Introduction
Breast cancer is currently the second most commonly
diagnosed cancer and the leading cause of cancer related death in
women in the United States. Epidemiological evidence indicates that
physical inactivity is linked to higher breast cancer risk, whereas
increased levels of physical activity in youth and throughout
adulthood are associated with a reduced risk for developing breast
cancer (1–4). Numerous animal studies have
investigated the relationship between physical activity and
reductions in mammary tumor growth and development; however,
conflicting findings have weakened the basis for inferring a causal
relationship. For example, many have reported benefits of exercise
on mammary growth and development (5–10)
while others have reported either no effect or negative outcomes
(11–14). The disparate results are likely due
to the variability in animal models as well as differences across
exercise protocols in which intensity, mode and duration often vary
making it difficult to compare across studies.
Previously, we reported that forced daily treadmill
running (1 h/day, 6 days/week for 20 weeks) in C3(1)/SV40Tag mice
significantly decreased tumor number and volume (15). To our knowledge, this was the first
investigation of the effects of any type of physical activity on
tumorigenesis in the C3(1)/SV40Tag mouse model of breast cancer and
only the second in a transgenic breast cancer mouse model (13). The C3(1)/SV40Tag mouse is a
representative model of the human disease; lesions that develop
between 8–12 weeks of age are histologically similar to mammary
intraepithelial neoplasia (MIN) and ductal carcinoma in situ
(DCIS) observed in humans (16,17).
Mammary tumors develop with a 100% incidence in transgenic female
mice and progress to invasive carcinomas at ∼16 weeks of age making
this a timely and appropriate model for interventional studies
(16,17).
In the present investigation, we sought to determine
whether voluntary physical activity, initiated prior to the
development of mammary tumors, could attenuate tumor development
and growth in the triple-negative C3(1)/SV40Tag mouse model of
breast cancer. These mice lack expression for estrogen receptor
(ER), progesterone receptor (PR) and ERBB2 (Her2, Neu), and the
absence of these has been associated with poor prognosis (18). Voluntary wheel running was used as
it evokes a significant training effect while minimizing stress
placed on the animal since the animal self-selects its own running
distance, time and speed (19).
Body composition was also assessed given that reductions in body
mass via increased energy expenditure or decreased energy intake
(i.e. caloric restriction) have been linked to decreased breast
cancer incidence (20).
It was hypothesized that voluntary wheel running
would attenuate mammary tumor incidence and growth in C3(1)/SV40Tag
mice. To our knowledge, this is the only report of a reduction in
tumor volume following voluntary wheel-running activity in a
transgenic mouse model of breast cancer. As such, it may carry
important implications for the development of specific exercise
protocols for slowing the progression of incident disease. These
findings may also have special relevance for women with triple
negative breast tumors who are more likely to have poorer prognoses
(21).
Materials and methods
Animals
Female FVB/N wild-type mice were originally
purchased from Harlan Sprague-Dawley Laboratories and bred with
male heterozygous C3(1)/SV40Tag transgenic mice (a gift from Dr
Jeffrey Green, Chief, Transgenic Oncogenesis and Genomics Section,
Laboratory of Cancer Biology and Genetics, National Cancer
Institute) in the animal research facility at the University of
South Carolina. Female offspring were genotyped using RT-PCR for
the C3(1)/SV40Tag gene by tail snips taken prior to weaning. Mice
were maintained on a 12/12-h light-dark cycle in a low-stress
environment (22°C, 50% humidity and low noise) and provided
standard rodent chow and water ad libitum. All animal care
and experimentation was approved by the University of South
Carolina's Institutional Animal Care and Use Committee.
Voluntary physical activity
treatment
At 4 weeks of age, female C3(1)/SV40Tag and
littermate (non-cancerous) FVB/N mice were randomly assigned to
either the physical activity (PA) or sedentary (Sed) treatment
(n=7–15) [FVB-Sed, n=7; FVB-PA, n=13; C3(1)-Sed, n=15; C3(1)-PA,
n=12]. Additional mice were included in the FVB-PA (compared to
FVB-Sed group) to increase the power for comparisons of wheel
running behavior to the C3(1)-PA group. Final sample size between
the C3(1) treatment groups varied as a result of misgenotyped mice
that were subsequently excluded from the analysis. All mice
included in the analysis survived the entire 20-week treatment
period.
All mice randomized to the PA condition were housed
individually and had continuous access to a voluntary running wheel
(Mini-Mitter, Bend, OR, USA) from 4–24 weeks of age. Sedentary mice
were individually housed in cages lacking a voluntary running
wheel. Voluntary wheel running activity was measured automatically
during the treatment period via a computer using Vital View
physiological and behavioral monitoring software (Mini-Mitter).
Total running distance, total time spent running on the wheel and
peak running speed were accessed and calculated as previously
described (22). Data are
presented as the sum of all 2-min intervals collected each day (24
h) that were then averaged per week for the 20 week treatment
period.
Body weight, body composition and food
intake
Body weight as well as food and water intake were
measured weekly throughout the treatment period. To assess
potential differences in body composition that may result from the
exercise intervention, a subset of animals from each group (n=4–7)
underwent body composition analysis at 12 weeks of age. Analysis
was performed on the Lunar PIXImus X-ray densitometer (DEXA) for
small animals. Animals were lightly anesthetized via isoflurane
inhalation using a nose cone. The 12-week measurement point was
chosen as animals had not yet developed palpable tumors; it is
possible that the presence of tumors would have skewed the
compositional data.
