Introduction
The annual incidence of head and neck squamous cell
carcinoma (HNSCC), the sixth most common non-skin cancer in the
world, is estimated to be >600,000 cases worldwide and the
estimated number of deaths per year due to HNSCC is ∼350,000
(1,2). Despite improvements in therapeutic
strategies including surgery, radiotherapy and/or chemotherapy, the
prognosis for patients with advanced-stage HNSCC remains poor,
especially owing to loco-regional recurrence (2,3).
Tobacco use, alcohol consumption and human papilloma virus
infection are recognized as major risk factors (2). Genetic mutation analysis data
indicate that the mutational profile of HNSCC is generally
consistent with those of other tumors with similar risk factors; in
addition, 30% of cases harbor mutations in genes related to
squamous differentiation (for example, NOTCH1, IRF6 and TP63)
(4). The deregulation of specific
signaling cascades such as epidermal growth factor receptor (EGFR),
Ras and Wnt/β-catenin signaling have also been reported in HNSCC
tumorigenesis (2,3,5).
Although several molecular targeting regimens such as cetuximab (an
EGFR inhibitor) and bevacizumab (a vascular endothelial growth
factor receptor inhibitor) have been developed, their clinical
trials have had limited efficacy and unexpected toxicities have
been reported; these outcomes have emphasized the difficulties in
controlling HNSCC (2,3). Further study is needed to understand
the fundamental molecular basis of HNSCC tumorigenesis.
One molecule that has been implicated in HNSCC
tumorigenesis is lysophosphatidic acid (LPA). LPAs are not only
membrane phospholipid metabolates consisting of both saturated and
unsaturated fatty acid chains but also extacellular lipid mediators
that activate specific G-protein-coupled receptors (GPCRs). LPAs
are ubiquitous bioactive molecules regulating various cellular
events such as proliferation, migration and anti-apoptotic effects
in various kinds of cells; they are thus widely involved in
development, homeostatic regulations and disease processes
(6–8). LPAs are produced through the
hydrolysis of lysophosphatidylcholine (LPC) by autotaxin (ATX),
which was initially discovered as a tumor cell motility factor
which exerts extracellular lysophospholipase D activity (6–9). LPA
can be also produced through the hydrolysis of phosphatidic acid by
soluble phospholipase A2 (6–8), but
it has been shown using ATX heterozygous knockout mice that ATX is
responsible for the bulk of LPA production in serum (10). Cancer cells of several types
secrete large amounts of LPC, whereupon recombinant ATX stimulates
proliferation and cell motility (9). In addition, overexpression of ATX has
been reported in various malignant tumors such as small cell lung
carcinoma, breast cancer and Hodgkin lymphoma (6–8).
Upregulated LPA production by ATX in the cancer microenvironment
has been implicated in malignant behavior of tumor cells. Thus, the
ATX-LPA axis is thought to be a promising target for
pharmacological intervention (6–8).
LPAs bind and activate GPCRs in the endothelial
differentiation gene (Edg) family (LPA1/Edg2, LPA2/Edg4, LPA3/Edg7)
as well as the phylogenetically distant non-Edg family
(LPA4/p2y9/GPR23, LPA5/GPR92/GPR93, LPA6/p2y5) (11–13).
The Edg-family LPA receptors bind to LPAs in a similar manner and
activate intracellular signaling pathways via Gi, Gq and
G12/13 proteins, which are supposed to be responsible
for the major tumorigenic processes mediated by the ATX-LPA axis
(11–13). The biological role of the more
recently discovered non-Edg-family receptors is not yet fully
understood. LPA4 (p2y9/GPR23) was identified through the ligand
screening of orphan GPCRs sharing high amino acid sequence homology
with the human platelet activating factor receptor, a known GPCR
(14). LPA activates
G12/13- and Rho-mediated signaling in LPA4-expressing
B103 neuroblastoma cells, which leads to neurite retraction and
stress fiber formation (15,16).
LPA4 signaling also evokes intracellular cAMP accumulation via Gs
activation and calcium ion mobilization via Gq and Gi activation
(15,16). Notably, Gs activation has not been
reported downstream of the classic Edg-family LPA receptors
(11–13,15,16).
LPA4-deficient mice, such as LPA1- and LPA2-deficient mice, display
no apparent phenotypic abnormalities, implicating the redundancy of
signaling of LPA receptors (17).
It has also been reported that LPA1- and LPA4-mediated signaling
interact in the osteoblastic differentiation of human mesenchymal
stem cells (18). LPA4 signaling
also attenuates LPA1-mediated migration and invasion of B103
neuroblastoma and DLD1 colon cancer cells, suggesting functional
antagonism between these two LPA receptors (17). Collectively, the expression
profiles of LPA receptors and their downstream signalings are
assumed to be related to malignant behavior of cancer cells, though
this link has not been fully investigated.
It has been reported that LPA stimulates
proliferation and motility in HNSCC cells (19). EGFR signaling has been shown to
play a central role in HNSCC biology, which can be trans-activated
by other receptor-mediated signaling cascades such as
platelet-derived growth factor, insulin-like growth factor and LPA
(2,3,5,19).
However, LPA also inhibits EGF-induced activation of signal
transducer and activator of transcription 1 (STAT1) in A431
esophageal squamous cell carcinoma cells (20). Thus not all the effects of LPAs can
be explained by trans-activation of EGFR. In the present study, we
hypothesized that LPA signaling mediated by both Edg- and non-Edg
receptor family members regulates malignant behavior of HNSCC
cells. Overexpression of LPA4 was attempted in SQ-20B HNSCC cells,
which natively express trivial levels of LPA4. LPAs, GPCR ligands
that are abundantly present in the serum and body fluids, may play
an important role in the establishment of the cancer
micro-environment and in the regulation of malignant behavior of
HNSCC (21).
Materials and methods
Cell culture and reagents
HEp-2, a human squamous cell carcinoma cell line,
was obtained as previously described (22). The SQ-20B cell line of laryngeal
squamous cell carcinoma was kindly provided by Professor Hideyuki
J. Majima (Kagoshima University Graduate School of Medical and
Dental Sciences) (23). Cells were
maintained in Dulbecco’s modified Eagle’s medium (DMEM)
(Sigma-Aldrich, St. Louis, MO) containing 10% fetal bovine serum
(FBS) (Nichirei Bioscience, Tokyo, Japan) together with antibiotics
(100 U/ml penicillin and 100 μg/ml streptomycin; Life
Technologies, Carlsbad, CA) at 37°C in a humidified atmosphere of
5% CO2 and passaged with trypsin-EDTA (Life
Technologies). LPA (oleoyl-l-α-LPA, 18:1) and mouse monoclonal
anti-β-actin antibody (AC-15) were purchased from Sigma-Aldrich,
and the goat polyclonal anti-LPA4 antibody (S-15) from Santa Cruz
Biotechnology (Santa Cruz, CA). Ki16425 was purchased from Cayman
Chemical Co. (Ann Arbor, Michigan MI). Rac1 inhibitor and Y-27632
were purchased from Wako (Osaka, Japan), AG1478 from Merck
(Darmstadt, Germany), and doxycycline (Dox) from Clontech (Mountain
View, CA).
