Introduction
Hepatitis virus infection is a major global health
problem. Acute hepatitis is sometimes serious and may be fatal in
children because of their immature immune system. Egypt has one of
the highest prevalence rates of hepatitis C virus (HCV) infection
owing to the vigorous public health campaigns conducted between the
1950s and 1982 to eradicate schistosomiasis (1). Hepatitis B virus (HBV)-related liver
disease is also common in Egypt, like many other countries.
Consequently, Egyptian children are at particularly high risk of
HBV and HCV infection.
Egypt was one of the first countries to introduce
universal HBV vaccination in 1992. The Ministry of Health and
Population conducted a wide range of prophylactic strategies to
control viral hepatitis. It was reported that the prevalence of
hepatitis B surface antigen (HBsAg) positivity among healthy
individuals decreased from 10.1% in 1985 to 1.18% in 2008, and the
frequency of acute HBV infection as a cause of symptomatic
hepatitis decreased significantly from 43.4% in 1983 to 28.5% in
2002 (2–4). Hepatitis virus infection,
particularly HCV infection, was reported to be an important risk
factor for acute hepatitis in Egyptian children (5). In Africa, acute hepatitis is still
common and is sometimes fatal. However, the reason for this is
unclear, and may be related to coinfection with Leptospira
or Rift Valley fever virus, for example (6,7).
Hepatitis A virus (HAV) is also an important
pathogen that is frequently associated with acute hepatitis. An
Egyptian earlier survey examined more than 5,000 patients with
acute hepatitis and showed that 40.2% of patients had HAV-related
acute hepatitis (8). In addition,
94.4% of children aged >5 years were reportedly positive for
anti-HAV IgG (9). These findings
also suggest that most Egyptian children were exposed to HAV in
their childhood.
In this study, we analysed the aetiology of
hepatitis virus using serological and genetic methods in 33
Egyptian children hospitalised with acute hepatitis.
Materials and methods
Study subjects
This study was conducted at the Children’s Hospital,
Mansoura University, Mansoura, Egypt. Thirty-three children with
acute hepatitis were identified and included in the study. The
study subjects were mostly male (n=26), with a mean ± standard
deviation (SD) age of 9.7±3.4 years. Overall, 60.6% of the children
lived in rural areas. All of the children enrolled in the study
underwent thorough clinical examinations and their medical history
was carefully reviewed. Acute hepatitis is defined as acute hepatic
injury, manifested by the release of cytoplasmic enzymes,
particularly alanine amino-transferase (ALT) and aspartate
aminotransferase (AST). In three of the patients, with mean ALT,
AST and total bilirubin (T-Bil) levels of 130.1±68.3 IU/l, 146±68.3
IU/l and 2.9±1.2 mg/dl, respectively, increases in these enzymes
was accompanied by symptoms such as fever, loss of appetite,
abnormal bilirubin metabolism-related jaundice, dark urine and pale
stools. All of the patients had AST and ALT levels over two times
the upper limit of normal at acute onset. Informed written consent
was obtained from the parents of all the children. The study was
approved by the Ethics Committee of Mansoura University.
Serological markers of HBV infection
HBsAg was assessed using a reversed passive
hemagglutination (R-PHA) test (Mycell II HBsAg; Institute of
Immunology, Tokyo, Japan). Anti-HCV antibody (HCV-Ab) was examined
using the passive Ortho HCV-Ab PA Test II (Fujirebio Inc., Tokyo,
Japan). Anti-hemagglutination (HA)-IgM, anti-hepatitis B surface
(HBs) and anti-hepatitis B core antigen (HBc)-IgM antibodies were
assessed using radioimmunoassays (SRL Inc., Tokyo, Japan).
Laboratory investigations, including liver function tests, were
performed using a Synchron autoanalyser (Beckman Coulter,
Fullerton, CA, USA). ALT, AST, albumin and T-Bil levels were
measured in all serum samples.
DNA/RNA extraction and viral load of
HBV
Viral DNA was extracted from 200 μl of serum
using a QIAamp DNA Blood mini kit and a QIAamp viral RNA kit
(Qiagen GmbH, Hilden, Germany), following the manufacturer’s
instructions. The viral load was assessed by real-time PCR using an
ABI Prism 7700 analyser (Applied Biosystems, Foster City, CA, USA).
HBV was amplified with a primer and probe set, as previously
described (10).
Amplification of the HBV/HCV genome and
identification of mutations
The sequence of the core promoter/precore (CP/PC)
region was amplified by PCR with nested primers (11). The amplified fragments were
directly sequenced and the G→A substitution at nucleotide (nt)
1,896 in the PC, A→T at nt 1,762, G→A at nt 1,764 in the basal CP,
and the Kozak sequence (CCACC; nt 1,809–1,813) were analysed.
