Introduction
CD133 was first presented as a key marker for brain
tumour stem cells (BTSCs) in 2003 when in vitro experiments
indicated that CD133+ cells possess significantly
enhanced ability to differentiate, proliferate and self-renew. The
research also showed CD133+ cells could generate new
malignancies that were phenotypically and histologically similar to
the tumour of origin (1). However,
since these findings were published, other researchers have been
able to demonstrate tumourigenesis by CD133− cells and
challenged the role of CD133 in tumour initiation (2,3).
Despite the controversy, the link between CD133 and tumourigenesis
has remained a ‘hot’ topic in CSC research (4–6).
Current evidence also indicates that CD133 expression in CSCs
differs according to cell cycle phases in vitro, although
stem cell potency and differentiating capabilities are preserved
(7). Indeed, CD133 expression, and
thus perhaps also glycosylation, fluctuates during the cell cycle,
being low in quiescent cells in G0 or G1 but higher in subsequent
phases (7). This has important
implications for using CD133 to enrich CSCs as non-dividing
invading cells and other quiescent cells within the tumour mass
could be missed by immunological detection of CD133.
Several splice variants of CD133 have been
identified in man (8). This has
potential impact on the CSC detection as CD133 may be present but
lacking the antibody specific epitope so cells could be described
falsely as CD133−. Indeed, it has been reported that
CD133− glioblastoma cells (determined by CD133/1
antibody) express a truncated variant of the protein (9). Post-translational glycosylation of
CD133 may also affect antibody detection since the commonly used
antibody, CD133/1, is likely to detect CD133-expressing cells only
when the epitope AC133 is glycosylated, causing concerns over the
limitations of glycosylated CD133 epitopes in isolating CSCs
(10). Furthermore, the role of
CD133 in brain tumour biology must be elucidated with consideration
of the tumour microenvironment. Hypoxic microenvironment is a
feature of glioblastoma multiforme (GBM), the most common and
malignant of primary glial tumours in the brain (11). Hypoxia is believed to trigger BTSC
proliferation and invasion as well as to promote resistance to
therapy (12,13). It has been demonstrated by us and
others, using the CD133/1 antibody, that CD133 expression increases
under hypoxic culture conditions (14–17),
suggesting a similar CD133 expression pattern in vivo. A
recent study shows that enhanced expression of OCT3/4
(octamer-binding transcription factor 3/4) and SOX2 (SRY-box
containing gene 2) by HIF-1α (hypoxia-inducible factor 1α) and
HIF-2α (hypoxia-inducible factor 2α) is required for
hypoxia-induced CD133 expression in cultured human lung cancer
cells (18). The study also
reveals the direct binding of OCT4 and SOX2 to the P1 promoter of
CD133 gene, further elucidating the underlying mechanism since P1
is most strongly associated with hypoxia-induced promoter activity
of CD133 transcription (18).
However, although it is widely accepted that hypoxia enhances CD133
expression, it is not yet well understood how the immunodetection
of CD133 may be affected by oxygen tension. A recent study
demonstrates inconsistent CD133 immunostaining patterns in
glioblastoma tissues using four different antibody clones,
reflecting different binding sites as well as glycosylation status.
Yet the implication of normoxic and hypoxic CD133+
niches remains unclear (19). In
this research we set out to investigate the possibility that CD133
glycosylation might be influenced by different oxygen tensions and
that this may, in turn, determine the true expression of CD133 in
glioma-derived cell populations.
We have previously reported the biological and
molecular characteristics of one unique paediatric GBM cell line,
IN699, which contains an unusually high population of
CD133-positive cells, where parent culture and the
CD133+/CD133− subpopulations derived
therefrom under normoxic and hypoxic culture conditions were
subject to thorough investigation (14). Our data from immunocytochemistry
and flow cytometry using neural stem cell specific antigenic
markers indicate similar stemness and differentiation capacities of
both CD133+ and CD133− cells. Molecular
cytogenetic study also shows identical genomic imbalances between
CD133+ and CD133− fractions. Under hypoxic
conditions, CD133+ cells demonstrated a significantly
enhanced proliferative rate whereas a significant increase in
invasive propensity was observed in the CD133− cells.
Furthermore, hypoxia promoted CD133 expression associated with
phenotype of poorly differentiated cells with cancer stem cell
markers (14). In the present
study, we further investigated whether CD133 glycosylation was
regulated by changes in oxygen tension and how hypoxia might affect
the immuno detection of CD133. Using western blotting (WB), flow
cytometry and immunocytochemistry (ICC), changes in CD133
expression and glycosylation under normoxic and hypoxic conditions
were demonstrated by CD133/1 and another commercially available
CD133 antibody (ab19898, see below) in early passage biopsy-derived
IN699 cells.
Materials and methods
Cell line
IN699 cells (passages 14–19) known to contain a high
proportion of CD133/1+ cells were used in this study
(14). Cells were cultured in
Neurobasal-A medium (Fisher Scientific, Loughborough, UK) with
supplements under normoxic (21% oxygen) and hypoxic (3% oxygen)
conditions as described in previous studies (14,20).
Cells were grown to 90% confluency before experimental use.
