Introduction
The pyrrolo-1,5-benzoxazepines (PBOXs) were
originally designed and synthesised as a novel series of
peripheral-type benzodiazepine receptor (PBR) ligands (1). Subsequent studies demonstrated novel
proapoptotic properties for the PBOXs that were independent of the
PBR (2). Tubulin was later
identified as the molecular target of a subset of the PBOX series
(3). The tubulin targeting PBOXs
demonstrated selective proapoptotic activity across a wide spectrum
of human tumour cell types and tumour models whilst displaying
minimal toxicity towards normal cells (4–8). The
most potent member of the series PBOX-15 effectively induced
apoptosis in ex vivo B cell chronic lymphocytic leukaemia
and chronic myeloid leukaemia cells derived from patients
displaying resistance to current first line therapies (8,9). We
recently described the anti-angiogenic activity of the PBOXs a
property that may augment its antineoplastic properties in
vivo(10). However, despite
the promising anticancer and anti-angiogenic properties of the
PBOXs some cell types display some inherent resistance to the
compounds.
Previous studies in colon cancer models demonstrated
activation of macroautophagy (hereafter referred to as autophagy)
as a cytoprotective response to nutritional deprivation (11), hydrogen sulphide exposure (12) and chemotherapeutics commonly used
in treatment of colon cancer (5-fluorouracil, topotecan,
irinotecan) (13–15). Autophagy is defined as a catabolic
mechanism mediating the recycling and turnover of cytoplasmic
components in response to starvation or stress (16). Autophagy is frequently activated in
tumour cells treated with chemotherapy, endocrine therapy and
irradiation (17–21). Despite the copious advances in
autophagy research the role played by autophagy in cell death and
cell survival still remains highly controversial. It is postulated
that autophagy constitutes an attempt to adapt to lethal stress
imposed by chemotherapeutics as opposed to a cell death mechanism
(18). However, some still argue
for the existence of genuine autophagic cell death (22). Targeting autophagy by genetic or
pharmacological means was shown to trigger the apoptotic pathway in
several preclinal studies (23,24).
Furthermore, inhibition of autophagy sensitises cancer cells to
chemotherapy (14), radiation
(25) and immunotherapy (26). To date there are 19 ongoing
clinical trials investigating the efficacy and toxicity of
autophagy inhibitors as an adjuvant therapy for the treatment of
solid and haematological cancers (27). The dual phosphatidylinositol
3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235 is
currently undergoing phase I/II clinical trials for the treatment
of advanced solid tumours (28)
and in radiosensitisation (29).
Also, the anti-malarial/autophagy inhibitor chloroquine is
undergoing phase I/II clinical trials as a single agent and also in
combination with various chemotherapeutics and/or radiation for a
diverse range of solid tumours and leukaemia (30).
In this report, we investigate the possibility of
cytoprotective macroautophagy (hereafter referred to as autophagy)
as a means of continued cell survival despite prolonged exposure to
the PBOXs in colon cancer cells. Manipulation of autophagy was also
explored as a means of augmenting the pro-apoptotic properties of
the PBOXs.
Materials and methods
Compounds
The pyrrolo-1,5-benzoxazepine compounds,
7-[(N,N-dimethylcarbamoyl)oxy]-6-(naphth-1-yl)pyrrolo[2,1-d][1,5]benzoxazepine
(PBOX-6) and
4-acetoxy-5-(1-(naphthyl)naphtho[2,3-b]pyrrolo[2,1-d][1,4]oxazepine
(PBOX-15) (Fig. 1A) were
synthesised as described previously (1) and were prepared as 5 mM stocks in
ethanol and stored at −20°C. Bafilomycin-A1 (BAF-A1) was purchased
from Sigma-Aldrich (Poole, UK). All general reagents unless stated
otherwise were purchased from Sigma-Aldrich. The general caspase
inhibitor Z-VAD-FMK was purchased from Merck Biosciences
(Nottingham, UK).
Cell culture
CT-26 and Caco-2 were originally obtained from
European Collection of Cell Cultures (Salisbury, UK). Cells were
grown in DMEM Glutamax media supplemented with 50 U/ml penicillin
and 50 μg/ml streptomycin. CT-26 media also contained 10% FBS, 1%
non-essential amino acids (NEAA) and 100 mg/l sodium pyruvate.
Caco-2 media contained 20% FBS and 1% NEAA. Cells were maintained
at 37°C in 5% CO2 in a humidified incubator. Cell
culture materials were supplied from Gibco, Invitrogen Corp (Grand
Island, NY, USA).
