Introduction
Prostate cancer is one of the most common male
cancers, representing 28% of all male cancers in 2010 (1). In addition, prostate cancer was the
second most common cause of cancer related death in US men
(1). Treatment of prostate cancer
can range from careful monitoring to treatment with prostatectomy
or radiation. In most cases radical radiation therapy is very
effective with 5 year local control rates of up to 89% (2). However, a proportion of patients will
fail initial therapy and present with recurrent advanced local and
distant metastatic disease. No curative treatment currently exists
for those patients who present with recurrent disease.
In recent years, there has been a rise in the use of
image guided radiation therapy (IGRT) in the treatment of prostate
cancer. Because the prostate is a mobile organ, inter and intra
fraction movement, as well as random deformation of the organ, can
occur throughout the course of daily radiation treatment (3). Crevoisier et al showed that
failure to compensate for daily prostate motion may lead to poorer
clinical outcomes (4). Prostate
position can be monitored through the use of fiducial markers that
are visible on radiographic images. By using daily imaging it is
possible to achieve accurate prostate localization and ensure
homogeneous dose distributions.
While the use of image-guidance has improved the
accuracy of radiotherapy, allowing safe and effective dose
escalation in the treatment of primary prostate cancer, there is
still no effective therapy for aggressive or recurrent disease. To
develop effective therapies for aggressive prostate cancer, whether
novel or a combination of several agents and treatment modalities,
it is imperative to first develop a model of radiation resistant
prostate cancer.
To facilitate the investigation of resistant
aggressive disease after radiation in a preclinical environment it
would be best to have a locally advanced orthotopic rodent model.
In this study we report the development of such a model for
resistant tumors after IGRT in rat prostates using a novel tumor
suppressor knockdown prostate cancer cell line.
Materials and methods
Cell culture
Human PCa cell line PC3 was modified by knocking
down the tumor suppressor protein DAB2IP (PC3-KD) and
co-transfected with luciferase reporter gene as described
previously by Kong et al(5). Cells were cultured in T medium
supplemented with 5% fetal calf serum, 100 U/ml penicillin, 100
μg/ml streptomycin, 900 μg/ml of G418, and 700 ng/ml
of puromycin in an atmosphere of 95% air/5% CO2 at
37°C.
Orthotopic model
A total of 1×105 PC3-KD cells were
diluted to a final volume of 30 μl. Male rats, either nude
or Copenhagen, were anesthetized using 1–2% isoflurane mixed with
100% O2. Cells were injected into the right lobe of
prostate and a gold fiducial marker was placed into the prostate
for subsequent image guided therapy. All the experiments were
conducted under UT Southwestern Institutional Animal Care and Use
Committee-approved guidelines for animal welfare.
Ultrasound
Ultrasound imaging was employed for i) minimally
invasive implantation of tumor cells; ii) measuring tumor volume in
the rat prostate. For implantation, rats were anesthetized and the
pelvis was shaved and sterilized. Animals were secured to the
handling table to ensure no movement. The prostate and bladder was
identified in the field of view by using an RMV716 transducer head
(VisualSonics Vevo® 770 Imaging System, Amsterdam, The
Netherlands). Once the prostate was visualized, a gold seed was
implanted into the prostate using an 18G needle mounted on a
trocar. The needle was aligned parallel to the transducer and
inserted parallel to the urethra. After confirming proper placement
of the fiducial, the needle was left in place and cells were
implanted using a 1cc syringe. Imaging was performed on the
VisualSonics Vevo 770 Imaging System using the real-time Micro
Visualization scan head RMV716 (11–24 MHz) specific for rats. Rats
were anesthetized and the transducer head was placed transverse to
the pelvis of the rats in supine position. Images were obtained by
resolving various depths of tissue into the center of optimum
resolution plane to ensure clear images throughout the tumor
volume.
