Introduction
The discovery of cancer stem cells (CSCs) in
hematopoietic malignancies (1) has
revealed that tumor tissues comprise a bulk of proliferating or
differentiated tumor cells derived from small populations of
self-renewing cells (2). Since
their identification in leukemia, CSCs have been detected in solid
tumors of the head and neck (3),
gastrointestinal system (4), colon
(5,6), breast (7) and brain (8,9).
CSCs are tumorigenic, which is evident from xenotransplantation in
immunodeficient mice, and are resistant to chemoradiation, whereas
daughter cells are chemoradiation-sensitive (10,11).
Recent studies have demonstrated that CSCs survive chemo- and
radiation therapy in hypoxic regions of tumors (10,11).
Studies of cell- autonomous mechanisms have revealed the
involvement of anaerobic glycolysis in CSC maintenance and
chemoradiation resistance (10,11).
For example, CD13/aminopeptidase N, a liver CSC marker, regulates
reactive oxygen species (ROS) through recycling reduced glutathione
(GSH), thus contributing to intracellular ROS decrease following
chemoradiation exposure (12).
Similarly, intracellular ROS are suppressed after chemoradiation
therapy through the activity of the hyaluronic acid receptor, CD44,
an adhesion molecule expressed in cancer stem-like cells that
directly interacts with pyruvate kinase M2, which is putatively
involved in anaerobic glycolysis in CSCs (13). Furthermore, the CD44 variant
(CD44v) has been shown to interact with xCT, a glutamate-cystine
transporter, and to control intracellular GSH levels (14). CD44 abrogation has been shown to
cause a loss of xCT from the cell surface, to suppress tumor growth
in a transgenic gastric cancer (GC) mouse model and stimulate the
p38 (mitogen- activated protein kinase) pathway (a downstream
target of ROS) and the expression of the cell cycle inhibitor,
p21(CIP1/WAF1), suggesting that CD44 plays a role in GSH synthesis
and protection against ROS in gastrointestinal cancers (14). Taken together, these data indicate
that cancer metabolism is critical for the initiation and
progression of gastrointestinal CSCs.
In the present study, we investigated cell surface
markers in gastric CSCs and after screening eight candidate markers
(CD13, CD26, CD44, CD90, CD117, CD133, EpCAM and ALDH), we
confirmed the involvement of aldehyde dehydrogenase (ALDH) in
sphere formation, tumorigenicity and chemoresistance. Throughout
the study of the ALDH pathway, a cancer metabolism regulator, we
encountered stemness genes, suggesting novel molecular therapeutic
targets.
Materials and methods
Cell lines and cell culture
The human GC cell lines, AGS, NUGC3, GSU, MKN1,
MKN7, MKN28, MKN45 and MKN74, were cultured in RPMI-1640 medium
(Sigma), supplemented with penicillin, streptomycin and 10% fetal
bovine serum, in plastic culture dishes (Corning). Spheres were
cultured in Gibco® Dulbecco’s modified Eagle’s medium
with nutrient mixture F-12 (Invitrogen), supplemented with 20 ng/ml
human recombinant epidermal growth factor (Promega), 20 ng/ml basic
fibroblast growth factor (PeproTech Inc.), B-27®
Supplement (Invitrogen) and N2 Supplement (Wako), in low-attachment
dishes (Corning).
Cell staining and flow cytometry
Cultured cells were harvested and stained using an
Aldefluor® stem cell detection kit (StemCell
Technologies) for 45 min at 37°C. To stain cell surface markers,
cells were incubated on ice with antibodies against CD44, CD26,
CD117, CD90 (all from BD Biosciences), EpCAM (BioLegend) and CD133
(Miltenyi). Isotype antibodies were used as the negative controls.
Discrimination between live and dead cells was carried out using
the Live/Dead® Fixable Yellow Dead Cell Stain kit
(Invitrogen). Mouse cells were identified by anti-H2kd
(eBioscience) and anti-mouse CD45 (eBioscience) antibodies.
Primary surgical specimens and
xenografts
Tumor tissues were digested into single cells with
collagenase (Roche) and DNase (Worthington) at 37°C for 1 h.