Cell proliferation assay
For cell viability assays cells, were seeded at
5×103 (CT-26) or 1×104 (Caco-2) per well in
96-well flat-bottomed plates and allowed to attach for 24 h.
Subsequently, cells were then exposed to either medium alone,
ethanol vehicle [1% ethanol (v/v)] or serial dilutions of PBOX
compounds (0.1–50 μM). After 72 h the number of viable cells was
estimated using the Alamar Blue assay (Invitrogen Corp, Carlsbad,
CA, USA) according to the manufacturer’s instructions. The results
were expressed as the percentage cell viability relative to
vehicle-treated control cells (100%). Dose response curves were
plotted and IC50 values were obtained using the
commercial software package Prism (GraphPad Software Inc., La
Jolla, CA, USA). Experiments were performed in triplicate on at
least three separate occasions.
Cell cycle analysis
Cells in the log phase of growth were treated with
relevant vehicle, drug or drug combination for the time indicated.
After treatment, cells were fixed with 70% (v/v) ethanol and stored
at −20°C. Cells were then centrifuged and stained with PBS
containing 0.5 mg/ml RNase and 0.15 mg/ml propidium iodide for 30
min at 37°C. The PI fluorescence was measured on a linear scale
using a FACSCalibur flow cytometer (Becton-Dickinson, San Jose, CA,
USA). The amount of PI fluorescence is directly proportional to the
amount of DNA present in each cell (6). Data collection was gated to exclude
cell debris and cell aggregates. At least 10,000 cells were
analysed per sample. All data were recorded and analysed using the
CellQuest software (Becton-Dickinson).
Quantification of autophagy by flow
cytometric analysis of acidic vesicular organelles (AVOs)
The formation of acidic compartments was quantified
by flow cytometric analysis of acridine orange stained cells. The
intensity of the red fluorescence is proportional to the amount of
acidity. Following treatment, cells were stained with acridine
orange (1 μg/ml) for 15 min at 37°C. BAF-A1 (Sigma-Aldrich) was
dissolved in DMSO and added to the cells 45 min prior to the
addition of acridine orange. Cells were then trypsinised and
collected in phenol-red free medium. Green (510–530 nm) and red
(>650 nm) fluorescence emission from 104 cells
illuminated with blue (488 nm) excitation light was measured with a
CyAn ADP Flow Cytometry Analyzer (Beckman Coulter, Nyon,
Switzerland). The red:green fluorescence ratio for individual cells
was calculated using FlowJo software (Tree Star Inc., San Carlos,
CA, USA).
Transmission electron microscopy
(TEM)
To morphologically examine PBOX-induced autophagy,
CT-26 cells were exposed to ethanol vehicle [0.2% (v/v)] or PBOX-15
(1 μM) for 48 h. Adherent cells were harvested by trypsinisation,
fixed for 1 h at room temperature in 4% paraformaldehyde, 2.5%
glutaraldehyde, 0.125 M HEPES pH 7.5. After washing in PBS the
cells were post-fixed in 2% osmium tetroxide solution and
dehydrated in a series of aqueous ethanol solutions. Samples were
embedded in epoxy resin. Ultrathin sections were cut on an
ultramicrotome (RMC MTXL) and collected on 300 mesh copper grids.
Each grid was stained with uranyl acetate and lead citrate and
stored for ultrastructural examination. Ultrastructural examination
was carried out in a Jeol 2100 transmission electron microscope
operating at 100 kV. A number of images were obtained as a
representative of each sample.
Western blot analysis
Cells were harvested in whole cell lysis buffer
containing 62.5 mM Tris (pH 6.8), 2% (w/v) SDS, 10% (v/v) glycerol,
0.00125% (w/v) bromophenol blue and 50 mM DTT. The proteins were
separated on a polyacrylamide gel and transfered to PVDF membrane,
probed overnight with the indicated primary antibody at 4°C and
relevant HRP-conjugated secondary antibody for 1 h at room
temperature. Rabbit anti-LC3B, anti-beclin-1, anti-Mcl-1,
lysosome-associated membrane protein 1 (LAMP-1) and anti-α-actin
were purchased from Cell Signaling (Danvers, MA, USA). The LC3B
(microtubule-associated protein light chain 3) antibody used has a
higher affinity for LC3B-II. Anti-PARP, anti-caspase-3, anti-Bcl-2,
anti-Bcl-xL, anti-GAPDH and anti-α-actin mAbs were purchased from
Merck Biosciences. Immunoreactive bands were detected by
autoradiography with enhanced chemiluminescence (Amersham
Biosciences, Buckinghamshire, UK).
