Introduction
Malignant gliomas, including the most common and
fatal form glioblastoma multiforme (GBM), remain a challenge to
treat (1–14). In the United States alone, more
than 10,000 patients per year are newly diagnosed with GBM
(15). Despite ongoing trials, the
best currently available multimodal treatment approaches include
surgical resection followed by adjuvant radiation and temozolomide
treatment (RT/TMZ), resulting in a low median overall survival (OS)
ranging from 12.2–15.9 months (5,9–11,16).
Previous studies have shown that survival rates are significantly
influenced by certain clinical and molecular factors (2,3,6,8,17–21).
Of those, surgical gross total resection (GTR), which is defined as
a complete removal of the contrast enhancing portion of the tumor
as measured on postoperative MRI by volumetric analysis, is one of
the important independent predictors of patient survival (5,9–11,13).
Stupp et al showed a median survival rate of 18.8 months
after GTR versus 13.5 months after subtotal resection (STR) versus
9.4 months after biopsy alone (13). These findings were confirmed by
McGirt et al who in a large series of 700 GBM patients
demonstrated a survival benefit of approximately 5–6 months after
primary and secondary GTR, when compared to STR (9). A more recent study by Sanai et
al demonstrated that pursuing a more aggressive EOR results in
increased survival, especially in patients with a higher degree of
tumor removal. Thus, STR of 78% or more still yielded a significant
survival benefit in patients afflicted with GBM (11).
Beyond the EOR, the postoperative adjuvant treatment
regimen is most relevant in determining patient outcome and
multiple studies indicate that a combination of radio-chemotherapy
and especially RT/TMZ further improves patient survival (3,13,14).
However, the majority of previous studies have been comparing
survival and EOR in GBM patients admitted to a neurosurgical
service and receiving RT/TMZ (11,13,14)
and hence only examined a selected group of GBM patients. The
recently published studies by Koshy et al and Johnson et
al were the first groups comparing survival of GBM patients
from the Surveillance, Epidemiology and End Results (SEER) program
before and during the TMZ era and showed a significant survival
benefit in TMZ treated patients (22,23).
In these studies, however, only patients from the last decade
(2000–2008) were included for analysis, without subgroup analysis
with regard to RT and EOR. In our here presented population-based
study, which spans over the past three decades, we provide a
comprehensive analysis of GBM patient data to demonstrate the
overall and age-specific impact of RT compared to EOR and its
relevance for prognosis.
Materials and methods
SEER and data extraction
The Surveillance, Epidemiology and End Results
(SEER) program of the National Cancer Institute, established in
1973, collects incidence and survival data for patients with
malignant tumors from selected population-based cancer registries
across the US. Since this data set is population-based, it captures
a defined geographical area with a demographically well-defined
population representing 28% of the US population; regions were
selected by NCI for inclusion into SEER for their ability to
operate a population-based cancer registry and for their diverse
population subgroups. Data for this study were downloaded from the
SEER public-use homepage (http://seer.cancer.gov/, NCI, Bethesda, MD) and were
converted to Microsoft Access and Excel databases for data
extraction and further analysis.
Patient population and variable
collection
A total of 21,783 patients diagnosed with GBM (SEER
code 9440/ICD-O-3) between 1973 and 2007 was identified and
included for analysis in this study. Of those, the following
patient variables were analyzed: year of diagnosis, age at
diagnosis, gender, race/ethnicity, laterality of lesion, number of
primaries (single versus multiple lesions), radiation treatment
(RT), extent of resection (EOR) and survival. For comparative
analysis the maximum common amount of available patients per set of
variables was chosen.
In a second step patients were binned in 10-year age
intervals (<20, 20–30, 30–40, 40–50, 50–60, 60–70, 70–80 and
>80 years) and data were analyzed for overall and treatment
specific survival (Table II).
 | Table IISEER overall and treatment specific
GBM patient survival across age intervals. |
Table II
SEER overall and treatment specific
GBM patient survival across age intervals.