Staining for fluorescence-activated cell sorting (FACS) analysis
was performed, as described above. For xenografting, cells were
injected subcutaneously with Matrigel® into NOD/SCID
mice. All the animal experiments were performed with approval of
Animal Experiments Committee of Osaka University.
RNA extraction, cDNA synthesis and
quantitative PCR
Total RNA was extracted using TRIzol®
reagent. cDNA was synthesized using SuperScript®
(Invitrogen). Quantitative PCR was performed using
LightCycler® 480 Real-Time PCR system. All procedures
were performed according to the manufacturer’s instructions.
Statistical analysis
Statistical significance was determined using the
Student’s t-test. Analyses were performed using JMP software.
Results
Screening of CSC markers in GC cell
lines
We examined novel markers in gastric CSCs, if: i)
they had been previously reported in other tumor types and for
which useful antibodies were available for FACS analysis; ii) they
were expressed in small populations (<50%) in the cell lines;
and iii) these observations were evident in more than half the cell
lines.
We investigated the functions of cell surface
markers and intracellular molecules (ALDH) to establish a
functional detection system. We screened six GC cell lines (AGS,
NUGC3, GSU, MKN7, MKN1 and MKN45) for gastric CSC markers using
eight candidate markers expressed in other CSCs (CD13, CD26, CD44,
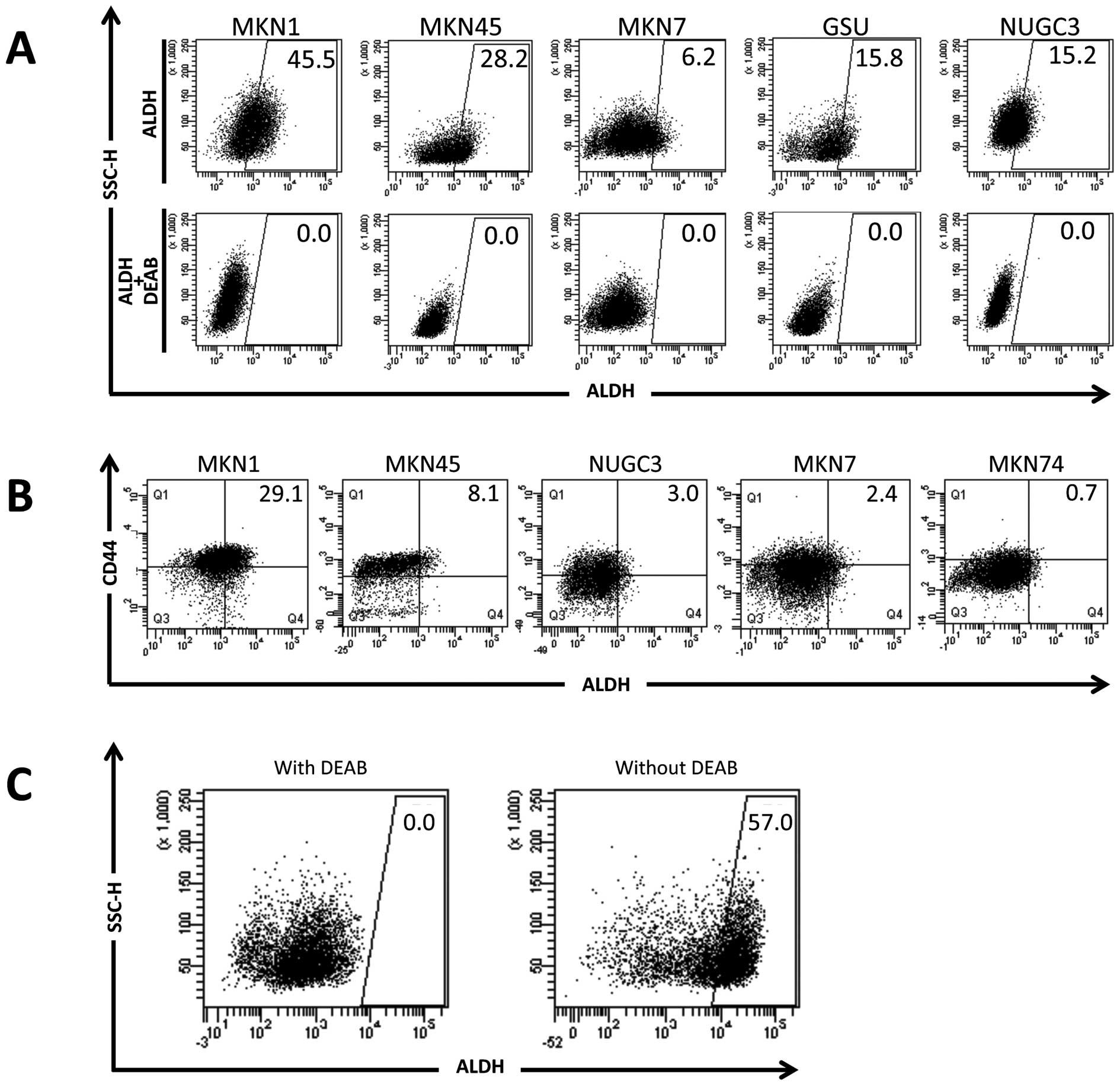
CD90, CD117, CD133, EpCAM and ALDH) (11). As shown in Table I, FACS analysis revealed a high
expression of EpCAM in all the cell lines (almost 100% postive
cells), whereas CD90, CD117 and CD133 expression was uniformly
undetectable or negative. CD26 expression was positive (>50%) in
four of the cell lines, but undetectable or negative in the other
two, suggesting that CD26 expression depends on individual cell
lines rather than the heterogeneous conditions of cell line
subpopulations. CD13 expression was detected in only one cell line,
GSU. Conversely, the investigation of ALDH indicated that the
proportion of cells highly expressing ALDH (ALDHhigh
cells) was relatively small (6.2–45.5%) compared with the other
markers (CD13, CD26, CD90, CD117, CD133 and EpCAM; Table I and Fig. 1A). Moreover, the
ALDHhigh cell populations reproducibly disappeared upon
