Introduction
Cervical cancer is the second most prevalent female
carcinoma worldwide after breast cancer (1). In 2012, the estimated number of new
cases of uterine cervix cancer was 12,170, accounting for 13.7% of
all gynaecological cancers in the United States (2). Although many therapy methods are
available, such as surgery therapy, radiotherapy, chemotherapy and
biotherapy and with much improvement in the application of these
techniques, death from cervical cancer still accounts for 14.3% of
all gynaecological cancers in the United States. According to the
reported deaths for the five leading cancers (breast cancer,
uterine cervix cancer, colorectal cancer, leukaemia and brain and
other nervous system cancers) by age and gender in the United
States in 2008, cervical cancer was the second leading cause of
cancer death among women aged 20–39 years, ranked second only to
breast cancer (2).
Radiotherapy is an effective therapy method for
cervical cancer, particularly when the disease is at an advanced
stage. Patients show different responses to this therapy method:
some are cured, whereas others suffer failed therapy (3) and are prone to relapse and distant
metastasis due to radioresistance. Many factors could impact tumour
cells responding to radiotherapy in the microenvironment. Ionising
radiation is able to activate sets of genes associated with
apoptosis, DNA damage repair, cell adhesion and angiogenesis
signalling pathways; these genes, in turn, may mediate cellular
responses to radiation (4,5), influencing the effects of
radiotherapy. p73 elicited apoptosis in response to DNA damage
induced by radiation and it demonstrated significantly higher
expression in radio-sensitive cancers than in radioresistant
cancers. Additionally, p73 might be important in regulating the
radiation response in cervical cancer cells (6). Ionising radiation was able to
activate transforming growth factor β1 (TGFβ); in turn, TGFβ
mediates the cellular and tissue radiation response (7,8).
Epithelial-mesenchymal transition (EMT) is a
morphogenesis process involved in embryonic and organ development.
This process is also involved in carcinoma progression (1). The features of EMT include loss of
cell adhesion molecules, downregulation of the expression of
epithelial differentiation markers and increased expression of
mesenchymal markers (9,10). Many factors control this process,
including several soluble factors, ion transport systems,
cytoskeletal modulators and transcriptional factors. Among these
signals, transcription factors Snail, slug and twist play an
important role (1) in inducing EMT
by repressing E-cadherin transcription (11). Data have shown that the recurrence
of breast cancer after therapy is accompanied by EMT (12). Tumourigenic EMT signalling pathways
that facilitate the migration and invasion of epithelial cancer
cells also contribute to apoptosis resistance and chemoresistant
malignant behaviour (1,13). Increasing evidence has suggested
that EMT contributes to chemotherapy resistance in cancer cells
(14–17). We proposed that EMT plays a role in
the low-dose radiation of cervical cancer cells, leading to
invasion and metastasis. We also tried to clarify the underlying
mechanism that was implicated in this process and we have
identified a new strategy to make therapy more specific.
Materials and methods
Cell lines and reagents
The human cervical cancer cell lines Siha and C33A
and mouse fibroblast cell line NIH3T3 were obtained from the
American Type Culture Collection (ATCC, Rockville, MD, USA).
Dulbecco’s modified Eagle’s medium (DMEM), minimum essential medium
(MEM) and fetal bovine serum (FBS) were purchased from Gibco-BRL
(Rockville, IN, USA). Rabbit anti-E-cadherin, anti-β-catenin,
anti-vimentin, anti-NF-κB p65 antibody and phospho-NF-κB p65
(Ser536) (93H1) rabbit mAb were purchased from Cell Signaling
Technology (Beverly, MA, USA). Mouse anti-β-actin antibody was
purchased from Sigma-Aldrich (St. Louis, MO, USA). TRIzol reagent
was purchased from Invitrogen (Carlsbad, CA, USA). Horseradish
peroxidase (HRP)-labelled goat anti-rabbit IgG (H+L) antibody and
HRP-labelled goat anti-mouse IgG (H+L) antibody were obtained from
Kirkegaard & Perry Laboratories, Inc. (KPL, Gaithersburg, MD,
USA). Cell lysis buffer for western blotting was purchased from
Biocolor BioScience & Technology Co. (Shanghai, China).
