Introduction
Cancer is a complex disease that requires the use of
different methods and treatment modes to cure. Surgery,
radiotherapy and chemotherapy, alone or in combination, are
currently the prevalent treatment modalities (1). However, there is an urgent need for
targeted therapies, which may provide more curative prospects.
Claudins (CLDNs) are a family of 17–27 kDa integral
membrane tight junction (TJ) proteins that pass through the
paracellular space in epithelial and endothelial tissues (2). The CLDN protein structure consists of
a cytoplasmic N-termini, a C-termini and two extracellular loops.
One of the loops interacts with CLDNs on adjacent cells to form an
adhesive structure with other TJ proteins (3). The other extracellular loop in CLDN3
and CLDN4 serves as a binding site for Clostridium
perfringens (C. perfringens) enterotoxin (CPE) (4).
As altered CLDN expression is frequently found in
cancer cells, the hypothesis of a correlation between altered CLDN
expression and cancer has been suggested (5). The loss of CLDNs and other TJ
proteins in cancer has been interpreted as a mechanism for the loss
of cell adhesion which is an important step in cancer progression
and metastasis. However, many CLDNs, such as CLDN3 and CLDN4, are
typically upregulated in many types of cancer, such as ovarian,
breast, prostate, colon and pancreatic cancer, suggesting that they
may have a positive effect on tumourigenesis and may lead to an
increase in invasion, motility and cell survival (5). The potential value of CLDN in cancer
therapy has been the subject of a number of studies (6) and is based on the fact that CLDNs are
expressed at the cell surface and contain two extracellular domains
that serve as potential target sites.
CPE is commonly associated with C.
perfringens type A food poisoning. CPE is a single polypeptide
of 35 kDa, which, upon binding to its receptors, causes cytolysis
through its effects on membrane permeability (5,7).
Both CLDN3 and CLDN4 are receptors for CPE. The intra-tumoural
administration of CPE in cancer cells has been shown to result in
tumour regression concomitant with a large degree of tumour
necrosis (5). It was first
demonstrated by Michl et al that the treatment of xenograft
pancreatic cancer cells with CPE, led to a significant reduction in
tumour burden, accompanied by necrosis, in CLDN4-expressing tumour
cells (8).
Pseudomonas aeruginosa exotoxin A (ETA)
functions by binding to nicotinamide and releasing the adenine
dinucleotide (ADP-ribose) in mammalian cells. This ADP-ribose unit
stays attached to ETA and is transferred to elongation factor-2
(EF-2), a protein involved in the translation and elongation of
proteins. This, in turn, blocks protein synthesis in host cells,
causing damage to target tissues (9). In this study, we demonstrate that HN5
head and neck squamous carcinoma cells overexpress CLDN4 and that
targeting the CLDN4 receptor by CPE-ETA’ (an immunotoxin created by
fusing the c-terminal CLDN4-binding domain of CPE to the ETA
domain) efficiently and specifically kills HN5 cells. Furthermore,
our findings show that CPE-ETA’ can be expressed and secreted by
the oncolytic bacterial strain, Clostridium ghonii (C.
ghonii), and that the secreted protein is potent against
CLDN4-expressing cells.
Materials and methods
Cell culture conditions
The cell lines used in this study were the HN5 human
head and neck squamous carcinoma, MCF-7 breast ductal carcinoma,
A549 non-small cell lung cancer, MRC-5 normal foetal lung
fibroblast, HT29 and HCT116 colon cancer, HeLa cervical cancer and
Huh-7 hepatocarcinoma cells. The cancer cells were cultured in
completed medium consisting of 500 ml DMEM (Gibco), 10% FBS, 12.5
ml HEPES buffer solution (1M) and 1 ml of penicillin (5,000 U) and
streptomycin (5,000 μg) antibiotic mixture. Culture flasks
were placed in a sterile tissue culture incubator under a
humidified atmosphere at 37°C and 5% CO2. Tissue culture
was performed by routine procedures (10). All cell lines were purchased from
the American Type Culture Collection, apart from HN5 which was
kindly provided by Dr Hong-Jian Zhu, University of Melbourne,
Melbourne, Australia.
Confirmation of CLDN4 expression in
cancer cell lines
Cells were seeded at appropriate densities and were
cultured until 80–90% confluency. Subsequently, the cells were
washed three times in PBS, scraped, centrifuged and resuspended in
100 μl of cell extraction buffer (Invitrogen) with 1 mM
phenylmethanesulfonylfluoride (PMSF) and protease inhibitor on ice
for 30 min while vortexing every 10 min. The lysate was clarified
by centrifugation at 13,000 rpm at 4°C and the supernatant stored
at −80°C until further use. Total protein concentration was
measured by spectrophotometry using the DC protein assay kit
(Bio-Rad), and equal amounts of proteins were loaded onto SDS-PAGE
gels for western blot analysis.
For qPCR analysis, RNA isolated from the cultured
cells was converted to cDNA using SuperScript III (Invitrogen).
Primers used for qPCR were as follows: CLDN4 forward, 5′-AGT GCA
AGG TGT ACG ACT CGC T-3′ and reverse, 5′-CGC TTT CAT CCT CCA GGC
AGT T-3′. GAPDH and β-actin were used as the internal reference
genes.
SDS-PAGE and western blot analysis
For SDS-PAGE, the following protein ladders were
used: Precision Plus Protein Dual Color Standards (Bio-Rad),
PageRuler Plus Prestained Protein Ladder (Fermentas). For western
blot analysis, the primary antibodies used were: anti-His antibody,
anti-CLDN4 antibody and anti-α-tubulin antibody. The secondary
antibody used was goat-anti-mouse IgG antibody. SDS-PAGE and
western blot analysis were performed according to standard
procedures (11).