Antibodies
Primary antibodies
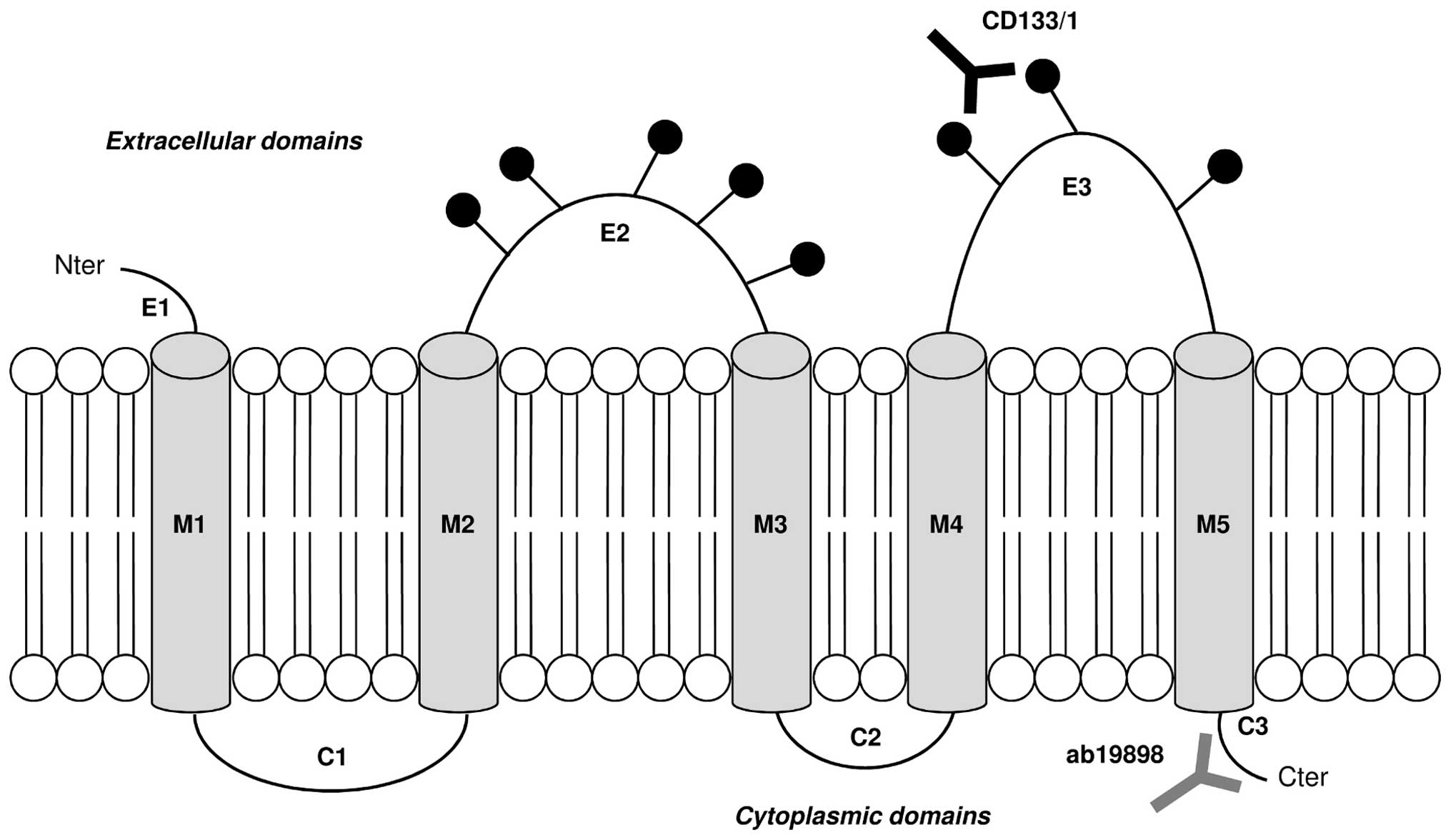
Ab19898 (Abcam, Cambridge, UK) recognises the
intracellular unglycosylated amino acid residues 848–865 of CD133
near the C-terminus. It was used in WB (1:100), ICC (1:200) and
flow cytometry (1:100) to investigate the presence of the CD133
protein.
CD133/1 (W6B3C1) pure for WB (1:200) and CD133/1
(AC133) pure for ICC (1:200) and flow cytometry (1:50) were
purchased from Miltenyi-biotec Ltd. (Surrey, UK). Both antibodies
recognise the glycosylated amino acid residues 508–792 on the
extracellular loops of CD133. They were used to detect changes in
CD133 glycosylation under normoxic and hypoxic conditions. The
binding sites of both CD133 primary antibodies are illustrated in
Fig. 1. Anti-actin (Sigma-Aldrich,
Dorset, UK) was used as a control in the WB experiment
(1:1000).
Secondary antibodies
Horseradish peroxidase (HRP)-conjugated IgG
(Invitrogen/Life Technologies Ltd., Paisley, UK) was used for
chemiluminescent detection in WB (1:1000). Fluorochrome-conjugated
AlexaFluor-488 and -568 (Invitrogen/Life Technologies Ltd.) were
used in flow cytometry (1:500) and ICC (1:500).