Cell culture and treatment
The human cervical cancer cell lines Siha and C33A
were cultured in MEM supplemented with 10% FBS and the mouse
fibroblast cell line NIH3T3 was cultured in DMEM supplemented with
10% FBS and 100 U of penicillin-streptomycin at 37°C in a
humidified 5% CO2 incubator.
Radiation treatment
The cervical cancer cells Siha and C33A cells were
cultured in 25-cm2 flasks. When the cells reached 75%
confluence, they were treated using the Faxitron Cabinet X-ray
System (Faxitron, Wheeling, IL, USA) as previously described
(18). The irradiated cells were
derived after irradiation of the parental cells at a dose rate of
0.36 Gy/min (given in 2-Gy fractions five times/week) with a
cumulative dose of 75 Gy. The control cells were treated using the
same procedure except that they were unirradiated. The culture
medium of the parental cells and X-ray-irradiated cells was changed
every 3 days and the cells were passaged every 7 days. After
reaching 75 Gy, the surviving cells were allowed to rest and
reorganise for 2 weeks before experiments.
RNA extraction and real-time qPCR
analysis
Total RNA was isolated using TRIzol reagent
according to the manufacturer’s protocol and reverse transcriptase
reactions were performed using 1 μg total RNA and the
PrimerScript RT reagent kit (Takara Biotechnology Co., Ltd.,
Dalian, China). For real-time PCR, the Applied Biosystems
StepOnePlus™ Real-Time PCR System was used according to the
manufacturer’s protocol. The relative gene expression ΔCt values of
mRNA were calculated by normalising to expression of the endogenous
control GAPDH. Each experiment was repeated three times.
Cell lysis and western blot analysis
The cells were washed three times with PBS, scraped
and lysed in RIPA buffer (Shennengbocai, Shanghai, China) (0.1%
SDS, 5 mM EDTA, 1% NP-40, 0.5% sodium deoxycholate and 1 mM sodium
vanadate) containing protease inhibitors (1 mM sodium fluoride, 1
mM PMSF). The lysates were harvested on ice for 30 min and
centrifuged at 12,000 rpm for 15 min at 4°C. The supernatants were
collected and the protein content was measured using the BCA
Protein Assay kit (Merck, Darmstadt, Germany). Seventy micrograms
of total protein was separated by 5–10% SDS-polyacrylamide gel
electrophoresis (SDS-PAGE) and transferred to a PVDF membrane
(Millipore) using a semi-dry blotting apparatus (Bio-Rad
Laboratories, Hercules, CA, USA). Membranes were blocked with 5%
(w/v) non-fat milk in TBST (100 mM NaCl, 50 mM Tris and 0.1%
Tween-20) at room temperature for 1 h and then they were incubated
overnight with primary antibodies at 4°C. After washing three times
with TBST, the membranes were incubated with appropriate
HRP-labelled secondary antibodies (KPL) for 2 h. Finally, the
signals of protein bands were detected with an ECL system (Merck)
according to the manufacturer’s instructions. β-actin was used as
the endogenous loading control.
Migration and invasion assay
For the migration assays, the lower chamber was
filled with 500 μl of conditional medium from NIHT3 cells.
The cells were digested with trypsin and 1×105 cells
suspended with serum-free MEM were seeded onto the upper chamber.
After 16 h, the non-migrating cells inside the filters were removed
with a cotton swab and the migrating cells on the other side of the
filter were fixed with methanol and stained with 0.1% crystal
violet. The number of cells was counted in at least 10 random
fields under a light microscope and data were analysed
statistically.
For the invasion assays, a Transwell system (8-μm
pore size; BD Biosciences, Bedford, MA, USA) precoated with 2 mg/ml
of basement membrane Matrigel (BD Biosciences) was used. The cells
were treated similar to the procedure used for the migration assay
described above. After 24 h, the filters were fixed and stained
similar to the procedure used for the migration assay. Experiments
were performed in triplicate.
