Introduction
Of all types of cancers, kidney cancer, which
accounts for >83,000 deaths per year worldwide, ranks the sixth
in the age-standardized incidence rate (per 100,000), with 5.8 in
females and 11.8 in males in developed countries and 2.5 in males
and 1.4 in females in developing countries (1). Renal cell carcinoma (RCC) constitutes
the most prevalent form of kidney neoplasm in the adult population.
Most RCC patients are found to have metastatic disease at initial
diagnosis and are, therefore, defined as patients with advanced
RCC. Immunotherapy with cytokines is the standard systemic
treatment for advanced RCC. However, advanced RCC is inherently
resistant to standard systemic treatment regimens (2). It has been reported that high-dose
interleukin-2 (IL-2) or interferon (IFN)-α shows only 10–15%
response rates in selected patients (3,4).
The increased knowledge of the molecular pathways
involved in the proliferation and angiogenesis of RCC has led to
the development of targeted therapy. Since 2005, six new targeted
therapy drugs (Sunitinib, Sorafenib, Pazopanib, Bevacizumab,
Temsirolimus and Everolimus) with proven efficacy have been
approved for treatment of metastatic RCC (5). However, the downside is that our
knowledge of the mechanisms of action of these drugs and the
intrinsic and extrinsic mechanisms of drug resistance do not evolve
equally fast and many questions remain unanswered.
Currently, targeted RCC therapy drugs suppress tumor
angiogenesis and abnormal proliferation of tumor cells mostly by
targeting to VHL/HIF signaling pathway and related growth factors
(6,7). As we know, kidney cancer cell
proliferation and angiogenesis are the results of the joint action
of multiple molecular pathways and there exists a compensatory
relationship among these pathways. When the VEGF/RTK signaling
pathway is suppressed, other compensation pathways may be
activated. Therefore, a single targeted drug often has limited
therapeutic effect. It is generally believed that better treatment
effect can be achieved only by the combined application of drugs
targeting to different angiogenesis and cell proliferation signal
pathways (8,9). In recent years, it has become a hot
spot to research on drugs targeting to the VEGF/RTK-independent
angiogenesis/cell proliferation pathways.
Comparative proteomic strategies have been
introduced to identification of targeted proteins in cancer
research (10–12). The two-dimensional polyacrylamide
gel electrophoresis (2-DE)/mass spectrometry (MS) approach is one
of the most popular tools for profiling the proteome in human
diseases. To date, numerous proteomics studies on RCC base on
2-DE/MS have been reported (11,13–15).
In our previous research, 31 proteins differentially expressed
between clear-cell RCC (ccRCC) cell line RLC-310 and renal normal
cell line HK-2 were identified by the 2-DE/MS method (16). RCCs comprise a heterogeneous group
of tumors with distinct genetic backgrounds and different
biological characteristics. The most common subtype of RCCs is
clear-cell RCC, which accounts for >80% of RCCs.
However, the intrinsic property of 2-DE leads to an
under-representation of the proteins that are highly hydrophobic or
highly basic or lowly abundant, or the proteins with high molecular
weight or extremely isoelectric points. Recently, several new
technologies have been developed to avoid 2-DE weakness. The PF-2D
separation technology employs isoelectric focusing chromatography
for the first dimension and reverse-phase chromatography for the
second dimension. The PF-2D combined with capillary LC-ESI-MS/MS
has been used to improve protease detection of the more acidic,
basic and hydrophobic proteins and to increase reproducibility and
throughput of the extremely isoelectric points typically observed
with 2-DE analysis.
In this study, we compared the global protein
profiles of ccRCC RLC-310 and normal renal cell line HK-2 using a
PF-2D/capillary LC-ESI-MS/MS based approach. Thirteen
differentially expressed proteins were newly discovered in RCC and
angiomotin (Amot) was a newly identified differentially expressed
protein in ccRCC cell line. We found that high levels of Amot
transcript were associated with poor differentiation, venous
invasion and decreased survival. Amot transcript was an independent
prognostic factor for ccRCC. These data suggest that Amot may serve
as a novel prognostic factor for ccRCC.
Materials and methods
Materials
The reagents used in the study are as follows:
RPMI-1640 and fetal bovine serum (FBS) (Gibco/Invitrogen, Carlsbad,
CA, USA); Transwell inserts (Costar, Cambridge, MA, USA);
ProteomeLab™ PF-2D system and its matched separation kit (Beckman
Coulter, Fullerton, CA, USA); LC-ESI-MS/MS instrument (Thermo
Finnigan US Companies, San Jose, CA, USA); rabbit anti-human Amot
polyclonal antibody (Abcam, Cambridge, MA, USA); goat anti-rabbit
secondary antibody and rabbit anti-human β-actin polyclonal
antibody (Jackson Co., Lansing, MI, USA); TRIzol reagent
(Invitrogen, Carlsbad, CA, USA); RNAfast200 (Flytech Biotechnology,
Shanghai, China); one-step SYBR RNA PCR kit II (Takara Biomedical,
Dalian, China); TPCK-trypsin, trifluoroacetic acid (TFA), n-octyl
glucoside and protease inhibitors (Sigma Co., St. Louis, MO,
USA).
Clinical samples
Fresh frozen surgical specimens of primary ccRCC
tissues paired with ANRT were obtained from 127 patients, who were
treated with nephrectomy at The First Affiliated Hospital of Xi’an
Jiaotong University between January 2005 and October 2007 and
stored at −80°C until use. The presence of tumor cells in the
collected tissues was verified by a consultant pathologist, who
examined H&E stained frozen sections. The histological types
were determined according to the World Health Organization
classification. The tumors were staged according to Robson staging
criteria and the histodifferentiation grading of the tumors was
assigned according to the criteria established by the World Health
Organization in 1997. This study was approved by the Institutional
Ethics Committee of Xi’an Jiaotong University. The informed consent
for the use of the samples was obtained from each patient. Patients
were routinely followed up on a regular basis and details were
stored in a database.
Methods
Cell line culture and sample
preparation
RLC-310 and HK-2 cell lines were cultured in
RPMI-1640 supplemented with 10% FBS at 37°C in 5% CO2.