RNA extraction, conventional and
real-time polymerase chain reaction (PCR)
Total RNA was isolated with TRIzol (Life
Technologies) according to the manufacturer’s instructions.
Extracted RNA (1 μg) was exposed to PrimeScript II reverse
transcriptase (RT) (Takara, Otsu, Japan) in a total volume of 20
μl. For conventional RT-PCR, complimentary DNA obtained in 1
μl of RT reaction mixture was amplified using AmpliTaq Gold
PCR Master Mix (Applied Biosystems, Carlsbad, CA). PCR products
were run and imaged on 1% agarose gels stained with ethidium
bromide. For real-time PCR, 1 μl of RT reaction mixture was
amplified using Fast SYBR-Green fluorescence dye and a StepOne
real-time PCR system (Applied Biosystems). Amplification reactions
were performed in duplicate and fluorescence curves were analyzed
with the included software. All PCR results were normalized for the
expression of β-actin. Primers were designed using Primer 3
software (http://frodo.wi.mit.edu/primer3/) running on a Windows
computer. A primer set for β-actin (XAHR20 and XAHR17) purchased
from Funakoshi (Tokyo, Japan) was used for conventional PCR. The
PCR primer sets used in the present study and the experimental
conditions are listed in Table
I.
 | Table ISequences of primers and experimental
conditions for PCR. |
Table I
Sequences of primers and experimental
conditions for PCR.
| A, Primers for the
conventional PCR |
| Genes | Sense primer
(5-3′) | Antisense primer
(5-3′) | Annealing
temperature (°C) | Amplified size
(bp) |
|
| LPA1 |
cgtgctggcctatgagaaat |
tgtgaactccagccaagatg | 60 | 209 |
| LPA2 |
ctgctcctggatggtttagg |
tgggcagaggatgtatagtgg | 60 | 209 |
| LPA3 |
ggacacccatgaagctaat |
tctgggttctcctgagagaa | 60 | 256 |
| LPA4 |
ctcttcgcaagcctgctact |
gttcagagttgcaaggcaca | 60 | 221 |
| LPA5 |
tctcccgtgtcctgactacc |
gccgtacatgttcatctgga | 60 | 286 |
| LPA6 |
cagaagccacatggaaaaca |
tgctgccactactgagcaat | 60 | 287 |
| β-actin |
acccacactgtgcccatcta |
cggaaccgctcattgcc | | |
| (XAHR20
primer) | (XAHR17
primer) | 60 | 289 |
|
| B, Primers for the
real-time PCR |
| Genes | Sense primer
(5-3′) | Antisense primer
(5-3′) | Annealing
temperature (°C) | Amplified size
(bp) |
|
| LPA1 |
tgcttggggcctttatcatc |
ttctcataggccagcacgtc | 60 | 94 |
| LPA2 |
atcatcctgggggcgttc |
cattgcaggactcacagccta | 60 | 85 |
| LPA3 |
taggggcgtttgtggtatgc |
caccttttcacatgctgcac | 60 | 97 |
| LPA4 |
ccatgggtgacagaagattca |
ggcagtagcattgcccaac | 60 | 83 |
| LPA5 |
tctctgctgctgatgaagctg |
agggaggtcatgggaatgtg | 60 | 92 |
| LPA6 |
ccagcggaaattttacagca |
gcaaattatctggatctttggatg | 60 | 99 |
| β-actin |
atccgcaaagacctgtacgc |
ccagggcagtgatctccttc | 60 | 97 |
Assays for proliferation and cell
motility
Viable cell numbers and proliferation rates were
measured by means of WST-1
[2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium,
a tetrazolim salt], assay (Roche, Indianapolis, IN) according to
the manufacturer’s instructions. Briefly, cells were inoculated on
a 96-well multi-titer plate at a density of 5×103 cells
per well. To equilibrate the cell cycle phase, the cells were
cultured in serum-free media (SFM) prior to LPA stimulation. The
plates were read at wavelength of 450 nm using a scanning
multi-well spectrophotometer (Bio-Rad, Model 680, Hercules, CA).
For the measurement of cell motility, a wound-healing assay was
performed (24). Briefly, cells
were seeded on each side of an Ibidi culture insert for live cell
analysis (Ibidi, Munich, Germany) and the area filled with migrated
cells was observed using an Olympus phase-contrast microscope
(model CKX41, Tokyo, Japan) connected to a DP50 digital camera
(Olympus). Image analysis was performed using Image J software
(NIH, Bethesda, MD).
Cell cycle analysis
For flow cytometric analysis of the cell-cycle
distribution, cells were harvested using trypsin-EDTA and fixed
with 70% ethanol. Fixed cells (1×105) were stained with
200 μl of Guava cell cycle reagent (Millipore, Billerica,
MA) and analyzed using the Guava Personal Cell Analysis System
(Millipore) according to the manufacturer’s instructions.
Recombinant adenovirus vector
Full-length cDNA of human LPA4 with C-terminal turbo
green fluorescence protein (tGFP) tag (Origene, Rockville, MD) was
subcloned into the recombinant adenovirus vector (AdvLPA4G) using
an Adeno-X Tet-On 3G system (Clontech) according to the
manufacturer’s instructions. AD293 cells (Agilent Technologies,
Santa Clara, CA) were used as a packaging cell line and a ViraBind™
adenovirus purification kit (Cell Biolabs, San Diego, CA) was used
for amplification. Working stocks of viruses were stored in
aliquots at −80°C. Titer was determined by means of a conventional
plaque assay using Noble Agar (Difco, Detroit, MI). Dox-negative
condition was used as a negative control. Transfection efficiencies
were tested with GFP fluorescence as observed with an Olympus
fluorescent microscope.
Western blotting
Whole cell extracts were obtained in RIPA buffer
(Santa Cruz Biotechnology) and were then subjected to the Quick
Start Bradford Protein Assay kit (Bio-Rad). Whole cell extracts (30
μg) were subsequently resolved in 10% sodium dodecyl sulfate
(SDS)-polyacrylamide gel electrophoresis (PAGE) and were
electronically transferred to a nitrocellulose membrane (Bio-Rad).
The membranes were probed with a 1:200 dilution of a goat
polyclonal anti-LPA4 followed by incubation with a 1:5000 dilution
of peroxidase-conjugated secondary antibody-like particle (supplied
in an XL-SAP kit for western blotting, APRO Life Science, Naruto,
Japan). The proteins were subsequently developed using ImmunoStar
LD reagents (Wako) and visualized with a luminescent imager
(Ez-Capture, ATTO Co., Tokyo, Japan). Alternatively, the blots were
incubated with Restore PLUS Western Blot Stripping Buffer (Thermo
Fisher Scientific, Waltham, MA) and re-probed with a 1:2000
dilution of anti-β-actin antibody.
