVKAII Eisai, Eisai, Tokyo, Japan).
Dynamic CT scans and/or MRI were obtained every 3–4 months after
SR. Chest CT, whole abdominal CT and bone scintigraphy were
performed when extrahepatic HCC recurrences were suspected.
Statistical analysis
The primary endpoints were overall survival (OS) and
recurrence-free survival (RFS), and the secondary endpoints were
procedure-related complications. Data were analyzed using
univariate and multivariate analyses. Continuous variables were
compared using unpaired t-tests and categorical variables were
compared using Fisher’s exact tests. Time to recurrence was defined
as the interval between surgery and first confirmed recurrence. For
analysis of RFS, follow-up ended at the time of first recurrence;
other patients were censored at their last follow-up visit or the
time of death from any cause without recurrence. For analysis of
OS, follow-up ended at the time of death from any cause, and the
remaining patients were censored at the last follow-up visit. The
cumulative OS and RFS rates were calculated using the Kaplan-Meier
method and tested using log-rank tests. The Cox proportional
hazards model was used for multivariate analysis of factors that
were considered significant in univariate analysis. These
statistical methods were used to estimate the interval from
surgery. Data were analyzed using SPSS software, version 9.0 (SPSS
Inc., Chicago, IL, USA) for Microsoft Windows. Data are expressed
as means ± standard deviation. Values of P<0.05 were considered
to be statistically significant.
Results
Patients
Baseline characteristics of the TACE and control
groups are shown in Table I. The
mean observation periods were 2.8±1.8 years in the TACE group and
2.9±2.1 years in the control group. There were significant
differences between the two groups in terms of HCC stage, tumor
number and pretreatment DCP-value, indicating that patients in the
TACE group had more advanced tumor characteristics. Anatomical
resection was performed in 54 patients in the TACE group, and
non-anatomical resection in 56 patients, compared with 57 and 68
patients in the control group (P=0.603).
 | Table I.Baseline characteristics between the
TACE group and the control group. |
Table I.
Baseline characteristics between the
TACE group and the control group.
| TACE group
(n=110) | Control group
(n=125) | P-value |
|---|
| Gender
(male/female) | 86/24 | 93/32 | 0.541a |
| Age (years) | 67.7±10.2 | 68.1±10.5 | 0.739b |
| HCC stage
(I/II/III/IV) | 5/57/38/10 | 11/82/25/7 | 0.029a |
| Etiology
(HBV/HCV/nBnC) | 15/63/32 | 15/74/36 | 0.922a |
| Child-Pugh
classification (A/B) | 108/2 | 120/5 | 0.453a |
| Tumor number
(single/multiple) | 65/45 | 94/31 | 0.012a |
| Maximum tumor size
(cm) | 5.0±3.2 | 4.5±2.2 | 0.138b |
| AST (IU/l) | 59.5±40.2 | 55.6±35.4 | 0.443b |
| ALT (IU/l) | 53.7±45.1 | 49.6±37.3 | 0.434b |
| Serum albumin
(g/dl) | 3.94±0.52 | 3.94±0.52 | 0.960b |
| Total bilirubin
(mg/dl) | 0.78±0.39 | 0.83±0.43 | 0.338b |
| Prothrombin time
(%) | 89.3±13.8 | 91.2±14.8 | 0.306b |
| Platelets
(×104/mm3) | 15.0±7.8 | 14.8±6.8 | 0.807b |
| ICGR 15 (%) | 12.7±9.3 | 14.6±10.4 | 0.173b |
| AFP (ng/ml) |
2,841.0±15,411.0 |
1,064.5±4,370.0 | 0.220b |
| DCP (mAU/ml) |
6,901.1±23,091.8 |
2,017.5±5,625.6 | 0.023b |
Histological findings
The histological findings in the TACE and control
groups are shown in Table II.
Complete necrosis occurred in 21 patients (19.1%) in the TACE
group. Microscopic vascular invasion was found in 32 patients
(29.1%) in the TACE group and 47 patients (37.6%) in the control
group.
 | Table II.Type of surgery, outcome of surgery
and histological findings between the TACE group and the control
group. |
Table II.
Type of surgery, outcome of surgery
and histological findings between the TACE group and the control
group.