The cells were harvested at the exponential growth phase by
trypsinization, washed with ice-cold PBS, counted and homogenized
in lysis buffer (6 M urea, 2 M thiourea, 10% glycerol, 50 mM Tris,
2% n-octyl glucoside, 5 mM TCEP and 1 mM protease inhibitors) on
ice. Suspensions were incubated for 1 h at 4°C and centrifuged at
20 000 × g for 60 min. The supernatants were stored at −80°C until
use. The total protein concentration was determined by the Bradford
method using bovine serum albumin as the standard.
Two-dimensional liquid phase
fractionation (PF-2D) separation and analysis
The ProteomeLab PF 2D Chemistry kit includes a
chromatofocusing high performance computing facility (HPCF) column
(first dimension), a nonporous high performance reversed-phase
(HPRP) column (second dimension), a start buffer (pH 8.5) and an
elution buffer (pH 4.0). The first dimension separation consists of
chromatofocusing, based on charge. Chromatofocusing was carried out
on the CF column by mixing two buffers with different pH values,
Start buffer (pH 8.5) and Eluent buffer (pH 4.0), to create a
linear pH gradient from 8.5 to 4.0, which was followed by a wash
buffer comprising 1 M NaCl. The pH gradient was achieved by
introducing increasing amounts of the eluent buffer (pH 4.0) at a
flow rate of 0.2 ml/min over 90 min. Protein samples (≤5.0 mg),
prepared in Start buffer, were loaded. Protein peaks in Eluent
buffer were monitored by absorbance at 280 nm. The first dimension
fractions were collected in 96-well plates (every 0.3 pH units
during the pH gradient portion of the run or every 5 min during the
other stages of the run, before the pH gradient and during salt
washing) and introduced into the second dimensional reversed phase
chromatography, which separated proteins based on
hydrophobicity.
In the second dimension, each fraction (500
μl) was sequentially analyzed by reversed phase HPLC at a
constant temperature of 50°C. Proteins were separated at a flow
rate of 0.75 ml/min on a non-porous C18 reversed phase column using
3.33% B/min linear gradient in which solvent A was 0.1% aqueous TFA
and solvent B was 0.08% TFA in acetonitrile. Proteins were
monitored at 214 nm. The reversed phase fractions were collected by
0.25 min/tube and stored at −80°C for further analysis. The
fractions were also collected into 96-deepwell plates for mass
spectrometry analysis.
The hardware was controlled by 32 Karat software.
With this system, the first and second dimensions occurred
sequentially in an automatic manner. For one-dimensional LC, the
percentages of the protein eluted on different columns and at
different pH conditions were determined by calculating the peak
area of the protein monitored at 214 nm, a wavelength at which the
peak area was directly proportional to the quantity of the
protein(s). Mass spectrometry was carried out to confirm the
composition of the protein peaks. Comparison of two separate UV/pI
maps consisting of the entire pH gradient was performed by a module
(DeltaVue) of the Mapping Tools data processing software. A second
module (MultiVue) enabled the analysis of a pH lane selected from
multiple sample runs.
Capillary LC-ESI-MS/MS analysis and
database searches
The reversed phase fractions (200 μl)
obtained from the two-dimensional LC were concentrated to 5–10
μl using a SpeedVac concentrator, and 1 M
NH4HCO3 was added to the residues to
neutralize the samples to pH 8.0. The samples were then digested at
37°C for 20 h with sequencing grade modified trypsin at an
enzyme-to-substrate ratio of 1:50. The digestion was stopped by
adding 10% TFA and the digestion product was freeze-dried; then
1D-LC ESI-MS/MS was performed using an LTQ linear IT mass
spectrometer with the CF column equilibrated with 0.1% formic acid
in 95% water and 5% acetonitrile. The system was fitted with a C18
RP column. The mobile phase A was 0.1% formic acid in water and the
mobile phase B was 0.1% formic acid in acetonitrile. Each sample
was dissolved to 5 μl in 0.1% formic acid in water and
auto-injected to the C18 Trap desalination column and then
separated on the C18 reversed-phase column. The system was set as
follows: injection mode, microspray; detection method, positive
ions; capillary temperature, 170°C. The gradients were set as
follows: 0–20 min, B fluid linear gradient from 4 to 50%; 20–24
min, B linear gradient of liquid from 50 to 100%; 24–30 min, B
solution maintained at 100%. The LTQ linear IT mass spectrometer
was set so that one full MS scan was followed by 20 MS/MS scans on
the 10 most intense ions from the MS spectrum.
MS/MS spectra were automatically searched against
the non-redundant International Protein Index (IPI) human protein
database (version 3.53) using the TurboSEQUEST program in the
Bioworks Browser software suite. The peptides were constrained to
be tryptic and up to two missed cleavages were allowed. The allowed
mass tolerance was 3.0 Da for the precursor ions and 1.0 Da for the
fragment ions. The stringent protein identification criteria were
based on Δ Cn ≥0.1 and cross-correlation scores (Xcorr, one charge
≥1.9, two charges ≥2.2, three charges ≥3.75). Only proteins
identified by at least two peptide matches were reported as
differentially expressed proteins.
Tissue processing and RNA and protein
extraction
Frozen sections of tissues were cut at a thickness
of 5–10 μm and kept for routine histology. Another 15–20
sections were mixed and homogenised in ice-cold RNA extraction
solution using a hand-held homogeniser. The concentration of RNA
was determined using a UV spectrophotometer. The rest of the
tissues were used for protein extraction.