Western blotting (WB)
Western blotting was performed according to standard
protocol. Briefly, cell lysates (20 μg of protein) from
normoxic and hypoxic IN699 cells were separated in the ‘any kDa’
pre-cast gel (Bio-Rad Laboratories Ltd., Hemel Hempstead, UK) and
transblotted onto a polyvinylidene difluoride membrane (GE
Healthcare Life Sciences, Little Chalfont, UK). Proteins were
detected using the corresponding primary antibody and horseradish
peroxidase-linked secondary antibodies; the blot was visualized by
chemiluminescence (Interchim SA/Cheshire Sciences Ltd., Chester,
UK) with the GBOX Chemi XT16 software (Syngene, Cambridge, UK).
Quantitative real-time PCR
(qRT-PCR)
The mRNA level of CD133 in IN699 cells
(normoxic and hypoxic) was quantified by qRT-PCR using TaqMan Gene
Expression Assay with predesigned probe spanning the exon 9–10
junction, which detects all CD133 transcript variants reported in
humans (8). The assay was
performed on the ABI 7500 Real-Time PCR System (Applied
Biosystems/Life Technologies Ltd.). The expression of CD133
was normalised to that of the endogenous gene, β-actin, served as
an internal control. The relative quantification of CD133 in
normoxic and hypoxic IN699 cells was carried out using the
2−ΔΔCt method (21).
ICC
ICC was carried out following established procedure
in our laboratory (14). For the
intracellular antibody (ab19898) detection, cells were
permeabilised with 0.02% Triton X-100 (Sigma-Aldrich) at 4°C for 10
min. Permeabilised and nonpermeabilised cells were then incubated
with the relevant primary antibody (ab19898 and CD133/1) at room
temperature for 1 h, following another 30-min incubation with the
corresponding secondary antibody. Hoechst Blue (1:200)
(Sigma-Aldrich) nuclear counterstain was added to each well and the
coverslips were then mounted on microscope slides with a small drop
of VectorShield mounting medium (Vector Laboratories Ltd., Orton
Southgate, UK). The slides were observed using a Zeiss Axio Imager
Epifluorescence microscope (Carl Zeiss Ltd., Welwyn Garden City,
UK). Images were captured using Velocity software (V5.2,
Perkin-Elmer, Cambridge, UK).
Flow cytometry
Flow cytometry was performed with CD133/1 and
ab19898 in three independent experiments as described previously
(14). For the extracellular
primary antibody CD133/1, normoxic and hypoxic IN699 cells were
incubated with CD133/1 at 4°C for 30 min excluding the control
samples. Then the anti-mouse secondary antibody AlexaFluor-488 was
added to all samples and the cells were incubated at 4°C for
another 15 min. After incubation the cells were resuspended in 1%
goat serum/PBS (Sigma-Aldrich) and transferred to
fluorescence-activated cell sorting (FACS) tubes. The analysis was
carried out on a FACSCalibur (BD Bioscience, Oxford, UK) and
propidium iodide (PI) (Sigma-Aldrich) was added to each tube
shortly before analysis in order to enable cell viability
correction. For the intracellular primary antibody ab19898, cells
were permeabilised with Cytofix/Cytoperm™ (BD Bioscience) at 4°C
for 20 min before adding ab19898. The remaining steps were
identical to those for the non-permeabilised cells, except that no
PI was added prior to visualisation.
Automated magnetic cell sorting
(autoMACS)
CD133-expressing cells were sorted using the
automated magnetic cell sorting system (autoMACS™) (Miltenyi
Biotec) (14). Briefly, IN699
cells were labelled with CD133/1 antibody conjugated to a magnetic
microbead (Miltenyi Biotec) and separated into CD133/1-positive and
negative populations. The negative cells were subcultured under
normoxic and hypoxic conditions then harvested at 24, 48 and 72-h
time points to analyse CD133/1 expression by flow cytometry.
Statistics
Statistical evaluation was carried out by Student’s
t-test for qRT-PCR and flow cytometry data analysis. A p-value of
<0.05 was taken as significant.