The complete nucleotide sequences of HBV from two
samples were sequenced using two overlapping amplicons with
specific primers (12). A second
PCR was performed to detect the full genome sequence in two virus
isolates and for pre-S1/S2/S gene detection using the previously
reported primers and PCR conditions (13).
The extracted RNA was reverse-transcribed to cDNA
using a Sensiscript RT kit (Qiagen GmbH) with oligo dT primers
(Promega, Madison, WI, USA). The transcribed cDNA was used for HCV
amplification by nested PCR. The 5′-non-coding regions of HCV-RNA
were amplified (14,15).
Genotyping of hepatitis virus by
phylogenetic analysis
The amplified products of the second PCR were
directly sequenced using the Taq Dye Deoxy Terminator cycle
sequencing kit with a 3100-Avant genetic analyser (Applied
Biosystems).
The two full-genome and S gene sequences of the HBV
strains determined in this study were compared with those of 20
reference sequences retrieved from the DDBJ/EMBL/GenBank database.
The subtypes of the strains used for comparison were obtained from
published articles (16).
The sequences were aligned using CLUSTAL X software
and the phylogenetic trees were constructed by the
neighbour-joining method (17). To
confirm the reliability of the phylogenetic tree analysis,
bootstrap resampling and reconstruction were carried out 1,000
times. These analyses were conducted using the Molecular
Evolutionary Genetics Analysis (MEGA) software program (available
at http://www.megasoftware.net) (18).
Results
Serological markers and laboratory
characteristics
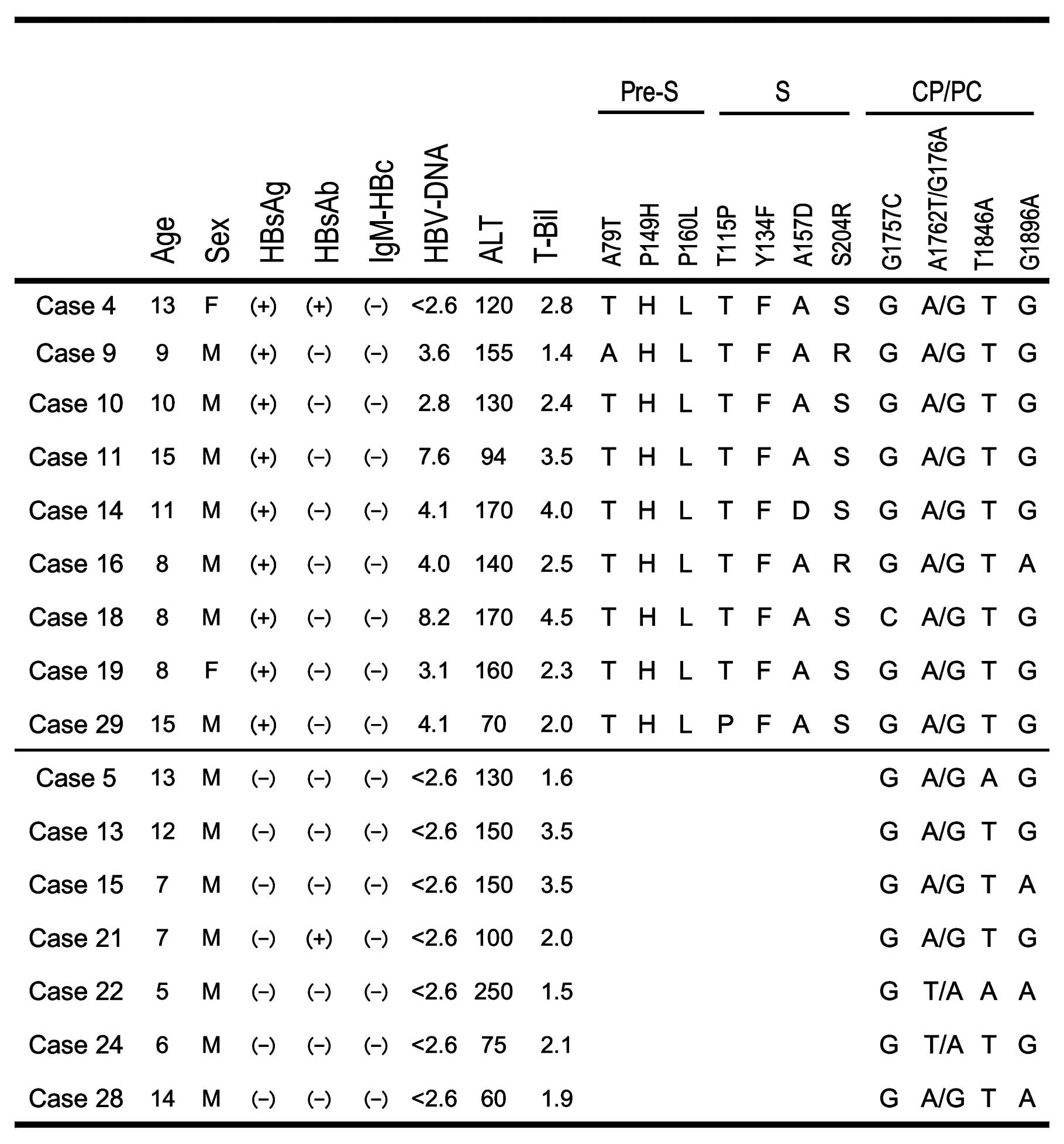
Serological data are summarized in Table I. Overall, 11 (33%) and 7 (21%)
children were positive for HA-IgM and anti-HCV antibodies,
respectively. HBsAg was detected in 9 children (27%) while the
other 24 (73%) were negative. There were no significant clinical
differences among children according to the type of hepatitis. Of
the HBsAg-positive children, one (case 4) was coinfected with HAV
and three (cases 9, 16 and 19) had HCV. On the other hand, only
three children were positive for anti-HBs antibodies and none was
positive for anti-HBc-IgM antibodies. HBV-DNA corresponding to the
pre-S/S and CP/PC regions was detected in all nine HBsAg-positive