RT-PCR and quantitative RT-PCR
The total RNA from cells and tissues was extracted
using the TRIzol Reagent according to the manufacturer’s
instructions. cDNA was prepared by reverse transcription of 1 mg
total RNA using oligo(dT) 15 primer and reverse transcriptase. The
primer sequences and the expected sizes of PCR products were as
follows: Amot, sense 5′-CAG CAG CAG CAG CCA CAG-3′ and antisense
5′-CCA CCT TCT CAT AGC ATC CTT CC-3′ (196 bp); β-actin, sense
5′-ATC GTG CGT GAC ATT AAG GAG AAG-3′ and antisense 5′-AGG AAG GAA
GGC TGG AAG AGT G-3′ (179 bp). RT-PCR was performed using a
one-step SYBR RNA PCR kit II according to the manufacturer’s
instructions in the following procedure: reverse transcription at
48°C for 30 min and denaturation at 95°C for 1 min; amplification
for 35 cycles at 94°C for 0.5 min, annealation at 60°C for 0.5 min
and extension at 70°C for 0.5 min; then a terminal elongation step
at 72°C for 5 min and a final holding stage at 4°C. Reactions were
run on an ABI 9700 Thermocycler (Applied Biosystems Inc., Foster
City, CA, USA). PCR products were separated by electrophoresis on
1.2% agarose gels. Ethidium bromide-stained bands were visualized
by UV illumination and quantified using the Dolphin-DOC Gel imaging
system (Molecular Dynamics, Sunnyvale, CA, USA).
The miRNA quantification was done using a previously
described method (17). RNA
samples used in qPCR validation experiments were isolated from 127
primary ccRCC tissues paired with ANRT. Primer 5 software was used
to design the primer sequences. The sequences of the respective
primers were: angiomotin (5′-AAG CGT TGC CTT GAC ATG GAG-3′ and
5′-GGA ACG CTG CTG GAG TAC TTT GA-3′), β-actin (5′-TGG CAC CCA GCA
CAA TGA A-3′ and 5′-CTA AGT CAT AGT CCG CCT AGA AGC A-3′). First,
100 ng of total RNA was reverse transcribed by incubation at 42°C
for 60 min and 70°C for 15 min in a 7900 Thermocycler (Applied
Biosystems, Carlsbad, CA, USA) using 100 U of M-MLV reverse
transcriptase (Takara: D2639A) and 1 μM stem-loop RT primer.
The samples were then held at 4°C. Real-time PCR was performed
using Platinum SYBR Green qPCR SuperMix-UDG (Invitrogen:
11733-038). All reactions were run in triplicate. The ΔΔCT method
was used to determine the expression differences between clinical
outcomes (18).
Western blot analysis
Tissue and cell protein extracts were collected
after sonication with 2X sample buffer (0.25 M Tris-HCl, 10%
2-mercaptoethanol, 4% sodium dodecyl sulphate and 10% sucrose). The
protein concentration was determined by Bradford method using
bovine serum albumin as the standard. A total of 20 μg of
each protein sample was run on a 12% SDS-PAGE gel and transferred
onto nitrocellulose membranes using a hygro-blotter. Non-specific
binding was blocked with a buffer containing 0.1% Tween-20 and 5%
non-fat dried milk for 1 h at room temperature and subsequently
incubated overnight at 4°C with anti-Amot antibody (1
μg/ml). β-actin (dilution at 1:1,000) was used as an
internal positive control. The antibody-bound membranes were then
incubated for 1 h at 37°C with anti-rabbit horseradish
peroxidase-conjugated IgG secondary antibody. Protein bands for
immunoblot analysis were detected on X-ray film using enhanced
chemiluminescence (ECL) chemiluminescence reagent. Gels and the