Bioluminescence imaging
BL imaging was performed weekly using an IVIS Lumina
Imaging System (Xenogen, Alameda, CA). Rats were anesthetized by
using isoflurane inhalation mixed in pure oxygen followed by an
i.p. injection of D-luciferin (80 mg/kg). BL images were acquired
10 min after luciferin injection using various exposure times.
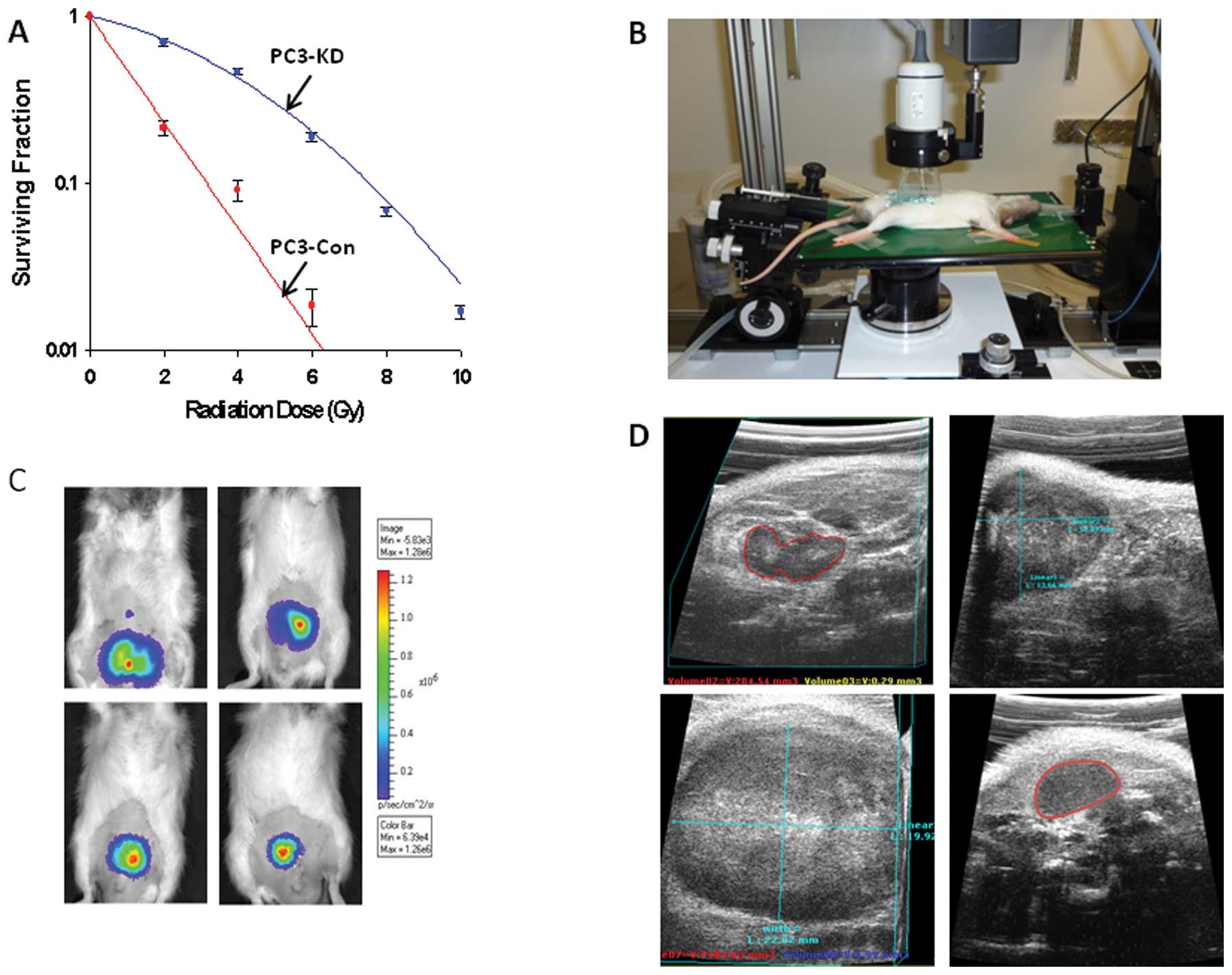
Colony formation assay
Surviving fraction (SF) analysis was performed using
PC3 Con (DAB2IP proficient) and PC3-KD (DAB2IP deficient) as
described by Kong et al(5,6). In
brief, cells were counted, serially diluted and plated in 60 mm
dishes. After 6 h, treated with increasing doses of radiation (0 to
8 Gy) and then incubated 10 days for colony formation. Colonies
were counted and SF curves were plotted using linear quadratic
equation (Sigma plot 11.0, Systat Software, Inc).