| All groups | <20 years | 20–30 years | 30–40 years | 40–50 years | 50–60 years | 60–70 years | 70–80 years | >80 years |
|---|
| Total patients | 6 (N=21,783) | 12 (N=235) | 20 (N=351) | 17 (N=745) | 13 (N=1,867) | 10 (N=3,654) | 7 (N=4,407) | 4 (N=3,808) | 2 (N=1,402) |
| GTR/RT | 11 (N=3,989) | 17 (N=62) | 20 (N=78) | 21 (N=207) | 16 (N=581) | 13 (N=1,018) | 10 (N=1,077) | 8 (N=825) | 6 (N=141) |
| STR/RT | 9 (N=6,614) | 16 (N=116) | 24 (N=193) | 17 (N=402) | 13 (N=853) | 10 (N=1,698) | 8 (N=1,959) | 6 (N=1,186) | 5 (N=207) |
| IR | 5 (N=1,417) | 9 (N=18) | 13 (N=24) | 11 (N=32) | 9 (N=129) | 7 (N=266) | 5 (N=356) | 4 (N=396) | 3 (N=196) |
| GTR/no RT | 3 (N=874) | 86 (N=11)a | 13 (N=13) | 10 (N=33) | 7 (N=55) | 5 (N=151) | 3 (N=201) | 2 (N=298) | 3 (N=112) |
| STR/no RT | 2 (N=1,939) | 8 (N=34) | 5 (N=23) | 2 (N=44) | 3 (N=155) | 2 (N=352) | 2 (N=554) | 2 (N=593) | 2 (N=184) |
| No surgery/no
RT | 1 (N=1,655) | 1 (N=12) | 1 (N=20) | 1 (N=27) | 1 (N=94) | 1 (N=169) | 1 (N=260) | 1 (N=510) | 1 (N=562) |
| Survival STR of GTR
(%) | 67 | 9 | 38 | 20 | 43 | 40 | 67 | 100 | 67 |
| Survival STR/RT of
GTR/RT (%) | 82 | 94 | 120 | 81 | 81 | 77 | 80 | 75 | 83 |
Statistical analysis
Factor analysis and Cox proportional hazards ratio
were used to determine the variables most closely correlated to
survival and most relevant for prognosis. Overall and treatment
specific survival was computed using the Kaplan-Meier method and
the log-rank test. Respective SEER data use policies have been
adhered to for this study. The statistical analysis was performed
using Microsoft Excel and Access 2010, JMP Pro 9.01 (SAS, Cary, NC)
statistical software packages.
Results
Demographics
The mean onset age of GBM in the analyzed patient
cohort of 21,783 individuals was 61.5 years (male, 60.5 years;
female, 63 years) and the gender distribution was 57% male
(N=12,447) and 43% female (N=9336). The ethnicity in the SEER
patient data set with GBM (SEER code 9440/ICD-O-3) was
predominantly Caucasian white with 92% (N=20,057) followed by
African American 0.05% (N=966), similar ethnical GBM data were
previously reported (24,25).
Cluster correlation and survival
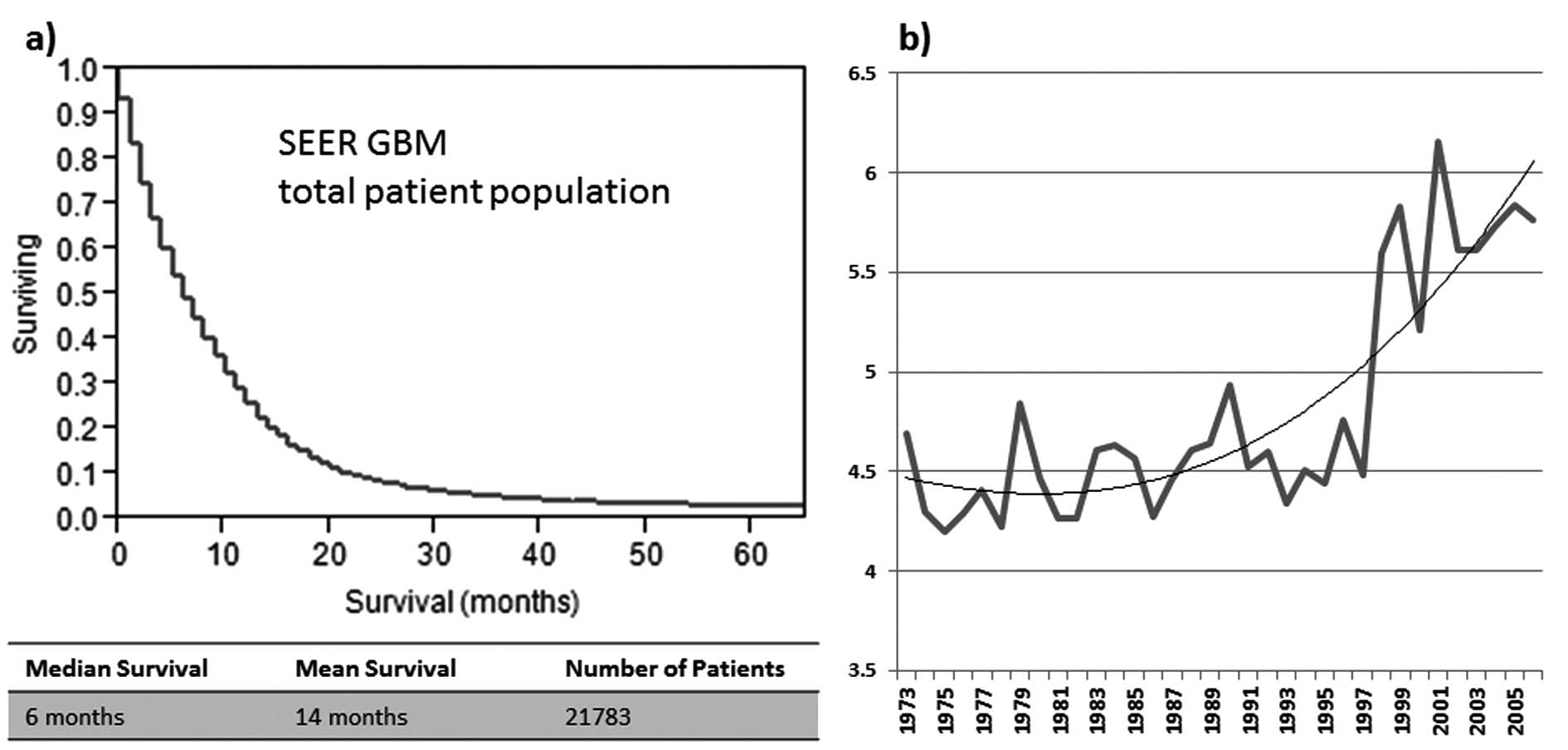
The median OS of all patients was 6 months for
individuals included in this study (women, 6 months; men, 7 months)
(Fig. 1a). The age-adjusted
survival development over time shows a marked increase of overall
median survival after 1997 (Fig.