film were scanned using a Personal Densitometer SI (Leica, Germany)
and analysed using Gel-Pro Analyzer 4 Image software.
Statistical analysis
Statistical analyses were conducted with SPSS 13.0
software. The differences between the cancer tissue and the
adjacent tissues in the western blotting of Amot were assessed
using the paired t-test. The relevance analysis of the Amot
transcript expression and clinicopathological parameters was
performed by the Kruskal-Wallis test and the cross-tabs
χ2 test. Survival curves were generated by using the
Kaplan-Meier method. The statistical analyses were performed by
using the log-rank test. Multivariate analyses were performed using
the Cox proportional hazard model. Statistical significance was
defined as p<0.05.
Results
Comparative proteomic analysis of
RLC-310 and HK-2
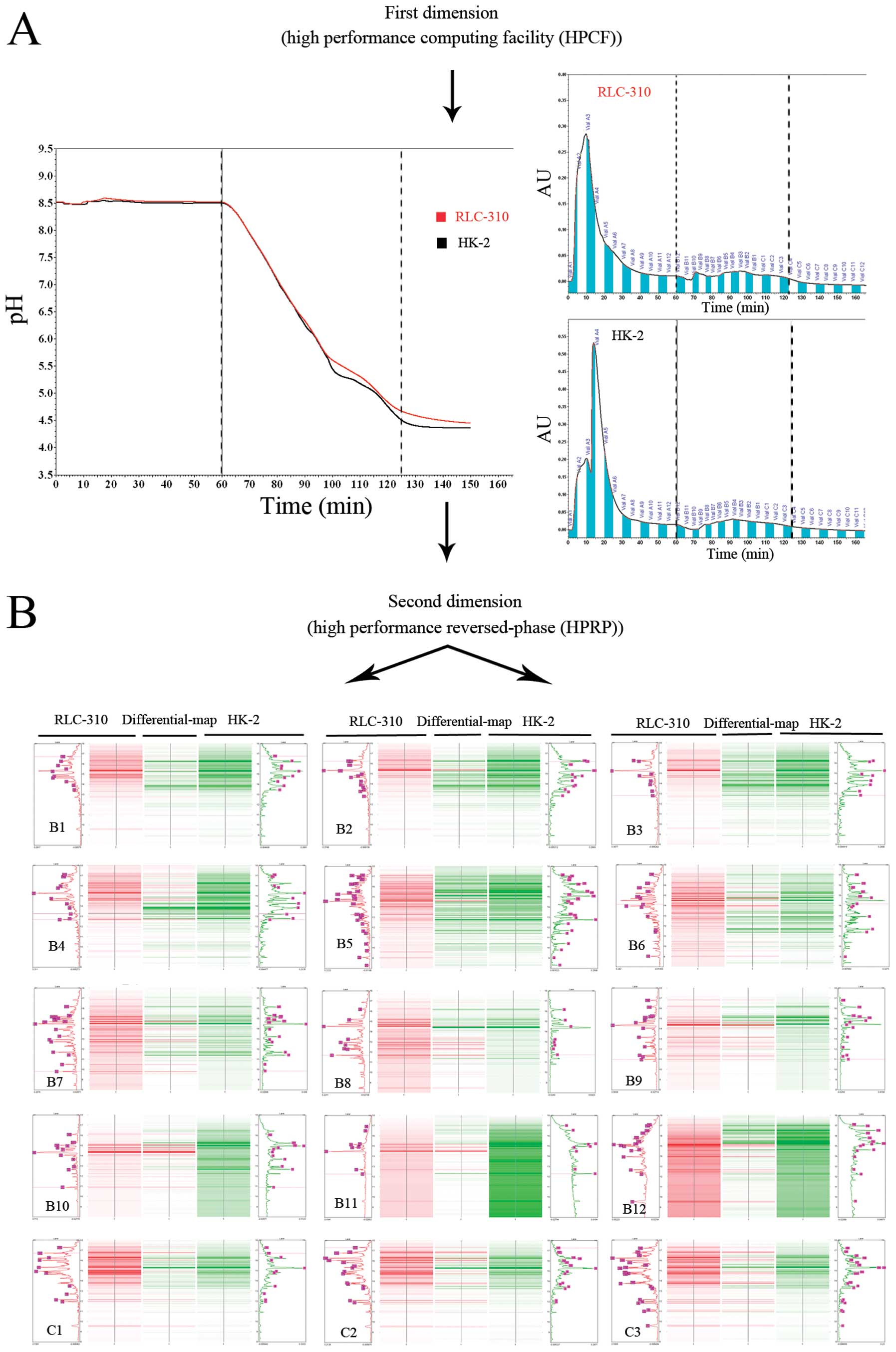
In the ProteomeLab PF-2D system, 2.5 mg of protein
extract from ccRCC cell line RLC-310 or normal renal cell line HK-2
was injected into the column. The virtual 2-D gel given by the
software ProteoVue showed the first- and second-dimension
separation of proteins by their isoelectric points (pI) (Fig. 1A) and hydrophobicity (Fig. 1B). For each cell line, 15 protein
fractions were collected in the 8.5-4.5-pH gradient in the second
dimension using the high performance reverse-phase chromatography
(buffer run: 60–125 min). Each obtained fraction showed ≤13 peaks
or bands on average and the protein fractions from the two cell
lines showed a total of ∼400 bands (Fig. 1). We only considered peaks or bands
that had a minimal absorbance (0.04 μA) and a protein peak
area ratio >2. Ultimately, 12 bands were excised for
identification using capillary liquid chromatography electrospray
ionization mass spectrometry/mass spectrometry (LC-ESI-MS/MS)
(Table I).
 | Table ISummary of fractions selected for
mass spectrometry analyses. |
Table I
Summary of fractions selected for
mass spectrometry analyses.
| Fraction
coordinates
| |
|---|
| Fraction ID | pH | Retention times
(RT) | Peak area ratio
(R/H) |
|---|
| RLC-310 (B5a-38b) | 6.53-6.23 | 12.64–12.91 | 2.378 |
| RLC-310 (B6a-44b) | 6.83–8.03 | 13.89–14.09 | 302.018 |
| RLC-310 (B9a-31b) | 7.73-7.43 | 11.21–11.33 | 302.018 |
| RLC-310 (B9a-32b) | 7.73-7.43 | 11.33–11.47 | 19.108 |
| RLC-310
(B10a-34b) | 8.03-7.73 | 10.86–11.03 | 19.108 |
| RLC-310
(B10a-47b) | 8.03-7.73 | 14.24–14.54 | 11.298 |
| RLC-310
(B10a-48b) | 8.03-7.73 | 14.69–14.92 | 3.828 |
| RLC-310 (C1a-63b) | 5.25–5.10 | 18.48–18.67 | 21.031 |
| HK-2 (B1a-42b) | 5.47-5.32 | 13.54–14.16 | 0.178 |
| HK-2 (B2a-41b) | 5.63-5.47 | 13.29–14.09 | 0.171 |
| HK-2 (B2a-65b) | 5.63-5.47 | 18.80–19.25 | 0.154 |
| HK-2 (B3a-45b) | 5.92-5.63 | 14.21–14.43 | 0.149 |
In the tandem mass spectrometry, eight mother-ions
were chosen from each protein sample for secondary mass
spectrometry according to the level of the mass spectrogram and
ultimately, the peptide mass of each protein sample was calculated
and the MS/MS map (fragment ion mass) was obtained. The amino acid
sequence of each peptide fragment was obtained by analyzing the
secondary mass spectrometry results of bn and yn ion series.
Finally, 196 differentially expressed proteins were identified by
searching the database ipi.HUMAN.v3.53.