children. HBV-DNA corresponding to the CP/PC region was detected in
7/24 HBsAg-negative children. There were no clinical differences
between the HBsAg-positive and HBsAg-negative children (Table II).
 | Table ISerological and clinical
characteristics of the patients. |
Table I
Serological and clinical
characteristics of the patients.
| HA-IgM | HBsAg | anti-HCV |
|---|
| Positive number
(%) | 11 (33%) | 9 (27%) | 7 (21%) |
| Male/female | 8/3 | 7/2 | 5/2 |
| Age (years) | 8.0±3.3 | 10.8±2.9 | 9.3±3.6 |
| ALT (IU/l) | 137±89 | 134±34 | 131±64 |
| T-Bil (mg/dl) | 3.0±1.2 | 2.8±1.0 | 2.0±0.4 |
 | Table IIPrevalence and characteristics of HBV
carriers. |
Table II
Prevalence and characteristics of HBV
carriers.
| HBV-DNA(+)
| HBV-DNA(−) | Total |
|---|
| HBsAg(+) | HBsAg(−) |
|---|
| Positive number
(%) | 9 (27%) | 7 (21%) | 17 (52%) | 33 |
| Age (years) | 10.8±2.9 | 9.1±3.7 | 9.4±3.5 | 9.7±3.4 |
| Gender
(male/female) | 7/2 | 7/0 | 12/5 | 26/7 |
| Residence
(rural/urban) | 5/4 | 5/2 | 10/9 | 20/13 |
| ALT (IU/l) | 134.4±34.7 | 127.9±47.6 | 128.9±88.6 | 130.2±68.3 |
| T-Bil (mg/dl) | 2.8±1.0 | 2.3±0.8 | 3.2±1.4 | 2.9±1.2 |
CP/PC mutations
Mutations in the pre-S/S and CP/PC regions were
detected by the PCR-direct sequencing method. All of the children,
except for cases 22 and 24, were double-wild for the A1762T/G1764A
double mutation. The G1896A mutation was found in 3/7 (43%)
HBsAg-negative children, compared with just 1/9 (11%)
HBsAg-positive children. No specific mutations were found in C1653,
T1753 or T1858.
Sequencing and phylogenetic analysis of
the pre-S/S gene
The entire pre-S/S gene was sequenced in the nine
HBsAg-positive children and was converted to the corresponding
amino acid sequence to identify amino acid variations. Fig. 1 summarises the amino acid
mutations/variations in the pre-S/S region. While A79T was found in
the pre-S region in one child (case 9), the P149H and P160L
variants were found in all of the children. The T115P and A157D
mutations were detected in one child and S204R mutation was
detected two children in the S region. The Y134F mutation in the
‘α’ determinant region was identified in all of the children, but
no specific mutations, such as T131I, K141E or G145R, were found in
the α loop (amino acids 111–156).