Statistical analysis
Experimental groups were compared using analysis of
variance (ANOVA) and, when appropriate, Student’s t-test. The data
are expressed as the mean ± SEM. A level of p<0.05 was
considered statistically significant.
Results
Expression of LPA1 and LPA4 in human
squamous cell carcinoma cells
The expression profiles of LPA receptors in various
cancer cell types were screened with conventional RT-PCR. HEp-2
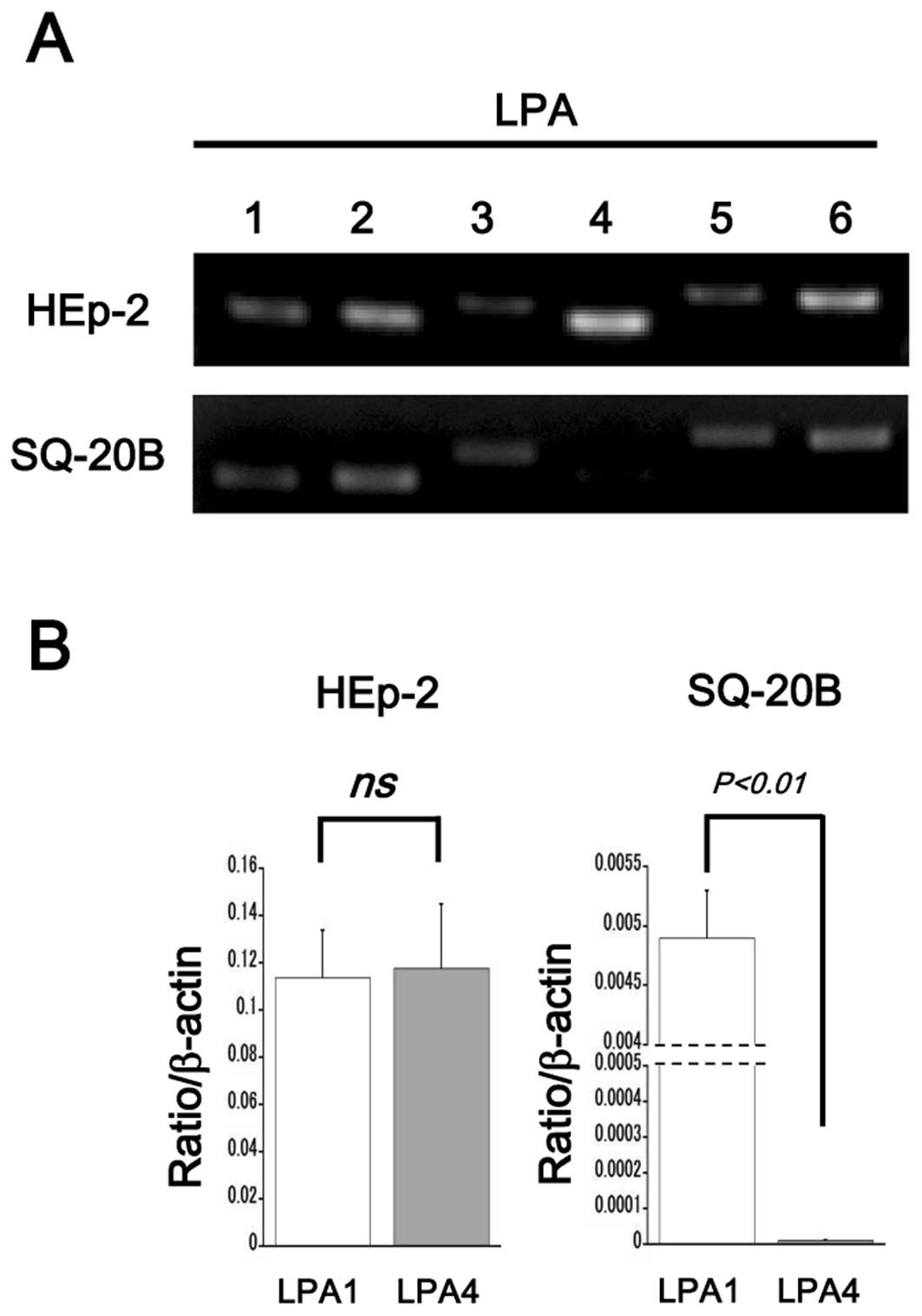
cells expressed all isoforms of LPA receptors (LPA1-6, Fig. 1A, upper panel). SQ-20B cells
expressed all Edg family LPA receptors (LPA1–3) and 2 isoforms of
the non-Edg-family LPA receptors (LPA5 and LPA6). Slight expression
of LPA4 was detected in SQ-20B cells (Fig. 1A, bottom panel). Real-time PCR
revealed that expression levels of LPA1 and LPA4 were similar in
HEp-2 cells, while only a trivial level of LPA4 expression was seen
in SQ-20B cells (Fig. 1B).
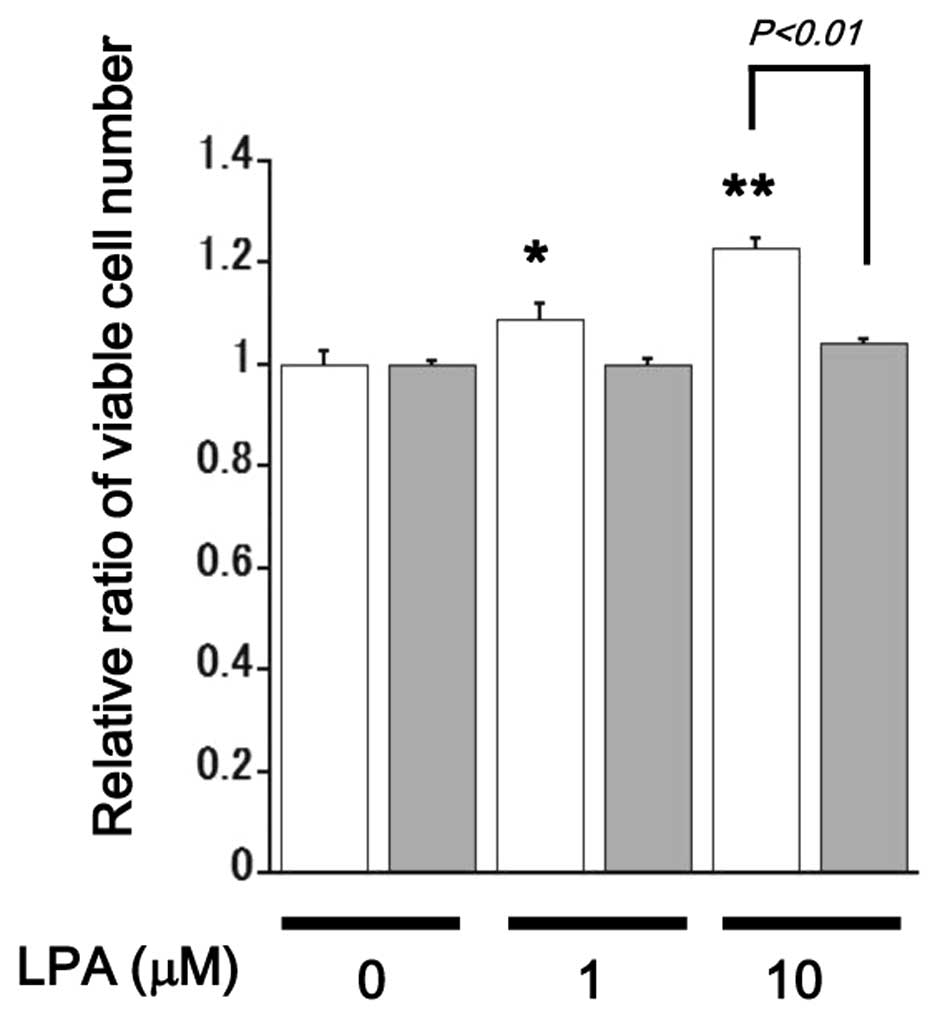
LPA stimulated proliferation in SQ-20B
cells but not in HEp-2 cells
In HEp-2 cells, WST-1 assay revealed no mitogenic
response against LPA. In SQ-20B cells, on the other hand, LPA
stimulated proliferation in a dose-dependent fashion (Fig. 2A). Thus, further experiments were
performed using LPA-responsive SQ-20B cells. Since it has been
reported that LPA-induced mitogenic response largely depends on the
transactivation of EGFR in some HNSCC cell lines (19), we tested the effect of AG1478, a
specific inhibitor for EGFR. In the absence of LPA, AG1478 reduced
proliferation of SQ-20B cells, suggesting the endogenous activation
of EGFR in this cell line. In the presence of LPA, however,
treatment with AG1478 did not result in reduced proliferation of
SQ-20B cells, suggesting that LPA signaling stimulates
proliferation of SQ-20B cells independently from EGFR activation
(Fig. 2B).