Identification and verification of
Amot overexpression in RLC-310
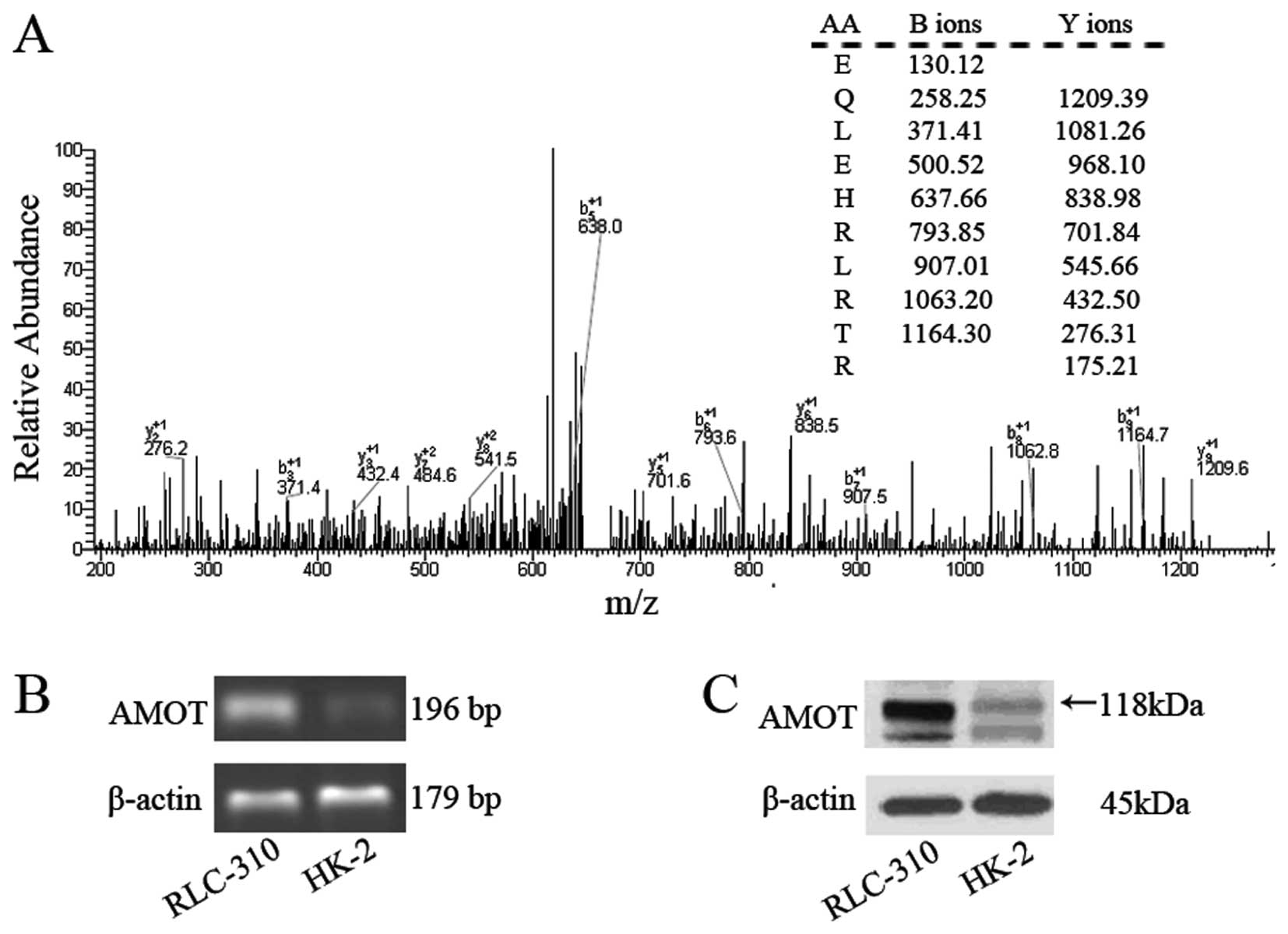
Interestingly, MS/MS analysis showed that the
protein Amot was differentially expressed in RLC-310
(B10a-47b), which has not been reported in
ccRCC studies before. The retention time of Amot in gradient
elution was 14.24–14.54 min and its experimental isoelectric point
was 8.03-7.73. Capillary LC-ESI-MS/MS analysis revealed a
preliminary score of 704.6 and 10 matched peptides as shown in
Fig. 2A. A significant difference
in the mRNA level of Amot was observed between RLC-310 and HK-2 by
semi-quantitative RT-PCR (Fig.
2B). Significantly high expression of Amot was detected in
RLC-310, compared with the Amot protein expression level in HK-2 by
western blot analyses (Fig. 2C).
Analyses of the Amot protein and mRNA levels further verified the
Amot expression difference displayed by the PF-2D system between
the two cell lines.
Overexpression of Amot in primary
ccRCC tissues
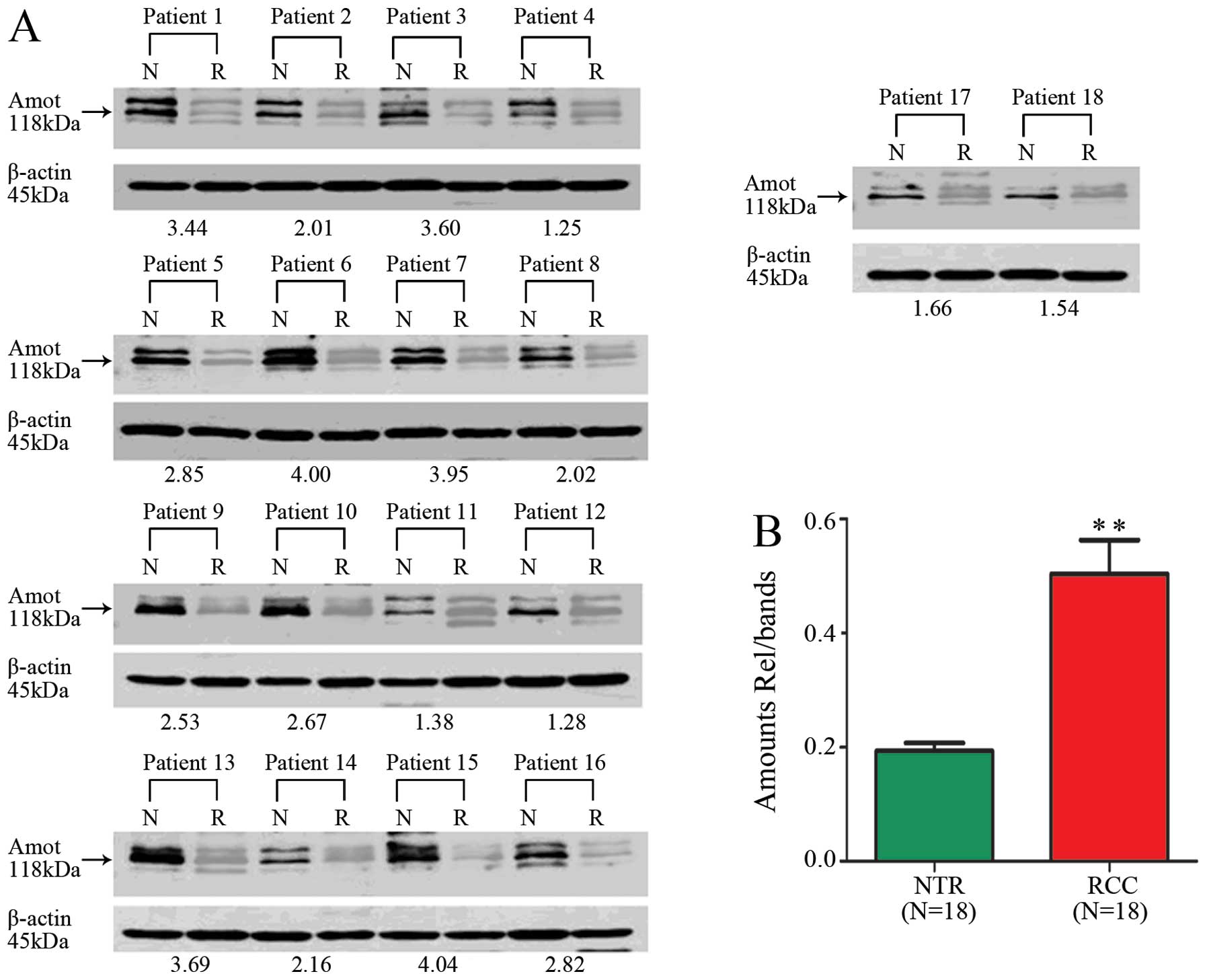
Western blot analysis of 18 tissues using anti-Amot
antibody showed an overexpression of Amot in ccRCC tissues,
compared with the Amot expression in adjacent normal renal tissues
(ANRT) (n=18: carcinoma tissues, 0.524±0.262; normal tissues,
0.183±0.067; Student’s t-test, p<0.01) (Fig. 3). A quantitative analysis of the
molecules indicated that the significant difference in the mRNA
level of Amot was also observed in all the carcinoma tissues
examined (n=127: carcinoma tissues, 6.03±4.46; normal tissues, 1;
Student’s t-test, p<0.01). Taken together, our data demonstrated
that Amot was overexpressed in ccRCC tissues at both mRNA and
protein levels, which is consistent with the observations made in
the ccRCC cell lines.