Magnetic resonance imaging
MRI studies were conducted using a 3 Tesla
whole-body human scanner (Achieva™, Philips Medical Systems, Best,
The Netherlands) with a small animal solenoid radio-frequency (RF)
coil (63 mm in diameter and 100 mm in length; Philips Research
Europe, Hamburg, Germany). Under anesthesia, animals were placed
supine where the thigh of the rat centered with respect to the
center of the RF coil. A volume containing the entire tumor was
subsequently obtained using T2 weighted multi-slice fast spin echo
sequences (repetition time, 5,700 msec; echo time, 70 msec; slice
thickness, 2 mm; field of view, 75 × 48 × 50 mm in-plain resolution
of 0.26 × 0.29 mm) and three dimensional gradient sequences
(repetition time, 7.7 msec; echo time, 4.5 msec; flip angle 15,
field of view = 75 × 51 × 50 mm resolution of 0.7 mm3
isotropic voxel).
Microirradiation
Radiation was carried out using an X-ray image
guided small animal irradiator as previously described (7,8). The
irradiator is characterized by a high dose rate, small beam size,
accurate and precise target localization facilitated through image
guidance, resulting in precision-high dose irradiation. The
collimation system consists of a 2.5-cm-thick brass alloy disk with
interchangeable apertures ranging from 1 to 20 mm in nominal
diameter.
H&E staining
Prostate tumors were removed and fixed in 4%
formalin. Tumors were mounted in paraffin and sections (10
μm) were prepared for standard H&E staining. Briefly,
sections were deparaffinized, rehydrated, stained with Harris’s
hematoxylin for 20 sec followed by treatment with Scott’s solution.
After washings, sections were stained with eosin for 2 min, rinsed,
dehydrated in ethanol and xylenes and mounted using permount.
Pimonidazole staining
Hypoxia staining in the rat prostate tumor was
performed using the Hypoxyprobe™-1 plus kit (Hypoxyprobe Inc.,
Burlington, MA). Hypoxyprobe-1 (pimonidazole HCl) was administered
i.p. (120 mg/kg) in tumor bearing rats. Two hours later, the animal
was sacrificed; tumor tissue was collected and fixed in 4% formalin
solution for 48 h. For detection, tumor sections were incubated
with FITC-conjugated mouse monoclonal antibody against pimonidazole
(1:50) for overnight at 4°C. After incubation with primary
antibodies, tumor sections were washed thoroughly and visualized
using a Zeiss Axio Imager 2 microscope (Carl Zeiss Microscopy, New
York, NY) using the FITC filter.
Results
Development of an orthotopic prostate
model for multimodal imaging
To appropriately facilitate the study of IGRT for
aggressive PCa, it is necessary to have a model that closely mimics
human disease, ideally with a tumor that is initially limited to
one lobe of the prostate. In this model, we implanted a human
prostate tumor cell line which is deficient in a tumor suppressor
protein DAB2IP (PC3-KD). This protein is a member of the Ras-GTPase
activating family and the loss of DAB2IP has been associated with
PI3K-Akt hyperactivation (9),
increased radiation resistance (5,6),
evasion of apoptosis (9),
epithelial-mesenchymal transition and poor clinical outcomes
(10). The PC3-KD cells were
implanted either using an open surgical method in nude rats
(Fig. 1A and B) or a minimally
invasive method using ultrasound guidance in Copenhagen rats. Using
the open surgical method cells were successfully implanted in all
animals (n=17), however when using the ultrasound guided method the
successful implantation rate was 70% (n=7). We also placed a gold
fiducial marker as shown in Fig.