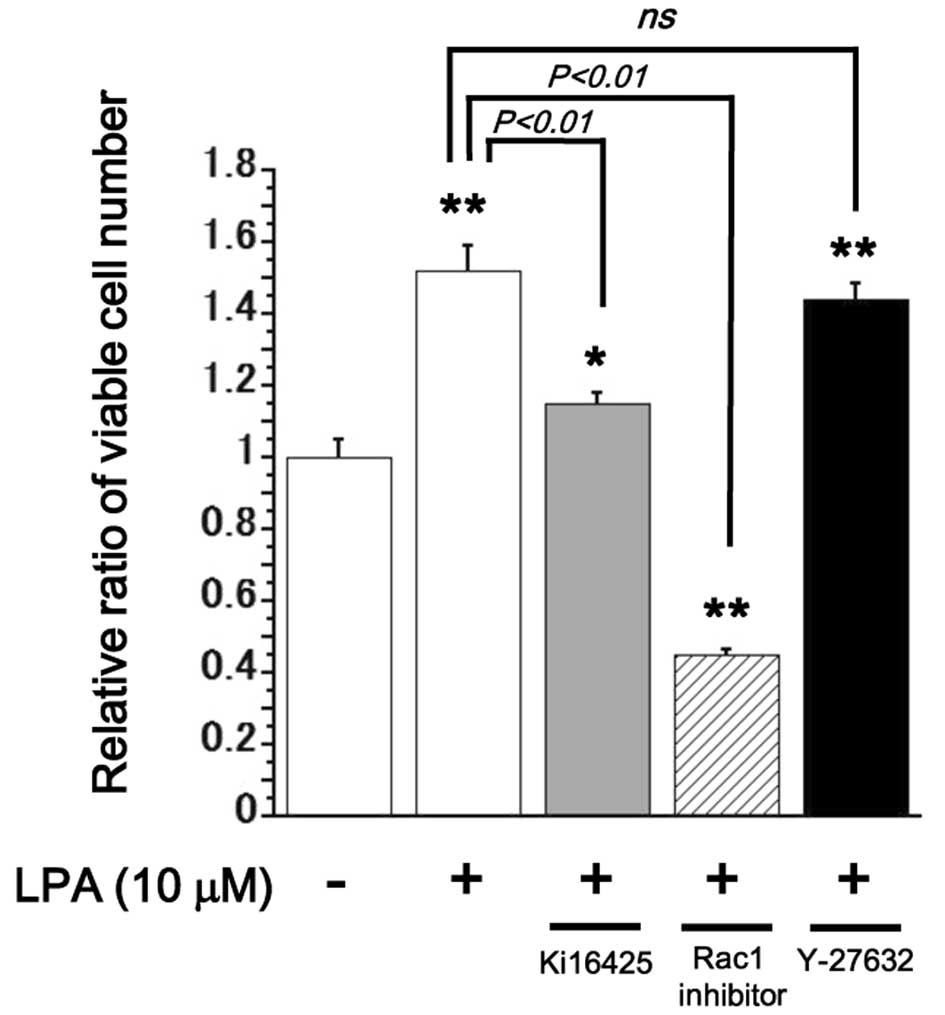
LPA stimulated proliferation of SQ-20B
cells via the activation of Ki16425-sensitive Edg family receptors
and Rac1
To investigate the intracellular signaling mechanism
responsible for LPA-stimulated proliferation in SQ-20B cells, the
effects of the LPA1 and LPA3 inhibitor Ki16425 (10 μM), Rac1
inhibitor (50 μM) and the Rho-associated coiled-coil forming
kinase (ROCK) inhibitor Y-27632 (10 μM) were tested
(25). Treatment with Ki16425 or a
Rac1 inhibitor inhibited proliferation of LPA (10
μM)-stimulated SQ-20B cell growth, whereas, treatment with
Y-27632 showed no significant effect on proliferation in these
cells (Fig. 3).
Overexpression of LPA4 in SQ-20B
cells
Next, we attempted overexpression of LPA4 in SQ-20B
cells which indigenously exhibit a trivial level of LPA4 expression
(Fig. 1). A fluorescent image
showed that AdvLPA4G (100 MOI, multiplicity of infection) infected
cells represented membranous and cytoplasmic expression of
GFP-associated LPA4 protein in the presence of Dox (100–500 ng/ml
of concentration was used in the present study) (Fig. 4A). Western blot analysis also
showed upregulated expression of LPA4 in Dox-treated
AdvLPA4G-infected cells (Fig.
4B).