Correlation of Amot expression with
clinical and pathological characteristics of ccRCC
One hundred and twenty-seven tissue samples from
ccRCC patients at different clinical stages were used to
investigate the correlation of the Amot expression level with the
clinical outcome of ccRCC. Of the 127 patients with ccRCC, 99 had a
higher level of Amot expression in ccRCC tissues (2−ΔΔct
>2) than in ANRT and 28 had a lower level of Amot expression
(2−ΔΔct ≤2). The overexpression rate of Amot in ccRCC
was 77.95% (99/127) (Table II). It
was shown that Amot overexpression had a significantly high
relationship with the poor histodifferentiation of carcinoma cells
(cross-tabs χ2 test, p<0.01) (Fig. 4A), a high relationship with venous
invasion (cross-tabs χ2 test, p<0.05) (Fig. 4B), but no apparent relationship
with the Robson stage (stages I, II and III/IV) for renal carcinoma
(cross-tabs χ2 test, p>0.05) (Fig. 4C). These results were also
confirmed by the Kruskal-Wallis test, a non-parametric test based
on completely randomized design for comparisons of more than two
samples.
 | Table IIUnivariate and multivariate analyses
of survival rate. |
Table II
Univariate and multivariate analyses
of survival rate.
| Cases (n) | 5-year survival
(%) | Log-rank test | Cox |
|---|
| Stage (Robson) | | | | |
| I | 63 | 77.8 | <0.0001 | 0.007 |
| II | 52 | 21.2 | | |
| III/ IV | 12 | 16.7 | | |
|
Histodifferentiation | | | | |
| Well | 51 | 80.4 | <0.0001 | <0.0001 |
| Moderately | 61 | 29.5 | | |
| Poorly | 15 | 20.0 | | |
| Venous
invasion | | | | |
| Negative | 67 | 53.7 | 0.039 | 0.677 |
| Positive | 60 | 43.3 | | |
| Amot RNA expression
(2−ΔΔct) | | | | |
| ≤2 | 28 | 78.6 | 0.001 | 0.036 |
| >2 | 99 | 40.4 | | |
Prognostic significance of Amot
expression in ccRCC
The survival analysis using the log-rank test
suggested that the overexpression of Amot transcript was probably
associated with the poor outcome of ccRCC patients (log-rank test,
p<0.01; Fig. 4D). The 5-year
survival rates of patients with low-level and high-level Amot
expressions were 78.6 and 40.4%, respectively. Multivariate
analysis was performed using the Cox proportional hazard model to
determine whether the prognostic value of the Amot transcript level
was independent of other risk factors associated with the clinical
outcome of ccRCC. The risk factors examined included Amot
transcript expression (low level and high level), venous invasion
(negative and positive), histodifferentiation (well, moderately and
poorly differentiated) and Robson stage (stages I, II and III/IV).
Table II shows that as a
prognostic factor for the survival of ccRCC patients, the Amot
transcript expression was independent of these risk factors
(p<0.05).
Discussion
In the present study, we compared the global protein
profiles of ccRCC RLC-310 and normal renal cell line HK-2 using a
PF-2D and capillary LC-ESI-MS/MS-based approach. More than 196
differentially expressed proteins were identified between the
carcinoma and normal cell lines. The differential expressions of
proteins in ccRCC cell line were functionally related to biological
pathways of cell proliferation and anti-apoptosis, energy
metabolism, mitochondria reduction and oxadation, oxidative stress
and resistance, cell signaling, invasion and adhesion, cytoskeleton
and motion, neovascularization and others. The extensive protein
profile indicated that multiple cellular pathways might be involved
in the process of tumorigenesis of ccRCC.
The most striking finding is the initial
identification of overexpressed Amot in ccRCC. Its increased
expression was also confirmed by RT-PCR and western blot analyses
in a cell line. Angiomotin was first identified as KIAA1071 by
Kikuno et al in 1999 from a set of size-fractionated human
adult and fetal brain cDNA libraries, but its function was unknown
(19). Troyanovsky et al
have reported that Amot is an angiostatin binding protein and can
mediate the angiostatin inhibition of migration and the tube
formation of endothelial cells (20). A further study found that the
expression of Amot in mouse aortic endothelial cells results in
stabilization of tubes in the Matrigel assay and promotes tumor
growth and invasion into surrounding muscle tissues in
vivo(21). The critical roles
of Amot in vascular patterning and endothelial polarization suggest
that it may be involved in oncogenesis. Amot has been found to be
highly expressed in human breast tumor tissues and linked to
angiogenesis (22). However, the
expression and role of Amot in RCC have not been experimentally
investigated.