Transient transfection assays
For the RNA interference assay, Lipofectamine 2000
(Invitrogen) was used according to the manufacturer’s instructions.
The cells were prepared at a density of 2×106
cells/flask and washed twice with PBS; then, a transfection mixture
of NF-κB p65 siRNA was added to the flasks. After a 4-h incubation
at 37°C in a 5% CO2 in a humidified incubator, the
transfection medium was discarded and replaced with complete
medium.
Statistical analysis
Statistical analysis was performed using Student’s
t-test. Results are presented as the mean ± standard deviation
(SD). P<0.05 was considered statistically significant and the
experiments were repeated at least three times.
Results
The effects of low-dose radiotherapy on
cellular morphology
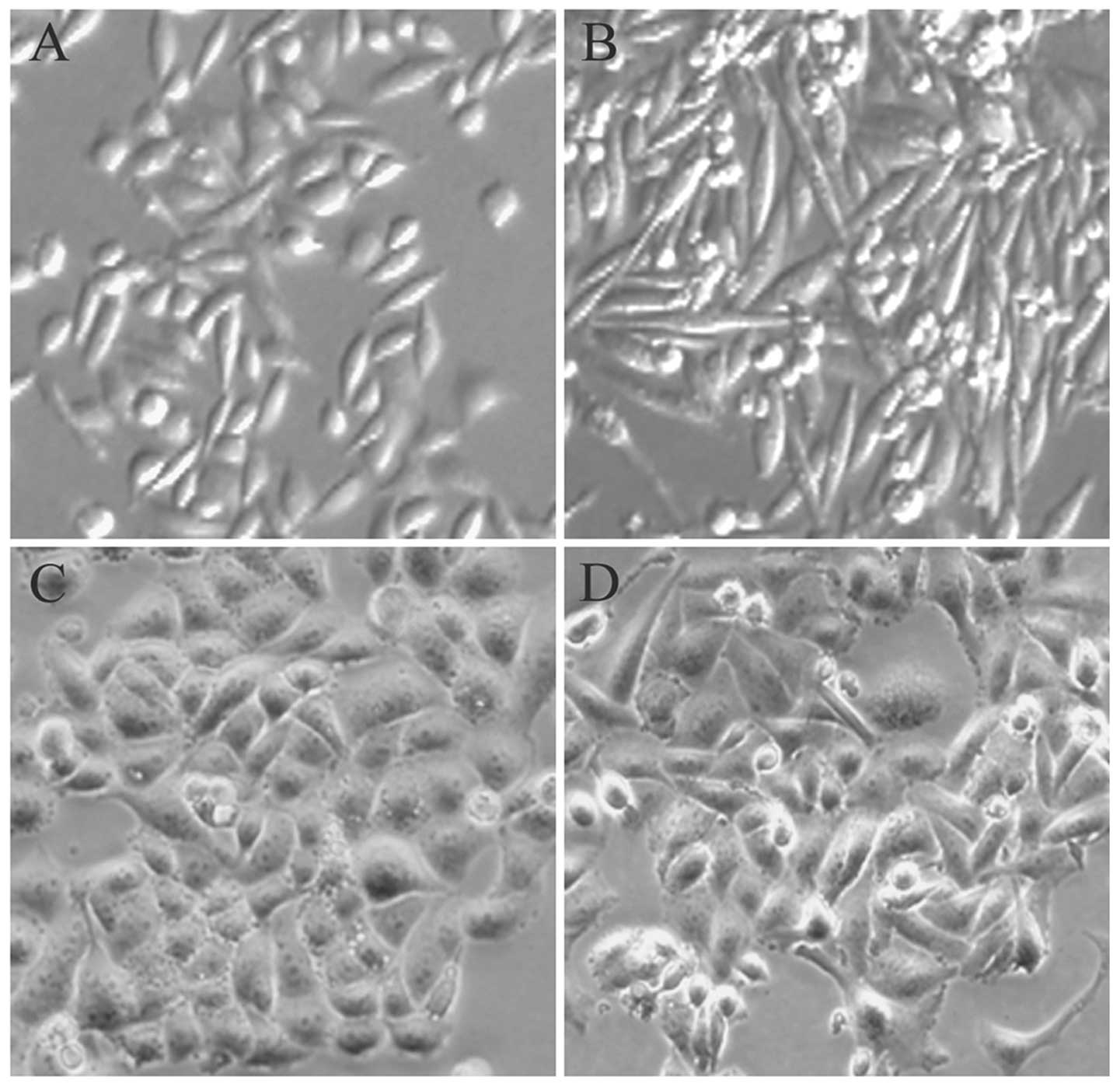
When the treatment of parental cervical cancer cells
with low-dose X-ray radiation reached the total of 20-Gy, the
morphology of cells changed from a polarised epithelial cell type
to a mesenchymal cell type. As shown in Fig. 1, the parental cells exhibited a
typical cobblestone-like shape and tight cell junctions, which are
traits of the epithelial phenotype. Additionally, the FIR cells
treated with low-dose X-ray radiation demonstrated a spindle-like,
elongated phenotype with loss of cell-to-cell contact, features
that are characteristic of fibroblasts.