Tumor progression
Beginning at 12 weeks of age, all C3(1)/SV40Tag mice
were examined twice a week for palpable tumors by the same trained
investigator. The number of tumors within each mouse was recorded
and the tumor volume was calculated using the formula: 0.52 ×
(largest diameter) × (smallest diameter)2(23). In order to account for differences
in tumor number, the total tumor volume was divided by the number
of tumors within that animal. This value was then used to represent
the average volume per tumor within each treatment group.
Sacrifice and tissue collection
At 24 weeks of age all mice were sacrificed via
isoflurane inhalation. Visible tumors were removed from all 10
mammary glands and measured to determine tumor volume. The heart
was removed and weighed to establish the effectiveness of the
exercise. Spleen weight was also recorded as it has been positively
associated with tumorigenesis (15).
Statistical analysis
All data were analyzed using commercial statistical
software (SigmaStat, SPSS, Chicago, IL, USA). Sacrifice data
including body weight, spleen weight, heart weight and DEXA data
were analyzed using a two-way ANOVA (genotype × physical activity)
with Student-Newman-Keuls post hoc analysis when appropriate.
Sacrifice tumor number, volume and volume per tumor, as well as
tumor latency and growth rate were analyzed using Student's
t-tests. Weekly wheel running data (distance, time and speed),
tumor data, body weight, food consumption and water intake were
analyzed using a two-way repeated measures ANOVA (time × dependent
variable) with Student-Newman-Keuls post hoc testing when
appropriate. Statistical significance was set at an α value of
P<0.05. Data are presented as mean (± SEM).
Results
Body weight, composition and food
intake
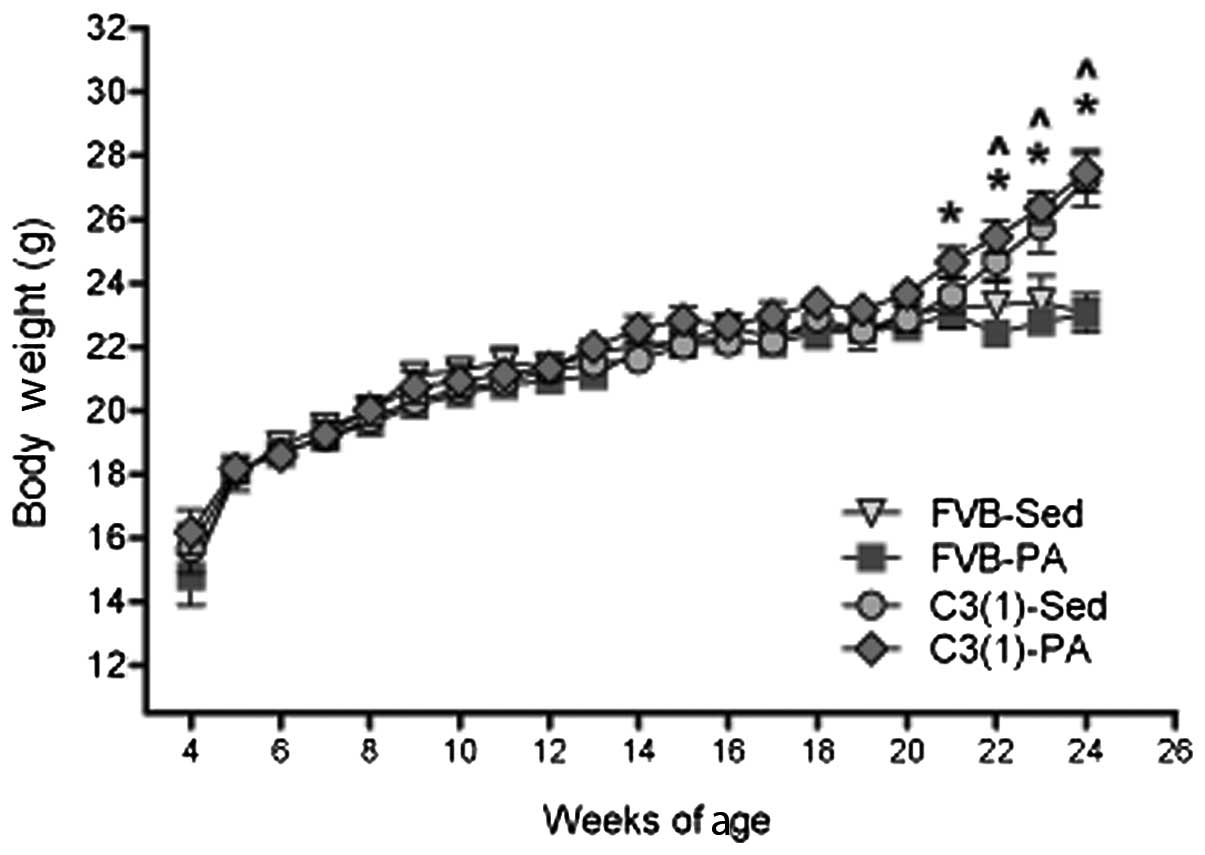
Body weight was measured weekly throughout the
treatment period (4–24 weeks of age) (Fig. 1). At 21 weeks of age, C3(1)-PA had
significantly elevated body weight compared with FVB-PA (P<0.05)
and from 22–24 weeks of age, both C3(1) groups (PA and Sed) had
significantly greater body weight than the FVB groups (PA and Sed)
(P<0.05). These differences however, were likely the result of
the increasing tumor burden in the C3(1) mice; body weight taken at
sacrifice following removal of all tumors showed no differences
between the groups. Body composition assessed by DEXA also
supported this finding; at 12 weeks of age there were no
differences in body fat percentage and body fat mass between the
groups. Percent body fat was 9.1±0.88% in the FVB-Sed mice;
10.5±0.39% in the FVB-PA mice; and 9.0±0.40 and 9.9±0.55% in the
C3(1)-Sed and C3(1)-PA mice, respectively. Total fat mass was also
similar between groups; 1.68±0.19 and 1.68±0.09 g in the FVB-Sed
mice and C3(1)-Sed mice; and 1.83±0.08 and 1.80±0.10 g in the
FVB-PA and C3(1)-PA mice. As expected, food and water intake,
measured weekly throughout the treatment period, was elevated in
the physical activity groups (P<0.05) (data not shown).