| Variables | TACE group
(n=110) | Control group
(n=125) | P-value |
|---|
| Hepatectomy | | | |
|
Anatomical/non-anatomical | 54/56 | 57/68 | 0.603a |
| Operation time
(min) | 259.5±74.5 | 269.1±87.8 | 0.881b |
| Blood loss during
surgery (ml) | 764.1±713.5 | 874.2±886.8 | 0.334b |
| Hospitalization
days | 17.9±15.8 | 16.6±10.4 | 0.447b |
| HCC histology | | | |
| Well | 7 | 14 | |
| Moderate | 47 | 73 | |
| Poorly | 35 | 38 | |
| Complete
necrosis | 21 | 0 | |
| Fibrous capsule
(yes) | 90 | 95 | |
| Capsular invasion
(yes) | 52 | 81 | |
| Microscopic
vascular invasion (yes) | 32 | 47 | |
| Microscopic
surgical margin (yes) | 24 | 40 | |
Cumulative OS and RFS in the TACE and
control groups
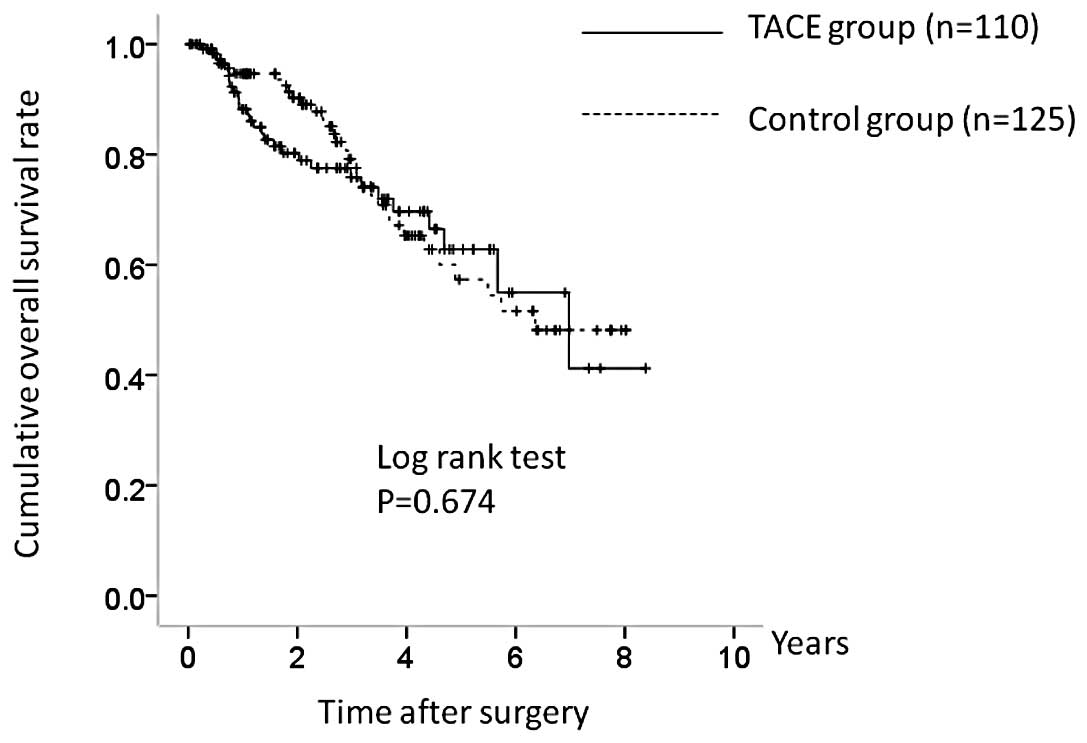
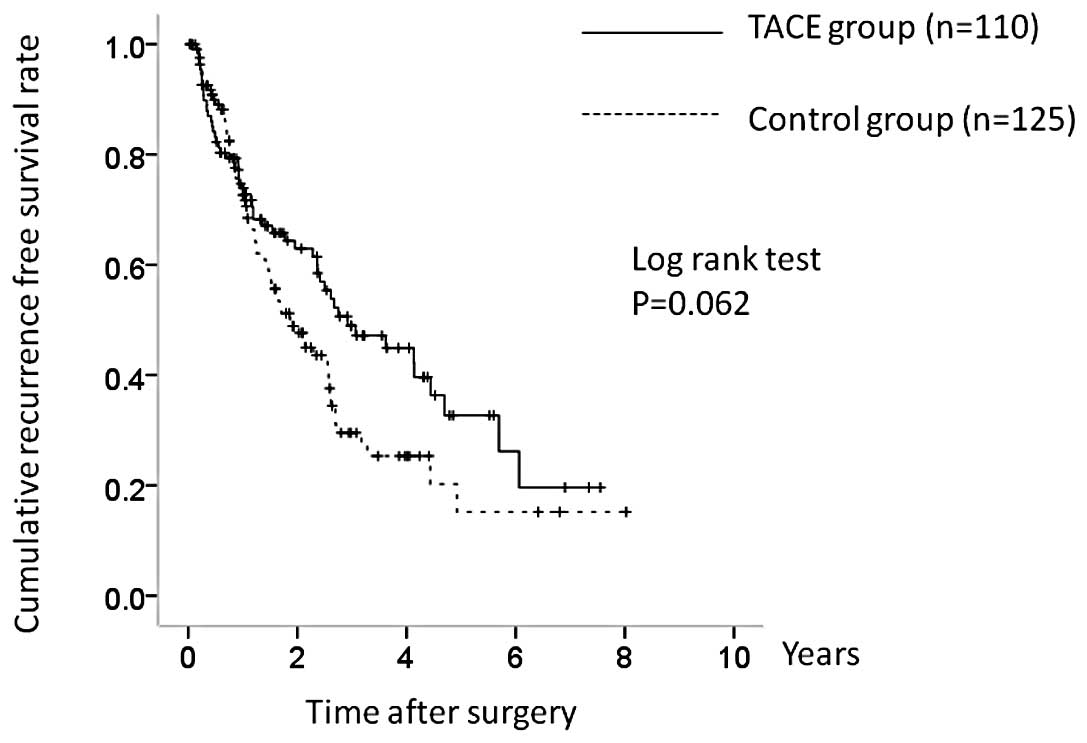
The 1-, 3- and 5-year OS rates in the two groups
were 87.4, 76.0 and 62.5%, respectively, in the TACE group and
94.9, 79.0 and 57.8%, respectively, in the control group (Fig. 2). There was no significant
difference in OS between the two groups (P=0.674). The
corresponding RFS rates at 1, 3 and 5 years were 73.3, 48.9 and
33.2%, respectively, in the TACE group and 73.3, 29.4 and 16.2%,
respectively, in the control group (Fig. 3). RFS was higher in the TACE group,
but the difference was not significant (P=0.062).