Bacterial strains and plasmids
The properties of the bacteria and plasmids used in
this study are listed in Table
I.
 | Table IBacterial strains and plasmids. |
Table I
Bacterial strains and plasmids.
| Strain or
plasmid | Relevant
characteristics | Source |
|---|
| Plasmids | | |
|
p10His-cCPE-ETA’ | Amp, T7, N-His-tag,
MCS | (12) |
| pMTL-555 | repL, traJ, ermB,
fac2 | (33) |
|
pMTL-10His-cCPE-ETA’ | repL, traJ, ermB,
fac2 | This study |
| Strains | | |
| E.
coli | | |
| DH5a | Φ80dlacZΔM15,
Δ(lacZYA-argF)U169 | Promega |
| CA434 | HB101 carrying the
IncPβ conjugative plasmid, R702 | (33) |
| BL21 A1 | F-ompT
hsdSB(rB-, mB-) gal dcm araB::T7RNAP-tetA | Invitrogen |
| Clostridia | | |
| C.
ghonii | | This study |
Construction of Clostridial CPE-ETA’
fusion vectors
To construct pMTL-10His-cCPE-ETA’,
p10His-cCPE-ETA’ (12)
was used as the template for PCR. The forward primer
SfiI_CPE (5′-GAG GGC CCA GCC GGC CCA TCA TCA TCA TCA
TCA TC-3′) and the reverse primer SmaI_ETA
(5′-GTC CCG GGA GTT
ACT TCA GGT CCT CGC-3′) were used to amplify
10His-cCPE-ETA’ which incorporated the SfiI and
SmaI sites, respectively. PCR products were amplified using
Phusion High-Fidelity DNA Polymerase (Finnzymes) to minimise
sequence errors. Finally, amplicons were excised from gels and
cloned into the Clostridial shuttle vector, pMTL-555, using the
restriction sites, SfiI and SmaI. The fusion protein
is preceded by a secretion signal which is cleaved upon
extracellular export of the protein. The recombinant plasmid
pMTL-10His-cCPE-ETA’ was verified by sequencing.
Conjugal transfer of plasmids into C.
ghonii
For conjugation Escherichia coli (E.
coli) cultures were grown aerobically at 37°C, while
Clostridium cultures were grown anaerobically at 37°C. After
25 μl spots of C. ghonii overnight cultures were
absorbed by HI agar (without antibiotic), CA434 donor cell
suspensions (E. coli strain able to transfer the pMTL-555
vector to Clostridium via its helper plasmid capabilities)
with recombinant vectors were spotted (25 μl) on these
Clostridium spots and grown at 37°C, overnight in anaerobic
conditions. To select Clostridia which had taken up the recombinant
plasmid, the spots were spread on HI agar plates selected by 10
μg/ml erythromycin, 250 μg/ml cycloserine and 10
μg/ml polymyxin B. The plates were grown in anaerobic
conditions until colonies were visible. To further verify that the
Clostridium had taken up the plasmids, 15 colonies were
selected from these plates and each colony was cultured under
aerobic and anaerobic conditions, respectively. Since
Clostridium can only grow under strict anaerobic conditions,
there should only be growth under anaerobic conditions.
Preparation of Clostridial secreted
proteins
Clostridia were cultured in 30 ml of HI medium with
erythromycin 10 μg/ml and D-cycloserine 250 μg/ml
overnight at 37°C under anaerobic conditions. The cultures were
spun at 5,000 × g and the supernatant (medium) was filtered through
a 0.2-micron Millex-HV Syringe-driven filter unit. The filtered
medium was concentrated using an Amicon ultra centrifugal filter
(ultracel-30k), at 5,000 rpm for at least 30 min at 4°C. The
proteins were washed by PBS and concentrated 30-fold to 500
μl. The DC protein assay kit (Bio-Rad) was used to measure
protein concentrations.
Expression of CPE-ETA’ in E. coli
To produce 10His-cCPE-ETA’, the
p10His-cCPE-ETA’ plasmid was used as previously reported
by us (12). After transfection
into E. coli, protein purification was performed using a
Ni-NTA Fast Start kit (Qiagen) following the manufacturer’s
instructions. Purified protein was desalted and concentrated in PBS
using an ultrafiltration filter (Amicon Ultra-15–30 kDa cut-off).
Finally, protein samples were stored at −80°C in 20% glycerol PBS
and when required protein concentrations were measured using the DC
protein assay kit (Bio-Rad).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
cytotoxicity assay
Cancer cells were seeded at a density of
1×104 cells per well of a 96-well plate. After overnight
incubation, recombinant proteins were added to the cells and
incubated for 48 h. To ascertain the specificity of CPE-ETA’ for
the CLDN4 receptor, HN5 cells were incubated with anti-CLDN4
antibodies (blocking of the CLDN4 receptor) for 1 h prior to the
addition of CPE-ETA’. MTT assays were performed according to a
standard procedure (13). The
absorbency was measured by using a POLARstar Omega
spectrophotometer from BMG Labtech. The results were converted to
percentage proliferation compared to the control PBS group.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 5 software. The significance level was 0.05 (P<0.05) and a
Student’s t-test was used to analyse the data. Experiments were
performed three times and the data are presented as the means ±
standard error of the mean.
Results
Identification of high levels of CLDN4
expression in cancer cell lines
Cell lysates from cancer cell lines were separated
by SDS-PAGE, transferred onto PVDF membranes and probed with
anti-human CLDN4 and α-tubulin antibodies. Subsequent analysis
revealed that CLDN4 expression in the MCF-7, HN5, HT29 and HCT116
cancer cell lines was significantly upregulated (Fig. 1A). Furthermore, a weak expression
was observed in the A549 cells, while CLDN4 expression was
undetectable in the HeLa, MRC-5 and Huh-7 cell lines. Therefore,
for all subsequent proliferation experiments, HeLa cells were used
as the negative control. In addition, even protein loading was
confirmed by the expression of the housekeeping protein, α-tubulin.
The results from real-time PCR analysis of the CLDN4 transcript
were consistent with those obtained from western blot analysis
(Fig. 1B).