1C whereas, Fig. 1D shows BL
imaging on weeks 1 and 3 after implantation.
PC3-KD cells are highly aggressive and demonstrate
significant radio-resistance (Fig.
2A). We also performed ultrasound guided PC3-KD cell
implantation in Copenhagen rats (Fig.
2B) and then tumor progression was followed by BL imaging
(Fig. 2C). Because of the poor
inherent contrast between the prostate tissue and tumor, CT is not
an optimal imaging modality for determination of size and location
of prostate tumors. Therefore, we used ultrasound (Fig. 2D) and MRI to monitor tumor growth,
size and location. By using ultrasound, it is possible to create
three dimensional reconstructions of the tumor and track the tumor
volume. Ultrasound images obtained from this model are notable for
areas of necrosis and diffuse calcification throughout the tumor as
represented by the varying echogeneity of the image (Fig. 2D).
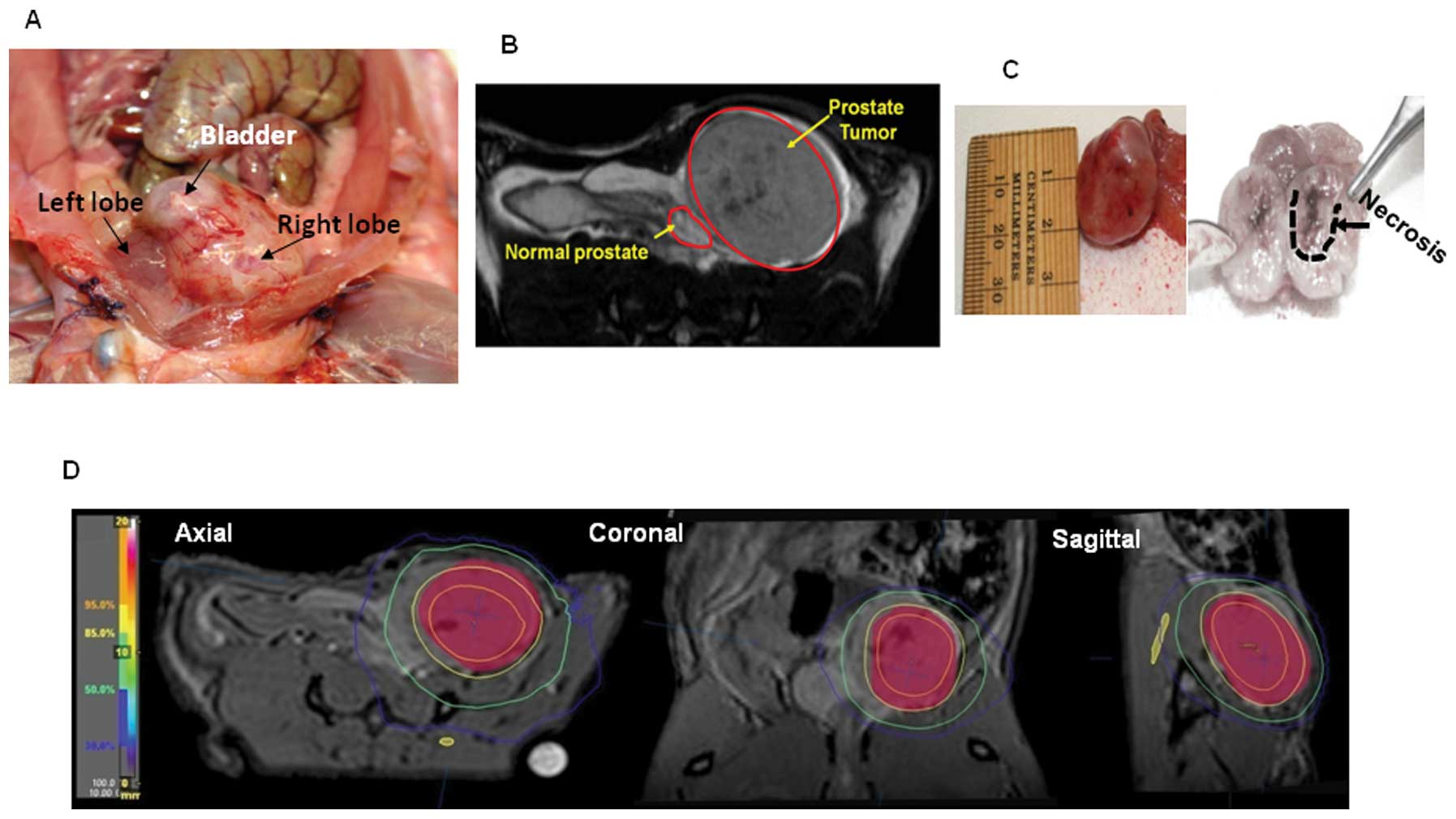
Fig. 3A displays
the high resolution digital image of the OT tumors in the right
prostate lobe of a nude rat. A specimen of such tumor was isolated
from the prostate with representative sizing and dissection showing
grossly visible areas of necrosis (Fig. 3C). We also performed MRI on nude
rats (Fig. 3B) and this imaging
modality in particular is highly useful for radiation treatment
planning (Fig. 3D).
Tumor growth and response to radiation
therapy
PC3-KD cells were implanted into the prostate of
Copenhagen (immune competent) and athymic nude rats (immune
deficient). Tumor growth was followed by BLI as described above.
Once the tumor size reached a certain size (approximately 5–7 mm in
diameter) based on ultrasound imaging, animals were split into
either a treatment (n=2 for Copenhagen; n=6 for Nude Rats,
respectively) or control arm (n=2 for both arms). Fig. 4A (upper and lower panel)