The effects of low-dose radiotherapy on
the expression of mesenchymal and epithelial markers
Preview results showed that the low-dose radiation
treatment induced the morphological changes of cells. To evaluate
if this change was the result of epithelial-mesenchymal transition
(EMT), we tested the expression of EMT markers in FIR cells and
their parental cells. Real-time qPCR results showed that expression
of the epithelial markers E-cadherin and CK-18 were remarkably
downregulated (P<0.01) and the mesenchymal markers N-cadherin
and vimentin were upregulated in FIR cells. Additionally, the
expression of N-cadherin in Siha FIR cells was highly significantly
upregulated (P<0.01, P=0.00066) and the expression of vimentin
in the two FIR cell lines was significantly upregulated compared
with that of their parental cells (P<0.05) (Fig. 2A). Next, we determined the protein
expression levels of the two FIR cell lines. Western blot analysis
was consistent with these results. The expression of E-cadherin was
decreased and N-cadherin and vimentin expression were increased
compared with their parental cells (Fig. 2B). These results indicated that
low-dose irradiation in cervical cancer cells induced EMT.
Low-dose ionising radiotherapy increases
migration and invasion of cervical cancer cells
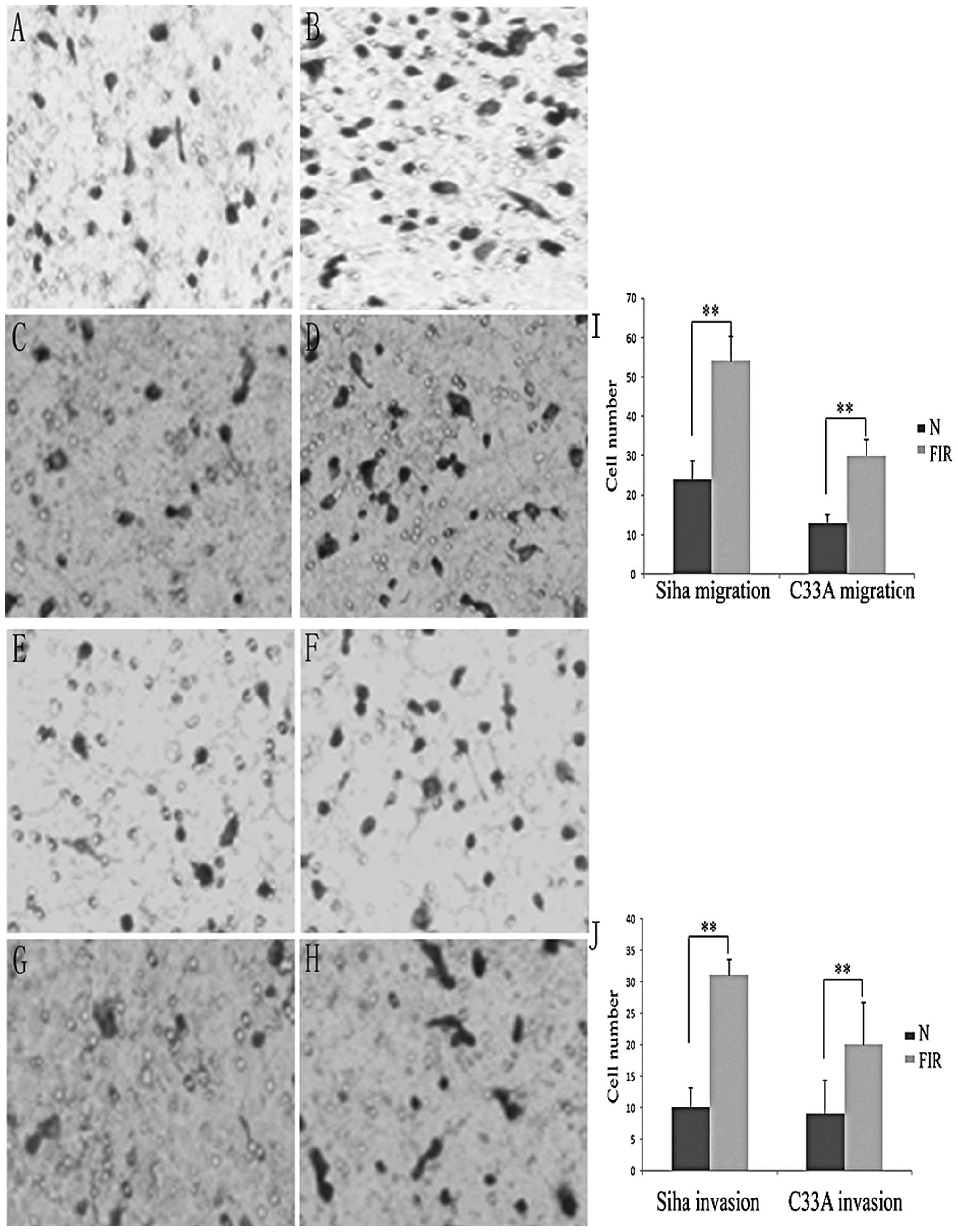
Cells that undergo EMT have demonstrated increased
migration and invasion ability (1). To determine whether these FIR cells
display such traits, we evaluated the migration and invasion
ability of FIR cells using the two-chamber assay. As shown in
Fig. 3, the migration ability of
the two FIR cell lines (P<0.01) was significantly enhanced
compared with that of their parental cells (P<0.05). The 16-h
invasion assay indicated that the two FIR cell lines acquired a
stronger invasive ability than their parental cells (Fig. 3B).
Low-dose radiation-induced EMT is related
to the NF-κB pathway
NF-κB was reported to be involved in Snail
gene-regulated EMT (19,20). Additionally, our lab previously
demonstrated that the p65 subunit of NF-κB plays a role in
MTDH-induced EMT (21). To
determine whether the p65 subunit of NF-κB was involved in the EMT
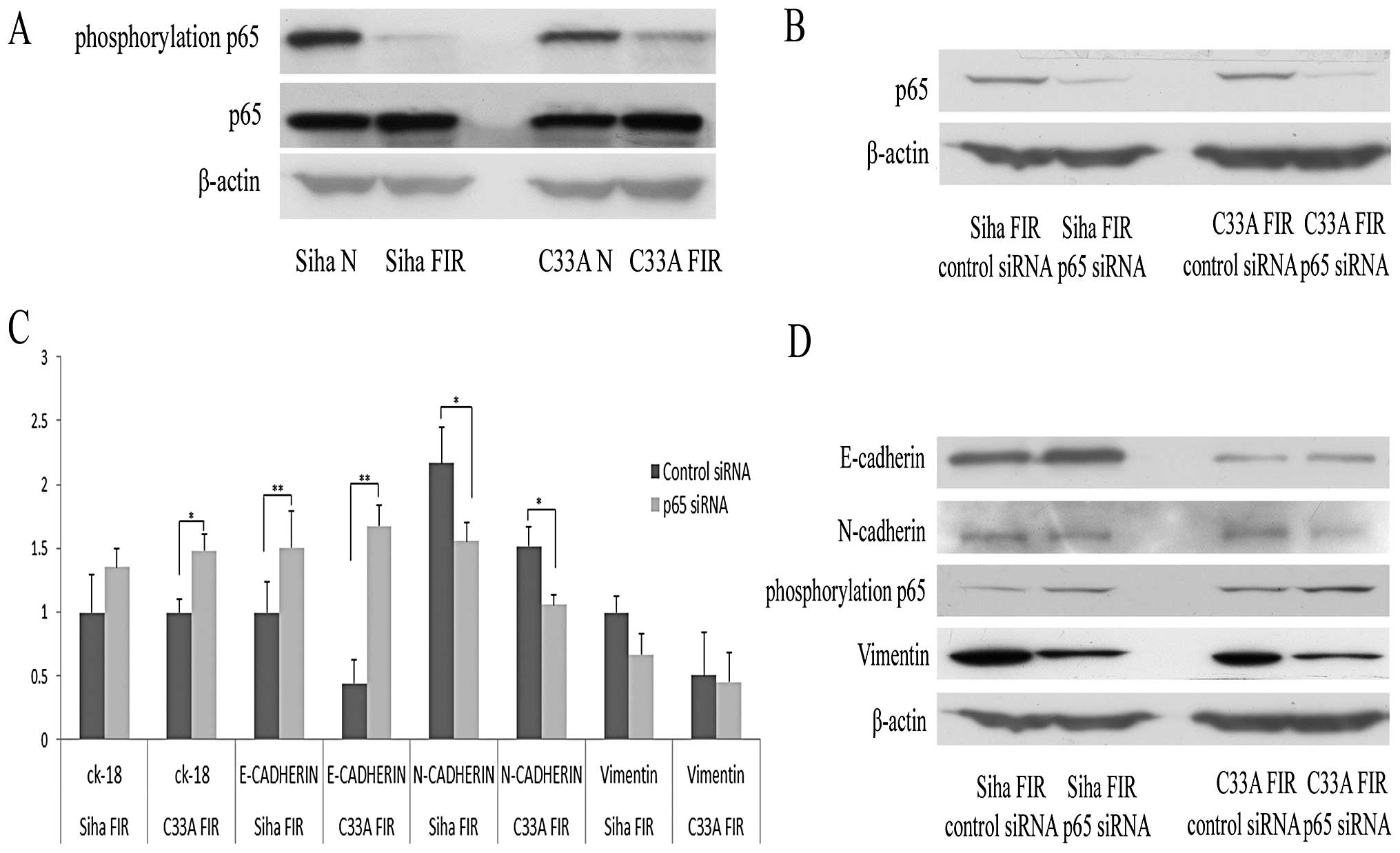
process induced by low-dose radiation, we evaluated the expression
of p65 and phosphorylated p65 by western blot analysis in parental
and FIR cervical cancer cells. As shown in Fig. 4A, p65 expression was upregulated
and phosphorylated p65 expression was downregulated in the two FIR
cell lines. We then further tested the roles of NF-κB in low-dose
radiation-induced EMT by transfecting NF-κB p65 siRNA into FIR
cells. The RNA interference efficiency of p65 was tested by western
blot analysis 48 h after transfection (Fig. 4B). Real-time qPCR results showed
that the miRNA expression of E-cadherin and CK-18 was upregulated
and that of N-cadherin and vimentin was downregulated after
transfection with NF-κB p65 siRNA (Fig. 4C). After transfection with NF-κB
p65 siRNA, E-cadherin expression was significantly upregulated
(P<0.01) and the expression of N-cadherin was downregulated
(P<0.05). The results of western blot analysis of the EMT
markers were consistent with the real-time qPCR results (Fig. 4D). These results suggested that p65
was involved in EMT induced by low-dose radiation.
Discussion
Radiotherapy is an effective curative method for
cervical cancer, even when the disease is at an advanced stage.