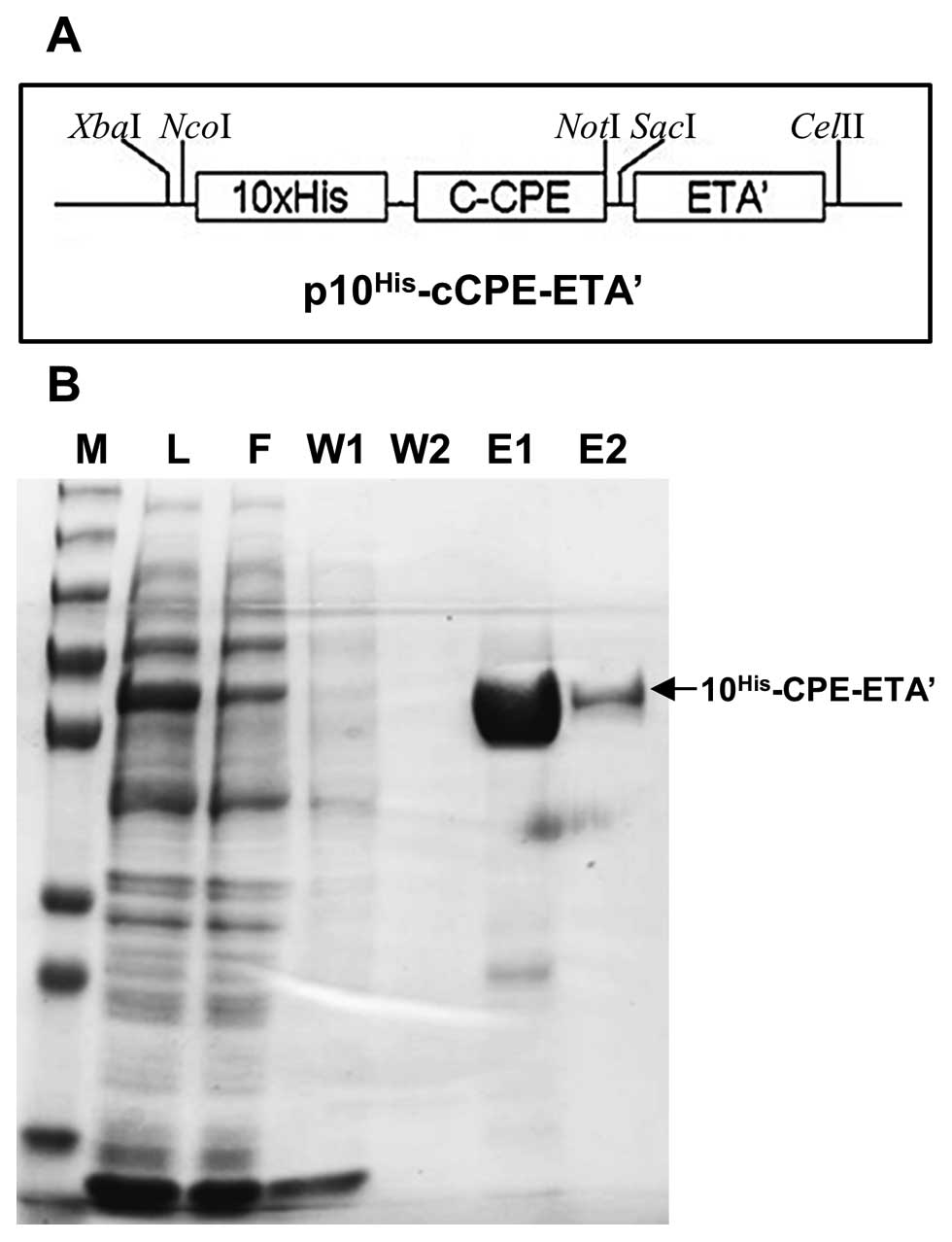
CPE-ETA’ protein expression and
purification
His-tag purification was employed to purify CPE-ETA’
(Fig. 2A) expressed in E.
coli. Analysis of the purified protein by SDS-PAGE revealed a
protein of the expected size (58 kDa) in elution 1 and elution 2
(Fig. 2B). The purification step
yielded 3 mg/ml of protein and a total of 6 mg of protein was
isolated.
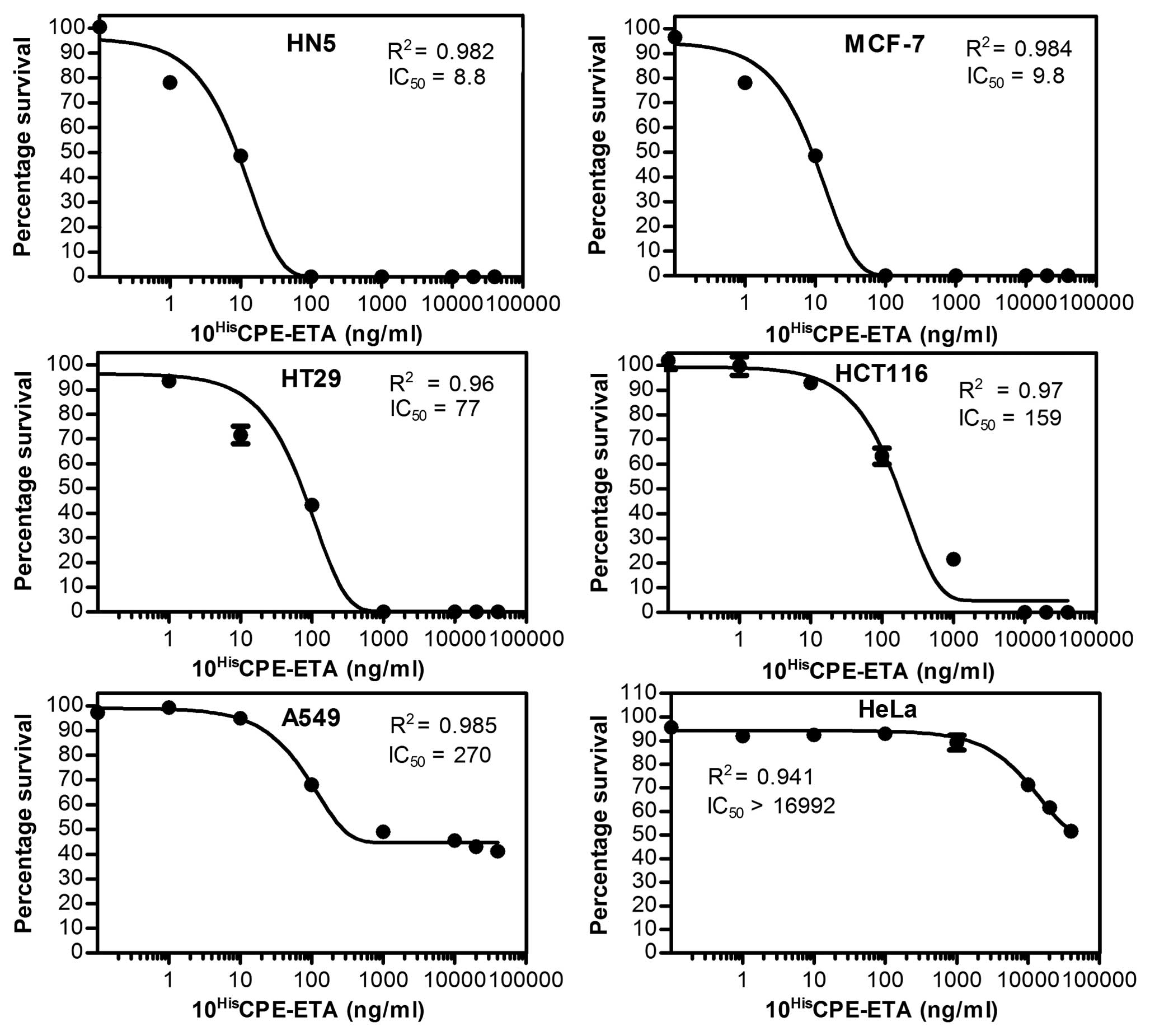
Efficacy of purified CPE-ETA’
The toxicity of purified CPE-ETA’ was examined by
employing MTT proliferation assays (13). MTT is converted to formazan by
living cells and can be detected by spectrophotometric
quantification. Purified protein was diluted from 0 to 40,000 ng/ml
to give a dose response and subsequent calculation of the 50%
inhibitory concentration (IC50) values (Fig. 3). PBS with 20% glycerol was used as