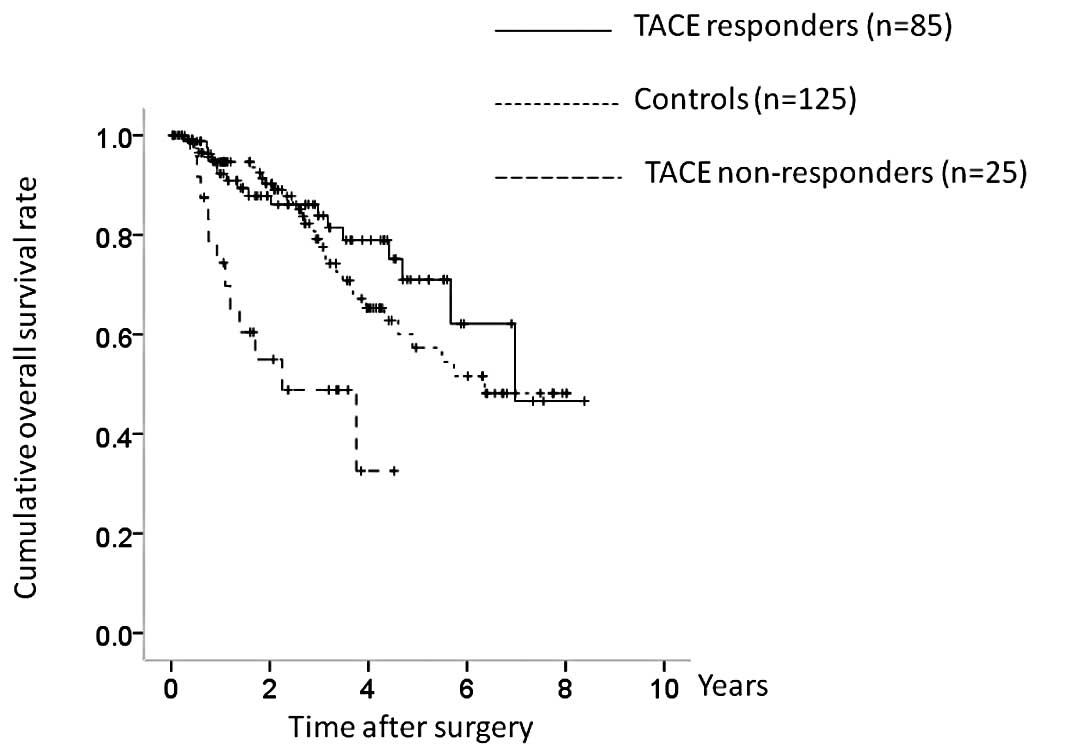
OS and RFS in TACE responders and
non-responders
In terms of the efficacy of TACE, 21 patients were
classified as TE4, 64 as TE3, 25 as TE2 and 0 as TE1. The TACE
group was further categorized into TACE responders (TE4 and TE3;
n=85) and TACE non-responders (TE2 and TE1; n=25), and OS and RFS
were compared between the TACE responders, TACE non-responders, and
the control group. There were significant differences in OS between
the three groups (TACE responders vs. controls, P= 0.381; controls
vs. TACE non-responders, P<0.001; TACE responders vs. TACE
non-responders, P<0.001; overall significance, P<0.001)
(Fig. 4). There were also
significant differences in RFS between the three groups (TACE
responders vs. controls, P=0.006; controls vs. TACE non-responders,
P=0.190; TACE responders vs. TACE nonresponders, P=0.004; overall
significance, P=0.004) (Fig.
5).
Factors contributing to OS after SR
Univariate analysis identified pretreatment therapy
(P<0.001), HCC stage (P= 0.012), maximum tumor size ≥4 cm (P=
0.003), tumor number (P=0.005), total bilirubin ≥1 mg/dl (P=0.003),
serum albumin ≥4.0 g/dl (P= 0.013), AFP ≥100 ng/ml (P<0.001),
DCP ≥100 mAU/ml (P= 0.020) and microscopic vascular invasion
(P=0.002) as significant factors contributing to OS after SR
(Table III). Multivariate analysis
of the nine factors found to be significant in univariate analysis
further identified pretreatment therapy (TACE responder, P=0.018),
pretreatment therapy (TACE non-responder, P=0.019), total bilirubin
≥1 mg/dl (P=0.003), serum albumin ≥4.0 g/dl (P=0.001), AFP ≥100
ng/ml (P= 0.011) and microscopic vascular invasion (P=0.021) as
significant contributors to OS. The hazard ratios (HRs) and 95%
confidence intervals (CIs) for these factors are detailed in
Table IV.
 | Table III.Univariate analyses contributing to
OS and RFS after surgical resection (n=235). |
Table III.
Univariate analyses contributing to
OS and RFS after surgical resection (n=235).
| Variables | n | OS
| RFS
|
|---|
| P-valuea | P-valuea |
|---|
| Age ≥70
(yes/no) | 119/116 | 0.229 | 0.794 |
| Gender
(male/female) | 179/56 | 0.504 | 0.848 |
| Pretreatment
therapy | | | |
|
TACE-R/TACE-NR/controls | 85/25/125 | <0.001 | 0.004 |
| Cause of liver
disease | | | |
| Hepatitis
B/hepatitis C/non-Bnon-C | 30/137/68 | 0.713 | 0.935 |
| HCC stage (I,
II/III, IV) | 155/80 | 0.012 | <0.001 |
| Maximum tumor size
≥4 cm (yes/no) | 115/120 | 0.003 | 0.049 |
| Tumor number
(single/multiple) | 159/76 | 0.005 | <0.001 |
| ICGR 15 ≥12% | 113/122 | 0.289 | 0.319 |
| Total bilirubin
≥1.0 mg/dl (yes/no) | 62/173 | 0.003 | 0.041 |
| Serum albumin ≥4.0
g/dl (yes/no) | 125/110 | 0.013 | 0.231 |
| AST ≥50 IU/l
(yes/no) | 106/129 | 0.353 | 0.005 |
| ALT ≥50 IU/l
(yes/no) | 90/145 | 0.263 | 0.012 |
| Platelets
≥10×104/mm3 (yes/no) | 172/63 | 0.502 | 0.549 |
| Prothrombin time
≥80% (yes/no) | 177/58 | 0.112 | 0.144 |
| AFP ≥100 ng/ml
(yes/no) | 70/165 | <0.001 | 0.036 |
| DCP ≥100 mAU/ml
(yes/no) | 151/84 | 0.020 | 0.180 |
| Microscopic capsule
(yes/no) | 185/50 | 0.696 | 0.171 |
| Microscopic capsule
invasion (yes/no) | 133/102 | 0.147 | 0.520 |
| Microscopic
vascular invasion (yes/no) | 80/155 | 0.002 | 0.021 |
| Microscopic
surgical margin (yes/no) | 64/171 | 0.818 | 0.951 |
 | Table IV.Multivariate analyses contributing to
OS after surgical resection. |
Table IV.