demonstrates tumor progression in Copenhagen rats after receiving
radiation treatment.
Control group tumors demonstrated aggressive, but
predictable growth (Fig. 4A, lower
panel). We initiated radiation treatment on the rats when the total
Flux (photon/sec) (integrated over an appropriate region of
interest, ROI) reached approximately 2.6–3.0×105 as
shown in Fig. 4B. We allowed one
Copenhagen rat to achieve a slightly larger tumor volume before
initiating treatment (Fig. 4B).
The rat was treated with 2 fractions of 10 Gy radiation as shown in
Fig 4A. Radiation was delivered
using image guidance to ensure that dose was delivered to the
tumor. Though PC3-KD is an aggressive radioresistant cell line
(Fig. 2A), the tumor demonstrated
a significant initial response to radiation therapy (Fig. 4A and B). In Copenhagen rats it
appears as if the tumors are initially controlled but begin to grow
a few weeks after radiation. We noted that BLI signal does not
completely resolve, remaining at a detectable level until week 7–8
(Fig. 4B). Between weeks 9–10, a
new focus of intensity reappears at the site of the original tumor
bed and ultimately grew uncontrollably and the animal died at the
end of week 14 (Fig. 4B). The
animal with a larger initial tumor (Rat 2) also received a similar
dose (total 20 Gy) however, died earlier at week 11. Tumor burden
led to compression of the urethra and partial obstruction of the
rectum. Therefore, death was caused either by post renal failure or
recto-sigmoid perforation secondary to fecalith impaction. However,
it is clear that primary tumor caused the complication which led to
death. It is also important to note that the peritoneum and
mesentery were free of metastatic disease as observed in Fig. 3A. In contrast, rats receiving no
radiation BLI signal increased continuously and the animals were
euthanized at week 8 (Fig. 4A,
lower panel, B).