the no-drug control. DMEM medium only (without cells and proteins)
was used as the blank for MTT assay. The results showed that
CPE-ETA’ was very effective against HN5, MCF-7, HT29 and HCT116
cells with an IC50 between 8–160 ng/ml (Fig. 3). Furthermore, the A549 cells
showed a moderate sensitivity against the targeted toxin
(IC50 ∼270 ng/ml), while the CLDN4-negative cell line,
HeLa, had an IC50 of ∼17,000 ng/ml.
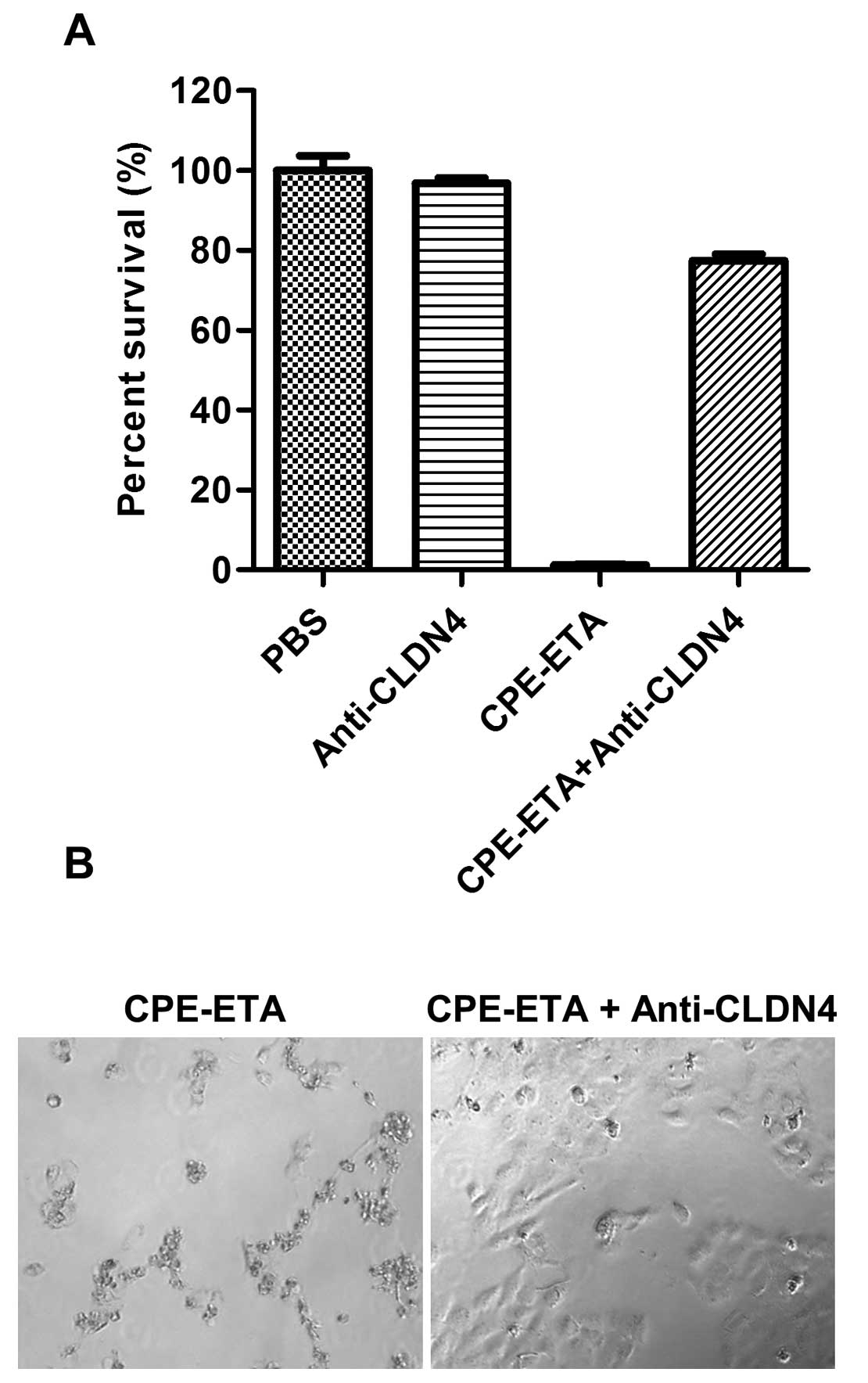
Specificity of CPE-ETA’ for CLDN4
receptors
To examine the specific biniding of CPE-ETA’ to the
CLDN4 receptor, HN5 cells were incubated with an anti-CLDN4
antibody prior to the addition of CPE-ETA’. It was found that
CPE-ETA’ had no effect on cells pre-treated with the antibody
compared to cells that were not treated with antibody (Fig. 4). It was also found that the CLDN4
antibody alone had no effect on the proliferation of the cells.