However, for some patients, it is easy to gain radioresistance
(3) during the therapy process,
causing therapy to become more difficult. Hence, radiation
resistance remains a challenge for radiotherapy and understanding
the molecular mechanism of radiation resistance is important to
develop new therapeutic strategies against cervical cancer. Our
results showed that low-dose radiation-induced EMT through NF-κB
p65 signalling enhanced the migration and invasion of cervical
cancer cells.
EMT is a morphogenesis process in multicellular
organisms (22) that has been
confirmed to cause the loss of cell adhesion molecules and to
enhance cell migration and invasion abilities (1,22,23),
promoting tumour progression (13,24).
Cancer cells that undergo EMT gain malignant behaviour such as
chemoresistance of cancer cells. In the present study, we showed
that low-dose radiation treatment could induce the cervical cancer
cell phenotype to switch from a cobblestone-like epithelial
morphology to a spindle-shaped mesenchymal morphology. At the same
time, expression of the epithelial markers E-cadherin and CK-18 was
downregulated and that of the mesenchymal markers N-cadherin and
vimentin was upregulated in the FIR cells versus their parental
cells. Our results further showed that the motility of FIR cells
was enhanced compared with that of their parental cells.
NF-κB is a transcription factor that plays an
important role in the regulation of cell apoptosis and
tumourigenesis. Additionally, NF-κB is essential for EMT induction
and maintenance (19,25). p38 suppresses TAK1-NF-κB signalling
to maintain E-cadherin expression, impeding the induction of EMT in
human primary mesothelial cells (26). NF-κB signalling was involved in
TNF-α-induced expression of Twist1 and overexpression of p65
induced upregulation of Twist1 expression together with EMT in
mammary epithelial cells (27).
Previous studies have shown that NF-κB p65 is involved in EMT
induced by MTDH in breast cancer cells (21). We further confirmed that NF-κB p65
was involved in low-dose radiation-induced EMT in cervical cancer
cells by RNA interference using NF-κB p65 siRNA. Our results showed
that when NF-κB p65 expression was silenced using siRNA, the EMT
process was reversed. E-cadherin expression was upregulated,
whereas expression of N-cadherin was downregulated. These results
suggested that p65 was associated with low-dose radiation-induced
EMT of human cervical cancer.
In conclusion, continuous low-dose radiation
enhanced the invasion potential of cervical cancer cells by
inducing EMT. p65 played an important role in this process and
silencing p65 could change this process. Targeting the reversion of
EMT or inhibiting EMT and its regulators may provide a new
therapeutic target for improving the effectiveness of radiotherapy
of cervical cancer.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (no. 81272857;
81072150), the Independent Innovation Foundation of Shandong
University (grant nos. 2010TS084 and 2010TS085) and the Natural
Science Foundation of Shandong (grant nos. ZR2009CQ038).
References
|
1.
|
Lee MY and Shen MR: Epithelial-mesenchymal
transition in cervical carcinoma. Am J Transl Res. 4:1–13.
2012.PubMed/NCBI
|
|
2.
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
3.
|
Kitahara O, Katagiri T, Tsunoda T, Harima
Y and Nakamura Y: Classification of sensitivity or resistance of
cervical cancers to ionizing radiation according to expression
profiles of 62 genes selected by cDNA microarray analysis.
Neoplasia. 4:295–303. 2002. View Article : Google Scholar
|
|
4.
|
Andarawewa KL, Erickson AC, Chou WS, et
al: Ionizing radiation predisposes nonmalignant human mammary
epithelial cells to undergo transforming growth factor beta induced
epithelial to mesenchymal transition. Cancer Res. 67:8662–8670.
2007. View Article : Google Scholar
|
|
5.
|
Qing Y, Yang XQ, Zhong ZY, et al:
Microarray analysis of DNA damage repair gene expression profiles
in cervical cancer cells radioresistant to 252Cf neutron and
X-rays. BMC Cancer. 10:712010. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Liu SS, Leung RC, Chan KY, et al: p73
expression is associated with the cellular radiosensitivity in
cervical cancer after radiotherapy. Clin Cancer Res. 10:3309–3316.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Barcellos-Hoff MH: Radiation-induced
transforming growth factor beta and subsequent extracellular matrix
reorganization in murine mammary gland. Cancer Res. 53:3880–3886.