Voluntary physical activity
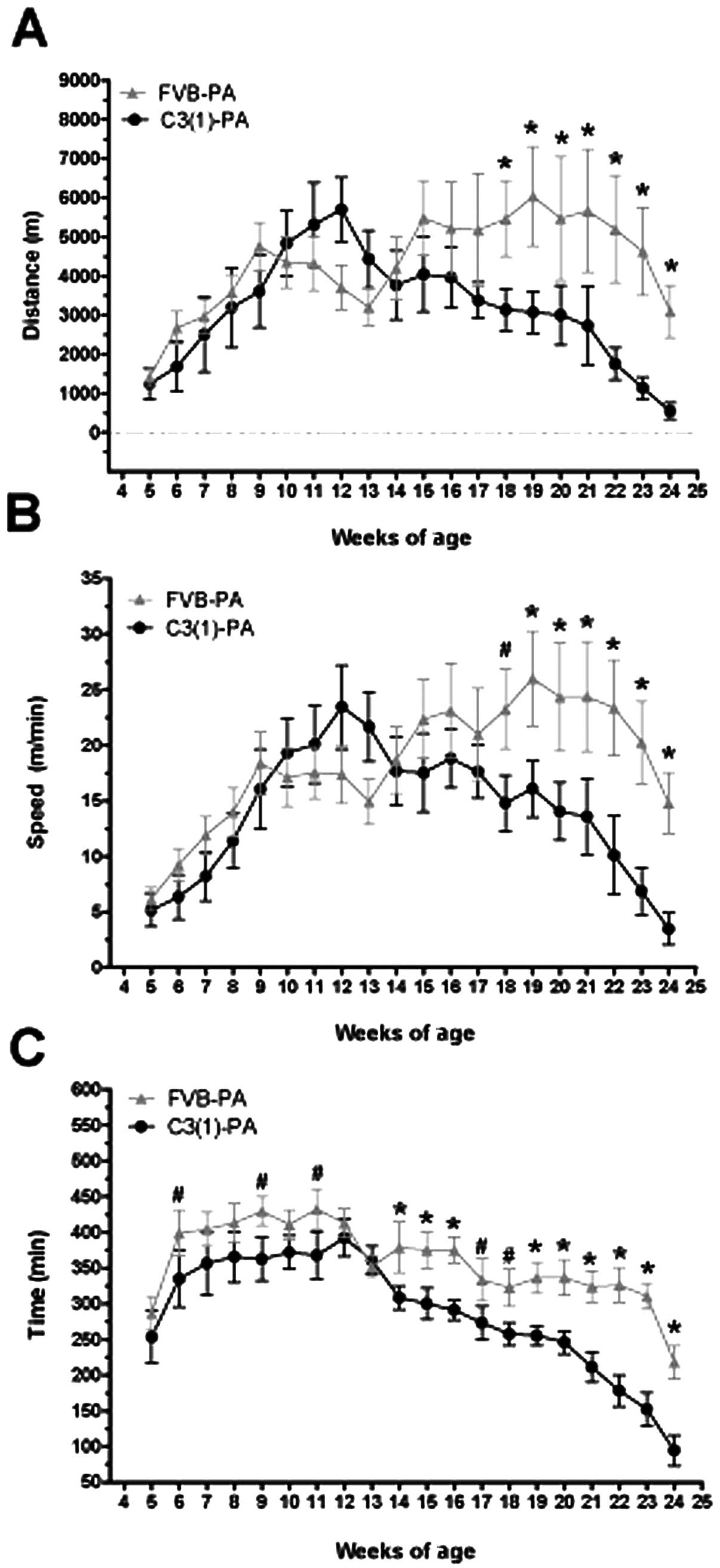
Mice randomized to the voluntary physical activity
intervention had continuous access to cage running wheels for the
duration of the experiment (4–24 weeks of age). Running distance,
peak speed and time increased over the first 8 weeks of the
intervention (Fig. 2A, B and C,
respectively) with no significant differences between the strains
(FVB/N and C3(1)/SV40Tag). However, by 18–19 weeks of age distance
and peak speed were significantly lower in the C3(1)/SV40Tag mice
(P<0.05), corresponding with increasing tumor growth. Similarly,
24-h running time was significantly lower in the C3(1)/SV40Tag mice
than FVB/N at 14–16 weeks and from 19–24 weeks of age (P<0.05).
Given that wheel running distance, speed and time declined
significantly in the last ∼4 weeks of the experiment, we did not
measure any common markers of training adaptations in the skeletal
muscle as it may not accurately reflect the magnitude of change
that occurred at earlier time-points during the treatment period.