After the overexpression of Amot in 18 frozen ccRCC
tissues was confirmed by western blot analysis, the expression
level of Amot was further investigated by real-time RT-RCR in 127
ccRCC tissues and their corresponding ANRT. The relationships
between the Amot expression and the clinical parameters such as
Robson stage, histodifferentiation and venous invasion of ccRCC
were established. It is notable that the Amot expression in ccRCC
was correlated with histodifferentiation and that the
overexpression of Amot significantly represented the poor
differentiation of ccRCC. On the whole, the ubiquitous
overexpression of Amot in ccRCC and its close relationship with
ccRCC cell differentiation suggest that Amot may be an important
component of ccRCC transformation and development.
The Amot expression showed a significant
relationship with venous invasion but no relationship with clinical
stages in ccRCC, which are similar to the results of Jiang et
al study on human breast cancer (22). Angiogenesis plays an important role
in the invasion and dissemination of RCC and is mediated by
numerous factors, such as HIF1α and VEGF. Amot also plays an
important role in angiogenesis. Holmgren et al(23) have reported that a combination of
DNA vaccines encoding Amot and the extracellular and transmembrane
domains of the human EGF receptor 2 (Her-2)/neu oncogene can
inhibit breast cancer progression and impaire tumor vascularization
in Her-2/neu transgenic mice. A further study found (24) that the anti-Amot B06 antibody can
significantly reduce the number of endothelial filopodia and
inhibit vessel migration during retinal angiogenesis in vivo
and that the systemic or local treatment with this antibody can
inhibit the pathological blood vessel formation associated with
tumor growth or laser-induced choroid neovascularization of the
eye. Recent studies (25) have
also shown that electroporation of plasmid coding for the human
Amot can significantly delay the progression of autochthonous
tumors in cancer prone BALB-neuT and PyMT genetically engineered
mice and transplantable TUBO tumor in wild-type BALB/c mice. These
studies suggest that the therapy targeting to Amot can restrain
pathological angiogenesis around the tumor and may be a new idea
for the development of antineoplastics.
Up to the present, the role of Amot in physiological
angiogenesis has remained unclear. Wells et al(26) have found that Rich1 binds the
scaffolding protein Amot and is thereby targeted to a protein
complex at tight junctions (TJs) containing the PDZ-domain proteins
and then maintain TJ integrity by the coordinate regulation of
Cdc42 and by linking specific components of the TJ to intracellular
protein trafficking. Aase et al(27) have confirmed that Amot is important
for endothelial polarization during migration and can control Rac1
activity in endothelial and epithelial cells. Recent research
(28) has also found that Amot
functions downstream of Merlin and upstream of Rich1 and that
depletion of angiomotin in Nf2(−/−) Schwann cells attenuates the
Ras-MAPK signaling pathway and impedes cellular proliferation in
vitro and tumorigenesis in vivo. However, the latest
studies (29,30) have found that Amot, as a component
of the Hippo pathway, can inhibit Yes-associated protein (YAP) and
transcriptional coactivator with PDZ-binding motif (TAZ)
oncoprotein by Amot-mediated Hippo-independent tight junction
localization. The results of these two studies lead to the
question, why did Amot inhibit rather than promote YAP and TAZ
oncoprotein? We speculated that it was probably because Amot may
play different roles due to its cellular locations and in these two
studies, the authors used the normal cell line (human embryonic
kidney cell line HEK293), but the biological significance of Amot
may differ between renal tumor cells and embryonic kidney
cells.
Among proangiogenic factors, vascular endothelial
growth factor (VEGF) is the mainstay of tumor angiogenesis
(31,32). Clear-cell RCCs (75%) are strongly
associated with mutations of Von Hippel Lindau (VHL) tumor
suppressor gene which induces the degradation of hypoxia-inducible
factor (HIF-1α and β) in the presence of oxygen. Therefore, the
VHL/HIF-1/VEGF pathway is deregulated in RCCs and it represents a
reasonable therapeutic target for RCCs (33,34).
Amot antibodies inhibit FGF-2 and VEGF-induced endothelial
migration in the Boyden chamber assay (24). However, Amot-deficient cells have
intact response to VEGF in regard to differentiation and
proliferation though the chemotactic response to VEGF is abolished
in Amot-deficient cells (27).
This means that Amot-related pathways may be VEGF-independent
angiogenesis pathways. Further studies need to be conducted to
elucidate the role of Amot in tumor pathological angiogenesis.
In this study, univariate and multivariate analysis
revealed that ccRCC patients with strongly positive Amot transcript
showed decreased survival, compared with other groups, which
indicates that the transcript expression of Amot in ccRCC may be an
independent predictor of survival. Although the molecular partners
of Amot that promote cancer development were not discovered, Amot
displays an unusually high expression in a few cancer types and is
correlated with poor outcome of the patients. Jiang et
al(22) have confirmed that
high levels of Amot transcript are associated with shorter overall
survival although they did not use the Cox statistic model to
perform multivariate analyses.
This study is the first to identify the
overexpression of Amot in ccRCC cells and tissues. Moreover, it is
initially reported that its overexpression in ccRCC was associated
with poor differentiation, venous invasion and prognosis. Our data
suggest that patients who undergo nephrectomy for localized
diseases (Robson stage I or II) and whose tumors express high
levels of Amot are at increased risk of death. Survival is a major
clinical determinant of the outcome of RCC. Thus, once Amot is
validated as a reliable prognostic marker for ccRCC, it will
contribute to the establishment of individualized follow-up
protocols as well as to the identification of patients suitable for
adjuvant therapy in clinical trials. Further studies are needed to
determine the prognostic value of this protein in a large spectrum
of ccRCC.