Multivariate analyses contributing to
OS after surgical resection.
| Variables | Hazard ratio | 95% CI | P-valuea |
|---|
| Pretreatment
therapy | | | |
| TACE
responders | 2.433 | 1.161–5.102 | 0.018 |
| TACE
non-responders | 0.374 | 0.164–0.851 | 0.019 |
| Controls | 1.000 | | |
| HCC stage | | | |
| Stage I, II | 1.000 | | |
| Stage III,
IV | 0.912 | 0.252–3.302 | 0.889 |
| Maximum tumor size
(cm) | | | |
| ≥4 | 0.766 | 0.416–1.411 | 0.393 |
| <4 | 1.000 | | |
| Tumor number | | | |
| Single | 1.000 | | |
| Multiple | 0.656 | 0.185–2.330 | 0.514 |
| Total bilirubin
(mg/dl) | | | |
| ≥1.0 | 0.413 | 0.231–0.740 | 0.003 |
| <1.0 | 1.000 | | |
| Serum albumin
(g/dl) | | | |
| ≥4.0 | 2.579 | 1.446–4.599 | 0.001 |
| <4.0 | 1.000 | | |
| AFP (ng/ml) | | | |
| ≥100 | 0.486 | 0.280–0.846 | 0.011 |
| <100 | 1.000 | | |
| DCP (mAU/ml) | | | |
| ≥100 | 0.627 | 0.320–1.229 | 0.174 |
| <100 | 1.000 | | |
| Microscopic
vascular invasion | | | |
| Yes | 0.491 | 0.269–0.899 | 0.021 |
| No | 1.000 | | |
Factors contributing to RFS after SR
Univariate analysis identified pretreatment therapy
(P= 0.004), HCC stage (P<0.001), maximum tumor size ≥4 cm (P=
0.049), tumor number (P<0.001), total bilirubin ≥1 mg/dl (P=
0.041), aspartate aminotransferase ≥50 IU/l (P= 0.005), alanine
aminotransferase ≥50 IU/l (P=0.012), AFP ≥100 ng/ml (P=0.036) and
microscopic vascular invasion (P=0.021) as significant factors
contributing to RFS after SR (Table
III). Multivariate analysis of the nine factors found to be
significant in univariate analysis confirmed pretreatment therapy
(TACE non-responder, P= 0.039), tumor number (P= 0.038) and AFP
≥100 ng/ml (P=0.043) as significant contributors to RFS. The HRs
and 95% CIs for these factors are detailed in Table V.
 | Table V.Multivariate analyses contributing to
RFS after surgical resection. |
Table V.
Multivariate analyses contributing to
RFS after surgical resection.
| Variables | Hazard ratio | 95% CI | P-valuea |
|---|
| Pretreatment
therapy | | | |
| TACE
responders | 1.075 | 0.593–1.949 | 0.811 |
| TACE
non-responders | 0.508 | 0.267–0.966 | 0.039 |
| Controls | 1.000 | | |
| HCC stage | | | |
| Stage I, II | 1.000 | | |
| Stage III,
IV | 0.845 | 0.319–2.238 | 0.735 |
| Maximum tumor size
(cm) | | | |
| ≥4 | 0.872 | 0.584–1.302 | 0.503 |
| <4 | 1.000 | | |
| Tumor number | | | |
| Single | 1.000 | | |
| Multiple | 0.513 | 0.094–0.953 | 0.038 |
| Total bilirubin
(mg/dl) | | | |
| ≥1.0 | 0.801 | 0.516–1.246 | 0.325 |
| <1.0 | 1.000 | | |
| AST (IU/l) | | | |
| ≥50 | 0.833 | 0.486–1.429 | 0.508 |
| <50 | 1.000 | | |
| ALT (IU/l) | | | |
| ≥50 | 0.751 | 0.434–1.300 | 0.307 |
| <50 | 1.000 | | |
| AFP (ng/ml) | | | |
| ≥100 | 0.616 | 0.379–0.970 | 0.043 |
| <100 | 1.000 | | |
| Microscopic
vascular invasion | | | |
| Yes | 0.857 | 0.568–1.292 | 0.462 |
| No | 1.000 | | |
Comparison of baseline characteristics
between TACE responders and non-responders
The baseline characteristics of the TACE responders
(n=85) and non-responders (n=25) are shown in Table VI. There were significant
differences between the groups in terms of HCC stage, maximum tumor
size, pretreatment AFP value and pretreatment DCP value, indicating
that TACE non-responders had more advanced tumor characteristics
than TACE responders.
 | Table VI.Baseline characteristics between the
TACE responder group and the TACE non-responder group. |
Table VI.