1993.
|
|
8.
|
Ewan KB, Henshall-Powell RL, Ravani SA, et
al: Transforming growth factor-beta1 mediates cellular response to
DNA damage in situ. Cancer Res. 62:5627–5631. 2002.PubMed/NCBI
|
|
9.
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Zeisberg M and Neilson EG: Biomarkers for
epithelial-mesenchymal transitions. J Clin Invest. 119:1429–1437.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Moody SE, Perez D, Pan TC, et al: The
transcriptional repressor Snail promotes mammary tumor recurrence.
Cancer Cell. 8:197–209. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Lee MY, Chou CY, Tang MJ and Shen MR:
Epithelialmesenchymal transition in cervical cancer: correlation
with tumor progression, epidermal growth factor receptor
overexpression and snail up-regulation. Clin Cancer Res.
14:4743–4750. 2008. View Article : Google Scholar
|
|
14.
|
Hsu DS, Lan HY, Huang CH, et al:
Regulation of excision repair cross-complementation group 1 by
Snail contributes to cisplatin resistance in head and neck cancer.
Clin Cancer Res. 16:4561–4571. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Yang AD, Fan F, Camp ER, et al: Chronic
oxaliplatin resistance induces epithelial-to-mesenchymal transition
in colorectal cancer cell lines. Clin Cancer Res. 12:4147–4153.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Cheng GZ, Chan J, Wang Q, Zhang W, Sun CD
and Wang LH: Twist transcriptionally up-regulates AKT2 in breast
cancer cells leading to increased migration, invasion and
resistance to paclitaxel. Cancer Res. 67:1979–1987. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Arumugam T, Ramachandran V, Fournier KF,
et al: Epithelial to mesenchymal transition contributes to drug
resistance in pancreatic cancer. Cancer Res. 69:5820–5828. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Li Z, Xia L, Lee LM, et al: Effector genes
altered in MCF-7 human breast cancer cells after exposure to
fractionated ionizing radiation. Radiat Res. 155:543–553. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Min C, Eddy SF, Sherr DH and Sonenshein
GE: NF-kappaB and epithelial to mesenchymal transition of cancer. J
Cell Biochem. 104:733–744. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Cho KB, Cho MK, Lee WY and Kang KW:
Overexpression of c-myc induces epithelial mesenchymal transition
in mammary epithelial cells. Cancer Lett. 293:230–239. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Li X, Kong X, Huo Q, et al: Metadherin
enhances the invasiveness of breast cancer cells by inducing
epithelial to mesenchymal transition. Cancer Sci. 102:1151–1157.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Thiery JP: Epithelial-mesenchymal
transitions in development and pathologies. Curr Opin Cell Biol.
15:740–746. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Schmalhofer O, Brabletz S and Brabletz T:
E-cadherin, beta-catenin and ZEB1 in malignant progression of
cancer. Cancer Metastasis Rev. 28:151–166. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Hsu YM, Chen YF, Chou CY, et al: KCl
cotransporter-3 down-regulates E-cadherin/beta-catenin complex to
promote epithelial-mesenchymal transition. Cancer Res.
67:11064–11073. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Huber MA, Azoitei N, Baumann B, et al:
NF-kappaB is essential for epithelial-mesenchymal transition and
metastasis in a model of breast cancer progression. J Clin Invest.
114:569–581. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Strippoli R, Benedicto I, Foronda M, et
al: p38 maintains E-cadherin expression by modulating TAK1-NF-kappa
B during epithelial-to-mesenchymal transition. J Cell Sci.
123:4321–4331. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Li CW, Xia W, Huo L, et al:
Epithelial-mesenchymal transition induced by TNF-alpha requires
NF-kappaB-mediated transcriptional upregulation of Twist1. Cancer
Res. 72:1290–1300. 2012. View Article : Google Scholar : PubMed/NCBI
|