the addition of the ALDH inhibitor, diethylaminobenzaldehyde
(DEAB), indicating the specificity of detection in
ALDHhigh cell populations. Reportedly, ALDH1A1, a
substrate for DEAB inhibition, has been shown to be responsible for
ALDH activity in CSCs (15). Thus,
we focused on ALDH activity.
 | Table IScreening for common CSC markers
using six GC cell lines. |
Table I
Screening for common CSC markers
using six GC cell lines.
Cells expressing high levels of ALDH also
express CD44
We examined CD44 expression, reportedly a CSC marker
in breast, colon, esophageal and gastric cancers (11,13,14).
Two-dimensional analysis data indicated that ALDHhigh
cell populations represented only a small subpopulation of
CD44-positive cells, suggesting that ALDH is a good candidate as a
CSC marker in GC (Table 1 and
Fig. 1B).
Cells expressing high ALDH exist in
xenografts in immunodeficient mice
We then examined the involvement of
ALDHhigh cell populations in human primary tumor
samples. Primary tumor tissues from surgical specimens were
obtained with written informed consent and inoculated into
immunodeficient NOD/SCID mice. Approximately 30% of inoculated
primary samples formed tumors in the mice after several weeks. The
probability of tumor formation is likely influenced by tumor tissue
viability (nutrients, necrosis and therapy-related damage),
vasculogenesis in the mice (dependent on local conditions) and CSC
conditions within primary samples. The tumor sample from a patient
was subjected to FACS analysis. The data indicated that 57% of the
human living tumor cells (separated by FACS using the Live/Dead
Fixable Yellow Dead Cell Stain system and distinguished from mouse
cells using anti-H2kd and CD45 antibodies) expressed active ALDH
(Fig. 1C), indicating that
ALDHhigh cells are present in primary human tumor
sample.
Sphere formation and tumorigenicity of
populations expressing high levels of ALDH
We then examined stemness in ALDHhigh
cells. A culture of FACS-sorted ALDHhigh cells in
serum-free medium resulted in the frequent formation of large
spheres compared with cells expressing low ALDH (ALDHlow
cells; Fig. 2A and B), suggesting
that ALDHhigh cells possess a greater self-renewal
ability, a critical characteristic of CSCs (2,11).