Results
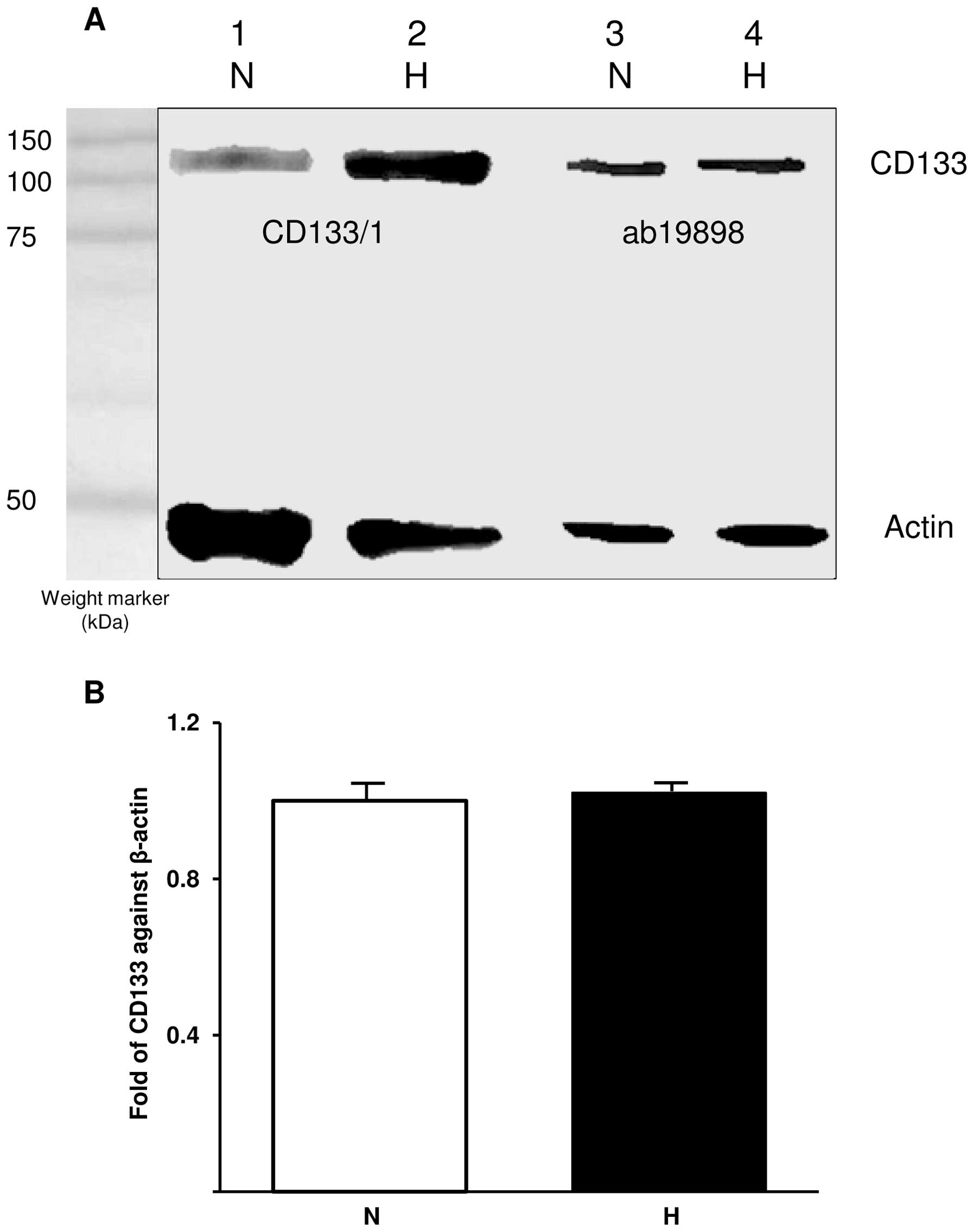
Analysis of CD133 expression by WB and
qRT-PCR
WB was used to semi-quantitatively determine the
presence of CD133 in normoxic and hypoxic cells. The blot was
probed with ab19898 and CD133/1 (W6B3C1) for CD133 detection and
actin was used as a control (Fig.
2A). Comparison between CD133 bands detected with CD133/1
indicates an increase in glycosylated CD133 under hypoxic
conditions whereas ab19898 detected similar amounts of CD133 under
both conditions. The expression of CD133 was further investigated
at mRNA level using qRT-PCR. In accordance with WB, CD133 mRNA was
detected at similar levels in both normoxic and hypoxic cells when
CD133 expression was normalised against that of the internal
control gene, β-actin (p=0.343; Fig.
2B).
Immunostaining of CD133
ICC images demonstrated the intracellular staining
by ab19898 whereas CD133/1 stained extracellularly giving a clear
outline of the cells (Fig. 3).