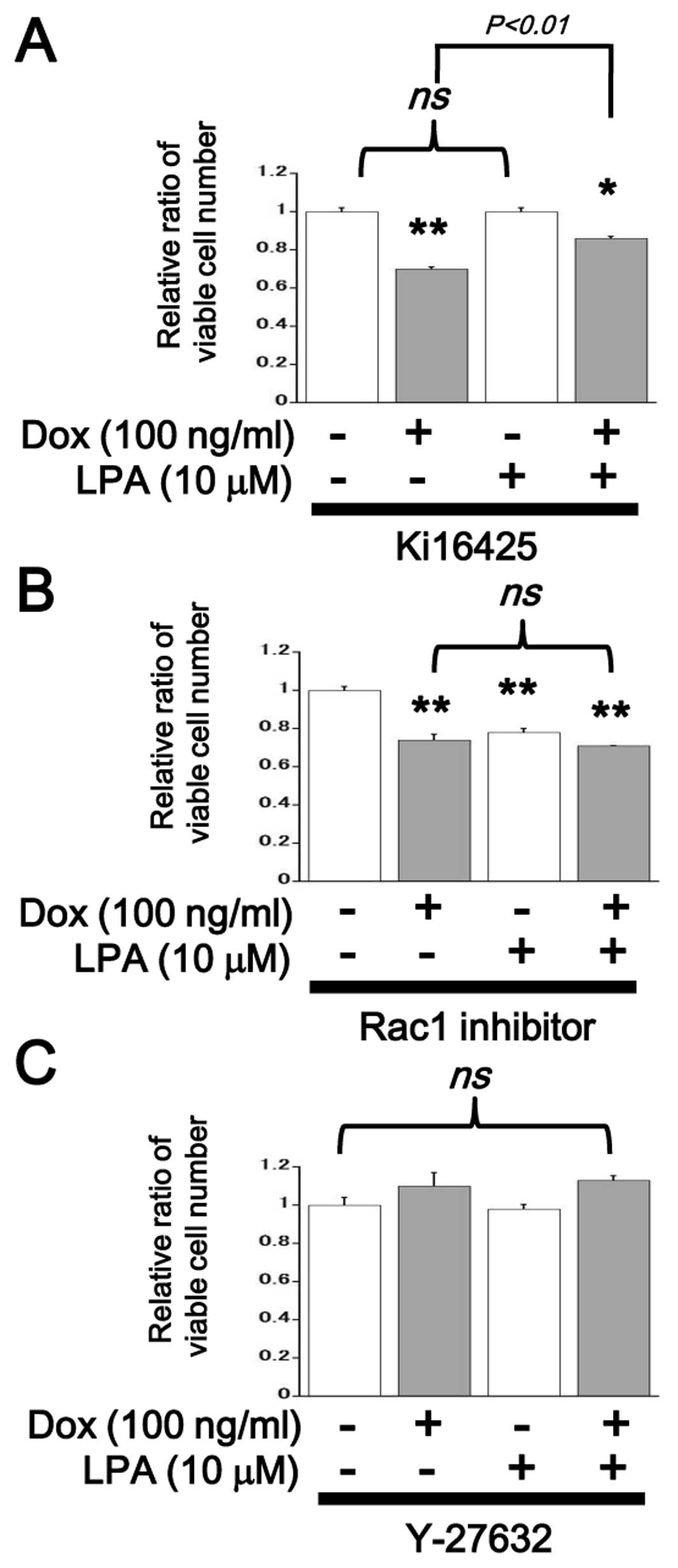
Overexpression of LPA4 inhibited
LPA-induced mitogenic response in SQ-20B cells
LPA induced a mitogenic response in SQ-20B cells in
the Dox-free control condition; this is consistent with the result
shown in Fig. 2A. In ectopic
LPA4-expressing cells, in contrast, LPA-induced mitogenic response
was completely inhibited (Fig. 5).
In the presence of Ki16425, proliferation of SQ-20B cells was
attenuated by the induction of ectopic LPA4 but could be partially
rescued by the addition of LPA (Fig.
6A). In the presence of Rac1 inhibitor, proliferation of SQ-20B
cells was suppressed irrespective of LPA treatment and no further
reduction resulted from ectopic LPA4 induction (Fig. 6B). In the presence of Y-27632, no
significant change in proliferation of SQ-20B cells was observed
upon LPA treatment or ectopic LPA4 induction (Fig. 6C). Flow cytometric cell cycle
analysis showed that the percentage of G2/M-phasic cells was
increased 6 h after LPA stimulation in ectopic LPA4-expressing
SQ-20B cells (Table II).
 | Table IIFlow cytometric cell cycle
analysis. |
Table II
Flow cytometric cell cycle
analysis.
| G0/G1 | S | G2/M |
|---|
| Dox(−) control | 52.8±1.14 | 11.4±0.33 | 35.6±0.78 |
| Dox(+) | 38.2±0.44a | 9.83±0.15a | 51.9±0.22a |
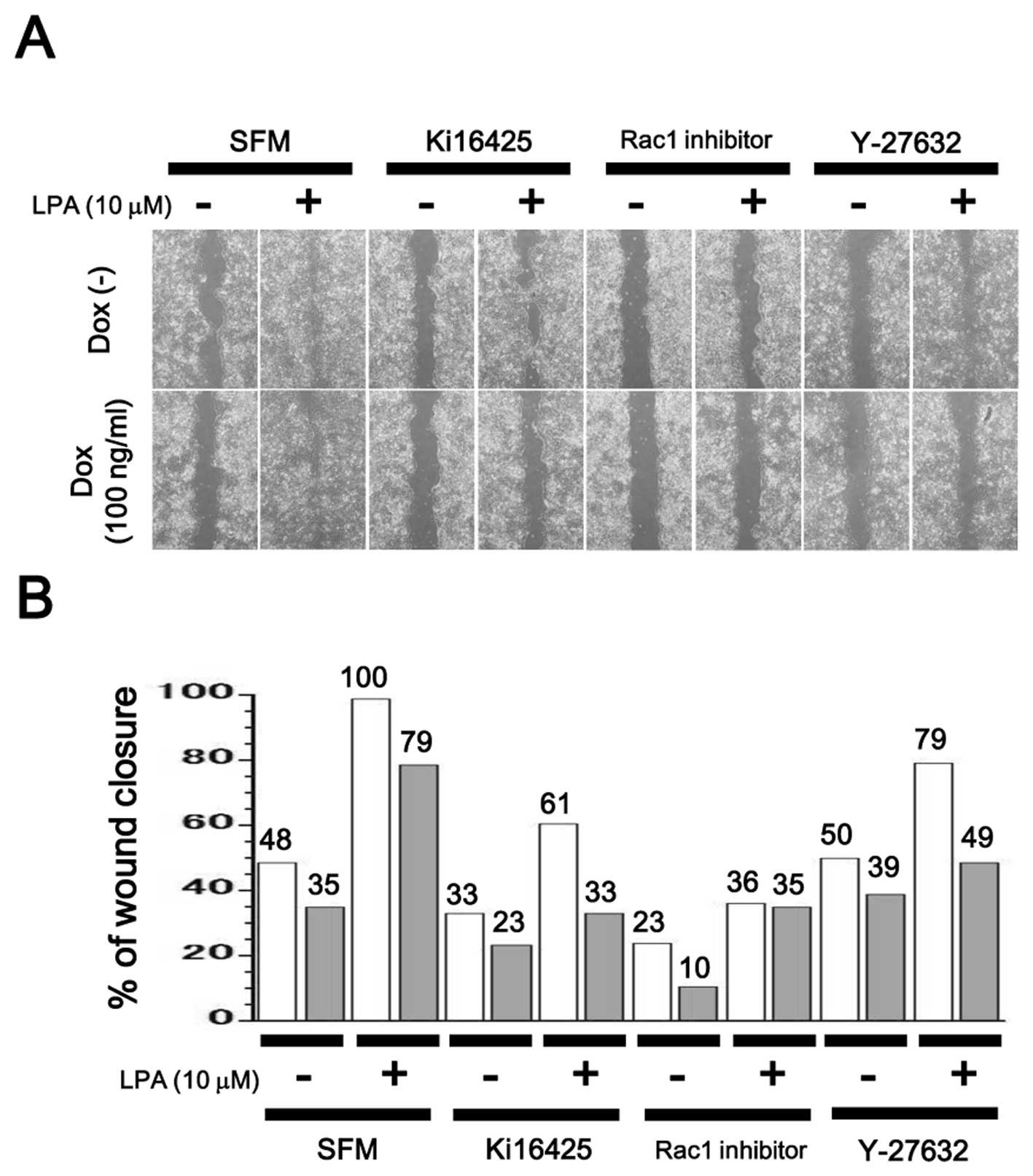
Inhibition of cell motility in
LPA4-expressing SQ-20B cells
Cell motility was measured through a wound healing
assay. LPA upregulated cell motility in SQ-20B cells, while
additional treatment with Ki16425, Rac1 inhibitor or Y-27632,
suppressed it. Ectopic induction of LPA4 also reduced cell motility
regardless of the presence or absence of inhibitors (Fig. 7).