Therefore, we can not make any conclusions about a potential
relationship between aerobic capacity and tumorigenesis in this
study. It has however, been previously reported that there is not a
direct effect of muscle citrate synthase activity on carcinogenesis
in a rat model of MNU-induced breast carcinogenesis (24).
Effects of voluntary physical activity on
tumorigenesis
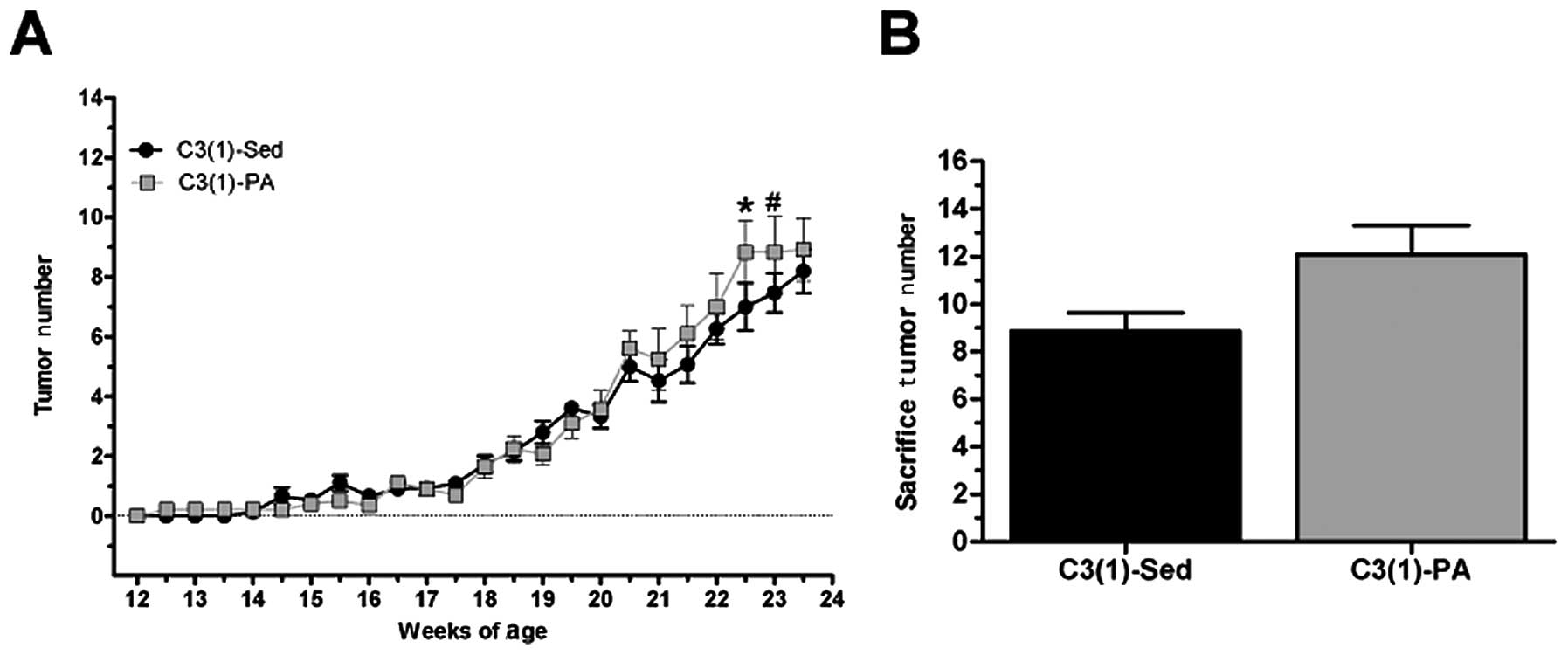
Beginning at 12 weeks of age all C3(1)/SV40Tag mice
were palpated twice a week for tumors, and tumor number and volume
were recorded. The C3(1)-PA mice had an increase in tumor number
compared to C3(1)-Sed mice at 22.5 weeks (P<0.05) and 23 weeks
(P=0.07) (Fig. 3). Specifically at
22.5 weeks, C3(1)-Sed mice had 7.0±0.8 tumors while the C3(1)-PA
mice had 8.8±1.1 tumors. At 23 weeks these values were almost
identical, but at sacrifice (24 weeks) C3(1)-Sed mice had 8.9±0.8
tumors while C3(1)-PA averaged 12.0±1.2 tumors. In addition, there
was no benefit of physical activity on the time to palpation of the
first tumor [C3(1)-Sed, 110.4±3.7 days; C3(1)-PA, 114.0±4.9
days].