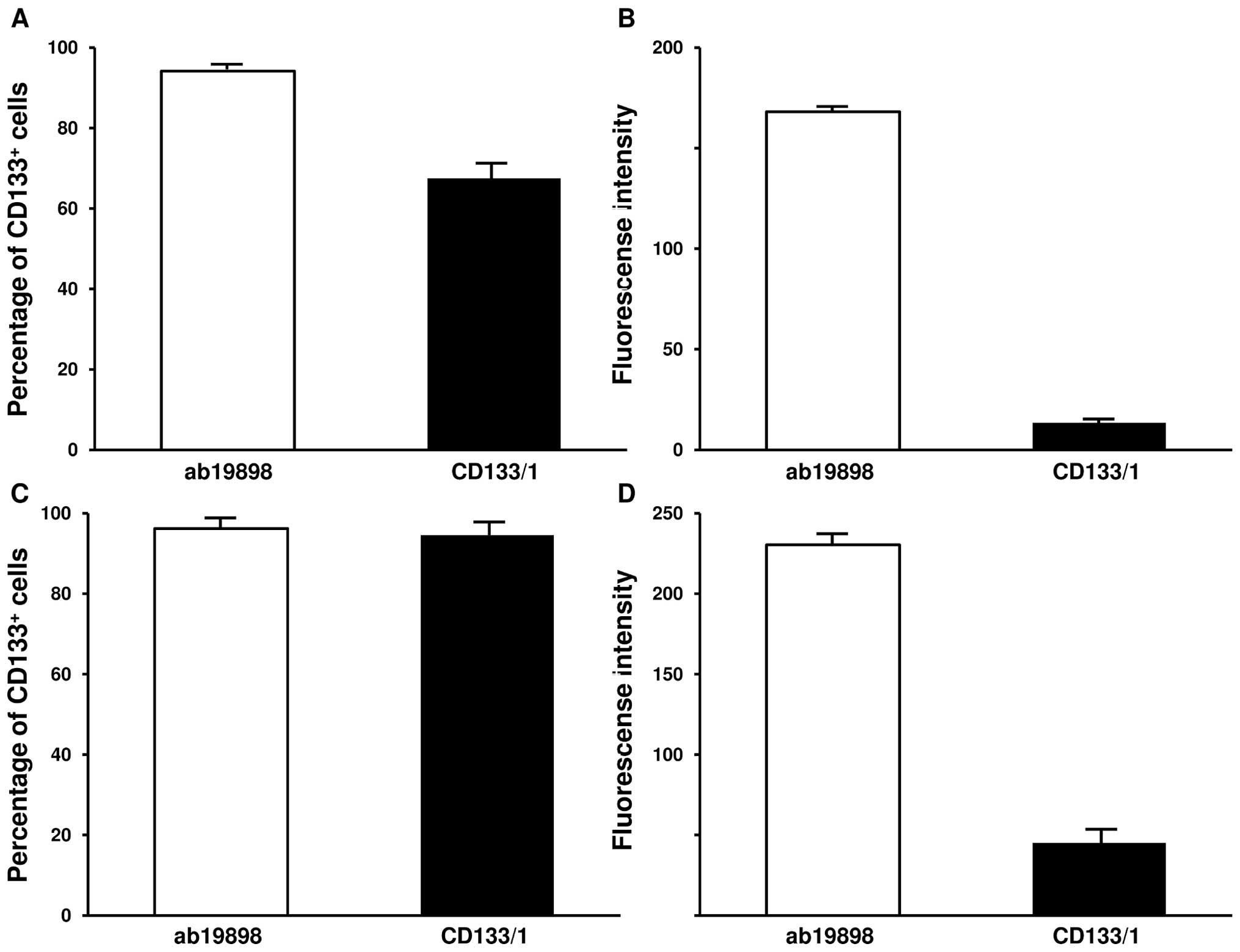
Quantification of CD133 by flow
cytometry
The IN699 cells were further characterised with
CD133/1 (AC133) and ab19898 antibodies by flow cytometry to
establish the percentage of CD133+ cells by the MFI
(mean fluorescence intensity) and the average expression level of
the protein in individual cells by GFI (geomean fluorescence
intensity). In accordance with previous data (14), CD133/1 detected 67.5% and 94.5% of
CD133+ cells under normoxic and hypoxic culture
conditions respectively (Fig. 4A and
C; p<0.005). The GFI associated with the antigen binding
sites significantly increased in hypoxia (Fig. 4B and D; p<0.005). On the
contrary, flow cytometry with ab19898 showed a similar percentage
of CD133+ cells under both culture conditions (Fig. 4A and C; p=0.157). Similarly, no
significant difference was demonstrated by GFI (Fig. 4B and D; p=0.0844). Compared with
CD133/1, ab19898 detected a higher percentage of CD133+
cells as well as a much stronger expression level of the protein
per cell in normoxic IN699 cells (Fig.
4A and B; p<0.005). Interestingly, under hypoxic conditions,
both antibodies detected a similar positive proportion as indicated
by MFI (p=0.0786) whereas GFI suggested that a significant increase
in the average expression level of CD133 was present using ab19898
(p<0.005) (Fig. 4C and D).
In order to further investigate the effect of oxygen
tension on CD133 glycosylation status, CD133/1-negative IN699 cells
were sorted by the autoMACS technique and cultured under normoxic
and hypoxic conditions over the period of 24, 48 and 72 h. Cells
were then analysed by flow cytometry using CD133/1 antibody.
Interestingly, 48.0%, 17.9% and 29.4% of the ‘negative’ population
were detected as positive respectively at each time point within
the normoxic cell group, using the same antibody used for cell
sorting, i.e., CD133/1. There was no obvious difference in GFI
amongst the time points, with the average value of 8.79 (p=0.422)
(data not shown). Under hypoxic conditions, 49.7%, 36.1% and 40.8%
of the ‘negative’ population were detected positive respectively,
with the average GFI of 8.48 (p=0.357) (data not shown). Thus, at
48 and 72-h hypoxia was shown to strongly promote the glycosylation
status of CD133.
Discussion
Collectively, our WB and qRT-PCR data suggest that
CD133 is constitutively expressed while flow cytometry results
indicate that the glycosylation status of the protein is enhanced
by hypoxia. Furthermore, glycosylation of CD133 fluctuates during
in vitro culture under both normoxic and hypoxic conditions.