Construction of CPE-ETA’
Fig. 5 shows the
map of CPE-ETA’ constructed in the pMTL-555 backbone. pMTL-555
allows for the expression of proteins in Clostridium under
the fac2 promoter. This plasmid is compatible in both E.
coli and Clostridium. Furthermore, it contains elements
for the conjugal transfer of plasmids from E. coli to
Clostridium. 10His-cCPE-ETA’ was amplified from
p10His-cCPE-ETA’ by PCR, SfiI and SmaI
restriction enzyme sites (Fig. 5A)
were incorporated into the amplicon for subsequent cloning into
pMTL-555. The PCR fragment was cloned into pMTL-555 (digested by
SfiI and SmaI) to produce
pMTL-10His-cCPE-ETA’ (Fig.
5A). Sequence analysis was used to verify the correct colonies
in pMTL-555.
Transfer and expression of
pMTL-10His-cCPE-ETA’ in C. ghonii
The E. coli donor strain, CA434, was used to
transfer plasmids into Clostridium by conjugation.
Clostridium is not easily amenable to heat- or
electro-transformation of plasmid DNA (14). However, we demonstrate that
conjugation can be used to transfer plasmid DNA into C.
ghonii and, more importantly, this is the first report of the
successful DNA transfer into the oncolytic C. ghonii strain.
Transfer of the plasmid was achieved from CA434 by conjugation into
C. ghonii after the selection of the plasmid by erythromycin
and counter selection of E. coli by cycloserine and
polymyxin. Clostridium is an obligate anaerobe and cannot
grow in the presence of O2. Therefore, to confirm the
identity of the recombinant Clostridium, 15 colonies were
selected, plated on HI agar and grown under aerobic and anaerobic
conditions, respectively. It was found that all colonies were able
to grow under anaerobic conditions but were unable to grow under
aerobic conditions (Fig. 5B),
suggesting that all colonies were Clostridium.
For confirmation of recombinant protein expression,
Clostridium strains were grown under anaerobic conditions
overnight in HI medium. Protein from cell lysates secreted into the
growth medium was analysed by western blot analysis using an
anti-His antibody. Subsequent western blot analysis showed that
10His-cCPE-ETA’ was expressed in Clostridium and
was secreted into the medim (Fig.
5C).
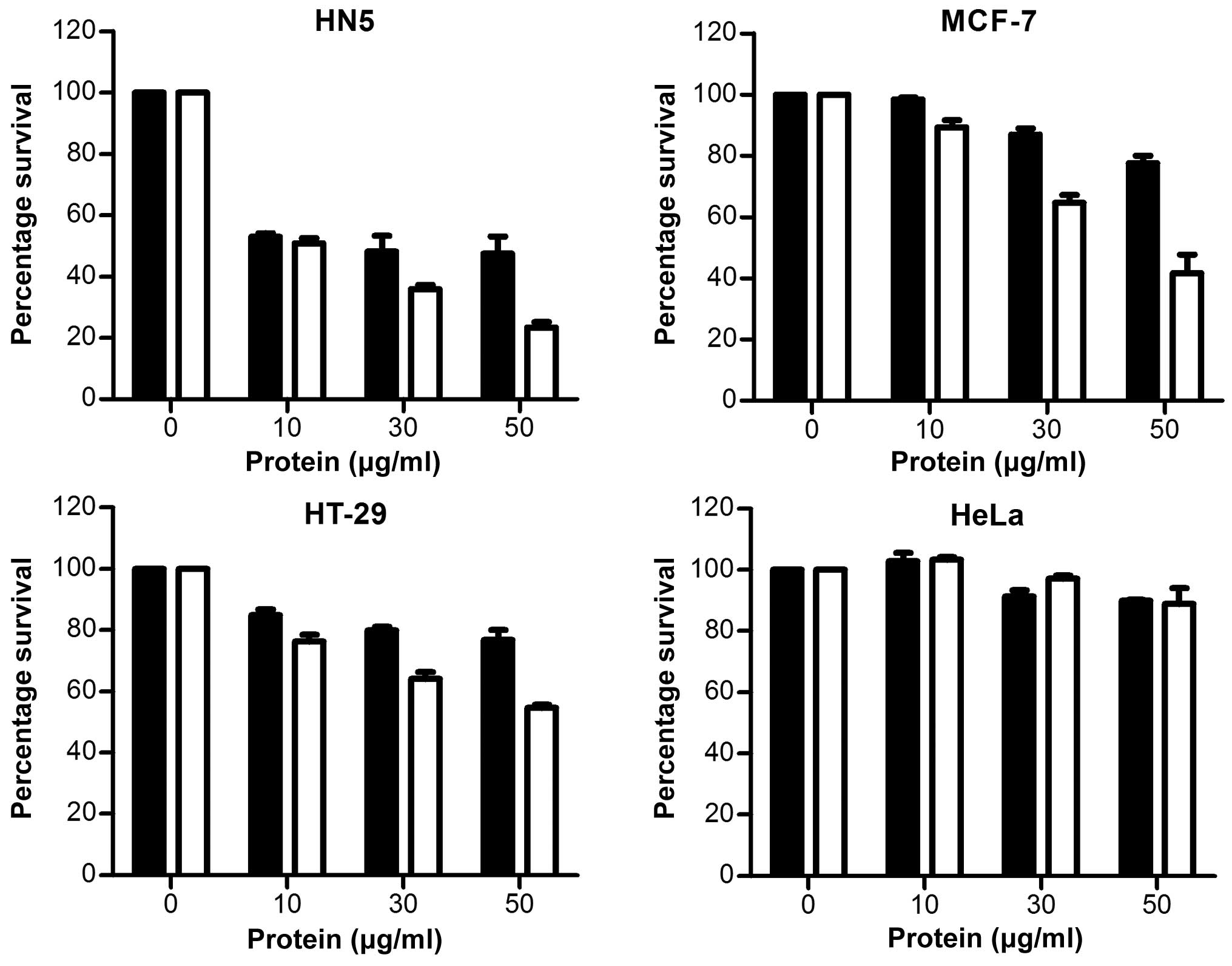
Efficacy of CPE-ETA’ secreted by C.