Discussion
In the present study, we demonstrated that
adenovirus-mediated ectopic induction of LPA4 signaling potentially
modulates malignant behavior of SQ-20B, HNSCC cells including
proliferation (Figs. 5 and
6) and cellular motility (Fig. 7). Activation of Ki16425-sensitive
Edg receptrors (LPA1 and LPA3) and Rac1 was identified as an
important mitogenic cascade with which LPA4 signaling may interfere
(Figs. 3 and 6). Signaling mediated by both Edg and
non-Edg receptors may be a determinant of malignant behavior of
HNSCC and may therefore be a promising therapeutic target.
It is known that Edg-family LPA receptors (LPA1,
LPA2, LPA3) have a ubiquitous distribution in most tissues. Non-Edg
LPA receptors, on the other hand, appear to have low expression
levels in many tissues (11–13).
Exceptionally, high levels of LPA4 expression have been observed in
the ovaries (11–14) and LPA5 expression has been
identified in the small intestine, spleen and dorsal root ganglion
cells (11–13,26).
LPA6 expression has been shown in the hair follicles and vascular
endothelium (11,27). In various other types of cells and
tissues, however, the expression profiles of LPA receptors have not
been investigated in detail. Here, we found that LPA4 was expressed
at different levels in two different SCC cell lines, HEp-2 and
SQ-20B (Fig. 1). More importantly,
LPA stimulated proliferation only in SQ-20B cells, which exhibit
trivial levels of LPA4 expression (Fig. 2). In our preliminary experiments,
Detroit-562, another HNSCC cell line and HCT116, a colorectal
cancer cell line, showed mild mitogenic responses against LPA in
accordance with low levels of LPA4 expression (data not shown).
It was also previously reported that LPA-induced
malignant behavior of cancer cells are largely dependent on
Edg-family receptor activation (6–8,11–13,21).
Consistently, we observed the inhibition of LPA-induced mitogenic
response in SQ-20B cells by Ki16425, an inhibitor for LPA1 and LPA3
(Fig. 3). On the contrary, LPA4, a
non-Edg LPA receptor, potentially acts as a negative regulator for
certain cellular events mediated by Edg-family receptors: for
example, during osteoblast differentiation, LPA1 and LPA4 have been
shown to exert distinct functions (18). Unfortunately, no specific inhibitor
for LPA4 is available to date (12), but the development of one would be
highly beneficial for shedding light on the role of LPA4 in
physiological and disease processes.
Rho-family GTPases including Rho, Rac and Cdc42 are
presumed to modulate various cellular functions such as
cytoskeletal reorganization, cell motility, invasion, proliferation
and apoptotic processes (28,29).
Rho-family GTPases are also major intracellular signaling molecules
downstream of GPCRs including the LPA receptors (11–13).
The mitogenic effect of LPA on SQ-20B cells was attenuated by
Ki16425 and Rac1 inhibitor. Thus, the Gi-Rac signaling axis may
play a role in LPA-induced proliferation downstream of
Ki16425-sensitive Edg receptors (LPA1 and LPA3) (11–13).
Y-27632, a Rho/ROCK inhibitor, had no significant effect on the
proliferation of LPA-stimulated SQ-20B cells (Fig. 3). Among known LPA receptors, LPA4
has been shown to bind only to G12/13 proteins and to
activate Rho (11–13). However, the
G12/13-Rho/ROCK pathway is not expected to be involved
in the regulation of proliferation in LPA-stimulated SQ-20B
cells.
Ectopic induction of LPA4 abolished LPA-induced
mitogenic response in SQ-20B cells (Fig. 5), suggesting that LPA4 signaling
acts as a negative regulator for proliferation. In the presence of
Ki16425, LPA mildly recovered cell proliferation of ectopic LPA4
expressing SQ-20B cells (Fig. 6A),
probably due to partial release from competitive inhibition by
Ki16425 against LPA1 and LPA3 (30). In the presence of Rac1 inhibitor,
ectopic expression of LPA4 no longer suppressed proliferation of
SQ-20B cells (Fig. 6B). We also
observed inhibition of LPA-induced Rac1 activation in ectopic LPA4
expressing SQ-20B cells using a pull-down assay (data not shown).
Thus, LPA4 signaling may interfere with Rac1 activation in
LPA-stimulated SQ-20B cells.
In our flow cytometric analysis, LPA-stimulated
SQ-20B cells showed an accumulation of G2/M-phasic cells with an
ectopic induction of LPA4 (Table
II). Similarly, Rat-2, a rat fibroblast cell line, expressing
dominant negative Rac1 (N17rac1) has been shown consistently to
exhibit growth arrest at the G2/M phase (31). In the presence of Y-27632, a
Rho/ROCK inhibitor, no significant changes were seen in the
proliferation of SQ-20B cells irrespective of LPA4 induction
(Fig. 6C). In various systems, it
has been indicated that the activities of Rac and Rho may be
antagonistic through their regulation of GEF (guanine nucleotide
exchange factors, which act as activators) and GAP
(GTPase-activating proteins, which act as inhibitors) (29,32–34).
In the present study, however, we could not confirm the involvement
of the G12/13-Rho/ROCK pathway in the regulation of
SQ-20B cell proliferation downstream of LPA4. Further study is
needed to identify the LPA4-mediated inhibitory pathway involved in
the LPA-induced and Gi/Rac-mediated mitogenic response in these
cells.
LPA stimulates not only proliferation but also cell
motility in HNSCC cells (19,35,36).