Previous studies using CD133/1 under normoxic conditions may have
only detected a fraction of CD133-expressing cells, implying that
the previously categorised CD133− cells with
tumour-initiating properties may have actually been undetected
CD133+ cells. Our flow cytometry data indicate that a
constitutive expression of CD133 exists in IN699 cells regardless
of oxygen stress but CD133 glycosylation is enhanced by oxygen
depletion although only a small proportion of the protein is
glycosylated in individual cells. This could be that the machinery
producing and moving mature molecules to the surface is saturated
or that there is only a certain amount of glycosylated CD133 on the
cell membrane at any given time.
In order to study CD133+ cells as BTSCs
their behaviour must be investigated under conditions close to
those in vivo. Analysing CD133+ cells under
hypoxic conditions, as in this study, advances knowledge in this
field. The broadly used CD133/1 antibody has been shown in this
study to rely on the glycosylation status of CD133, which in
normoxia would yield a much lower population of CD133+
cells than in hypoxia. This study supports the results that hypoxia
elevates the number of CD133+ cells (17), although the connection with
shortcomings of the CD133/1 antibody approach was not made in
previous studies. Such studies have also failed to show whether the
total amount of CD133 expressed per cell increases under hypoxic
conditions, irrespective of glycosylation status. This could have
been demonstrated effectively if a non-glycosylation dependent
antibody like ab19898 had been used, as our data suggest that
although hypoxia enhances CD133 glycosylation, a proportion of the
molecule remains unglycosylated in the CD133-expressing IN699
cells. Therefore this study shows that the immunodetection of CD133
using CD133/1 is affected by glycosylation status of the protein,
providing an explanation for the previous contradictory results
regarding tumourigenic properties of CD133+ and
CD133− cells (2,3).
The present study also questions the widely used
CD133/1 as the antibody of choice to isolate entire
CD133+ populations, although CD133/1 may still be useful
in elucidating the biological function of the glycosylated CD133.
Based on our observations, ab19898 should be more effective than
CD133/1 in enriching CD133+ cells in glioma, be they
CSCs or not, as the former is not only glycosylation-independent,
but also binds the intracellular, and thus probably immature,
protein in cells that may not be expressing cell surface CD133 at
the time. Moreover, different splice variants of CD133 may have
different glycosylation patterns and it is unknown how this could
affect the immunological detection of CD133 using
glycosylation-dependent antibodies (22). The suggestion that CD133/1 has
limitations in detecting CD133 is supported by findings that the
AC133 epitope recognised by CD133/1 is lost during cancer stem cell
differentiation, although the expression level of CD133 protein
remains the same (23), as well as
by a recent study in which a truncated variant of the protein was
detected in glioblastoma cells negative for CD133/1 antibody
(9). On the other hand, the
glycosylated CD133 protein on the cell surface is more likely to be
mature with defined biological function and capability of
interacting with extracellular messenger molecules (24), e.g., the ganglioside GD3 which is
overexpressed on glioma cells and plays important regulatory roles
in apoptosis and invasion (25,26).
Therefore CD133/1 could be valuable in elucidation of the
functional significance of CD133 glycosylation and its impact on
CSC biology, filling the knowledge gap surrounding glycosylation
patterns during cell proliferation and differentiation.
To our knowledge, this is the first time that
hypoxia has been shown to enhance CD133 glycosylation, although
hypoxia-induced CD133 expression has been demonstrated previously
(14–17,27).
Although a previous study using tissue sections of glioma revealed
differences in CD133 expression based upon antibody used (19), ours is the first study whereby the
defined population of CD133+ or CD133−
glioblastoma cells was used to establish the influence of hypoxic
conditions on CD133 glycosylation using different antibodies.
Moreover, it is known that hypoxia promotes cancer cell invasion
and distant metastasis, including that in gliomas (28–30),
and recently upregulation of CD133 under hypoxic conditions has
also been associated with increased aggressiveness of pancreatic
cancer cells (31). In addition,
the critical roles that mitochondria play in cancer development and
progression have long been recognised (32–35)
and accumulating evidence supports the ‘cross-talking’ relationship
between mitochondria and HIF-1 in protecting cancer cells from
hypoxia-mediated apoptosis (36–40).
Therefore, based on studies by us and other researchers, we
hypothesise that glycosylated CD133 may interact with GD3 (as
mentioned above) and/or other unknown modulators to promote glioma
stem cell survival and invasiveness during hypoxia-triggered
apoptosis, mediated by signal pathways involving HIF-1α and
mitochondria (16,25–27,36–42).
This hypothetic involvement of glycosylated CD133 in the
‘pro-survival’ feature of hypoxic CSCs is further mirrored by
observations where CD133-positive cells show enhanced resistance
against pro-apoptotic chemotherapy drugs (43,44).
Furthermore, it is unlikely that there is one ideal
CSC marker which can be detected on all cells at any given time and
under any condition. In the case of CD133, rather than evaluating
it as a CSC marker, it might be more pertinent to understand its
biological function in tumour development and progression.
Acknowledgements
The authors wish to express their
gratitude for support of this work by Ali’s Dream, Brainstrust,
Brain Tumour Research, Brain Tumour UK and Charlie’s Challenge.
References
|
1
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks PB: Identification of a cancer stem
cell in human brain tumours. Cancer Res. 63:5821–5828.