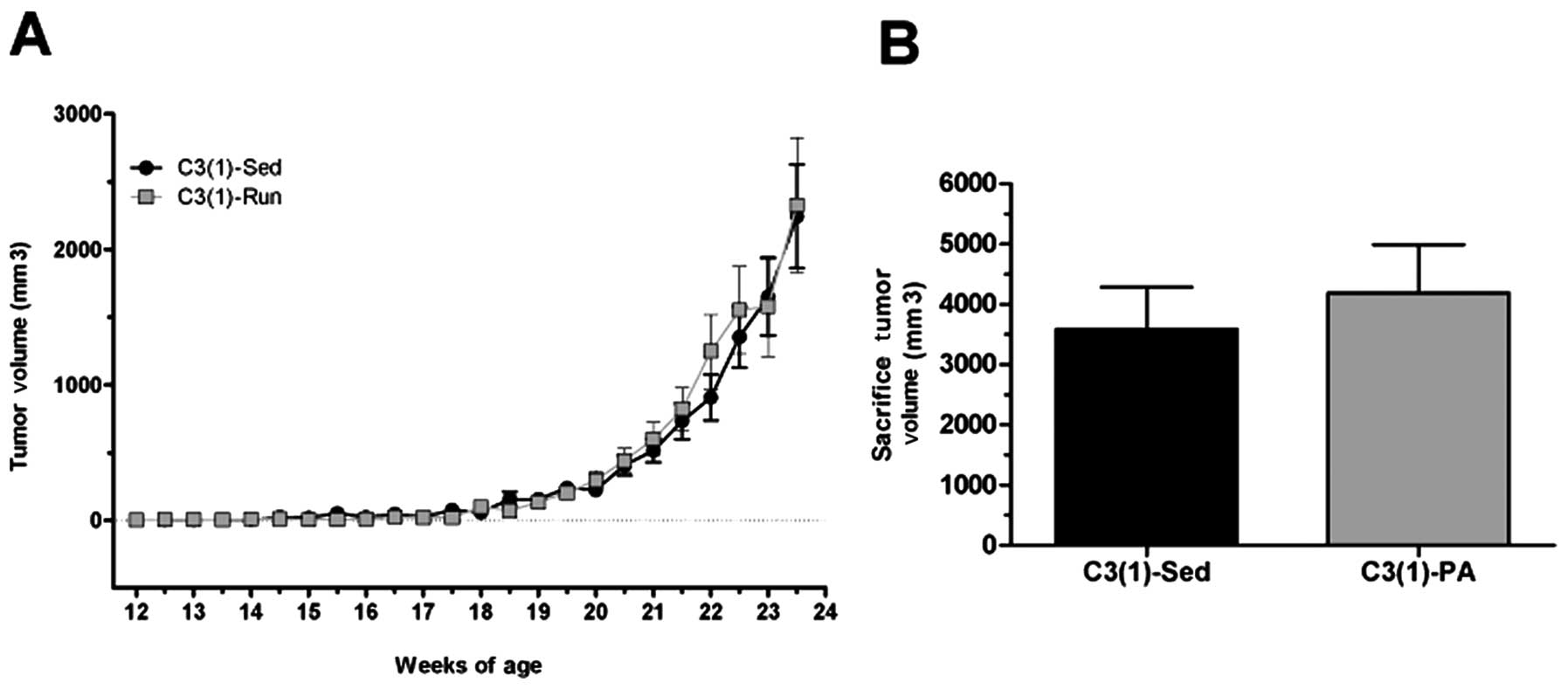
On the contrary, average tumor volume was reduced at
sacrifice (24 weeks) in the C3(1)-PA mice (3,581.6±705.6
mm3 compared with 4,190.8±797.9 mm3 in the
sedentary mice) (Fig. 4).
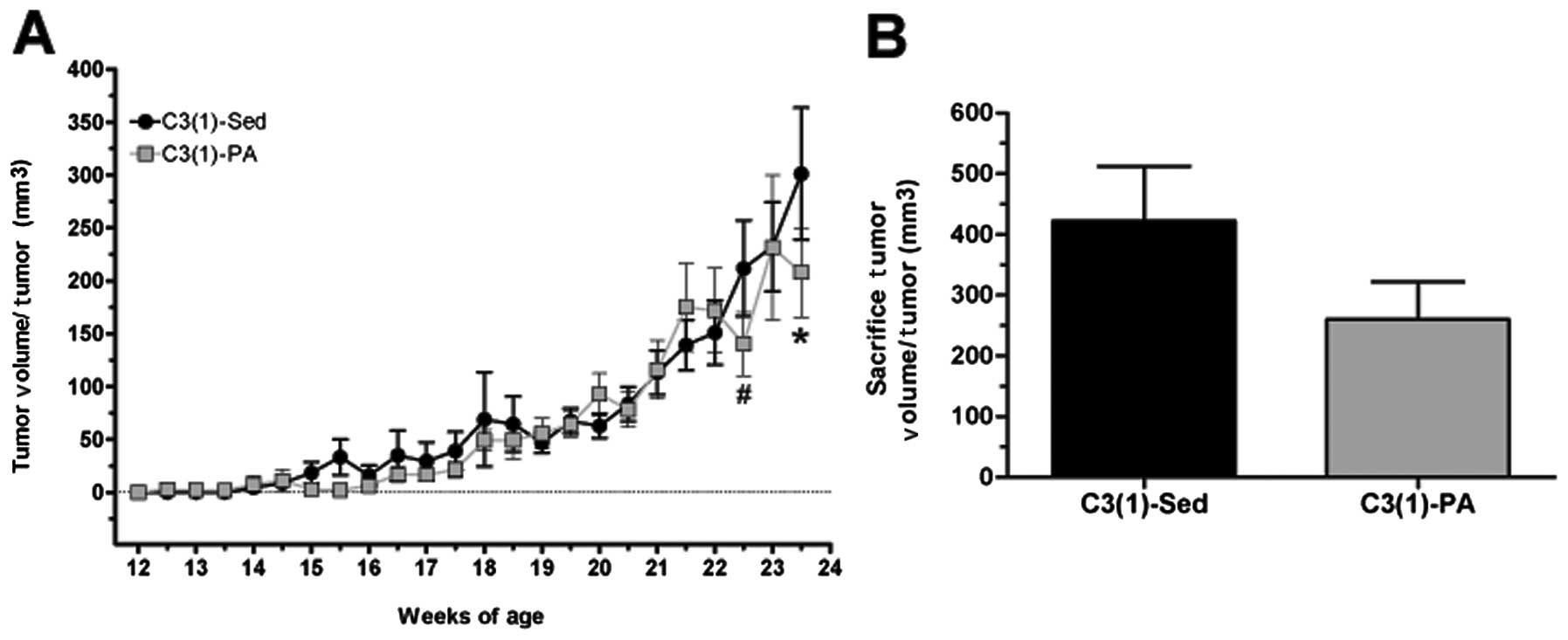
Furthermore, when adjusted for tumor number, average tumor volume
was lower in the C3(1)-PA group at 23.5 weeks (P<0.05) and at
sacrifice (Fig. 5). Specifically,
at 23.5 weeks C3(1)-Sed had an average tumor volume per tumor of
301.2±62.4 mm3 compared to 207.9±42.3 mm3 in
the C3(1)-PA mice. By sacrifice at 24 weeks of age, the average
tumor volume per tumor was almost 40% lower in the C3(1)-PA mice
compared to C3(1)-Sed mice as tumor volume in the C3(1)-Sed mice
had risen to 422.3±89.9 mm3 versus just 260.2±61.7
mm3 in the C3(1)-PA mice. Similarly, tumor growth rate
expressed as the change in tumor volume (in mm3) per day
from time of palpation to 23.5 weeks of age was decreased ∼20% by
physical activity [C3(1)-Sed, 9.4±2.2; C3(1)-PA, 7.6±1.78].