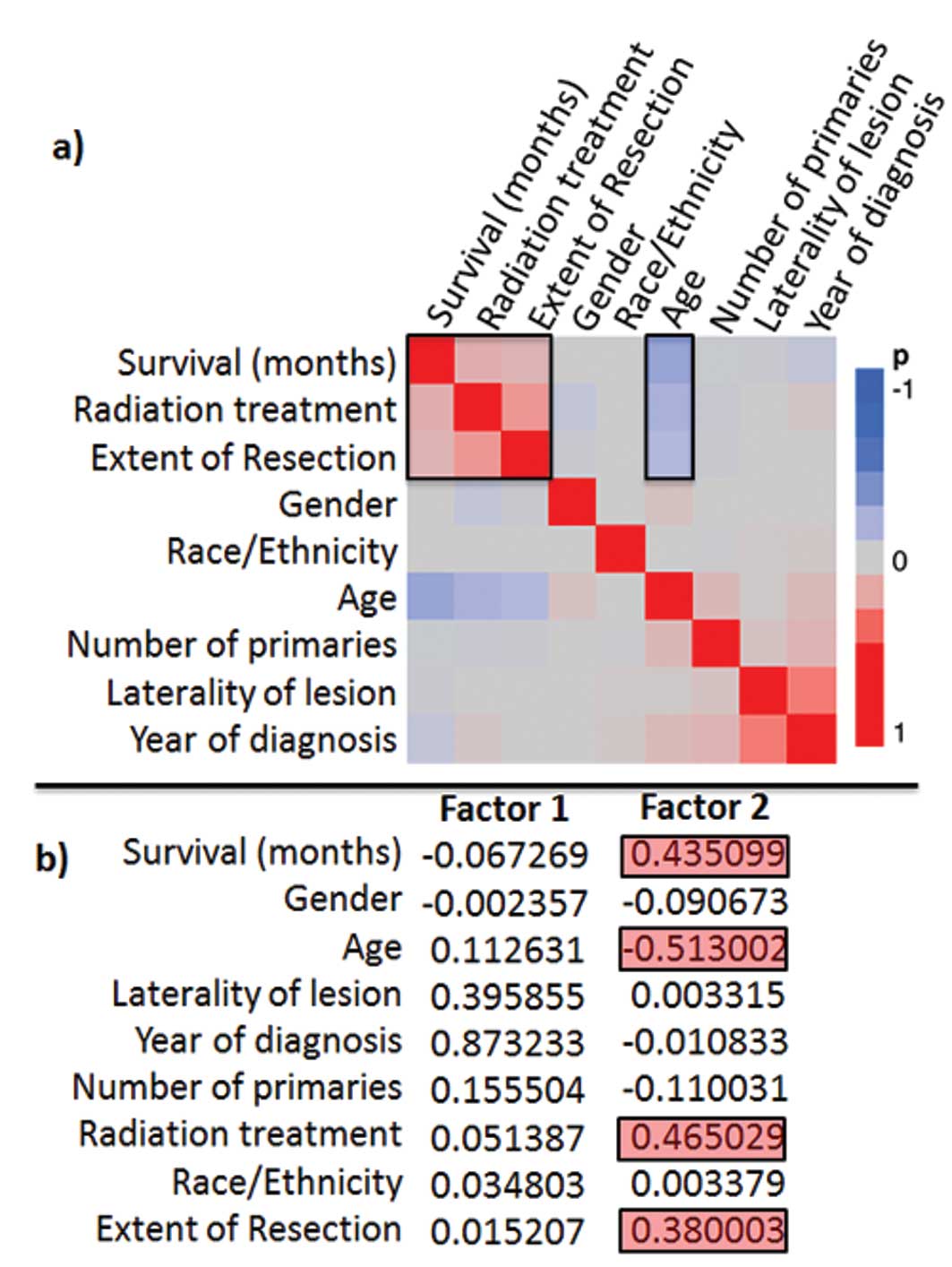
1b). In the factor analysis the best negative correlation to
survival was observed for age, the best positive correlation for
EOR and RT (Fig. 2a and b). The
most significant (p<0.00001) prognostic survival variables were:
RT, age and EOR and in the proportional hazard’s ratio test, these
3 variables were found to be significant independent predictors for
patient survival (Table I).