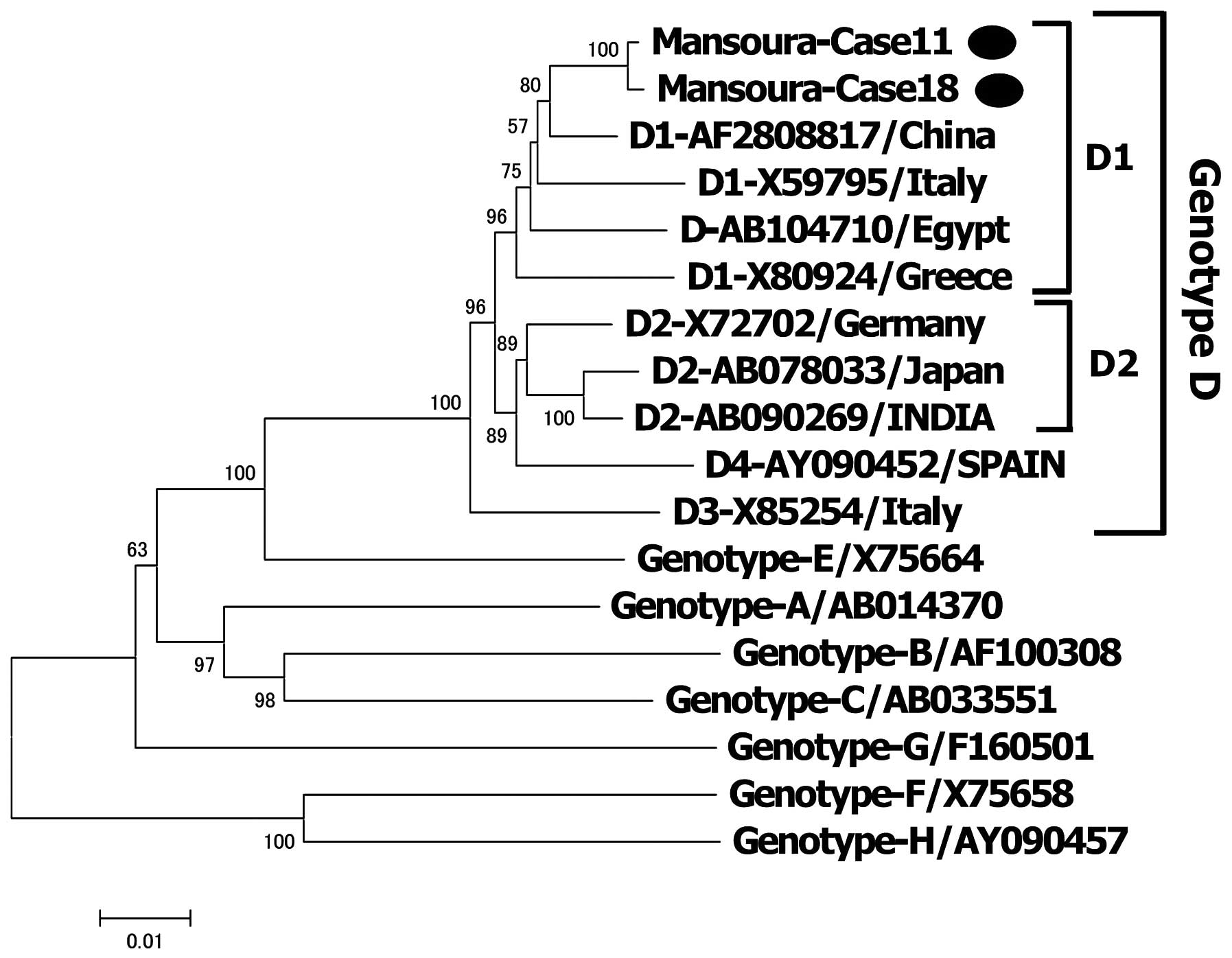
A phylogenetic tree was constructed for the 9
children using 15 reference sequences of HBV isolates from various
countries. HBV was classified as genotype D1 in all of the children
(Fig. 2).
HBV genotypes and complete nucleotide
sequence of HBV
To confirm the genotyping, a phylogenetic tree was
constructed using two complete genome sequences with 16 reference
sequences of HBV isolates derived from various countries. The two
complete genomes were 3,182 bp long with a common deletion of 33
nucleotides in the pre-S1 region. Both genotypes were classified as
genotype D (subgenotype D1) (Fig.
3).