We examined tumorigenicity in vivo by inoculating
FACS-sorted ALDHhigh and ALDHlow MKN45 cells
subcutaneously into NOD/SCID mice. We performed a limiting dilution
experiment by reducing the number of inoculating cells. The
inoculation of 500 ALDHhigh, but not ALDHlow
cells resulted in tumor formation in three out of four mice
(Fig. 2C). The inoculation of
5,000 cells resulted in tumors being formed from the
ALDHhigh and ALDHlow cells (Fig. 2C). Taken together, these
observations indicate that, although multiple factors may be
involved, ALDH function is closely associated with the initiation,
maintenance and progression of CSCs in vitro and in
vivo.
Chemoresistance in cells highly
expressing ALDH and the underlying mechanisms
Our aim was the identification of novel molecular
therapeutic targets. Reportedly, CSCs can survive toxic injuries
and chemoradiation therapy (2,10,11).
To combat this, we explored the effect of chemotherapeutic agents
commonly used to treat GC. The exposure of the cell cultures to
cisplatin and 5-fluorouracil (5-FU) increased the number of
ALDHhigh cells (3.1–4.4% with cisplatin and 31.0% with
5-FU treatment; Fig. 3A),
indicating that exposure to these chemotherapeutic agents causes an
accumulation of surviving ALDHhigh CSCs. To elucidate
the molecular mechanisms underlying this chemoresistance, we
examined gene expression changes in candidate pathways (11). We found that Notch1 and
Sonic hedgehog (Shh) expression was increased in
ALDHhigh, compared to ALDHlow cells,
suggesting that the survival of ALDHhigh cells following
chemotherapy is associated with increased Notch1 and
Shh signaling.
Discussion
The demonstration of an association between ALDH and
tumors was first shown in breast cancer (16) and subsequently in pancreatic
(17), liver (18), colorectal (19), head and neck (19), thyroid (20) and lung (21) cancers. In lung cancer, ALDH
activity has been shown to be selective for adenocarcinoma stem
cells, depending on Notch signaling (21), in agreement with our observations
of gastric CSCs. We demonstrated that in GC, Notch and
Shh signaling may be important for both CSC maintenance and
the generation of chemoresistance, providing the rationale for
further study of therapy-resistant ALDHhigh CSCs. ALDH
is widely used as a marker to identify and isolate various types of
normal stem cells and CSCs (22).
In GC, several markers reportedly characterize CSCs: CD133
(23), CD44 (23–26),
side-populations identified by FACS (27), CD44 and EpCAM (25), CD54 (26) and CD90 (28). Of these markers, CD44 and ALDH are
involved in aerobic glycolysis during cancer metabolism. Although
an association between ALDH and the clinicopathlogical features of
GC has been reported (29), the
relevance of ALDH to chemoresistance has yet to be fully
investigated; another study detected no association between
immunohistochemical staining for ALDH and prognosis in GC patients
(23). In this study, we examined
for the first time the involvement of ALDH in chemoresistance and
identified a candidate underlying molecular mechanism for this
resistance.
GC is the second major cause of cancer-related
mortality worldwide and is prevalent across Asia. Helicobacter
pylori (H. pylori) infection was identified in 1982 by
Marshall and Warren in patients with chronic gastritis and gastric
ulcers (30). H.
pylori-associated GC has been investigated in order to
elucidate the mechanisms underlying gastric tissue damage. In
general, the two mechanisms by which H. pylori promotes
cancer are as follows: i) enhanced production of free radicals
proximal to the H. pylori infection site, increasing the
host cell mutation rate; and ii) pregenetic factors that transform
host cell phenotypes by altering adhesion proteins or
inflammation-related cytokines/chemokines, such as tumor necrosis
factor-α or interleukin-6. Thus, H. pylori infection causes
enhanced migration or invasion of damaged epithelial cells, without
additional tumor suppressor gene mutations (31). Those non-cell autonomous mechanisms
are likely facilitated by the hypoxic microenvironment of tumors,
since recent studies have implicated hypoxia in inflammatory
reactions provoked by H. pylori infection (32). Indeed, hypoxia-inducible factor-1α
is mediated by the induction of a ROS-inducible protein
(apurinic/apyrimidinic endonuclease 1) and its enhanced interaction
with the transcriptional coactivator, p300, leads to transformed
phenotypes in H. pylori-infected gastric epithelia (33). Although H. pylori infection
and related atrophic gastritis are closely associated with GC,
hypoxia and its related metabolism play a critical role in tumor
initiation and progression in the stomach and likely in other
organs (34). Further studies are
warranted to elucidate the association between H. pylori
infection and ALDH-positive CSCs in hypoxic areas and to evaluate
the eradication of H. pylori infection and GC treatment by
surgery, chemotherapy and molecular targeting of therapy-resistant
CSC functions.