ghonii
To examine the effects of CPE-ETA’ secreted protein
from C. ghonii, bacteria were grown in HI mediun overnight
at 37°C under anaerobic conditions. The growth medium was
concentrated using ultrafiltration and buffered in PBS. The
proteins were used directly in MTT assays and the results expressed
as the percentage survival. It was found that medium from
non-recombinant Clostridium (pMTL-555) was able to kill all
cells tested (Fig. 6), possibly
due to endogenous toxins, protease and lipases produced by C.
ghonii(15). Previous data
from our group has shown that C. ghonii has oncolytic
activity when administered in vivo in tumour-bearing mice
with high specificity and safety profiles (unpublished data).
Furthermore, CPE-ETA’ increased the killing capacity
of C. ghonii-secreted protein in HN5, MCF-7 and HT29 cells
(Fig. 6). It was found that
CPE-ETA’ did not affect the proliferation of CLDN4-negative HeLa
cells.
Discussion
The current study investigated the expression
profiles of CLDN4 in a number of cancer cell lines. CLDN4
overexpression has been implicated in a variety of cancers,
including breast, colon, prostate, pancreatic and ovarian cancers
(8,16–19).
Furthermore, we investigated potential therapeutic strategies to
target CLDN4. To this end, the binding domain of CPE (20), a natural toxin with high binding
affinity for CLDN4, was employed to ‘piggyback’ the ETA toxin
domain of Pseudomonas aeruginosa(21) to cancers overexpressing CLDN4
(12). The engineered immunotoxin
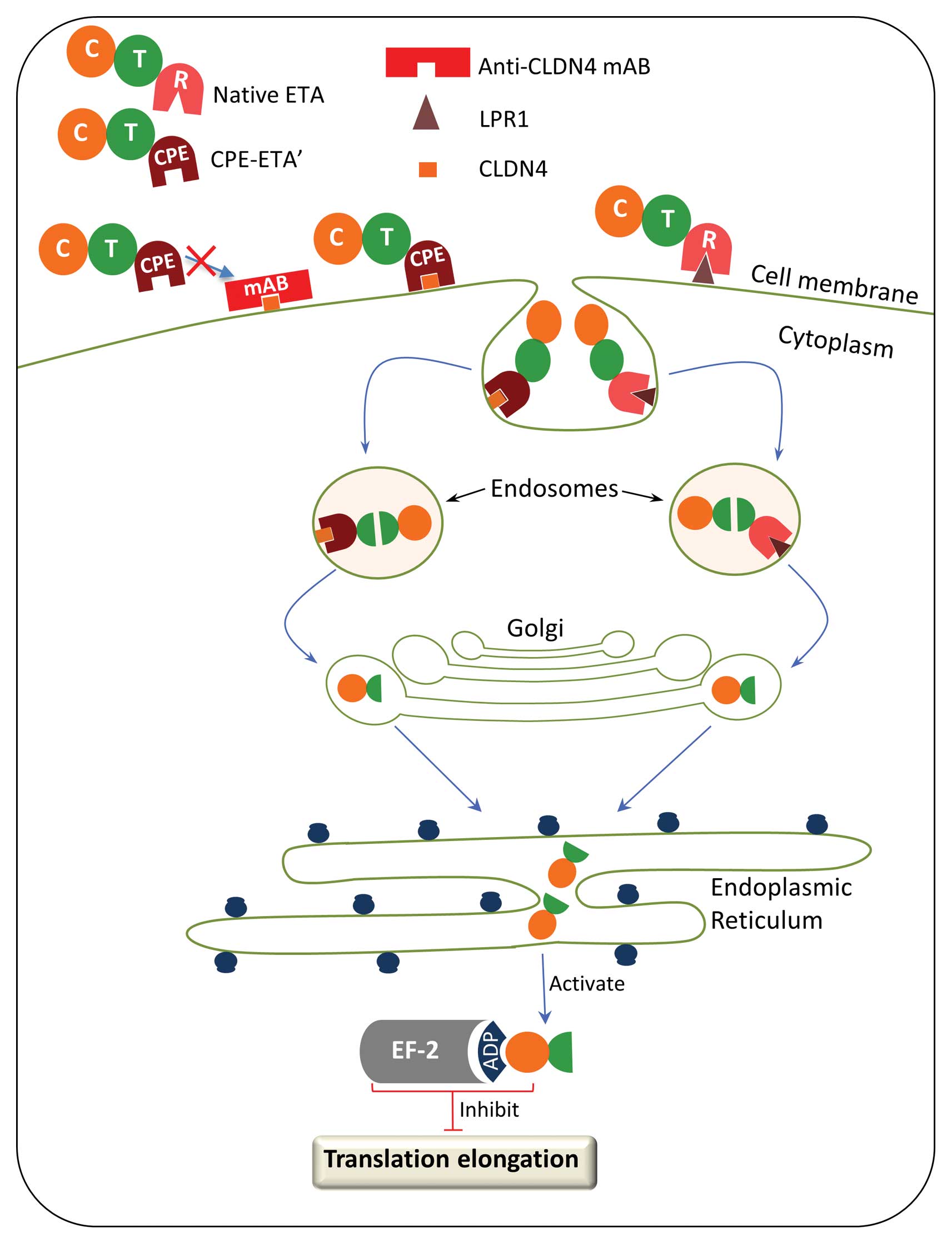
upon binding to the CLDN4 receptor is expected to translocate into
the cytoplasm via the Golgi apparatus and endoplasmic reticulum
where it will bind to EF-2 and inhibit protein synthesis (Fig. 7). The fusion toxin was transfected
ino E. coli and the purified protein was assayed against
CLDN4-expressing cells. Finally, an oncolytic
Clostridial(22) strain was
engineered to secrete the CPE-ETA’ fusion protein and the secreted
protein was tested on several cancer cell lines.