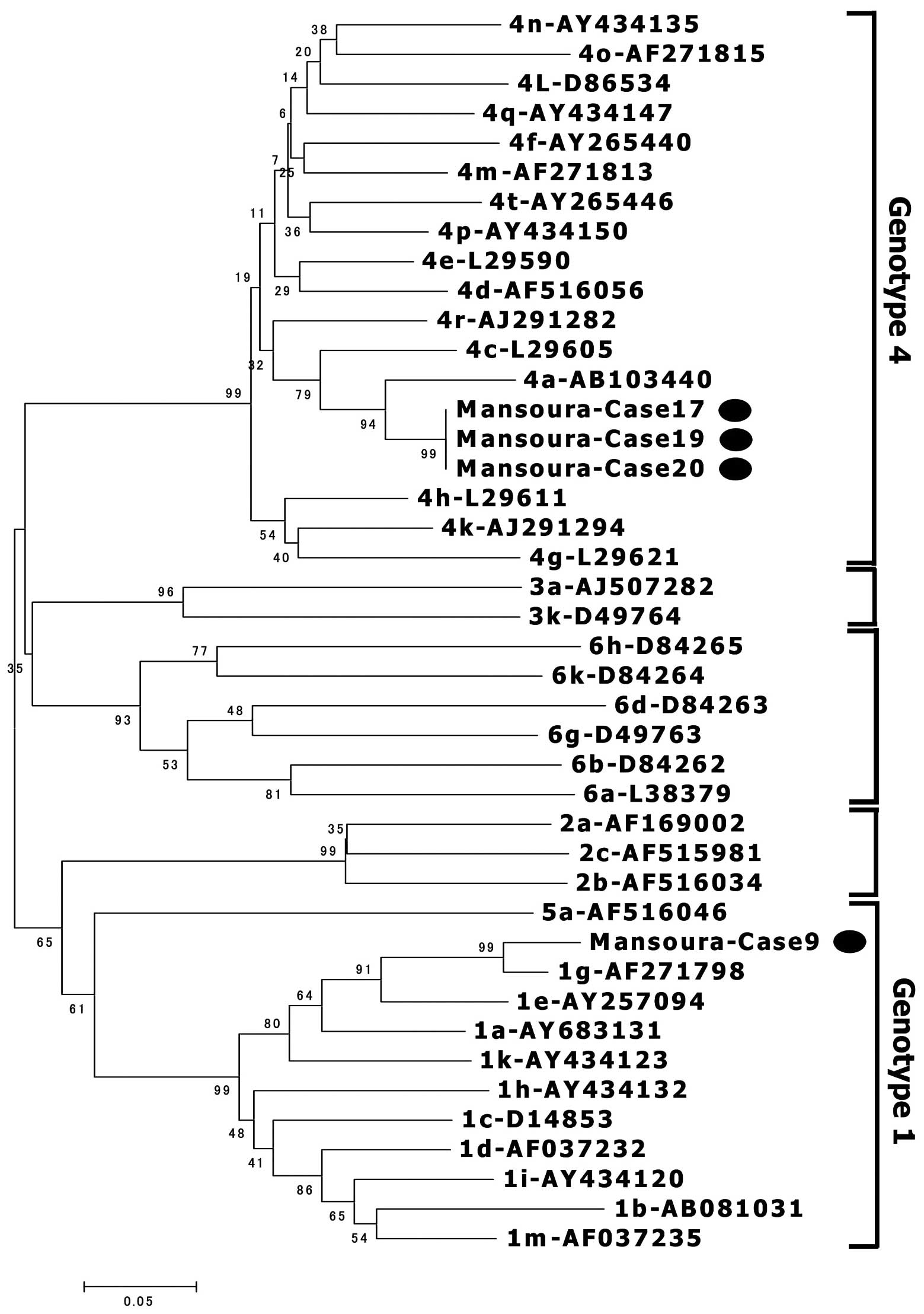
Detection of HCV-RNA and phylogenetic
analysis
HCV-RNA was detected in 4/7 children with anti-HCV
antibodies. The HCV genomes from four children were amplified and
sequenced, and three were classified as subgenotype 4a and one as
subgenotype 1g (Fig. 4).
Discussion
The cause of acute hepatitis usually shows
geographic differences. In particular, environmental factors (e.g.,
aflatoxin) and endemic infections (e.g., schistotomiasis) are
associated with acute and chronic liver diseases in Egypt (19). Regarding viral hepatitis, it was
reported that HCV infection is an important predictor of acute
hepatitis among Egyptian children (5). Although HCV infection was relatively
common, HAV infection was more prevalent in this study. Although
the seroprevalence of HAV in children is associated with
socioeconomic status in Egypt, the location of Mansoura may also
partly explain the high prevalence in this study (20). El Mansoura is a city in Egypt with
a population of 420,000. It is the capital of the Ad-Daqahliyah
Governate. Mansoura lies on the east bank of the Damietta branch of
the River Nile, in the delta region, about 120 km northeast of
Cairo. Acute HAV infection is usually curable and its clinical
course differs from those of HBV and HCV infections. This also
explains why long-term follow-up is necessary for HBV and HCV
infections.
HBV infection is a significant global health problem
and may cause both acute and chronic infection in humans (21). The World Health Organization
recently estimated that there are at least 350 million individuals
worldwide with HBV infection (22). Infection with HBV can lead to
progressive liver disease, including liver cirrhosis and
hepatocellular carcinoma (HCC), and approximately 1 million people
with HBV die from HCC annually. HBV is associated with
socioeconomic conditions, and Southeast Asia, China and Africa have
the highest rates of infection (23).