Baseline characteristics between the
TACE responder group and the TACE non-responder group.
| TACE responders
(n=85) | TACE non-responders
(n=25) | P-value |
|---|
| Gender
(male/female) | 66/19 | 20/5 | 1.000a |
| Age (years) | 67.2±10.7 | 69.1±8.0 | 0.452b |
| HCC stage
(I/II/III/IV) | 5/47/29/4 | 0/10/9/6 | 0.019a |
| Etiology
(HBV/HCV/non-Bnon-C) | 17/50/18 | 2/14/9 | 0.178a |
| Child-Pugh
classification (A/B) | 84/1 | 24/1 | 0.405a |
| Tumor number
(single/multiple) | 54/31 | 11/14 | 0.106a |
| Maximum tumor size
(cm) | 4.1±1.9 | 8.1±4.5 | <0.001b |
| AST (IU/l) | 60.5±40.9 | 56.0±38.4 | 0.627b |
| ALT (IU/l) | 55.5±48.5 | 47.6±31.0 | 0.446b |
| Serum albumin
(g/dl) | 3.97±0.49 | 3.82±0.61 | 0.183b |
| Total bilirubin
(mg/dl) | 0.78±0.37 | 0.80±0.45 | 0.781b |
| Prothrombin time
(%) | 89.0±14.1 | 90.3±12.7 | 0.667b |
| Platelets
(×104/mm3) | 15.0±8.0 | 15.0±7.2 | 0.983b |
| ICGR 15 (%) | 12.8±8.5 | 12.5±11.9 | 0.897b |
| AFP (ng/ml) |
1,114.0±3,917.9 |
8,712.7±31,280.1 | 0.030b |
| DCP (mAU/ml) |
3,197.0±11,343.4 |
19,495.1±41,923.6 | 0.002b |
Causes of death
Twenty-nine patients in the TACE group (26.4%) died
during the follow-up period. The causes of death were HCC
recurrence in 21 patients, liver failure in 6 patients and other
causes in 2 patients. Thirty-two patients in the control group
(25.6%) died during the follow-up period, and the causes of death
were HCC recurrence in 21 patients, liver failure in 7 patients and
other causes in 4 patients.
HCC recurrence in the TACE and control
groups
Fifty-three patients in the TACE group (48.2%) and
69 (55.2%) in the control group had HCC recurrence during the
follow-up period. The patterns of HCC recurrence after SR in the
TACE group were as follows: single HCC recurrence in the liver in
12 patients; multiple HCC recurrences in the liver in 29 patients;
multiple HCC recurrences in the liver with lung metastases in 7
patients; multiple HCC recurrences in the liver with brain
metastases in 1 patient; and multiple HCC recurrences in the liver
with multiple lymph node (LN) metastases in 4 patients. The
patterns of HCC recurrence after SR in the control group were:
single HCC recurrence in the liver in 32 patients; single HCC
recurrence with invasion of the right hepatic vein in 1 patient;
multiple HCC recurrences in the liver in 33 patients; multiple HCC
recurrences in the liver with LN metastases in 1 patient; multiple
HCC recurrences in the liver with portal vein invasion in 1
patient; and multiple HCC recurrences in the liver with lung
metastases in 1 patient.
The treatment methods for the first HCC recurrence
in the TACE group were SR in 2 patients, radiofrequency ablation
(RFA) in 23 patients, TACE in 16 patients, percutaneous ethanol
injection (PEI) in 2 patients, systemic chemotherapy in 7 patients
and no specific treatment in 3 patients. The treatment methods used
in the control group were SR in 6 patients, RFA in 36 patients,
TACE in 15 patients, PEI in 2 patients, systemic chemotherapy in 4
patients and no specific treatment in 6 patients.
Operation time, surgical blood loss and
hospitalization period in the TACE and control groups
The mean operation times were 259.5±74.5 min in the
TACE group and 269.1±87.8 min in the control group (P=0.881). The
mean surgical blood loss was 764.1±713.5 ml in the TACE group and
874.2±886.8 ml in the control group (P=0.334). The mean periods
from surgery until discharge were 17.9±15.8 days in the TACE group
and 16.6±10.4 days in the control group (P=0.447) (Table II).
TACE and surgery-related serious adverse
events (SAEs)
The interval from TACE until surgery in the TACE
group was 38.0±17.6 days, and no patient was prevented from
undergoing SR as a result of TACE-related complications.
Surgery-related SAEs in the TACE group included abscess formation
in 5 patients, bile leakage in 4 patients, refractory ascites in 2
patients, aspiration pneumonia in 2 patients, gastrointestinal
bleeding in 2 patients, acute heart failure in 1 patient and
perforation of the small intestine in 1 patient. Equivalent
complications in the control group included abscess formation in 3
patients, bile leakage in 4 patients, refractory ascites in 6
patients, aspiration pneumonia in 2 patients, acute respiratory
distress syndrome in 1 patient and brain infarction in 1 patient.
All these SAEs improved during the same hospitalization. There was
no significant difference between the groups in terms of SAEs
related to surgery (P=0.714).
Subgroup analyses of OS and RFS in
patients with HCC stage I or II
TACE non-responders had more advanced tumor
characteristics than TACE responders, and we therefore performed
subgroup analyses in patients with HCC stage I or II (n=155) and
HCC stage III or IV (n=80). Patients with HCC stage I or II
comprised 52 TACE responders, 10 TACE non-responders and 93
controls. Although the TACE nonresponders had a poorer prognosis in
terms of OS, there was no overall significant difference between
the three groups (TACE responders vs. controls, P=0.523; controls
vs. TACE non-responders, P= 0.118; TACE responders vs. TACE
nonresponders, P=0.040; overall significance, P=0.148) (Fig. 6). However, there were significant
differences between the three groups in terms of RFS (TACE
responders vs. controls, P=0.003; controls vs. TACE non-responders,
P=0.992; TACE responders vs. TACE non-responders, P= 0.105; overall
significance, P=0.013) (Fig.
7).