2003.PubMed/NCBI
|
|
2
|
Wang J, Sakariassen PØ, Tsinkalovsky O,
Immervoll H, Bøe SO, Svendsen A, Prestegarden L, Røsland G, Thorsen
F, Stuhr L, Molven A, Bjerkvig R and Enger PØ: CD133 negative
glioma cells form tumors in nude rats and give rise to CD133
positive cells. Int J Cancer. 122:761–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Clément V, Dutoit V, Marino D, Dietrich PY
and Radovanovic I: Limits of CD133 as a marker of glioma
self-renewing cells. Int J Cancer. 125:244–248. 2009.PubMed/NCBI
|
|
4
|
Mizrak D, Brittan M and Alison MR: CD133:
molecule of the moment. J Pathol. 214:3–9. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cheng JX, Liu BL and Zhang X: How powerful
is CD133 as a cancer stem cell marker in brain tumors? Cancer Treat
Rev. 35:403–408. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Campos B and Herold-Mende CC: Insight into
the complex regulation of CD133 in glioma. Int J Cancer.
128:501–510. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun Y, Kong W, Falk A, Hu J, Zhou L,
Pollard S and Smith A: CD133 (prominin) negative human neural stem
cells are clonogenic and tripotent. PLoS One. 4:1–10.
2009.PubMed/NCBI
|
|
8
|
Fargeas CA, Huttner WB and Corbeil D:
Nomenclature of prominin-1 (CD133) splice variants - an update.
Tissue Antigens. 69:602–606. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Osmond TL, Broadley KW and McConnell MJ:
Glioblastoma cells negative for the anti-CD133 antibody AC133
express a truncated variant of the CD133 protein. Int J Mol Med.
25:883–888. 2010.PubMed/NCBI
|
|
10
|
Bidlingmaier S, Zhu X and Liu B: The
utility and limitations of glycosylated human CD133 epitopes in
defining cancer stem cells. J Mol Med. 86:1025–1032. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oliver L, Olivier C, Marhuenda FB, Campone
M and Vallette FM: Hypoxia and the malignant glioma
microenvironment: regulation and implications for therapy. Curr Mol
Pharmacol. 2:263–284. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Evans SM, Judy KD, Dunphy I, Jenkins WT,
Hwang WT, Nelson PT, Lustig RA, Jenkins K, Magarelli DP, Hahn SM,
Collins RA, Grady MS and Koch CJ: Hypoxia is important in the
biology and aggression of human glial brain tumors. Clin Cancer
Res. 10:8177–8184. 2004. View Article : Google Scholar
|
|
13
|
Amberger-Murphy V: Hypoxia helps glioma to
fight therapy. Curr Cancer Drug Targets. 9:381–390. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Donovan LK, Potter NE, Warr T and
Pilkington GJ: A Prominin-1-rich paediatric glioblastoma:
biological behaviour is determined by oxygen tension modulated
CD133 expression but not accompanied by underlying molecular
profiles. Transl Oncol. 5:141–154. 2012. View Article : Google Scholar
|
|
15
|
McCord AM, Jamal M, Shankavaram UT, Lang
FF, Camphausen K and Tofilon PJ: Physiologic oxygen concentration
enhances the stem-like properties of CD133+ human
glioblastoma cells in vitro. Mol Cancer Res. 7:489–497. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Griguer CE, Oliva CR, Gobin E, Marcorelles
P, Benos DJ, Lancaster JR Jr and Gillespie GY: CD133 is a marker of
bioenergetic stress in human glioma. PLoS One. 3:e36552008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Platet N, Liu SY, Atifi ME, Oliver L,
Vallette FM, Berger F and Wion D: Influence of oxygen tension on
CD133 phenotype in human glioma cell cultures. Cancer Lett.
258:286–290. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Iida H, Suzuki M, Goitsuka R and Ueno H:
Hypoxia induces CD133 expression in human lung cancer cells by
up-regulation of OCT3/4 and SOX2. Int J Oncol. 40:71–79.
2012.PubMed/NCBI
|
|
19
|
Hermansen SK, Christensen KG, Jensen SS
and Kristensen BW: Inconsistent immunohistochemical expression
patterns of four different CD133 antibody clones in glioblastoma. J
Histochem Cytochem. 59:391–407. 2011. View Article : Google Scholar
|
|
20
|
Günther HS, Schmidt NO, Phillips HS,
Kemming D, Kharbanda S, Soriano R, Modrusan Z, Meissner H, Westphal
M and Lamszus K: Glioblastoma-derived stem cell-enriched cultures
form distinct subgroups according to molecular and phenotypic
criteria. Oncogene. 27:2897–2909. 2008.PubMed/NCBI
|
|
21
|
An Q, Burke GA, Dainton M, Harrison CJ,
Kempski H, Konn Z, Myooren W, Stewart A, Taj M, Webb D, Strefford
JC and Martineau M: Haploinsufficiency of the MLL and TOB2 genes in
lymphoid malignancy. Leukemia. 24:649–652. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fargeas CA, Fonseca A, Huttner WB and
Corbeil D: Prominin-1 (CD133): from progenitor cells to human
diseases. Future Lipidology. 1:213–255. 2006. View Article : Google Scholar
|
|
23
|
Kemper K, Sprick MR, De Bree M, Vermeulen
L, Hoek M, Zeilstra J, Pals ST, Mehmet H, Stassi G and Medema JP:
The AC133 epitope, but not the CD133 protein, is lost upon cancer
stem cell differentiation. Cancer Res. 70:719–729. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gahmber CG and Tolvanen M: Why mammalian
cell surface proteins are glycoproteins. Trends Biochem Sci.