The age at which HBV infection occurs influences the
long-term outcomes and determines the primary targets of
vaccination programmes. Perinatal transmission from a mother to
child at or soon after birth occurs in about 90% of children, with
long-term complications of chronic hepatitis, cirrhosis and
hepatocellular carcinoma, leading to death in middle age,
particularly in men. This has serious economic consequences for
both the family and country as a whole. In 1991, the Child Survival
Project/Expanded Program on Immunization implemented a nationwide
plan to support immunization of all infants against HBV. In the
Expanded Program of Immunization, infants were vaccinated with a
2.5 μg dose of a recombinant vaccine, together with a
combined vaccine for diphtheria, tetanus and pertussis, at 2, 4 and
6 months of age. This recommended series of 3 intramuscular doses
of the HBV vaccine induces a protective antibody response (i.e.,
anti-HBs antibody) in 90% of healthy adults and 95% of infants,
children and adolescents (24).
However, despite the introduction of successful infant and
adolescent immunisation programs in many countries, the burden of
HBV-related disease remains high. More than 90% of young Egyptians
have been immunised and a large proportion of older Egyptians are
resistant to HBV infection because they have either been immunised
or were previously infected (4,5). In
this study, 3 children (9%) were positive for anti-HBs antibodies,
suggesting incomplete protection against HBV infection. On the
other hand, the prevalence of HBV infection among children is
rapidly decreasing, with a significantly lower frequency of acute
HBV infection in 2002 (5%) than in 1983 (11.9%) among those aged
12–19 years. It is probable that the level of immunity against
HBsAg is so low that anti-HBs could be diminished soon after
infancy, although the HBV vaccine is useful to protect against HBV
infection in early life. This may explain why the prevalence of
acute HBV infection among adults aged 20–39 years was higher in
2002 (20.5%), compared with the same age group in 1983 (16.2%).
In this study, HBsAg was detected in 9 children
(27%). As none of the children was positive for anti-HBc-IgM, we
think that these 9 children had acute-on-chronic HBV infection. The
impact of HBV vaccination in Egyptian school children aged over 10
years in an endemic area of the Nile Delta was evaluated, but the
prevalence of HBsAg did not change, even among vaccinated children
(25). Therefore, the high
prevalence of HBsAg in vaccinated and non-vaccinated children could
be due to intrauterine HBV infection, a weak immune response, or
infection with escape mutant variants (25).
In this cohort of patients with symptomatic HAV,
acute HBV infection was not apparent in children at 9 years of age
(i.e., children who had been vaccinated), compared with an
infection rate of 6.8% in the same age group at the same hospital
in 1983.
In this study, seven children were negative for
HBsAg and positive for HBV-DNA and were therefore classified as
having occult HBV infection. The prevalence of occult HBV varies
considerably and greatly depends on the prevalence of HBV in the
general population and the methods used to detect HBV DNA (26). Occult HBV infection has been
reported in patients with resolved acute-on-chronic HBV infection
and in patients lacking serological markers for past HBV infection
(27). Two common findings and
possible explanations for occult HBV are low levels of viral
replicative activity and/or mutations in the ‘α’ epitope of the S
gene encoding amino acid residues 100–160 of HBsAg (so-called S
mutants or variants) (26). These
seven children had low HBV-DNA levels, preventing us from
amplifying the S region. The S gene of HBV has three open reading
frames (i.e., the pre-S1, pre-S2 and S regions). The surface gene
contains a neutralizing epitope, the ‘α’ determinant region, which
is located at nt 124–147. Mutations in this region could alter the
antigenicity of HBsAg, causing the failure of anti-HBs to
neutralize HBsAg, allowing its escape from the host’s immune
system, resulting in active viral replication and liver disease
(28). In this study, we found no
specific mutation in the ‘α’ determinant region, such as T131I,
K141E or G145R, although there were seven amino acid mutations in
the pre-S and S regions in HBsAg-positive children. It is generally
thought that the escape mutant is rare in Egypt. It was reported
that the pre-S variant was associated with immune escape and
mutations of some epitopes located downstream of the ‘α’
determinant region might affect the neutralisation domain (29). Although the A157D and S204R
variants were detected in this study, they did not affect the
production of HBsAg.
HBV is classified into seven genotypes, A–G, based
on sequence divergence of the entire genome of >8% (30,31).