Acknowledgements
We thank Miyuki Ozaki and Yuko Noguchi
for technical support. The current study was partly supported by a
Core Research Grant-in-Aid for Scientific Research from the
Ministry of Education, Culture, Sports, Science and Technology,
Japan (to H.I. and M.M.); a Grant-in-Aid from the Third Term
Comprehensive 10-year Strategy for Cancer Control of the Ministry
of Health, Labour and Welfare, Japan (to H.I. and M.M.); a grant
from the Kobayashi Cancer Research Foundation (to H.I.); a grant
from the Princess Takamatsu Cancer Research Fund, Japan (to H.I.);
and a grant from the SENSHIN Medical Research Foundation (to
H.I.).
References
|
1
|
Bonnet D and Dick JE: Human acute leukemia
is organized as a hierarchy that originates from a primitive
hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Prince ME, Sivanandan R, Kaczorowski A, et
al: Identification of a subpopulation of cells with cancer stem
cell properties in head and neck squamous cell carcinoma. Proc Natl
Acad Sci USA. 104:973–978. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Haraguchi N, Utsunomiya T, Inoue H, Tanaka
F, Mimori K, Barnard GF and Mori M: Characterization of a side
population of cancer cells from human gastrointestinal system. Stem
Cell. 24:506–513. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
Biffoni M, Todaro M, Peschle C and De Maria R: Identification and
expansion of human colon-cancer-initiating cells. Nature.
445:111–115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
O’Brien CA, Pollett A, Gallinger S and
Dick JE: A human colon cancer cell capable of initiating tumour
growth in immunodeficient mice. Nature. 445:106–110.
2007.PubMed/NCBI
|
|
7
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Piccirillo SG, Reynolds BA, Zanetti N, et
al: Bone morphogenetic proteins inhibit the tumorigenic potential
of human brain tumour-initiating cells. Nature. 444:761–765. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bao S, Wu Q, McLendon RE, et al: Glioma
stem cells promote radioresistance by preferential activation of
the DNA damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ishii H, Iwatsuki M, Ieta K, Ohta D,
Haraguchi N, Mimori K and Mori M: Cancer stem cells and
chemoradiation resistance. Cancer Sci. 99:1871–1877. 2008.
View Article : Google Scholar
|
|
11
|
Dewi DL, Ishii H, Kano Y, et al: Cancer
stem cell theory in gastrointestinal malignancies: recent progress
and upcoming challenges. J Gastroenterol. 46:1145–1157. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Haraguchi N, Ishii H, Mimori K, et al:
CD13 is a therapeutic target in human liver cancer stem cells. J
Clin Invest. 120:3326–3339. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tamada M, Nagano O, Tateyama S, et al:
Modulation of glucose metabolism by CD44 contributes to antioxidant
status and drug resistance in cancer cells. Cancer Res.
72:1438–1448. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ishimoto T, Nagano O, Yae T, et al: CD44
variant regulates redox status in cancer cells by stabilizing the
xCT subunit of system xc(−) and thereby promotes tumor growth.
Cancer Cell. 19:387–400. 2011.PubMed/NCBI
|
|
15
|
Marcato P, Dean CA, Giacomantonio CA and
Lee PW: Aldehyde dehydrogenase: its role as a cancer stem cell
marker comes down to the specific isoform. Cell Cycle.
10:1378–1384. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ginestier C, Hur MH, Charafe-Jauffret E,
et al: ALDH1 is a marker of normal and malignant human mammary stem
cells and a predictor of poor clinical outcome. Cell Stem Cell.