Acknowledgements
This study was supported by National
Natural Science Foundation of China (no. 81172171).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Drucker BJ: Renal cell carcinoma: current
status and future prospects. Cancer Treat Rev. 31:536–545. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fisher RI, Rosenberg SA and Fyfe G:
Long-term survival update for high-dose recombinant interleukin-2
in patients with renal cell carcinoma. Cancer J Sci Am. 6(Suppl 1):
S55–S57. 2000.PubMed/NCBI
|
|
4
|
Messing EM, Manola J, Wilding G, et al:
Phase III study of interferon alfa-NL as adjuvant treatment for
resectable renal cell carcinoma: an Eastern Cooperative Oncology
Group/Intergroup trial. J Clin Oncol. 21:1214–1222. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gonzalez LJ, Espinosa E, Garcia CI, et al:
Sequential therapy in metastatic renal cell carcinoma: pre-clinical
and clinical rationale for selecting a second- or subsequent-line
therapy with a different mechanism of action. Cancer Metastasis
Rev. 31(Suppl 1): S11–S17. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Patard JJ, Pouessel D, Bensalah K and
Culine S: Targeted therapy in renal cell carcinoma. World J Urol.
26:135–140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rathmell WK and Chen S: VHL inactivation
in renal cell carcinoma: implications for diagnosis, prognosis and
treatment. Expert Rev Anticancer Ther. 8:63–73. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Audenet F, Yates DR, Cancel-Tassin G,
Cussenot O and Roupret M: Genetic pathways involved in
carcinogenesis of clear cell renal cell carcinoma: genomics towards
personalized medicine. BJU Int. 109:1864–1870. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cho IC and Chung J: Current status of
targeted therapy for advanced renal cell carcinoma. Korean J Urol.
53:217–228. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Simpson RJ and Dorow DS: Cancer
proteomics: from signaling networks to tumor markers. Trends
Biotechnol. 19:S40–S48. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Banks RE, Craven RA, Harnden P, Madaan S,
Joyce A and Selby PJ: Key clinical issues in renal cancer: a
challenge for proteomics. World J Urol. 25:537–556. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Petricoin EF, Zoon KC, Kohn EC, Barrett JC
and Liotta LA: Clinical proteomics: translating benchside promise
into bedside reality. Nat Rev Drug Discov. 1:683–695. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Arsanious A, Bjarnason GA and Yousef GM:
From bench to bedside: current and future applications of molecular
profiling in renal cell carcinoma. Mol Cancer. 8:202009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nogueira M and Kim HL: Molecular markers
for predicting prognosis of renal cell carcinoma. Urol Oncol.
26:113–124. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wood SL, Rogers M, Cairns DA, et al:
Association of serum amyloid A protein and peptide fragments with
prognosis in renal cancer. Br J Cancer. 103:101–111. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang J, Li A, Yang Y and Li X:
Identification of cyclophilin A as a potential prognostic factor
for clear-cell renal cell carcinoma by comparative proteomic
analysis. Cancer Biol Ther. 11:535–546. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen C, Ridzon DA, Broomer AJ, et al:
Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic
Acids Res. 33:e1792005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chang JT, Chen IH, Liao CT, et al: A
reverse transcription comparative real-time PCR method for
quantitative detection of angiogenic growth factors in head and
neck cancer patients. Clin Biochem. 35:591–596. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kikuno R, Nagase T, Ishikawa K, et al:
Prediction of the coding sequences of unidentified human genes.
XIV. The complete sequences of 100 new cDNA clones from brain which
code for large proteins in vitro. DNA Res. 6:197–205. 1999.
View Article : Google Scholar
|
|
20
|
Troyanovsky B, Levchenko T, Mansson G,
Matvijenko O and Holmgren L: Angiomotin: an angiostatin binding
protein that regulates endothelial cell migration and tube
formation. J Cell Biol. 152:1247–1254. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Levchenko T, Bratt A, Arbiser JL and
Holmgren L: Angiomotin expression promotes hemangioendothelioma
invasion. Oncogene. 23:1469–1473. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiang WG, Watkins G, Douglas-Jones A,
Holmgren L and Mansel RE: Angiomotin and angiomotin like proteins,
their expression and correlation with angiogenesis and clinical
outcome in human breast cancer. BMC Cancer. 6:162006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Holmgren L, Ambrosino E, Birot O, et al: A
DNA vaccine targeting angiomotin inhibits angiogenesis and
suppresses tumor growth. Proc Natl Acad Sci USA. 103:9208–9213.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Levchenko T, Veitonmaki N, Lundkvist A, et
al: Therapeutic antibodies targeting angiomotin inhibit
angiogenesis in vivo. FASEB J. 22:880–889. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Arigoni M, Barutello G, Lanzardo S, et al:
A vaccine targeting angiomotin induces an antibody response which
alters tumor vessel permeability and hampers the growth of
established tumors. Angiogenesis. 15:305–316. 2012. View Article : Google Scholar
|
|
26
|
Wells CD, Fawcett JP, Traweger A, et al: A
Rich1/Amot complex regulates the Cdc42 GTPase and apical-polarity
proteins in epithelial cells. Cell. 125:535–548. 2006. View Article : Google Scholar
|
|
27
|
Aase K, Ernkvist M, Ebarasi L, et al:
Angiomotin regulates endothelial cell migration during embryonic
angiogenesis. Genes Dev. 21:2055–2068. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yi C, Troutman S, Fera D, et al: A tight
junction-associated Merlin-angiomotin complex mediates Merlin’s
regulation of mitogenic signaling and tumor suppressive functions.
Cancer Cell. 19:527–540. 2011.PubMed/NCBI
|
|
29
|
Zhao B, Li L, Lu Q, et al: Angiomotin is a
novel Hippo pathway component that inhibits YAP oncoprotein. Genes
Dev. 25:51–63. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chan SW, Lim CJ, Chong YF, Pobbati AV,
Huang C and Hong W: Hippo pathway-independent restriction of TAZ
and YAP by angiomotin. J Biol Chem. 286:7018–7026. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rini BI: VEGF-targeted therapy in renal
cell carcinoma: active drugs and active choices. Curr Oncol Rep.
8:85–89. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ferrara N and Kerbel RS: Angiogenesis as a
therapeutic target. Nature. 438:967–974. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hu Y, Liu J and Huang H: Recent agents
targeting HIF-1alpha for cancer therapy. J Cell Biochem.
114:498–509. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rathmell WK, Wright TM and Rini BI:
Molecularly targeted therapy in renal cell carcinoma. Expert Rev
Anticancer Ther. 5:1031–1040. 2005. View Article : Google Scholar : PubMed/NCBI
|