The athymic nude rat tumors displayed the same
aggressive growth pattern, however, they grew significantly faster
than the tumors in Copenhagen rats. Control group displayed
aggressive, but predictable growth; rats were euthanized on day 16.
We initiated radiation as soon as signal appeared in the treatment
rats (total Flux photons/sec; 5×105 to 1×106)
(Fig. 4C). These rats were also
treated with 2×10 Gy on days 13 and 16. Tumors display little
response to radiation; ultimately tumor growth was delayed for a
matter of days before resuming growth. By day 22 tumors were large
enough to warrant euthanasia. Our previous mouse model study also
showed that PC3-KD subcutaneous tumors are highly radioresistant
when treated with fractionated radiation (6). These results clearly demonstrate that
orthotopically implanted PC3-KD cells can recapitulate aggressive
prostate tumors and furthermore, if not treated at an early stage,
local control is difficult to achieve and this necessitates the use
of pathway specific inhibitors in combination with radiation
treatment. While possessing intrinsic radiation resistance, we
demonstrate that DAB2IP deficient tumors can recur after initial
response to appropriate IGRT.
To correlate the imaging with biological events we
performed IHC analysis. H&E stained sections confirm the
placement of the tumor into the rat prostate; PC3-KD cells are
highly anaplastic and aggressive (Fig.
5A). Orthotopically implanted tumors are very similar to human
disease, tumors are locally aggressive as shown in Fig. 5C. Radiated rat prostates display
gross necrosis and cell death, some areas of the tumor show changes
indicative of apoptosis (Fig. 5B and
D) these sections agree with the ultrasound findings which were
indicative of necrosis. Changes seen in the irradiated rat
prostates were consistent with reactive inflammation, neutrophils
can be seen infiltrating the perivascular space as well as
infiltrating the areas of necrosis (Fig. 5E). Using pimonidazole, we show that
tumors develop large areas of hypoxia heterogeneously spread
throughout the tumor (Fig. 5F).
These areas of hypoxia may explain the high amount of resistance to
radiation therapy.
Discussion
While several other OT prostate tumors have been
reported in the literature (11–13),
there are currently no models that can accurately represent tumors
that fail initial RT. With the increased use of genetic
manipulation leading towards the addition of luciferase reporters
to cell lines, it is now feasible to track tumor growth and
response to treatment. Given that there is a lack of effective
therapies for patients who present with biochemical failure, we
felt that developing an aggressive locally advanced model that did
not respond to radiation therapy alone was necessary to develop
combined modality therapies capable of controlling aggressive
tumors.
There are several requirements necessary for the
development of a locally advanced prostate tumor specially for IGRT
or combined modality therapy. Current OT prostate models for
preclinical studies are primarily developed in mouse, however, to
delineate tumors and track tumor response animals with larger
prostates need to be used, hence we used rats. Secondly, the major
difficulty in creating an OT model that accurately demonstrates
human disease, specifically a tumor that is radiation resistant, is
reliability. In order to facilitate resistance we used a DAB2IP
knockdown prostate cell line. By using the DAB2IP knockdown cell
line resistant tumors are reliably formed. Orthotopically implanted
cells were able to grow large tumors in immune-competent male
Copenhagen rats as well as nude rats.
Copenhagen rats are used primarily to study
metastatic progression of prostate cancer as first described by
Dunning (14–16). Several of the models developed to
study prostate carcinoma did involve the injection of cells into
the prostate (16), however,
previous studies were done using syngeneic models which did not
invoke a strong response when orthotopically implanted (17,18).