 | Table IEffect likelihood ratio table: EOR, RT
and age are significant independent predictor for patient survival
in GBM. |
Table I
Effect likelihood ratio table: EOR, RT
and age are significant independent predictor for patient survival
in GBM.
| Variable | Nparm | DF | Chi-square | p-value |
|---|
| Extent of
resection | 4 | 4 | 897.256437 | <0.00001 |
| Radiation treatment
(RT) | 3 | 3 | 2,739.97669 | <0.00001 |
| Age | 1 | 1 | 1,015.88061 | <0.00001 |
With regard to EOR, the median OS reported for GTR
patient was 10 months compared to 8 months for STR or 5 months for
biopsy only (all p<0.00001) (Fig.
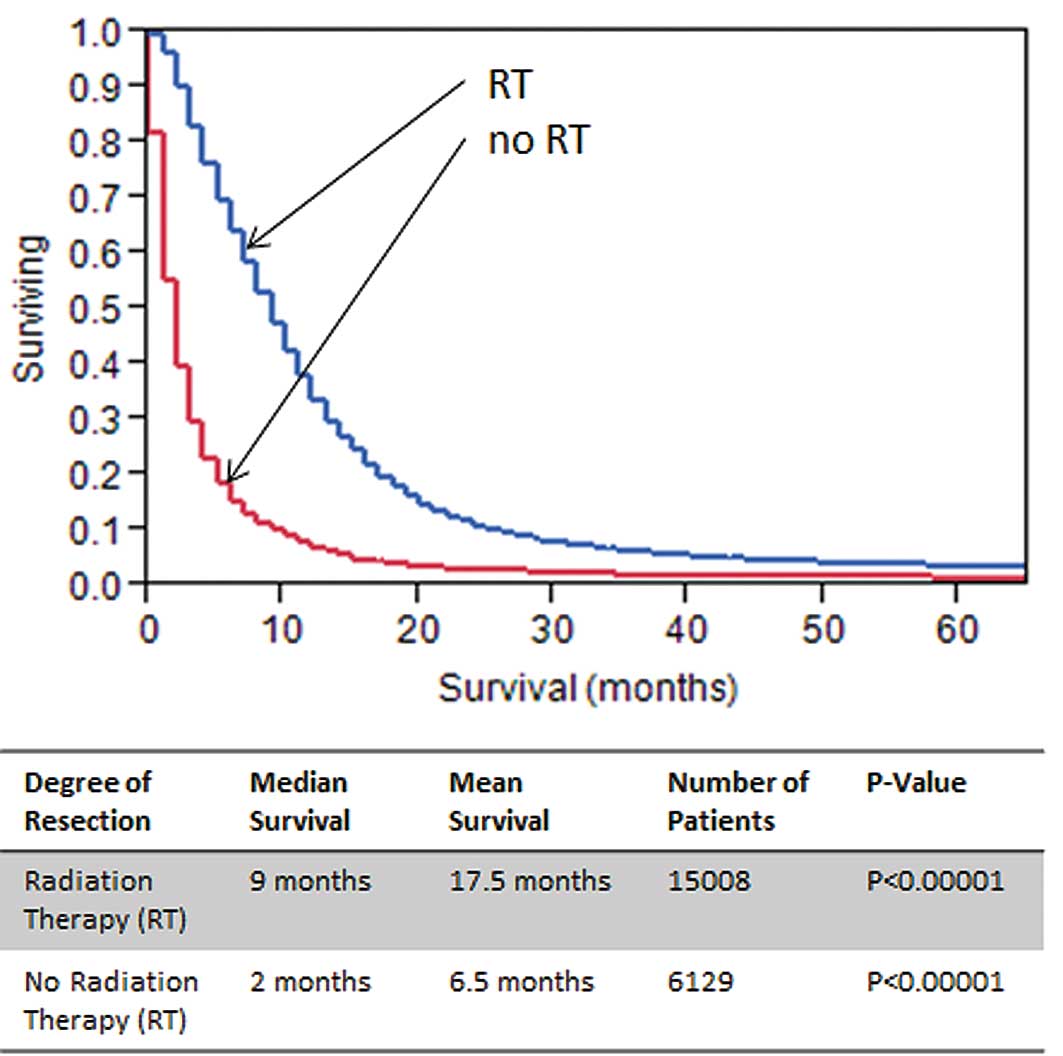
3). Kaplan-Meier analysis of patients with and without RT
reported a survival benefit of approximately 7 months for patients
in the RT group (p<0.00001; Fig.