Subgroup analyses of OS and RFS in
patients with HCC stage III or IV
The patients with HCC stage III or IV (n=80)
included 33 TACE responders, 15 TACE non-responders and 32
controls. There were significant differences in OS between the
three groups (TACE responders vs. controls, P=0.267; controls vs.
TACE non-responders, P=0.025; TACE responders vs. TACE
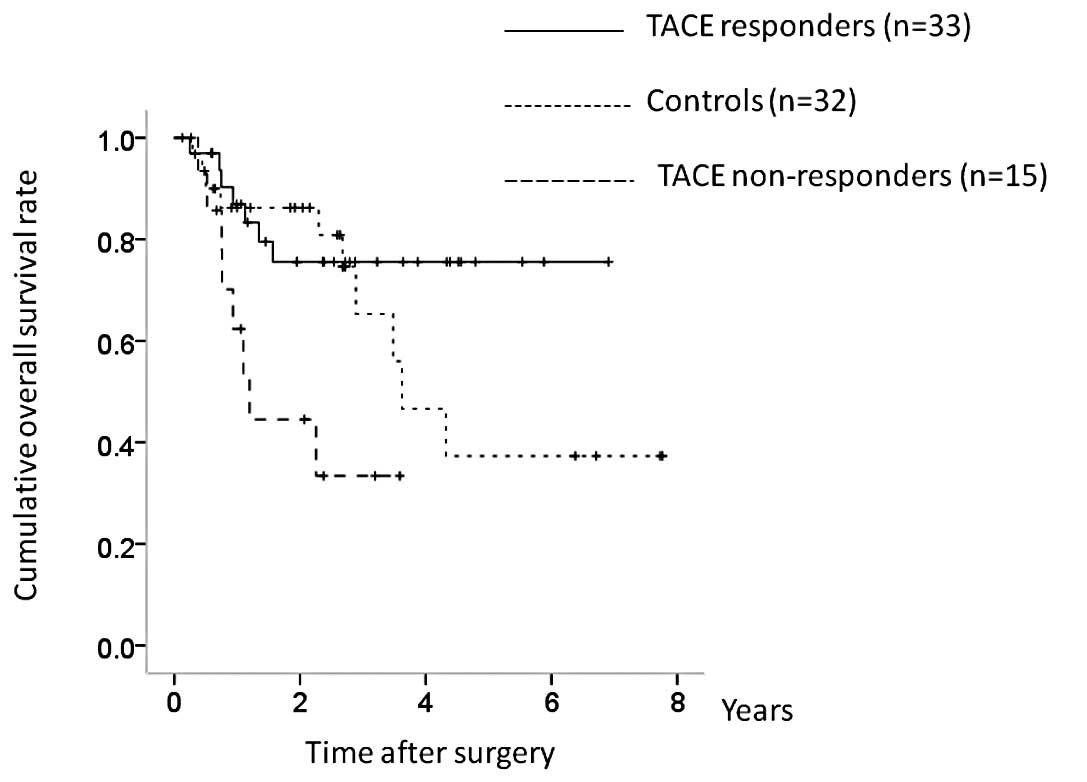
non-responders, P= 0.010; overall significance, P= 0.011) (Fig. 8). In terms of RFS, although TACE
non-responders had a poorer prognosis, the difference was not
significant (TACE responders vs. controls, P=0.106; controls vs.
TACE non-responders, P= 0.462; TACE responders vs. TACE
non-responders, P=0.060; overall significance, P=0.116) (Fig. 9).
Discussion
To the best of our knowledge, the present study
represents one of the largest comparative studies on the influence
of preoperative TACE on the survival of patients with resectable
HCC (3,10,12,13,19–21).
Although four randomized controlled trials have investigated the
effects of pretreatment TACE on survival after SR (3,10,12,13)
and similarly concluded that pretreatment with TACE did not improve
survival after SR, the sample sizes, TACE procedures and baseline
clinical characteristics differed among these studies. Hence, the
efficacy of pretreatment TACE on survival after SR remains
unclear.
In this study, multivariate analysis identified TACE
responder and TACE non-responder in terms of OS, and TACE
non-responder in terms of RFS, as significant independent
prognostic factors after SR. Moreover, in terms of RFS in stage I
or II HCC patients and OS in stage III or IV HCC patients, the
overall differences reached significance in univariate analysis.
These results suggest that the therapeutic efficacy of pretreatment
TACE is associated with clinical outcome after SR.
The extent of tumor vascularization is significantly
associated with the degree of TACE efficacy, and a high degree of
vascularization is thus considered to be a predictive sign for
response to TACE (22).
Preoperative TACE may thus be recommended in HCC patients with a
high degree of tumor vascularity (23), although Adachi et al
reported that preoperative TACE should be avoided as incomplete
tumor necrosis promotes the hematogenous spread of residual tumor
cells during SR (24).
Several studies have reported that serum vascular
endothelial growth factor (VEGF) can act as a prognostic factor for
the treatment of HCC (25–27). Sergio et al(22) also demonstrated that when TACE for
HCC was ineffective, it might induce a significant increase in
serum VEGF levels and affect patient survival. TACE non-responders
in the present study may thus have had a poor prognosis associated
with increased serum VEGF levels. It has also been suggested that
preoperative TACE should not be performed in patients with a low
degree of vascularization. Further studies are needed to clarify
this issue.
In terms of tumor histology, the current study
included 13 patients (52.0%) with poorly differentiated HCC in the
TACE non-responder group and 22 patients (25.9%) with poorly
differentiated HCC in the TACE responder group (P= 0.017). Patients
with poorly differentiated HCC would be expected to have a poorer
prognosis because of their poor response to TACE.
Liver function parameters reflected by serum
bilirubin and serum albumin were significant independent factors
linked to OS in the present study. Several studies have
investigated the importance of maintaining liver function on
survival after surgery for HCC (28,29).