Previous studies also investigated the radiation sensitivity of the
Copenhagen rat prostate tumor model, which consists of anaplastic
high grade tumors (15), and found
that the tumors had radioresistant subpopulations in vitro
but could not find correlating aggressive radiation resistant
tumors in vivo(19).
Furthermore, previous studies could not recapitulate recurrent
disease (19).
It is very interesting to note the differences in
growth rates between immune competent Copenhagen rats and athymic
nude rats. Tumors in nude rats grow much quicker causing mass
effect within days rather than weeks. Paradoxically, the rapidly
growing nude rat tumors should be more radiation sensitive,
however, our model shows that they are much more radiation
resistant. It is possible that innate immunity, rather than humoral
immunity, response of Copenhagen rats plays a significant role in
controlling tumor proliferation. However, the Copenhagen study
remains a pilot and this requires a larger more in depth study.
Once the tumors were successfully implanted they
exhibited several characteristics pertinent to aggressive tumor
growth. Radiation response also seems to correlate to initiation of
treatment. In Copenhagen rats, treatment arm that received RT early
regrowth is delayed by several weeks. However, the animal with the
larger starting volume relapse was significantly shorter indicating
that if treatment is delayed the tumor becomes more difficult to
control. It is also important to note that based on the calculated
α- and β-values of PC3-KD 2 fractions of 10 Gy leads to an LQED
(Linear Quadratic Equivalent Dose) 2 Gy of 60 Gy, a dose that is
clinically relevant in the treatment of human PCa. Furthermore,
rapidly growing tumors often display heterogeneous areas of
necrosis as a result of insufficient vascular supply (20). Insufficient blood supply leads to
hypoxia which correlates to poor response. Ultrasound imaging of
large tumors demonstrate large areas of necrosis as well as diffuse
calcification and pimonidazole staining confirms that implanted
tumors rapidly develop several large hypoxic areas.
In radiation resistant models, the ability to track
tumor growth and response to therapy is essential. BLI was the
primary imaging modality in this study and has been correlated with
both CT as well as MRI (21). We
further evaluated our model through the use of ultrasound. Here we
demonstrate that ultrasound technology can be used successfully for
the determination of tumor volume as well as to aid in tumor cell
implantation. Ultrasound was also helpful in revealing additional
information regarding the accurate localization, calcification,
necrosis and the effects of the tumor on proximal pelvic organs
such as bladder, which are not appreciable on BLI. While both
imaging modalities could be used individually, the complementary
information provided using both modalities creates a complete image
of the tumor.
Acknowledgements
We thank Ralph Mason, at UT
Southwestern Medical Center for providing the Copenhagen Rats;
Ramona Lopez and Thomas Boike for imaging assistance; Scott Buttars
at Visual Sonics for technical assistance in ultrasound. This study
was supported by the funding from Flight Attendant Medical Research
Institute (D.S.), W81XWH-11-1-0270 (D.S.). Additional support for
this study in part through the UT Southwestern Small Animal Imaging
Research Program (UTSWSAIRP; U24 CA12660801, P20 Pre-ICMIC CA86354)
and UT Southwestern Clinical and Translational Science Award grant
5TL1 RR024984 (V.T.).
References
|
1.
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
2.
|
Coen JJ, Zietman AL, Thakral H and Shipley
WU: Radical radiation for localized prostate cancer: local
persistence of disease results in a late wave of metastases. J Clin
Oncol. 20:3199–3205. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Li T, Thongphiew D, Zhu X, et al: Adaptive
prostate IGRT combining online re-optimization and re-positioning:
a feasibility study. Phys Med Biol. 56:1243–1258. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
De Crevoisier R, Tucker SL, Dong L, et al:
Increased risk of biochemical and local failure in patients with
distended rectum on the planning CT for prostate cancer
radiotherapy. Int J Radiat Oncol Biol Phys. 62:965–973. 2005.
|
|
5.
|
Kong Z, Xie D, Boike T, et al:
Downregulation of human DAB2IP gene expression in prostate cancer
cells results in resistance to ionizing radiation. Cancer Res.