4). Because both RT and EOR were significant and independent
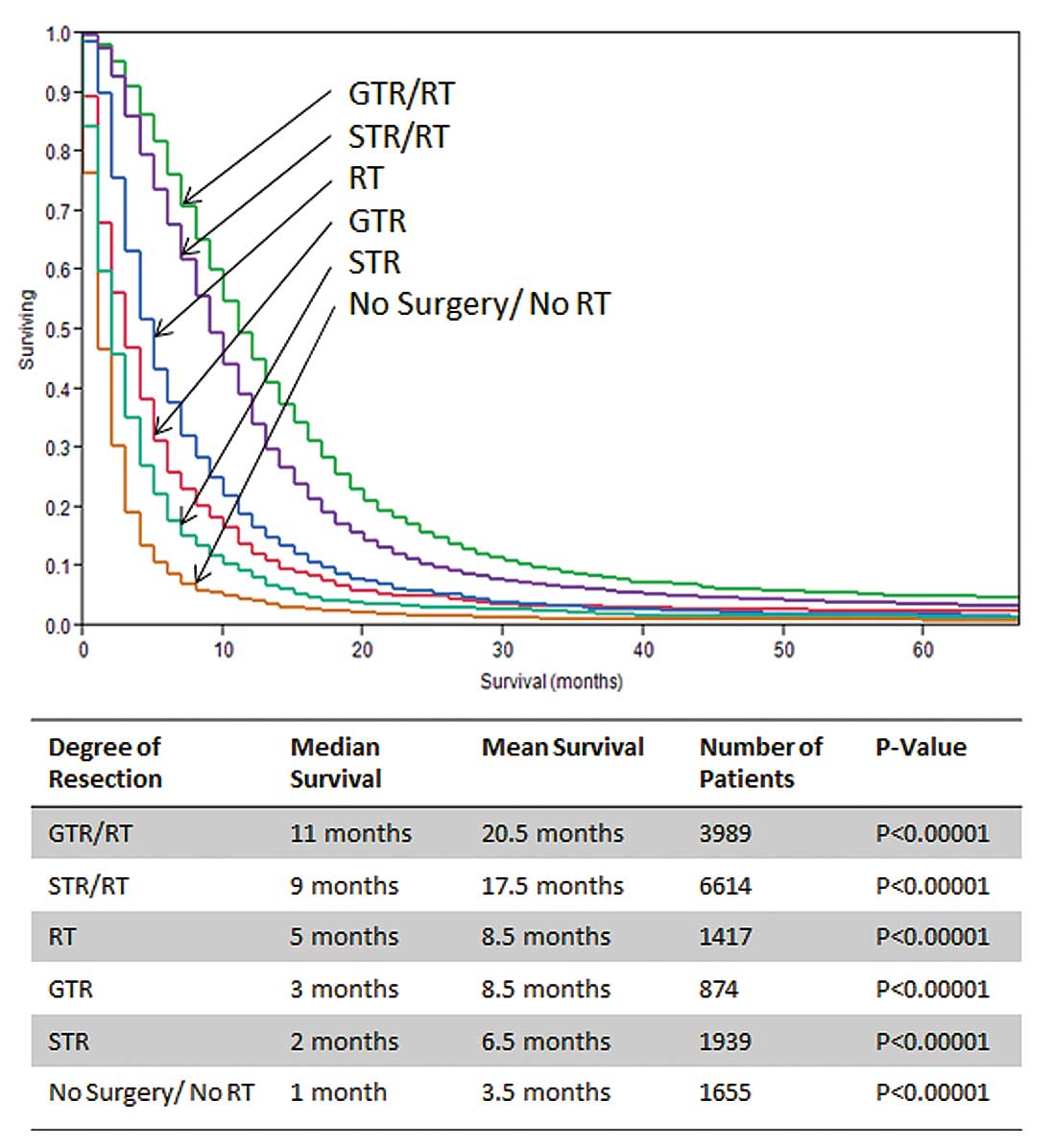
prognostic variables, we further subcategorized the patient cohort
as follows: i) GTR/RT, ii) GTR alone, iii) STR/RT, iv) STR alone,
v) RT alone and vi) no surgery/no RT. In this subset analysis,
patients with neither surgery nor RT showed a median survival of
only 1 month. Likewise STR survival without RT demonstrated a low
survival (2 months) and GTR without RT showed a relatively low
median survival of 3 months. Interestingly, patients treated with
RT without any surgical intervention had a higher median survival
(5 months) than patients treated with any surgical monotherapy.
When surgery and RT was combined, median survival markedly
increased for both GTR/RT (11 months) and STR/RT (9 months)
(Fig. 5, Table II).