Our results were consistent with previous reports; in HCC patients
with poor liver function, branched chain amino acid therapy to
maintain liver function may be effective to optimize the clinical
outcomes (7).
High pretreatment AFP level was an independent
prognostic factor in terms of both OS and RFS in our study. HCC
patients with high AFP levels had poorer tumor histology and larger
tumor mass (30), which may be
associated with their poorer clinical outcome. Microvascular
invasion was a significant prognostic factor in univariate and
multivariate analyses in terms of OS, and in the univariate
analysis in terms of RFS. Lim et al reported that
microvascular invasion was an adverse predictor of OS and RFS
following SR for HCC (31). Our
results were in agreement with their reports. Careful monitoring
for HCC recurrence will thus be needed in patients with
microvascular invasion, and patients with high pretreatment AFP
levels.
Zhou et al reported that several patients in
their randomized controlled trial could not undergo definitive
surgery because of tumor progression after TACE or because of
TACE-related SAEs. However, no patients in the current study were
unable to undergo surgery (3).
Moreover, there were no significant differences between the TACE
and control groups in terms of operation time, blood loss during
surgery and hospitalization period. Our study results, therefore,
suggest that the TACE procedure was safe in patients with
resectable HCC.
The present study has several limitations. First, it
is a retrospective study. Second, the follow-up period was
relatively short for survival analysis. Third, the sample sizes of
the TACE responder, TACE non-responder, and control groups were not
balanced. However, despite these limitations, the results of this
study demonstrated that the therapeutic efficacy of preoperative
TACE may be associated with clinical outcome after SR in patients
with HCC. Preoperative TACE may thus be a prognostic factor in
patients with resectable HCC after SR.
Acknowledgements
The authors would like to thank all
the staff in the angiography room and the operation room of the
Osaka Red Cross Hospital for their valuable support.
References
|
1.
|
Livraghi T, Mäkisalo H and Line PD:
Treatment options in hepatocellular carcinoma today. Scand J Surg.
100:22–29. 2011.PubMed/NCBI
|
|
2.
|
El-Serag HB: Epidemiology of viral
hepatitis and hepatocellular carcinoma. Gastroenterology.
142:1264–1273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Zhou WP, Lai EC, Li AJ, Fu SY, Zhou JP,
Pan ZY, Lau WY and Wu MC: A prospective, randomized, controlled
trial of preoperative transarterial chemoembolization for
resectable large hepatocellular carcinoma. Ann Surg. 249:195–202.
2009. View Article : Google Scholar
|
|
4.
|
Nishikawa H, Osaki Y, Kita R, Kimura T,
Inuzuka T, Takeda H, Nakajima J, Matsuda F, Sakamoto A, Henmi S,
Hatamaru K, Saito S and Nasu A: Transcatheter arterial infusion
chemotherapy prior to radiofrequency thermal ablation for single
hepatocellular carcinoma reduces the risk of intrahepatic distant
recurrence. Int J Oncol. 41:903–909. 2012.
|
|
5.
|
de Lope CR, Tremosini S, Forner A, Reig M
and Bruix J: Management of HCC. J Hepatol. 56(Suppl 1): S75–S87.
2012.
|
|
6.
|
Takayasu K, Arii S, Ikai I, Omata M, Okita
K, Ichida T, Matsuyama Y, Nakanuma Y, Kojiro M and Makuuchi M:
Prospective cohort study of transarterial chemoembolization for
unresectable hepatocellular carcinoma in 8510 patients.
Gastroenterology. 131:461–469. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Nishikawa H, Osaki Y, Inuzuka T, Takeda H,
Nakajima J, Matsuda F, Henmi S, Sakamoto A, Ishikawa T, Saito S,
Kita R and Kimura T: Branched-chain amino acid treatment before
transcatheter arterial chemoembolization for hepatocellular
carcinoma. World J Gastroenterol. 18:1379–1384. 2012. View Article : Google Scholar
|
|
8.
|
Llovet JM and Bruix J: Systematic review
of randomized trials for unresectable hepatocellular carcinoma:
chemoembolization improves survival. Hepatology. 37:429–442. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Bruix J and Sherman M; Practice Guidelines
Committee, American Association for the Study of Liver Diseases:
Management of hepatocellular carcinoma. Hepatology. 42:1208–1236.
2005. View Article : Google Scholar
|
|
10.
|
Kaibori M, Tanigawa N, Kariya S, Ikeda H,
Nakahashi Y, Hirohara J, Koreeda C, Seki T, Sawada S, Okazaki K and
Kwon AH: A prospective randomized controlled trial of preoperative
whole-liver chemolipiodolization for hepatocellular carcinoma. Dig
Dis Sci. 57:1404–1412. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Yamanaka N, Okamoto E, Fujihara S, Kato T,
Fujimoto J, Oriyama T, Mitsunobu M, Toyosaka A, Uematsu K and
Yamamoto K: Do the tumor cells of hepatocellular carcinomas
dislodge into the portal venous stream during hepatic resection?
Cancer. 70:2263–2267. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Yamasaki S, Hasegawa H, Kinoshita H,
Furukawa M, Imaoka S, Takasaki K, Kakumoto Y, Saitsu H, Yamada R,
Oosaki Y, Arii S, Okamoto E, Monden M, Ryu M, Kusano S, Kanematsu
T, Ikeda K, Yamamoto M, Saoshiro T and Tsuzuki T: A prospective
randomized trial of the preventive effect of pre-operative
transcatheter arterial embolization against recurrence of
hepatocellular carcinoma. Jpn J Cancer Res. 87:206–211. 1996.