However, this did not reach statistical significance.
Heart and spleen weight
At sacrifice heart weight was recorded as an
indicator of aerobic training. A main effect of both strain and
physical activity was detected when expressed relative to body
weight and post hoc comparisons revealed a significant elevation in
both the FVB-PA (0.49±0.01%) and C3(1)-PA (0.48±0.01%) groups
compared with C3(1)-Sed (0.39±0.012%) (P<0.05). Spleen weight
was also recorded at sacrifice as this has been associated with
tumor burden (15). When expressed
relative to body weight, there was a main effect of strain [C3(1),
0.96±0.10%; FVB/N, 0.40±0.005%] but no effects of exercise and no
interaction.
Discussion
Epidemiological evidence supports a reduction in
breast cancer risk for physically active women (2). Animal models provide a useful tool to
study this relationship; however, inconsistencies among breast
cancer models and physical activity protocols have hampered the
reproducibility of the association reported in the epidemiological
literature. Therefore we sought to establish the relationship
between voluntary wheel running and tumorigenesis in the
representative, triple-negative C3(1)/SV40Tag transgenic mouse
model of human breast cancer. Results show that voluntary wheel
running, initiated prior to tumor development, significantly
reduces tumor volume per tumor independent of changes in body
weight or body composition. Consistent with this, we found a slight
improvement in tumor growth rate with physical activity, but this
did not reach statistical significance. On the other hand however,
tumor number was significantly elevated following 20 weeks of
physical activity and there was no benefit of physical activity on
tumor latency in this mouse model.
Several studies have examined the effects of
physical activity or exercise on mammary carcinogenesis in animal
models, but the results remain variable when voluntary wheel
running is employed (13,20,24,25).
Thompson et al(26) has
consistently shown decreased carcinogenic incidence after both free
and motorized wheel running in the chemically-induced rat model of
mammary tumorigenesis (20,24,26).
Conversely, benefits as well as no effect of exercise have been
reported in mouse implantation models using MDA-MB-231 human breast
cancer cells (14,25). However, chemical, implantation and
hormone-induced cancer models may not accurately portray the
development and progression of the human disease making it
difficult to specifically assess the effects of exercise on disease
initiation and progression. Use of genetically engineered mouse
models may more closely mimic spontaneous tumor development and
therefore provide clinically relevant insight into the relationship
between physical activity and breast cancer growth. However, to our
knowledge, only one other study has examined the effects of
voluntary physical activity on breast cancer progression in a
transgenic mouse model (13).
In the current investigation, we report a ∼40%
reduction in tumor size, but no benefits on tumor establishment
(measured as tumor number), following voluntary wheel running. We
interpret this to mean that in the C3(1)/SV40Tag mouse, which
develop tumors with 100% incidence, voluntary wheel running
activity is more effective at preventing progression of tumor
growth as opposed to inhibiting tumor initiation. The lack of a
benefit of physical activity on tumor number is likely due to the
inability of voluntary exercise to overcome the highly tumorigenic
phenotype induced by the inactivation of two primary tumor
suppressors, p53 and pRb, in this model (16). Alternatively, the potential for an
interaction between p53 status and exercise also exists as p53,
cardiovascular fitness and cancer-free survival have been linked in
investigations of p53 knockout mice undergoing exercise protocols
(27). Previously, Colbert et
al hypothesized that their findings of increased mammary tumor
incidence in p53+/−:MMTV-Wnt-1 mice with both voluntary
wheel running and treadmill running may have been due to the lack
of functional p53 (13). In
contrast, C3(1)/SV40Tag mice express wild-type p53 which is
inactivated by Tag binding. Therefore, given the role of p53 in the
promotion of aerobic metabolism (vs. glycolytic), and the
mitochondrial adaptive response to exercise, it is certainly
plausible that these alterations in p53 may be linked to the
divergent effects of exercise on tumorigenesis (28). Lastly, an interaction between the
numerous physiological effects of physical activity and either
C3(1) or SV40Tag expression could also have contributed to the
increase in tumor number following exercise. However, such
relationships were not examined in the current investigation and
therefore we can only speculate that these genetic alterations of
our mouse model may have contributed to the reported effects.