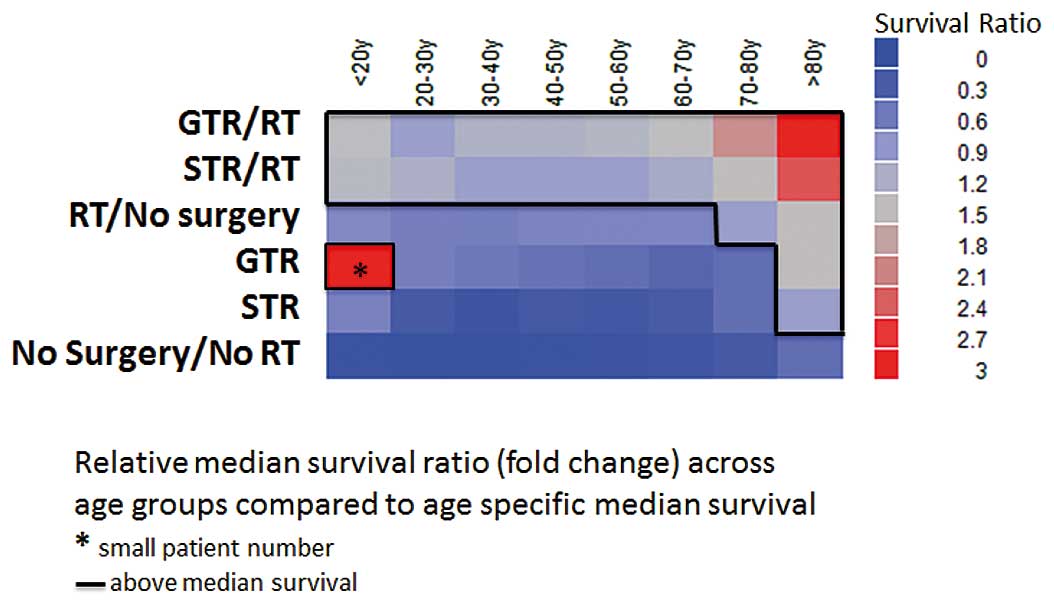
Age distribution and survival
variables
Analysis of patients binned into 10-year age
subgroups demonstrates that patients receiving neither surgical
treatment nor RT showed a median survival of only 1 month across
all age groups. Furthermore, patients undergoing STR without RT
showed a survival advantage of 1 month when 30 years and older,
whilst STR in patients below age 30 showed an average of more than
5 months of increased survival when compared to patient receiving
no treatment at all.
GTR alone improved survival when compared to both no
treatment and STR in all age groups. However, relative to the age
specific overall median survival, GTR alone results in below
average survival in patients younger than 70 years (Table II, Fig. 6). RT, when employed as a
monotherapy, is superior to both GTR and STR alone across all age
groups. But it results in significantly decreased median survival
compared to overall age-specific survival, except in elderly
patients above 70 years of age. Either combined GTR or STR with
adjuvant RT yielded the highest median survival across all examined
age groups. With increasing age, the relative survival advantage
compared with the overall age specific median survival advantage
increases with more aggressive therapy (Fig. 6). When comparing median survival
times in patients undergoing STR or GTR monotherapies across age
intervals, less distinct survival differences are demonstrated with
increasing age. Of note, in the setting of adjuvant RT; STR and GTR
yielded very similar effects on survival in the age groups below 30
years (STR/GTR, 94–120%). This relative survival contribution
remained stable at 75–83% when examined across advancing age groups
(Table II).
Discussion
In this study, we identified RT, age and EOR to be
significant and independent prognostic factors in a cohort of
21,783 GBM patients. From the variables selected for analysis, RT,
age and EOR were most closely correlated with outcome as reflected
in survival times (Fig. 2). These
three variables were all highly prognostic (p<0.00001) as well
as independent predictors for survival in the Cox proportional
hazards ratio (Table I). The fact
that age continues to be a strong predictor of survival is
concordant with previously published literature. Among various
other age-related general factors, this is thought to reflect that
GBM in older patients are most commonly of the primary (de
novo) type. This type is more aggressive, invasive, and has a
different genomic and molecular microenvironment (26,27)
when compared to secondary GBM seen more commonly in younger
patients.
With regard to the relevance of EOR, the scientific
debate is ongoing, but our study clearly demonstrates an overall
median survival advantage, which is significantly higher for GTR
(10 months) when compared to STR (8 months) or biopsy (5 months)
(p<0.00001; Fig. 3). The
survival benefit of GTR compared to STR is concordant with the
literature; however, studies disagree on a threshold of resection
leading to a significant survival advantage (10,11,13).
Nevertheless, our analysis indicates that any
reasonable surgical resection improves survival by 8–10 months in
the setting of adjuvant RT. This is of great clinical significance
and challenges the previous widely held belief, which suggests that
only GTR or near total resections of greater than 98% of tumor
volume offers a significant survival benefit (5).