Therefore, we examined the role of LPA4 signaling on cell motility
in SQ-20B cells. Our wound healing assay data suggested that
LPA-induced cell motility is mediated by Ki16425-sensitive Edg
family receptor activation and the exogenously induced LPA4
signaling negatively regulates cell motility in SQ-20B cells
(Fig. 7). Given that the inhibitor
for either Rac1 or Rho/ROCK attenuated cell motility, these small
G-proteins must play an important role in promoting cell motility
in these cells. Rho proteins induce stress fiber and focal adhesion
contact formation, whereas Rac and Cdc42 are involved in the
formation of lamellipodia and filopodia (28,29,32–34).
Irrespective of any antagonistic relationship between the Rac and
Rho/ROCK pathways (32–34), these small G-proteins would
coordinately promote changes in cell motility (37). It has been reported that cell
motility induced by LPA is associated with activation of RhoA and
inhibition of Akt and Rac in embryonic fibroblasts derived from
LPA4-deficient mice (17). It has
also been reported that ATX promotes invasion in HT1080,
fibrosarcoma cells via the activation of cyclic AMP/EPAC (exchange
protein directly activated by the cyclic AMP)/Rac1 pathway at the
downstream of LPA4 (38). Our data
suggest that LPA4 signaling negatively modulates cell motility in
HNSCC. The regulatory mechanism involved in this process, including
the Rac and Rho/ROCK pathways, should be clarified in further
investigations.
Known LPA receptors (LPA1–6) have been shown to
mediate major cellular events through their effects on LPAs, though
some LPA-mediated cellular functions may be mediated by the
intracellular signaling molecule peroxisome proliferator-activated
receptor (PPARγ) (39,40). Moreover, 2,3-cyclic phosphatidic
acid, an endogenously produced PPARγ antagonist, that is similar in
structure to LPA, inhibits cancer cell invasion and metastasis
in vitro and in vivo (41). In IMR-32 human neuroblastoma cells,
however, LPA antagonizes 15-deoxy-Δ12,14-prostaglandin J2-mediated
PPARγ activation (42). Although
we did not test the activation level of PPARγ in LPA-stimulated
SQ-20B cells, the possibility of an interaction between
trans-membrane LPA receptors and the intracellular targets of LPA
in HNSCC needs to be addressed in a future study.
Acknowledgements
We would like to thank Professor
Hideyuki J. Majima (Department of Oncology and Department of Space
Environmental Medicine, Kagoshima University Graduate School of
Medical and Dental Sciences), for the generous gift of the SQ-20B
laryngeal squamous cell carcinoma cell line. We would like to thank
Professor Satoshi Ishii (Department of Immunology, Graduate School
of Medicine, Akita University), for helpful discussions and
suggestions. We also gratefully acknowledge the technical
assistance of Ms. Junko Shiroma (Department of Pathology and Cell
Biology, Graduate School of Medicine, University of the Ryukyus).
This study was supported in part by a Grant-in-Aid for Scientific
Research (C) to S.K. (no. 24590462) and a Grant-in-Aid for Young
Scientists (B) to S.M. (no. 24791792) from the Ministry of
Education, Culture, Sports, Science and Technology (MEXT), Japan.
This work was also supported in part by Grants for the Project of
Young Investigator Research (2010 and 2011) to S.C. from the
University of Ryukyus.
References
|
1.
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Argiris A, Karamouzis MV, Raben D and
Ferris RL: Head and neck cancer. Lancet. 371:1695–1709. 2008.
View Article : Google Scholar
|
|
3.
|
Matta A and Ralhan R: Overview of current
and future biologically based targeted therapies in head and neck
squamous cell carcinoma. Head Neck Oncol. 1:62009. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Stransky N, Egloff AM, Tward AD, Kostic
AD, Cibulskis K, Sivachenko A, Kryukov GV, Lawrence MS, Sougnez C,
McKenna A, Shefler E, Ramos AH, Stojanov P, Carter SL, Voet D,
Cortés ML, Auclair D, Berger MF, Saksena G, Guiducci C, Onofrio RC,
Parkin M, Romkes M, Weissfeld JL, Seethala RR, Wang L,
Rangel-Escareño C, Fernandez-Lopez JC, Hidalgo-Miranda A,
Melendez-Zajgla J, Winckler W, Ardlie K, Gabriel SB, Meyerson M,
Lander ES, Getz G, Golub TR, Garraway LA and Grandis JR: The
mutational landscape of head and neck squamous cell carcinoma.
Science. 333:1157–1160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Molinolo AA, Amornphimoltham P, Squarize
CH, Castilho RM, Patel V and Gutkind JS: Dysregulated molecular
networks in head and neck carcinogenesis. Oral Oncol. 45:324–334.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Gendaszewska-Darmach E: Lysophosphatidic
acids, cyclic phosphatidic acids and autotaxin as promising targets
in therapies of cancer and other diseases. Acta Biochim Pol.
55:227–240. 2008.PubMed/NCBI
|
|
7.
|
Mills GB and Moolenaar WH: The emerging
role of lysophosphatidic acid in cancer. Nat Rev Cancer. 3:582–591.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Moolenaar WH: Lysophospholipids in the
limelight: autotaxin takes center stage. J Cell Biol. 158:197–199.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Umezu-Goto M, Kishi Y, Taira A, Hama K,
Dohmae N, Takio K, Yamori T, Mills GB, Inoue K, Aoki J and Arai H:
Autotaxin has lysophospholipase D activity leading to tumor cell
growth and motility by lysophosphatidic acid production. J Cell
Biol. 158:227–233. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Tanaka M, Okudaira S, Kishi Y, Ohkawa R,
Iseki S, Ota M, Noji S, Yatomi Y, Aoki J and Arai H: Autotaxin
stabilizes blood vessels and is required for embryonic vasculature
by producing lysophosphatidic acid. J Biol Chem. 281:25822–25830.
2006. View Article : Google Scholar
|
|
11.
|
Yanagida K and Ishii S: Non-Edg family LPA
receptors: the cutting edge of LPA research. J Biochem.
150:223–232. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Choi JW, Herr DR, Noguchi K, Yung YC, Lee
CW, Mutoh T, Lin ME, Teo ST, Park KE, Mosley AN and Chun J: LPA
receptors: subtypes and biological actions. Annu Rev Pharmacol
Toxicol. 50:157–186. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Meyer Zu Heringdorf D and Jakobs KH:
Lysophospholipid receptors: signalling, pharmacology and regulation
by lysophospholipid metabolism. Biochim Biophys Acta. 1768:923–940.
2007.PubMed/NCBI
|
|
14.
|
Noguchi K, Ishii S and Shimizu T:
Identification of p2y9/GPR23 as a novel G protein-coupled receptor
for lysophosphatidic acid, structurally distant from the Edg
family. J Biol Chem. 278:25600–25606. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Lee CW, Rivera R, Dubin AE and Chun J:
LPA(4)/GPR23 is a lysophosphatidic acid (LPA) receptor utilizing
G(s)-, G(q)/G(i)-mediated calcium signaling and G(12/13)-mediated
Rho activation. J Biol Chem. 282:4310–4317. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Yanagida K, Ishii S, Hamano F, Noguchi K
and Shimizu T: LPA4/p2y9/GPR23 mediates rho-dependent morphological
changes in a rat neuronal cell line. J Biol Chem. 282:5814–5824.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Lee Z, Cheng CT, Zhang H, Subler MA, Wu J,
Mukherjee A, Windle JJ, Chen CK and Fang X: Role of LPA4/p2y9/GPR23
in negative regulation of cell motility. Mol Biol Cell.