In contrast to our present findings, we previously
showed that treadmill running reduced both tumor volume and number
in C3(1)/SV40Tag mice (15). This
difference is likely due to variations in the exercise protocols.
In the present investigation, running distances, times and speeds
declined at later ages presumably due to the increasing tumor
burden and sickness. On the other hand the treadmill running
protocol forced animals to maintain a constant running volume and
intensity for the duration of the study. Therefore, the decline in
running behavior in the current study may have occurred at a
critical time for maximizing the benefits of physical activity on
tumor multiplicity and/or growth in this mouse model. However, a
direct comparison between these studies is somewhat hampered by
allelic variation between the sets of C3(1)/SV40Tag mice as the
present investigation used heterozygous mice while the treadmill
study included only homozygous C3(1)/SV40Tag mice.
These results also provide evidence of a potential
intensity dependent effect of exercise on tumorigenesis in
C3(1)/SV40Tag mice. Previously, Thompson et al found very
low intensity exercise to enhance tumorigenesis (11,12),
while higher intensities (>35% max) were related to reductions
in tumor multiplicity independent of exercise duration (5). Therefore, the lack of more pronounced
benefits of physical activity on tumorigenesis in this mouse model
may be related to the decline in exercise intensity as tumors
developed and progressed in size. Further experimentation including
testing of additional exercise protocols and increased group sizes
is necessary to determine the strength of this model for
mechanistic research on the benefits of physical activity in breast
cancer.
The focus of this study was not to determine
biological mechanisms, however, we did assess body weight and body
composition as it is well known that caloric restriction and body
mass reductions decrease breast cancer risk and incidence (6,29,30).
Therefore, it was important to confirm that the effects of exercise
were independent of an energy imbalance. We found no differences in
body weight (once tumor weight was accounted for) nor body fat,
between the sedentary and physically active wild-type and
C3(1)/SV40Tag mice after 8 weeks of voluntary wheel running,
implying that any benefits of physical activity were via mechanisms
independent of differences in fat mass. Body composition analyses
were only performed at 12 weeks of age in an attempt to limit the
disruption of running behavior and to eliminate any potential
influence of tumor composition. As a result, we can only speculate
that body composition was not different between the groups as tumor
burden increased. In support of our findings, several other
investigations have also shown benefits of physical activity on
breast cancer independent of changes in body composition (5,9,10).
Therefore, additional hypotheses explaining the changes induced by
voluntary wheel running may include alterations in energy sensing
pathways, growth related proteins and/or myokines and cytokines
(14,15,31).
In fact, we have previously shown that treadmill exercise can
reduce circulating levels of the inflammatory, pro-tumorigenic
cytokines, MCP-1 and IL-6, in accordance with decreased tumor
volume in C3(1)/SV40Tag mice (15). Further, exercise induced changes in
the availability of metabolic fuels, due at least in part to
decreased tumor blood flow, may limit tumor growth in this mouse
model (31). Future studies should
focus on investigating the effects of exercise on these mechanisms
specifically in the C3(1)/SV40Tag mouse.
This is the first report of a benefit of voluntary
wheel running on tumor growth in a transgenic mouse model of breast
cancer. Although voluntary wheel running activity was associated
with an increase in tumor number, the average volume per tumor was
significantly reduced implying that this physical activity paradigm
may be more effective at preventing tumor growth as opposed to
inhibiting tumor initiation in the C3(1)/SV40Tag breast cancer
mouse model. These findings contribute to the growing body of
literature on the benefits of physical activity on breast cancer
and provide support for the continued development of the
C3(1)/SV40Tag mouse model for investigation of the relationship
between breast cancer progression and physical activity.
References
|
1
|
Mittendorf R, Longnecker MP, Newcomb PA,
et al: Strenuous physical activity in young adulthood and risk of
breast cancer (United States). Cancer Causes Control. 6:347–353.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Friedenreich CM: Physical activity and
breast cancer: review of the epidemiologic evidence and biologic
mechanisms. Recent Results Cancer Res. 188:125–139. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Verloop J, Rookus MA, van der Kooy K and
van Leeuwen FE: Physical activity and breast cancer risk in women
aged 20–54 years. J Natl Cancer Inst. 92:128–135. 2000.
|
|
4
|
Thune I and Furberg AS: Physical activity
and cancer risk: dose-response and cancer, all sites and
site-specific. Med Sci Sports Exerc. 33:S530–S610. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thompson HJ, Westerlind KC, Snedden J,
Briggs S and Singh M: Exercise intensity dependent inhibition of
1-methyl-1-nitrosourea induced mammary carcinogenesis in female
F-344 rats. Carcinogenesis. 16:1783–1786. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lane HW, Teer P, Keith RE, White MT and
Strahan S: Reduced energy intake and moderate exercise reduce
mammary tumor incidence in virgin female BALB/c mice treated with
7,12-dimethylbenz(a)anthracene. J Nutr. 121:1883–1888.