A recent study by Sanai et al(11), similarly challenged this doctrine
by demonstrating that STR of more than 78% of tumor volume already
benefitted the patient with respect to post-treatment survival. Our
results as well as those from the latter study suggest that EOR is
a significant predictor of survival and offers hope to those
patients in whom GTR is not achievable. Surgical resection in
absence of adjuvant RT improves median survival by 1–2 months only
and thus confers a minimal advantage compared to patients who did
neither undergo resection nor RT. Conversely, RT alone resulted in
a 2–3 months prolonged survival period when compared to monotherapy
with GTR or STR (Fig. 5). The
relative shorter survival times in either the surgery alone (2–3
months) or RT alone groups (5 months) compared to concurrent
treatment (9–11 months) suggests a beneficial treatment synergy,
but may also reflect the fact that healthier patients are more
likely to receive more comprehensive therapy.
The presented median OS of 6 months derived from the
whole SEER GBM patient cohort is relatively low, when compared to
other published studies presenting a median OS range of 12.2–15.9
months (5,9–11).
This might be due to the fact that these data sets, unlike the
SEER, for the most part represent a highly preselected group of
patients (e.g. admitted to an expert neurosurgical oncology
service, also receiving adjuvant TMZ/RT at different intervals)
(9–11).
The strength of this study clearly is the dataset
spanning more than 3 decades with the advantage of not being
influenced by selection biases associated with treatment and
referral patterns seen in many of the previous studies.
Furthermore, a clear upward trend for survival (Fig. 1b) can be seen starting in 1997,
this may coincide with the introduction of novel chemotherapeutic
agents, refined irradiation protocols, and advanced surgical
techniques (5,11,28,29).
These results are also in agreement to the recently published
studies comparing the adjuvant era before and after introduction of
temozolomide therapy (22,23). Both studies showed a significant
patient survival benefit after the presented EORTC/NCIC trial in
2004. Regardless of this study’s limitations inherent to SEER (such
as lack of adjuvant chemotherapy and quantitative EOR data, as well
as less standardized data collection/documentation compared to
smaller single center studies) the strength of our study lies
within the large scale population-based approach.
We clearly corroborated data that patients, who
receive postoperative RT survived 7 months longer than non-radiated
patients (p<0.00001; Fig. 4).
It was also obvious that RT without surgery resulted in
consistently better survival than survival in those patients who
were treated by surgery alone, whether GTR or STR (Fig. 5). This difference was confirmed
across all age groups (Table II).
This is of great clinical significance since elderly patients or
those with surgical contraindications can benefit from treatment,
even when receiving RT alone. RT as a suitable treatment modality
for GBM patients was analyzed in the elderly before and our results
confirm the findings of these studies with a significant survival
benefit of patients receiving RT compared to patients who do not
(12,30,31).
However, the more aggressive the treatment regimen, the higher the
relative survival gain compared to the age specific median survival
and this is seen particularly in the elderly patient (Fig. 6). Thus, we were able to show that
even patients of 80 years and older, if eligible, should undergo
aggressive treatment since this can result in prolonged survival.
In general, our study demonstrates that a comprehensive treatment
regimen is of utmost importance to prolong survival in GBM patients
across all age groups.
Age specific analysis suggested GTR to be superior
to STR particularly in younger patients. Interestingly, in patients
below 30 years of age this advantage was lost when RT was added to
the treatment regimen, stressing the importance of adjuvant RT.
Acknowledgements
Surveillance Research Program,
National Cancer Institute SEER*Stat software (seer.cancer.gov/seerstat).
References
|
1
|
Hess KR, Broglio KR and Bondy ML: Adult
glioma incidence trends in the United States, 1977–2000. Cancer.
101:2293–2299. 2004.PubMed/NCBI
|
|
2
|
Buckner JC: Factors influencing survival
in high-grade gliomas. Semin Oncol. 30:10–14. 2003. View Article : Google Scholar
|
|
3
|
Hegi ME, Diserens AC, Gorlia T, et al:
MGMT gene silencing and benefit from temozolomide in glioblastoma.
N Engl J Med. 352:997–1003. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iwamoto FM, Reiner AS, Nayak L, Panageas
KS, Elkin EB and Abrey LE: Prognosis and patterns of care in
elderly patients with glioma. Cancer. 115:5534–5540. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lacroix M, Abi-Said D, Fourney DR, et al:
A multivariate analysis of 416 patients with glioblastoma
multiforme: prognosis, extent of resection, and survival. J
Neurosurg. 95:190–198. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lamborn KR, Chang SM and Prados MD:
Prognostic factors for survival of patients with glioblastoma:
recursive partitioning analysis. Neuro Oncol. 6:227–235. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lefranc F, Rynkowski M, DeWitte O and Kiss
R: Present and potential future adjuvant issues in high-grade
astrocytic glioma treatment. Adv Tech Stand Neurosurg. 34:3–35.