70:2829–2839. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Kong Z, Raghavan P, Xie D, et al:
Epothilone B confers radiation dose enhancement in DAB2IP gene
knock-down radioresistant prostate cancer cells. Int J Radiat Oncol
Biol Phys. 78:1210–1218. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Pidikiti R, Stojadinovic S, Speiser M, et
al: Dosimetric characterization of an image-guided stereotactic
small animal irradiator. Phys Med Biol. 56:2585–2599. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Saha D, Watkins L, Yin Y, et al: An
orthotopic lung tumor model for image-guided microirradiation in
rats. Radiat Res. 174:62–71. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Xie D, Gore C, Zhou J, et al: DAB2IP
coordinates both PI3K-Akt and ASK1 pathways for cell survival and
apoptosis. Proc Natl Acad Sci USA. 106:19878–19883. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Xie D, Gore C, Liu J, et al: Role of
DAB2IP in modulating epithelial-to-mesenchymal transition and
prostate cancer metastasis. Proc Natl Acad Sci USA. 107:2485–2490.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Raheem O, Kulidjian AA, Wu C, et al: A
novel patient-derived intra-femoral xenograft model of bone
metastatic prostate cancer that recapitulates mixed osteolytic and
osteoblastic lesions. J Transl Med. 9:1852011. View Article : Google Scholar
|
|
12.
|
Shikanov S, Shikanov A, Gofrit O, Nyska A,
Corn B and Domb AJ: Intratumoral delivery of paclitaxel for
treatment of orthotopic prostate cancer. J Pharm Sci. 98:1005–1014.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Tse BW, Russell PJ, Lochner M, Forster I
and Power CA: IL-18 inhibits growth of murine orthotopic prostate
carcinomas via both adaptive and innate immune mechanisms. PLoS
One. 6:e242412011. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Dunning WF: Prostate cancer in the rat.
Natl Cancer Inst Monogr. 12:351–369. 1963.
|
|
15.
|
Lubaroff DM and Culp DA: Experience with
an animal model for the study of prostatic carcinoma. Trans Am
Assoc Genitourin Surg. 69:72–77. 1977.PubMed/NCBI
|
|
16.
|
Lubaroff DM, Canfield L, Feldbush TL and
Bonney WW: R3327 adenocarcinoma of the Copenhagen rat as a model
for the study of the immunologic aspects of prostate cancer. J Natl
Cancer Inst. 58:1677–1689. 1977.PubMed/NCBI
|
|
17.
|
Lopez DM and Voigt W: Adenocarcinoma
R-3327 of the Copenhagen rat as a suitable model for immunological
studies of prostate cancer. Cancer Res. 37:2057–2061.
1977.PubMed/NCBI
|
|
18.
|
Vieweg J, Rosenthal FM, Bannerji R, et al:
Immunotherapy of prostate cancer in the Dunning rat model: use of
cytokine gene modified tumor vaccines. Cancer Res. 54:1760–1765.
1994.PubMed/NCBI
|
|
19.
|
Rao BR, Slotman BJ, Geldof AA and Perez
CA: Radiation sensitivity of Copenhagen rat prostatic carcinoma
(R3327-AT and R3327-MATLyLu). Int J Radiat Oncol Biol Phys.
20:981–985. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Vaupel P, Kallinowski F and Okunieff P:
Blood flow, oxygen and nutrient supply, and metabolic
microenvironment of human tumors: a review. Cancer Res.
49:6449–6465. 1989.PubMed/NCBI
|
|
21.
|
Szentirmai O, Baker CH, Lin N, et al:
Noninvasive bioluminescence imaging of luciferase expressing
intracranial U87 xenografts: correlation with magnetic resonance
imaging determined tumor volume and longitudinal use in assessing
tumor growth and antiangiogenic treatment effect. Neurosurgery.
58:365–372. 2006.
|