21:308–311. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Taïeb N, Maresca M, Guo X, Garmy N, Fatini
J and Yahi N: The first extracellular domain of the tumour stem
cell marker CD133 contains an antigenic ganglioside-binding motif.
Cancer Lett. 278:164–173. 2009.PubMed/NCBI
|
|
26
|
Birks SM, Danquah JO, King L, Vlasak R,
Gorecki DC and Pilkington GJ: Targeting the GD3 acetylation pathway
selectively induces apoptosis in glioblastoma. Neuro Oncol.
13:950–960. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bar EE, Lin A, Bar EE, Lin A, Mahairaki V,
Matsui W and Eberhart CG: Hypoxia increases the expression of
stem-cell markers and promotes clonogenicity in glioblastoma
neuro-spheres. Am J Pathol. 177:1491–1502. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ruan K, Song G and Ouyang G: Role of
hypoxia in the hallmarks of human cancer. J Cell Biochem.
107:1053–1062. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lu X and Kang Y: Hypoxia and
hypoxia-inducible factors: master regulators of metastasis. Clin
Cancer Res. 16:5928–5935. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fujiwara S, Nakagawa K, Harada H, Nagato
S, Furukawa K, Teraoka M, Seno T, Oka K, Iwata S and Ohnishi T:
Silencing hypoxia-inducible factor-1alpha inhibits cell migration
and invasion under hypoxic environment in malignant gliomas. Int J
Oncol. 30:793–802. 2007.
|
|
31
|
Hashimoto O, Shimizu K, Semba S, Chiba S,
Ku Y, Yokozaki H and Hori Y: Hypoxia induces tumor aggressiveness
and the expansion of CD133-positive cells in a hypoxia-inducible
factor-1α-dependent manner in pancreatic cancer cells.
Pathobiology. 78:181–192. 2011.PubMed/NCBI
|
|
32
|
Lee HC and Wei YH: Mitochondrial DNA
instability and metabolic shift in human cancers. Int J Mol Sci.
10:674–701. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gogvadze V, Zhivotovsky B and Orrenius S:
The Warburg effect and mitochondrial stability in cancer cells. Mol
Aspects Med. 31:60–74. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Grandemange S, Herzig S and Martinou JC:
Mitochondrial dynamics and cancer. Semin Cancer Biol. 19:50–56.
2009. View Article : Google Scholar
|
|
35
|
Ishikawa K and Hayashi J: A novel function
of mtDNA: its involvement in metastasis. Ann NY Acad Sci.
1201:40–43. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Denko NC: Hypoxia, HIF1 and glucose
metabolism in the solid tumour. Nat Rev Cancer. 8:705–713. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Semenza GL: Regulation of cancer cell
metabolism by hypoxiainducible factor 1. Semin Cancer Biol.
19:12–16. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chiche J, Rouleau M, Gounon P,
Brahimi-Horn MC, Pouysségur J and Mazure NM: Hypoxic enlarged
mitochondria protect cancer cells from apoptotic stimuli. J Cell
Physiol. 222:648–657. 2010.PubMed/NCBI
|
|
39
|
Klimova T and Chandel NS: Mitochondrial
complex III regulates hypoxic activation of HIF. Cell Death Differ.
15:660–666. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
De Haan C, Habibi-Nazhad B, Yan E, Salloum
N, Parliament M and Allalunis-Turner J: Mutation in mitochondrial
complex I ND6 subunit is associated with defective response to
hypoxia in human glioma cells. Mol Cancer. 3:192004.PubMed/NCBI
|
|
41
|
Soeda A, Park M, Lee D, Mintz A,
Androutsellis-Theotokis A, McKay RD, Engh J, Iwama T, Kunisada T,
Kassam AB, Pollack IF and Park DM: Hypoxia promotes expansion of
the CD133-positive glioma stem cells through activation of
HIF-1alpha. Oncogene. 28:3949–3959. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lluis JM, Llacuna L, von Montfort C,
Bárcena C, Enrich C, Morales A and Fernandez-Checa JC: GD3 synthase
overexpression sensitizes hepatocarcinoma cells to hypoxia and
reduces tumor growth by suppressing the cSrc/NF-kappaB survival
pathway. PLoS One. 4:e80592009. View Article : Google Scholar
|
|
43
|
Kolenda J, Jensen SS, Aaberg-Jessen C,
Christensen K, Andersen C, Brünner N and Kristensen BW: Effects of
hypoxia on expression of a panel of stem cell and chemoresistance
markers in glio blastoma-derived spheroids. J Neurooncol.
103:43–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cortes-Dericks L, Carboni GL, Schmid RA
and Karoubi G: Putative cancer stem cells in malignant pleural
mesothelioma show resistance to cisplatin and pemetrexed. Int J
Oncol. 37:437–444. 2010.PubMed/NCBI
|