View Article : Google Scholar
|
|
13.
|
Wu CC, Ho YZ, Ho WL, Wu TC, Liu TJ and
P’eng FK: Preoperative transcatheter arterial chemoembolization for
resectable large hepatocellular carcinoma: a reappraisal. Br J
Surg. 82:122–126. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Yamasaki T, Kurokawa F, Shirahashi H,
Kusano N, Hironaka K and Okita K: Percutaneous radiofrequency
ablation therapy with combined angiography and computed tomography
assistance for patients with hepatocellular carcinoma. Cancer.
91:1342–1348. 2001. View Article : Google Scholar
|
|
15.
|
Liver Cancer Study Group of Japan: The
general rules for the clinical and pathological study of primary
liver cancer. Jpn J Surg. 19:98–129. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Kudo M and Okanoue T: Management of
hepatocellular carcinoma in Japan: consensus-based clinical
practice manual proposed by the Japan Society of Hepatology.
Oncology. 72(Suppl 1): 2–15. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Kudo M, Kubo S, Takayasu K, Sakamoto M,
Tanaka M, Ikai I, Furuse J, Nakamura K and Makuuchi M; Liver Cancer
Study Group of Japan (Committee for Response Evaluation Criteria in
Cancer of the Liver, Liver Cancer Study Group of Japan): Response
Evaluation Criteria in Cancer of the Liver (RECICL) proposed by the
Liver Cancer Study Group of Japan (2009 Revised Version). Hepatol
Res. 40:686–692. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Hu BS, Chen K, Tan HM, Ding XM and Tan JW:
Comparison of laparoscopic vs open liver lobectomy (segmentectomy)
for hepatocellular carcinoma. World J Gastroenterol. 17:4725–4728.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Sasaki A, Iwashita Y, Shibata K, Ohta M,
Kitano S and Mori M: Preoperative transcatheter arterial
chemoembolization reduces long-term survival rate after hepatic
resection for resectable hepatocellular carcinoma. Eur J Surg
Oncol. 32:773–779. 2006. View Article : Google Scholar
|
|
20.
|
Choi GH, Kim DH, Kang CM, Kim KS, Choi JS,
Lee WJ and Kim BR: Is preoperative transarterial chemoembolization
needed for a resectable hepatocellular carcinoma? World J Surg.
31:2370–2377. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Sugo H, Futagawa S, Beppu T, Fukasawa M
and Kojima K: Role of preoperative transcatheter arterial
chemoembolization for resectable hepatocellular carcinoma: relation
between postoperative course and the pattern of tumor recurrence.
World J Surg. 27:1295–1299. 2003. View Article : Google Scholar
|
|
22.
|
Sergio A, Cristofori C, Cardin R, Pivetta
G, Ragazzi R, Baldan A, Girardi L, Cillo U, Burra P, Giacomin A and
Farinati F: Transcatheter arterial chemoembolization (TACE) in
hepatocellular carcinoma (HCC): the role of angiogenesis and
invasiveness. Am J Gastroenterol. 103:914–921. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Zhang Z, Liu Q, He J, Yang J, Yang G and
Wu M: The effect of preoperative transcatheter hepatic arterial
chemoembolization on disease-free survival after hepatectomy for
hepatocellular carcinoma. Cancer. 89:2606–2612. 2000. View Article : Google Scholar
|
|
24.
|
Adachi E, Matsumata T, Nishizaki T,
Hashimoto H, Tsuneyoshi M and Sugimachi K: Effects of preoperative
transcatheter hepatic arterial chemoembolization for hepatocellular
carcinoma. The relationship between postoperative course and tumor
necrosis. Cancer. 72:3593–3598. 1998. View Article : Google Scholar
|
|
25.
|
Chao Y, Li CP, Chau GY, Chen CP, King KL,
Lui WY, Yen SH, Chang FY, Chan WK and Lee SD: Prognostic
significance of vascular endothelial growth factor, basic
fibroblast growth factor, and angiogenin in patients with
resectable hepatocellular carcinoma after surgery. Ann Surg Oncol.
10:355–362. 2003. View Article : Google Scholar
|
|
26.
|
Poon RT, Lau C, Yu WC, Fan ST and Wong J:
High serum levels of vascular endothelial growth factor predict
poor response to transarterial chemoembolization in hepatocellular
carcinoma: a prospective study. Oncol Rep. 11:1077–1084. 2004.
|
|
27.
|
Shim JH, Park JW, Kim JH, An M, Kong SY,
Nam BH, Choi JI, Kim HB, Lee WJ and Kim CM: Association between
increment of serum VEGF level and prognosis after transcatheter
arterial chemoembolization in hepatocellular carcinoma patients.
Cancer Sci. 99:2037–2044. 2008.
|
|
28.
|
Ikai I, Arii S, Kojiro M, Ichida T,
Makuuchi M, Matsuyama Y, Nakanuma Y, Okita K, Omata M, Takayasu K
and Yamaoka Y: Reevaluation of prognostic factors for survival
after liver resection in patients with hepatocellular carcinoma in
a Japanese nationwide survey. Cancer. 101:796–802. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Gluer AM, Cocco N, Laurence JM, Johnston
ES, Hollands MJ, Pleass HC, Richardson AJ and Lam VW: Systematic
review of actual 10-year survival following resection for
hepatocellular carcinoma. HPB. 14:285–290. 2012.PubMed/NCBI
|
|
30.
|
Zhou L, Liu J and Luo F: Serum tumor
markers for detection of hepatocellular carcinoma. World J
Gastroenterol. 12:1175–1181. 2006.PubMed/NCBI
|
|
31.
|
Lim KC, Chow PK, Allen JC, Chia GS, Lim M,
Cheow PC, Chung AY, Ooi LL and Tan SB: Microvascular invasion is a
better predictor of tumor recurrence and overall survival following
surgical resection for hepatocellular carcinoma compared to the
Milan criteria. Ann Surg. 254:108–113. 2011. View Article : Google Scholar
|