19:5435–5445. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Liu YB, Kharode Y, Bodine PV, Yaworsky PJ,
Robinson JA and Billiard J: LPA induces osteoblast differentiation
through interplay of two receptors: LPA1 and LPA4. J Cell Biochem.
109:794–800. 2010.PubMed/NCBI
|
|
19.
|
Gschwind A, Prenzel N and Ullrich A:
Lysophosphatidic acid-induced squamous cell carcinoma cell
proliferation and motility involves epidermal growth factor
receptor signal transactivation. Cancer Res. 62:6329–6336.
2002.
|
|
20.
|
Suzuki Y, Ozawa Y, Murakami K and Miyazaki
H: Lysophosphatidic acid inhibits epidermal-growth-factor-induced
Stat1 signaling in human epidermoid carcinoma A431 cells. Biochem
Biophys Res Commun. 240:856–861. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Rolin J and Maghazachi AA: Effects of
lysophospholipids on tumor microenvironment. Cancer Microenviron.
4:393–403. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Toyozumi Y, Arima N, Izumaru S, Kato S,
Morimatsu M and Nakashima T: Loss of caspase-8 activation pathway
is a possible mechanism for CDDP resistance in human laryngeal
squamous cell carcinoma, HEp-2 cells. Int J Oncol. 25:721–728.
2004.PubMed/NCBI
|
|
23.
|
Weichselbaum RR, Dahlberg W, Beckett M,
Karrison T, Miller D, Clark J and Ervin TJ: Radiation-resistant and
repair-proficient human tumor cells may be associated with
radiotherapy failure in head- and neck-cancer patients. Proc Natl
Acad Sci USA. 83:2684–2688. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Msaki A, Sánchez AM, Koh LF, Barré B,
Rocha S, Perkins ND and Johnson RF: The role of RelA (p65)
threonine 505 phosphor-ylation in the regulation of cell growth,
survival and migration. Mol Biol Cell. 22:3032–3040. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Uehata M, Ishizaki T, Satoh H, Ono T,
Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M
and Narumiya S: Calcium sensitization of smooth muscle mediated by
a Rho-associated protein kinase in hypertension. Nature.
389:990–994. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Lee CW, Rivera R, Gardell S, Dubin AE and
Chun J: GPR92 as a new G12/13- and Gq-coupled lysophosphatidic acid
receptor that increases cAMP, LPA5. J Biol Chem. 281:23589–23597.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Yanagida K, Masago K, Nakanishi H, Kihara
Y, Hamano F, Tajima Y, Taguchi R, Shimizu T and Ishii S:
Identification and characterization of a novel lysophosphatidic
acid receptor, p2y5/LPA6. J Biol Chem. 284:17731–17741. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Bhave SL, Teknos TN and Pan Q: Molecular
parameters of head and neck cancer metastasis. Crit Rev Eukaryot
Gene Expr. 21:143–153. 2011. View Article : Google Scholar
|
|
29.
|
Hall A: Rho GTPases and the control of
cell behaviour. Biochem Soc Trans. 33:891–895. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Routhier A, Astuccio M, Lahey D, Monfredo
N, Johnson A, Callahan W, Partington A, Fellows K, Ouellette L,
Zhidro S, Goodrow C, Smith A, Sullivan K, Simone P, Le L, Vezuli B,
Zohni M, West E, Gleason D and Bryan B: Pharmacological inhibition
of Rho-kinase signaling with Y-27632 blocks melanoma tumor growth.
Oncol Rep. 23:861–867. 2010.PubMed/NCBI
|
|
31.
|
Moore KA, Sethi R, Doanes AM, Johnson TM,
Pracyk JB, Kirby M, Irani K, Goldschmidt-Clermont PJ and Finkel T:
Rac1 is required for cell proliferation and G2/M progression.
Biochem J. 326:17–20. 1997.PubMed/NCBI
|
|
32.
|
Mack NA, Whalley HJ, Castillo-Lluva S and
Malliri A: The diverse roles of Rac signaling in tumorigenesis.
Cell Cycle. 10:1571–1581. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Shoval I and Kalcheim C: Antagonistic
activities of Rho and Rac GTPases underlie the transition from
neural crest delamination to migration. Dev Dyn. 241:1155–1168.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Guilluy C, Garcia-Mata R and Burridge K:
Rho protein crosstalk: another social network? Trends Cell Biol.
21:718–726. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Kramer RH, Shen X and Zhou H: Tumor cell
invasion and survival in head and neck cancer. Cancer Metastasis
Rev. 24:35–45. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Abraham MT, Kuriakose MA, Sacks PG, Yee H,
Chiriboga L, Bearer EL and Delacure MD: Motility-related proteins
as markers for head and neck squamous cell cancer. Laryngoscope.
111:1285–1289. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Abe M, Sogabe Y, Syuto T, Yokoyama Y and
Ishikawa O: Evidence that PI3K, Rac, Rho and Rho kinase are
involved in basic fibroblast growth factor-stimulated
fibroblast-collagen matrix contraction. J Cell Biochem.
102:1290–1299. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Harper K, Arsenault D, Boulay-Jean S,
Lauzier A, Lucien F and Dubois CM: Autotaxin promotes cancer
invasion via the lysophosphatidic acid receptor 4: participation of
the cyclic AMP/EPAC/Rac1 signaling pathway in invadopodia
formation. Cancer Res. 70:4634–4643. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Zhang C, Baker DL, Yasuda S, Makarova N,
Balazs L, Johnson LR, Marathe GK, McIntyre TM, Xu Y, Prestwich GD,
Byun HS, Bittman R and Tigyi G: Lysophosphatidic acid induces
neointima formation through PPARgamma activation. J Exp Med.
199:763–774. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Liliom K, Tsukahara T, Tsukahara R,
Zelman-Femiak M, Swiezewska and Tigyi G: Farnesyl phosphates are
endogenous ligands of lysophosphatidic acid receptors: inhibition
of LPA GPCR and activation of PPARs. Biochim Biophys Acta.
1761:1506–1514. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Tsukahara T: The role of PPARγ in the
transcriptional control by agonists and antagonists. PPAR Res.
2012:3623612012.
|
|
42.
|
Rodway HA, Hunt AN, Kohler JA, Postle AD
and Lillycrop KA: Lysophosphatidic acid attenuates the cytotoxic
effects and degree of peroxisome proliferator-activated receptor
gamma activation induced by 15-deoxyDelta12,14-prostaglandin J2 in
neuroblastoma cells. Biochem J. 382:83–91. 2004. View Article : Google Scholar
|