Analysis of the expression levels of CLDN4 in the
following cancer cell lines were found to be consistent with data
from previous reports using these cell lines: MCF-7 human breast
cancer (4), A549 human lung cancer
(23), HeLa human cervical cancer
(19), HT29 human colon cancer
(24), HCT116 human colon cancer
(25) and Huh-7 human liver cancer
cells (26). On the other hand,
the expression of CLDN4 in the HN5 human head and neck cancer and
the MRC-5 human fibroblast cell line has not been previously
reported.
Our findings show that purified CPE-ETA’ was highly
toxic to almost all the cancer cell types apart from the negative
control cell line, HeLa. In addition, blocking the CLDN4 receptor
with an anti-CLDN4 antibody abrogated the effects of CPE-ETA’,
suggesting that the killing capacity of CPE-ETA’ is dependent on
binding to the CLDN4 receptor (Fig.
7). The IC50 data indicated that this fusion protein
had a significant effect on the HN5 human head and neck squamous
carcinoma cancer cells, the MCF-7 human breast cancer and the HT29
human colon cancer cells. Furthermore, the high sensitivity of HN5
cells opens up the possibility of further research for the clinical
application of CPE-ETA’ as a therapeutic agent for the treatment of
head and neck cancers.
Fifty percent of patients presenting with head and
neck cancer are at an advanced stage of the disease, limiting
effective treatment regimes (27).
The standard therapy for head and neck cancer is chemo-radiotherapy
which is often associated with serious side-effects (28). Targeted therapies have been
developed which target the EGFR receptor, disrupting angiogenesis
(an important process in the growth and progression of solid
tumours) (29). Cetuximab, a
monoclonal antibody, is clinically used for the targeted therapy of
head and neck cancer (29). In
this study, we show that CLDN4 is overexpressed in HN5 head and
neck cancer cells and that CPE-ETA’ is a potent inhibitor of HN5
cells by targeting the CLDN4 receptor. Furthermore, this killing
capacity of CPE-ETA’ was found to be specific and targeted to
CLDN4-expressing cells, as shown by the inability of CPE-ETA’ to
inhibit the proliferation of CLDN4-negative HeLa cells (Fig. 3).
Having established the killing capacity of CPE-ETA’
in HN5 cells, we sought to increase the specificity and
availability of the CPE-ETA’ in the tumour microenvironment.
Clostridial strains have shown promise in seeking and colonising
solid tumours. Since Clostridial species are obligate anaerobes,
they can only replicate under hypoxic conditions, a hallmark of all
solid tumours (22,30). Furthermore, Clostridial species are
spore-forming, which makes them ideal for carrying therapeutic
payloads to solid tumours, as spores are known to elicit minimal
immune response in the host (31).
In order to examine the expression of functional CPE-ETA’ fusion
proteins in Clostridium, CPE-ETA’ was used to construct the
Clostridium expression vector, pMTL-555 (32). A Clostridium secretion
signal was incorporated at the N-terminal of CPE-ETA’ for
extracellular secretion. The final construct was transferred to
C. ghonii by conjugal transfer. Clostridium is not
easily amenable to genetic modifications and is not readily
transformed by heat shock or electroporation. This is the first
report of successfully transforming C. ghonii and expressing
functional therapeutic proteins.
Proteins secreted in the medium by recombinant C.
ghonii were examined for cytotoxicity using MTT assay and were
found to exert effects on several CLDN4-positive cancer lines.
Furthermore, C. ghonii modified to express CPE-ETA’ showed
increased killing capacity in the HN5, HT29 and MCF-7 cells, but
had little effect on HeLa cells (CLDN4-negative). These data
suggest that CPE-ETA’ has high specificity for CLDN4-expressing
cells. Therefore, in the tumour environment, the expression of this
protein in C. ghonii will have little effect on
CLDN4-negative cells. Furthermore, secretion of this protein from
the tumour may be useful in eliminating distant small metastatic
tumours that cannot be colonised by C. ghonii, as these
tumours will not have the level of hypoxia to sustain the growth of
C. ghonii.
In conclusion, this study confirms the upregulation
of CLDN4 expression in certain cancer cell lines and identifies
CLDN4 overexpression in the HN5 head and neck squamous carcinoma
cell line. Furthermore, we demonstrate that the targeting of HN5
cells with the CLDN4 binding protein, CPE-ETA’, not only shows
extreme potency, but high specificity. In addition, we show that
oncolytic Clostridia are capable of expressing and secreting
the functional CPE-ETA’ fusion protein and that this protein is
capable of eliciting cell death in a number of CLDN4-positive
cancer cells. The data presented in this study warrant further
investigation for using oncolytic Clostridia to deliver
therapeutic proteins locally to head and neck and breast cancer
cells with high specificity, efficacy and safety.
Abbreviations:
|
CLDN4
|
claudin-4;
|
|
ETA
|
Pseudomonas aeruginosa exotoxin
A;
|
|
CPE
|
Clostridium perfringens
enterotoxin;
|
|
EF-2
|
elongation factor-2;
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
Acknowledgements
This study was supported by the Dr
Jian Zhou Smart State Fellowship from the Queensland state
government and by grants from the National Health and Medical
Research Council and Cancer Council of Queensland to M.Q.W. We
would like to thank other members of the Wei Laboratory for their
support and helpful comments.
References
|
1
|
Lucas R and Keisari Y: Innovative cancer
treatments that augment radiotherapy or chemo-therapy by the use of
immunotherapy or gene therapy. Recent Pat Anticancer Drug Discov.
1:201–208. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Krause G, Winkler L, Mueller SL, Haseloff
RF, Piontek J and Blasig IE: Structure and function of claudins.
Biochim Biophys Acta. 1778:631–645. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lal-Nag M and Morin PJ: The claudins.
Genome Biol. 10:2352009. View Article : Google Scholar
|
|
4
|
Saeki R, Kondoh M, Kakutani H, et al: A
novel tumor-targeted therapy using a claudin-4-targeting molecule.