1991.PubMed/NCBI
|
|
7
|
Thompson HJ, Westerlind KC, Snedden JR,
Briggs S and Singh M: Inhibition of mammary carcinogenesis by
treadmill exercise. J Natl Cancer Inst. 87:453–455. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Whittal KS and Parkhouse WS: Exercise
during adolescence and its effects on mammary gland development,
proliferation, and nitrosomethylurea (NMU) induced tumorigenesis in
rats. Breast Cancer Res Treat. 37:21–27. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Westerlind KC, McCarty HL, Schultheiss PC,
et al: Moderate exercise training slows mammary tumour growth in
adolescent rats. Eur J Cancer Prev. 12:281–287. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cohen LA, Kendall ME, Meschter C, Epstein
MA, Reinhardt J and Zang E: Inhibition of rat mammary tumorigenesis
by voluntary exercise. In Vivo. 7:151–158. 1993.PubMed/NCBI
|
|
11
|
Thompson HJ, Ronan AM, Ritacco KA,
Tagliaferro AR and Meeker LD: Effect of exercise on the induction
of mammary carcinogenesis. Cancer Res. 48:2720–2723.
1988.PubMed/NCBI
|
|
12
|
Thompson HJ, Ronan AM, Ritacco KA and
Tagliaferro AR: Effect of type and amount of dietary fat on the
enhancement of rat mammary tumorigenesis by exercise. Cancer Res.
49:1904–1908. 1989.PubMed/NCBI
|
|
13
|
Colbert LH, Westerlind KC, Perkins SN, et
al: Exercise effects on tumorigenesis in a p53-deficient mouse
model of breast cancer. Med Sci Sports Exerc. 41:1597–1605. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jones LW, Viglianti BL, Tashjian JA, et
al: Effect of aerobic exercise on tumor physiology in an animal
model of human breast cancer. J Appl Physiol. 108:343–348. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Murphy EA, Davis JM, Barrilleaux TL, et
al: Benefits of exercise training on breast cancer progression and
inflammation in C3(1)SV40Tag mice. Cytokine. 55:274–279. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Green JE, Shibata MA, Yoshidome K, et al:
The C3(1)/SV40 T-antigen transgenic mouse model of mammary cancer:
ductal epithelial cell targeting with multistage progression to
carcinoma. Oncogene. 19:1020–1027. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maroulakou IG, Anver M, Garrett L and
Green JE: Prostate and mammary adenocarcinoma in transgenic mice
carrying a rat C3(1) simian virus 40 large tumor antigen fusion
gene. Proc Natl Acad Sci USA. 91:11236–11240. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bennett CN and Green JE: Genomic analyses
as a guide to target identification and preclinical testing of
mouse models of breast cancer. Toxicol Pathol. 38:88–95. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Davidson SR, Burnett M and Hoffman-Goetz
L: Training effects in mice after long-term voluntary exercise. Med
Sci Sports Exerc. 38:250–255. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu Z, Jiang W, Zacher JH, Neil ES,
McGinley JN and Thompson HJ: Effects of energy restriction and
wheel running on mammary carcinogenesis and host systemic factors
in a rat model. Cancer Prev Res (Phila). 5:414–422. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reis-Filho JS and Tutt AN: Triple negative
tumours: a critical review. Histopathology. 52:108–118. 2008.
View Article : Google Scholar
|
|
22
|
Ottenweller JE, Natelson BH, Gause WC, et
al: Mouse running activity is lowered by Brucella abortus
treatment: a potential model to study chronic fatigue. Physiol
Behav. 63:795–801. 1998.PubMed/NCBI
|
|
23
|
Calvo A, Yokoyama Y, Smith LE, et al:
Inhibition of the mammary carcinoma angiogenic switch in C3(1)/SV40
transgenic mice by a mutated form of human endostatin. Int J
Cancer. 101:224–234. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mann PB, Jiang W, Zhu Z, Wolfe P,
McTiernan A and Thompson HJ: Wheel running, skeletal muscle aerobic
capacity and 1-methyl-1-nitrosourea induced mammary carcinogenesis
in the rat. Carcinogenesis. 31:1279–1283. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Welsch MA, Cohen LA and Welsch CW:
Inhibition of growth of human breast carcinoma xenografts by energy
expenditure via voluntary exercise in athymic mice fed a high-fat
diet. Nutr Cancer. 23:309–318. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thompson HJ, Wolfe P, McTiernan A, Jiang W
and Zhu Z: Wheel running-induced changes in plasma biomarkers and
carcinogenic response in the 1-methyl-1-nitrosourea-induced rat
model for breast cancer. Cancer Prev Res (Phila). 3:1484–1492.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang PY, Zhuang J and Hwang PM: p53:
exercise capacity and metabolism. Curr Opin Oncol. 24:76–82. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Saleem A, Carter HN, Iqbal S and Hood DA:
Role of p53 within the regulatory network controlling muscle
mitochondrial biogenesis. Exerc Sport Sci Rev. 39:199–205.
2011.PubMed/NCBI
|
|
29
|
Ligibel J: Obesity and breast cancer.
Oncology (Williston Park). 25:994–1000. 2011.PubMed/NCBI
|
|
30
|
Cohen LA, Choi K, Backlund JY, Harris R
and Wang CX: Modulation of N-nitrosomethylurea induced mammary
tumorigenesis by dietary fat and voluntary exercise. In Vivo.
5:333–344. 1991.PubMed/NCBI
|
|
31
|
Thompson HJ, Jiang W and Zhu Z: Candidate
mechanisms accounting for effects of physical activity on breast
carcinogenesis. IUBMB Life. 61:895–901. 2009. View Article : Google Scholar : PubMed/NCBI
|