2009.PubMed/NCBI
|
|
8
|
Li SW, Qiu XG, Chen BS, et al: Prognostic
factors influencing clinical outcomes of glioblastoma multiforme.
Chin Med J (Engl). 122:1245–1249. 2009.PubMed/NCBI
|
|
9
|
McGirt MJ, Chaichana KL, Gathinji M, et
al: Independent association of extent of resection with survival in
patients with malignant brain astrocytoma. J Neurosurg.
110:156–162. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sanai N and Berger MS: Glioma extent of
resection and its impact on patient outcome. Neurosurgery.
62:753–764. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sanai N, Polley MY, McDermott MW, Parsa AT
and Berger MS: An extent of resection threshold for newly diagnosed
glioblastomas. J Neurosurg. 115:3–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Scott J, Tsai YY, Chinnaiyan P and Yu HH:
Effectiveness of radiotherapy for elderly patients with
glioblastoma. Int J Radiat Oncol Biol Phys. 81:206–210. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stupp R, Hegi ME, Mason WP, et al: Effects
of radiotherapy with concomitant and adjuvant temozolomide versus
radiotherapy alone on survival in glioblastoma in a randomised
phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet
Oncol. 10:459–466. 2009.
|
|
14
|
Stupp R, Mason WP, van den Bent MJ, et al:
Radiotherapy plus concomitant and adjuvant temozolomide for
glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
CBTRUS: Central Brain Tumor registry of
the United States. http://www.cbtrus.org/.
2008.
|
|
16
|
Zinn PO, Sathyan P, Mahajan B, Bruyere J,
Hegi M, Majumder S and Colen RR: A novel volume-age-KPS (VAK)
glioblastoma classification identifies a prognostic cognate
microRNA-gene signature. PLoS One. 7:e415222012.PubMed/NCBI
|
|
17
|
Marko NF, Toms SA, Barnett GH and Weil R:
Genomic expression patterns distinguish long-term from short-term
glioblastoma survivors: a preliminary feasibility study. Genomics.
91:395–406. 2008. View Article : Google Scholar
|
|
18
|
Phillips HS, Kharbanda S, Chen R, et al:
Molecular subclasses of high-grade glioma predict prognosis,
delineate a pattern of disease progression, and resemble stages in
neurogenesis. Cancer Cell. 9:157–173. 2006. View Article : Google Scholar
|
|
19
|
Colman H, Zhang L, Sulman EP, et al: A
multigene predictor of outcome in glioblastoma. Neuro Oncol.
12:49–57. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cooper LA, Gutman DA, Long Q, et al: The
proneural molecular signature is enriched in oligodendrogliomas and
predicts improved survival among diffuse gliomas. PLoS One.
5:e125482010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zinn PO, Majadan B, Sathyan P, et al:
Radiogenomic mapping of edema/cellular invasion MRI-phenotypes in
glioblastoma multiforme. PLoS One. 6:e254512011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Johnson DR and O’Neill BP: Glioblastoma
survival in the United States before and during the temozolomide
era. J Neurooncol. 107:359–364. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koshy M, Villano JL, Dolecek TA, et al:
Improved survival time trends for glioblastoma using the SEER 17
population-based registries. J Neurooncol. 107:207–212. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Barnholtz-Sloan JS, Sloan AE and Schwartz
AG: Racial differences in survival after diagnosis with primary
malignant brain tumor. Cancer. 98:603–609. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barnholtz-Sloan JS, Maldonado JL, Williams
VL, et al: Racial/ethnic differences in survival among elderly
patients with a primary glioblastoma. J Neurooncol. 85:171–180.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ang C, Guiot MC, Ramanakumar AV, Roberge D
and Kavan P: Clinical significance of molecular biomarkers in
glioblastoma. Can J Neurol Sci. 37:625–630. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kanu OO, Hughes B, Di C, et al:
Glioblastoma multiforme oncogenomics and signaling pathways. Clin
Med Oncol. 3:39–52. 2009.PubMed/NCBI
|
|
28
|
Chang JE, Khuntia D, Robins HI and Mehta
MP: Radiotherapy and radiosensitizers in the treatment of
glioblastoma multi-forme. Clin Adv Hematol Oncol. 5:894–915.
2007.PubMed/NCBI
|
|
29
|
Clarke J, Butowski N and Chang S: Recent
advances in therapy for glioblastoma. Arch Neurol. 67:279–283.
2010. View Article : Google Scholar
|
|
30
|
Keime-Guibert F, Chinot O, Taillandier L,
et al: Radiotherapy for glioblastoma in the elderly. N Engl J Med.
356:1527–1535. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lai R, Hershman DL, Doan T and Neugut AI:
The timing of cranial radiation in elderly patients with newly
diagnosed glioblastoma multiforme. Neuro Oncol. 12:190–198. 2010.
View Article : Google Scholar : PubMed/NCBI
|