An eighth genotype, designated H, was recently reported in Central
America (32), but it has not been
fully characterised. Therefore, eight genotypes of HBV (A–H) are
currently recognized and subgenotypes, differing by ≥4%, have been
described (30). A few reports
have described the frequency of HBV genotypes in Egypt and revealed
that HBV genotype D is the most prevalent. One explanation for this
is that Egypt receives many tourists and visitors from countries
where genotype D is prevalent, particularly other Mediterranean
countries, with a high degree of nt homology (33). We recently reported that genotype D
was prevalent among HBV carriers in Ismailia City (34). In the present study, all of the
samples were classified as genotype D. A recent study showed that
HBV infection exhibited some genotypic variation among children
with cancer, and genotypes B and D were more frequently associated
with malignancies than were genotypes A and C (35). Because very few studies in Egypt
have focused on children with cancer, we must carefully follow-up
these patients.
In conclusion, hepatitis viral infection, including
acute-on-chronic infection by HCV and HBV, is common among children
hospitalised for acute hepatitis in Egypt. A large proportion of
children were positive for HBV-DNA, possibly because of genetic
variability and/or low-level immunity. Future studies should focus
on improvements in immunisation programmes.
Acknowledgements
This study was supported by a
Grant-in-Aid from the Japan Initiative for Global Research Network
on Infectious Disease (J-GRID) supported by The Ministry of
Education, Culture, Sports, Science and Technology, Japan.
References
|
1
|
Frank C, Mohamed MK, Strickland GT, et al:
The role of parenteral antischistosomal therapy in the spread of
hepatitis C virus in Egypt. Lancet. 11:887–891. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sherif MM, Abou-Aita BA, Abou-Elew MH and
el-Kafrawi AO: Hepatitis B virus infection in upper and lower
Egypt. J Med Virol. 15:129–135. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
El-Zayadi AR, Ibrahim EH, Badran HM, et
al: Anti-HBc screening in Egyptian blood donors reduces the risk of
hepatitis B virus transmission. Transfus Med. 18:55–61. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zakaria S, Fouad R, Shaker O, et al:
Changing patterns of acute viral hepatitis at a major urban
referral center in Egypt. Clin Infect Dis. 44:30–36. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meky FA, Stoszek SK, Abdel-Hamid M, et al:
Active surveillance for acute viral hepatitis in rural villages in
the Nile Delta. Clin Infect Dis. 42:628–633. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bird BH, Githinji JW, Macharia JM, et al:
Multiple virus lineages sharing recent common ancestry were
associated with a Large Rift Valley fever outbreak among livestock
in Kenya during 2006–2007. J Virol. 82:11152–11166. 2008.PubMed/NCBI
|
|
7
|
Ismail TF, Wasfy MO, Abdul-Rahman B, et
al: Retrospective serosurvey of leptospirosis among patients with
acute febrile illness and hepatitis in Egypt. Am J Trop Med Hyg.
75:1085–1089. 2006.PubMed/NCBI
|
|
8
|
Talaat M, El-Sayed N, Kandeel A, et al:
Sentinel surveillance for patients with acute hepatitis in Egypt,
2001–04. East Mediterr Health J. 16:134–140. 2010.PubMed/NCBI
|
|
9
|
Al-Aziz AM and Awad MA: Seroprevalence of
hepatitis A virus antibodies among a sample of Egyptian children.
East Mediterr Health J. 14:1028–1035. 2008.PubMed/NCBI
|
|
10
|
Abe A, Inoue K, Tanaka T, et al:
Quantitation of hepatitis B virus genomic DNA by real-time
detection PCR. J Clin Microbiol. 37:2899–2903. 1999.PubMed/NCBI
|
|
11
|
Yamaura T, Tanaka E, Matsumoto A, et al: A
case-control study for early prediction of hepatitis B e antigen
seroconversion by hepatitis B virus DNA levels and mutations in the
precore region and core promoter. J Med Virol. 70:545–552. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sugauchi F, Mizokami M, Orito E, et al: A
novel variant genotype C of hepatitis B virus identified in
isolates from Australian Aborigines: complete genome sequence and
phylogenetic relatedness. J Gen Virol. 82:883–892. 2001.
|
|
13
|
Cui C, Shi J, Hui L, et al: The dominant
hepatitis B virus genotype identified in Tibet is a C/D hybrid. J
Gen Virol. 83:2773–2777. 2002.PubMed/NCBI
|
|
14
|
Abdel-Hamid M, El-Daly M, Molnegren V, et
al: Genetic diversity in hepatitis C virus in Egypt and possible
association with hepatocellular carcinoma. J Gen Virol.