Mol Pharmacol. 76:918–926. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Morin PJ: Claudin proteins in human
cancer: promising new targets for diagnosis and therapy. Cancer
Res. 65:9603–9606. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Suzuki M, Kato-Nakano M, Kawamoto S, et
al: Therapeutic antitumor efficacy of monoclonal antibody against
claudin-4 for pancreatic and ovarian cancers. Cancer Sci.
100:1623–1630. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ling J, Liao H, Clark R, Wong MS and Lo
DD: Structural constraints for the binding of short peptides to
claudin-4 revealed by surface plasmon resonance. J Biol Chem.
283:30585–30595. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Michl P, Buchholz M, Rolke M, et al:
Claudin-4: a new target for pancreatic cancer treatment using
Clostridium perfringens enterotoxin. Gastroenterology.
121:678–684. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pier GB, Boyer D, Preston M, et al: Human
monoclonal antibodies to Pseudomonas aeruginosa alginate
that protect against infection by both mucoid and nonmucoid
strains. J Immunol. 173:5671–5678. 2004.PubMed/NCBI
|
|
10
|
Phelan MC: Basic techniques in mammalian
cell tissue culture. Curr Protoc Cell Biol. Chapter 1: Unit 1 1.
2007. View Article : Google Scholar
|
|
11
|
Kurien BT and Scofield RH: Introduction to
protein blotting. Methods Mol Biol. 536:9–22. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yao Q, Cao S, Li C, et al: Turn a
diarrhoea toxin into a receptor-mediated therapy for a plethora of
CLDN-4-overexpressing cancers. Biochem Biophys Res Commun.
398:413–419. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dong H, Zhang Y, Dai Z and Li Y:
Engineering Clostridium strain to accept unmethylated DNA.
PLoS One. 5:e90382010.
|
|
15
|
Barbé S, Van Mellaert L and Anné J: The
use of clostridial spores for cancer treatment. J Appl Microbiol.
101:571–578. 2006.
|
|
16
|
Litkouhi B, Kwong J, Lo CM, et al:
Claudin-4 overexpression in epithelial ovarian cancer is associated
with hypomethylation and is a potential target for modulation of
tight junction barrier function using a C-terminal fragment of
Clostridium perfringens enterotoxin. Neoplasia. 9:304–314.
2007. View Article : Google Scholar
|
|
17
|
Lanigan F, McKiernan E, Brennan DJ, et al:
Increased claudin-4 expression is associated with poor prognosis
and high tumour grade in breast cancer. Int J Cancer.
124:2088–2097. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ueda J, Semba S, Chiba H, et al:
Heterogeneous expression of claudin-4 in human colorectal cancer:
decreased claudin-4 expression at the invasive front correlates
cancer invasion and metastasis. Pathobiology. 74:32–41. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Landers KA, Samaratunga H, Teng L, et al:
Identification of claudin-4 as a marker highly overexpressed in
both primary and metastatic prostate cancer. Br J Cancer.
99:491–501. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kominsky SL, Vali M, Korz D, et al:
Clostridium perfringens enterotoxin elicits rapid and
specific cytolysis of breast carcinoma cells mediated through tight
junction proteins claudin 3 and 4. Am J Pathol. 164:1627–1633.
2004. View Article : Google Scholar
|
|
21
|
Barth S, Huhn M, Matthey B, et al:
Recombinant anti-CD25 immunotoxin RFT5(SCFV)-ETA’ demonstrates
successful elimination of disseminated human Hodgkin lymphoma in
SCID mice. Int J Cancer. 86:718–724. 2000.
|
|
22
|
Wei MQ, Mengesha A, Good D and Anné J:
Bacterial targeted tumour therapy-dawn of a new era. Cancer Lett.
259:16–27. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Frederick BA, Helfrich BA, Coldren CD, et
al: Epithelial to mesenchymal transition predicts gefitinib
resistance in cell lines of head and neck squamous cell carcinoma
and non-small cell lung carcinoma. Mol Cancer Ther. 6:1683–1691.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rangel LB, Agarwal R, D’Souza T, et al:
Tight junction proteins claudin-3 and claudin-4 are frequently
overexpressed in ovarian cancer but not in ovarian cystadenomas.
Clin Cancer Res. 9:2567–2575. 2003.PubMed/NCBI
|
|
25
|
Li J, Sherman-Baust CA, Tsai-Turton M,
Bristow RE, Roden RB and Morin PJ: Claudin-containing exosomes in
the peripheral circulation of women with ovarian cancer. BMC
Cancer. 9:2442009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Meertens L, Bertaux C, Cukierman L, et al:
The tight junction proteins claudin-1, -6, and -9 are entry
cofactors for hepatitis C virus. J Virol. 82:3555–3560. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gourin CG and Podolsky RH: Racial
disparities in patients with head and neck squamous cell carcinoma.
Laryngoscope. 116:1093–1106. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Al-Sarraf M: Treatment of locally advanced
head and neck cancer: historical and critical review. Cancer
Control. 9:387–399. 2002.PubMed/NCBI
|
|
29
|
Bonner JA, Harari PM, Giralt J, et al:
Radiotherapy plus cetuximab for squamous-cell carcinoma of the head
and neck. N Engl J Med. 354:567–578. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wei MQ, Ipe D, Cao S and Hashimi S:
Genetic Modification to Improve the Therapeutic Potential of
Oncolytic Clostridia. Advances in Genetics Research. Urbano KV: 8.
Nova Publishers; Hauppauge, NY: pp. 65–82. 2011
|
|
31
|
Cao S, Cripps A and Wei MQ: New strategies
for cancer gene therapy: progress and opportunities. Clin Exp
Pharmacol Physiol. 37:108–114. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Theys J, Pennington O, Dubois L, et al:
Repeated cycles of Clostridium-directed enzyme prodrug
therapy result in sustained antitumour effects in vivo. Br J
Cancer. 95:1212–1219. 2006.
|
|
33
|
Groot AJ, Mengesha A, van der Wall E, van
Diest PJ, Theys J and Vooijs M: Functional antibodies produced by
oncolytic clostridia. Biochem Biophys Res Commun. 364:985–989.
2007. View Article : Google Scholar : PubMed/NCBI
|