1:555–567. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Feldmann G, Dhara S, Fendrich V, et al:
Blockade of hedgehog signaling inhibits pancreatic cancer invasion
and metastases: a new paradigm for combination therapy in solid
cancers. Cancer Res. 67:2187–2196. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ma S, Chan KW, Lee TK, Tang KH, Wo JY,
Zheng BJ and Guan XY: Aldehyde dehydrogenase discriminates the
CD133 liver cancer stem cell populations. Mol Cancer Res.
6:1146–1153. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang EH, Hynes MJ, Zhang T, et al:
Aldehyde dehydrogenase 1 is a marker for normal and malignant human
colonic stem cells (SC) and tracks SC overpopulation during colon
tumorigenesis. Cancer Res. 69:3382–3389. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Todaro M, Iovino F, Eterno V, et al:
Tumorigenic and metastatic activity of human thyroid cancer stem
cells. Cancer Res. 70:8874–8885. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sullivan JP, Spinola M, Dodge M, et al:
Aldehyde dehydrogenase activity selects for lung adenocarcinoma
stem cells dependent on notch signaling. Cancer Res. 70:9937–9948.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma I and Allan AL: The role of human
aldehyde dehydrogenase in normal and cancer stem cells. Stem Cell
Rev. 7:292–306. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wakamatsu Y, Sakamoto N, Oo HZ, et al:
Expression of cancer stem cell markers ALDH1, CD44 and CD133 in
primary tumor and lymph node metastasis of gastric cancer. Pathol
Int. 62:112–119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Takaishi S, Okumura T, Tu S, et al:
Identification of gastric cancer stem cells using the cell surface
marker CD44. Stem Cell. 27:1006–1020. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Han ME, Jeon TY, Hwang SH, et al: Cancer
spheres from gastric cancer patients provide an ideal model system
for cancer stem cell research. Cell Mol Life Sci. 68:3589–3605.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen T, Yang K, Yu J, et al:
Identification and expansion of cancer stem cells in tumor tissues
and peripheral blood derived from gastric adenocarcinoma patients.
Cell Res. 22:248–258. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fukuda K, Saikawa Y, Ohashi M, et al:
Tumor initiating potential of side population cells in human
gastric cancer. Int J Oncol. 34:1201–1207. 2009.PubMed/NCBI
|
|
28
|
Jiang J, Zhang Y, Chuai S, et al:
Trastuzumab (herceptin) targets gastric cancer stem cells
characterized by CD90 phenotype. Oncogene. 31:671–682. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Katsuno Y, Ehata S, Yashiro M, Yanagihara
K, Hirakawa K and Miyazono K: Coordinated expression of REG4 and
aldehyde dehydrogenase 1 regulating tumourigenic capacity of
diffuse-type gastric carcinoma-initiating cells is inhibited by
TGF-β. J Pathol. 228:391–404. 2012.PubMed/NCBI
|
|
30
|
Marshall BJ and Warren JR: Unidentified
curved bacilli in the stomach of patients with gastritis and peptic
ulceration. Lancet. 16:1311–1315. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Suganuma M, Yamaguchi K, Ono Y, et al:
TNF-alpha-inducing protein, a carcinogenic factor secreted from
H. pylori, enters gastric cancer cells. Int J Cancer.
123:117–122. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sinkovics JG: Molecular biology of
oncogenic inflammatory processes. I. Non-oncogenic and oncogenic
pathogens, intrinsic inflammatory reactions without pathogens and
microRNA/DNA interactions (Review). Int J Oncol. 40:305–349.
2012.
|
|
33
|
Bhattacharyya A, Chattopadhyay R, Hall EH,
Mebrahtu ST, Ernst PB and Crowe SE: Mechanism of hypoxia-inducible
factor 1 alpha-mediated Mcl1 regulation in Helicobacter
pylori-infected human gastric epithelium. Am J Physiol
Gastrointest Liver Physiol. 299:G1177–G1186. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Barker HE, Cox TR and Erler JT: The
rationale for targeting the LOX family in cancer. Nat Rev Cancer.
12:540–552. 2012. View Article : Google Scholar : PubMed/NCBI
|