88:1526–1531. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ohno T, Mizokami M, Wu RR, et al: New
hepatitis C virus (HCV) genotyping system that allows for
identification of HCV genotypes 1a, 1b, 2a, 2b, 3a, 3b, 4, 5a, and
6a. J Clin Microbiol. 35:201–207. 1997.PubMed/NCBI
|
|
16
|
Norder H, Courouce AM, Coursaget P, et al:
Genetic diversity of hepatitis B virus strains derived worldwide:
genotypes, subgenotypes, and HBsAg subtypes. Intervirology.
47:289–309. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Saitou N and Nei M: The neighbor-joining
method: a new method for reconstructing phylogenetic trees. Mol
Biol Evol. 4:406–425. 1987.PubMed/NCBI
|
|
18
|
Kumar R and Agrawal B: Novel treatment
options for hepatitis B virus infection. Curr Opin Investig Drugs.
5:171–178. 2004.PubMed/NCBI
|
|
19
|
Anwar WA, Khaled HM, Amra HA, El-Nezami H
and Loffredo CA: Changing pattern of hepatocellular carcinoma (HCC)
and its risk factor in Egypt: possibilities for prevention. Mutat
Res. 659:176–184. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Salama II, Samy SM, Shaaban FA, Hassanin
AI and Abou Ismail LA: Seroprevalence of hepatitis A among children
of different socioeconomic status in Cairo. East Mediterr Health J.
13:1256–1264. 2007.PubMed/NCBI
|
|
21
|
Maddrey WC: Hepatitis B: an important
public health issue. J Med Virol. 61:362–366. 2001. View Article : Google Scholar
|
|
22
|
Lee WM: Hepatitis B virus infection. N
Engl J Med. 337:1733–1745. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Custer B, Sullivan SD, Hazlet TK, Iloeje
U, Veenstra DL and Kowdley KV: Global epidemiology of hepatitis B
virus. J Clin Gastroenterol. 38:158–168. 2004. View Article : Google Scholar
|
|
24
|
Mansour E, Abdul-Rahim S, Batouty G,
Zaghloul I and Abdel-Hadi S: Integration of hepatitis B
immunization in the Expanded Program on Immunization of the Child
Survival Project. J Egypt Public Health Assoc. 68:487–494.
1993.
|
|
25
|
El Sherbini A, Mohsen SA, Seleem Z, Ghany
AA, Moneib A and Abaza AH: Hepatitis B virus among school children
in an endemic area in Egypt over a decade: impact of hepatitis B
vaccine. Am J Infect Control. 34:600–602. 2006.PubMed/NCBI
|
|
26
|
Brechot C, Thiers V, Kremsdorf D, Nalpas
B, Pol S and Paterlini-Brechot P: Persistent hepatitis B virus
infection in subjects without hepatitis B surface antigen:
clinically significant or purely ‘occult’? Hepatology. 34:194–203.
2001.
|
|
27
|
Carreno V, Bartolome J, Castillo I and
Quiroga JA: Occult hepatitis B virus and hepatitis C virus
infections. Rev Med Virol. 18:139–157. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zheng X, Weinberger KM, Gehrke R, et al:
Mutant hepatitis B virus surface antigens (HBsAg) are immunogenic
but may have a change specificity. Virology. 329:454–464. 2004.
View Article : Google Scholar
|
|
29
|
Tai PC, Suk FM, Gerlich WH, Neurath AR and
Shih C: Hypermodification and immune escape of an internally
deleted middle-envelope (M) protein of frequent and predominant
hepatitis B virus variants. Virology. 292:44–58. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Norder H, Courouce AM and Magnius LO:
Complete genomes, phylogenetic relatedness, and structural proteins
of six strains of the hepatitis B virus, four of which represent
two new genotypes. Virology. 198:489–503. 1994. View Article : Google Scholar
|
|
31
|
Okamoto H, Tsuda F, Sakugawa H, et al:
Typing hepatitis B virus by homology in nucleotide sequence:
comparison of surface antigen subtypes. J Gen Virol. 69:2575–2583.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Arauz-Ruiz P, Norder H, Robertson BH and
Magnius LO: Genotype H: a new Amerindian genotype of hepatitis B
virus revealed in Central America. J Gen Virol. 83:2059–2073.
2002.PubMed/NCBI
|
|
33
|
Saudy N, Sugauchi F, Tanaka Y, et al:
Genotypes and phylogenetic characterization of hepatitis B and
delta viruses in Egypt. J Med Virol. 70:529–536. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Youssef A, Yano Y, Utsumi T, et al:
Molecular epidemiological study of hepatitis viruses in Ismailia,
Egypt. Intervirol. 52:123–131. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zekri AR, Hafez MM, Mohamed NI, et al:
Hepatitis B virus (HBV) genotypes in Egyptian pediatric cancer
patients with acute and chronic active HBV infection. Virol J.
4:742007. View Article : Google Scholar : PubMed/NCBI
|