Statistical analysis
The statistical analysis of experimental data was
performed using a Student’s paired t-test (comparison of two
groups) or one-way ANOVA (comparison of more than two groups) were
appropriate and results were presented as mean ± SEM. A value of
P<0.05 was considered to be significant.
Results
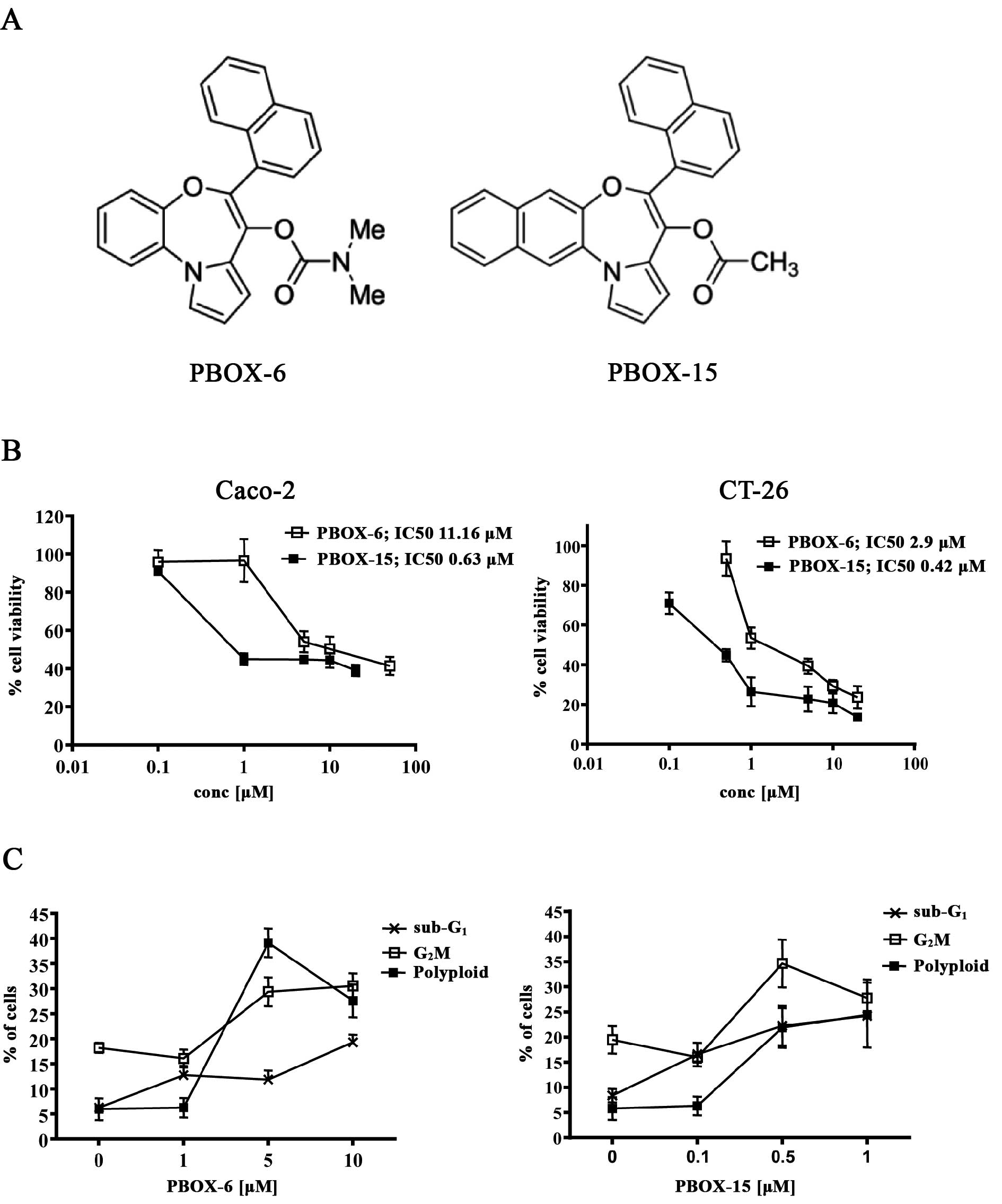
PBOX-induced cytotoxicity
The chemical structures of selected PBOXs are
illustrated in Fig. 1A. As shown
in Fig. 1B, the PBOXs induced a
dose-dependent inhibition of cell growth in both the CT-26 and
Caco-2 colon adenocarcinoma derived cells with the half-maximal
inhibitory concentration (IC50) ranging from 0.42 to
11.16 μM. As anticipated for microtubule targeting drugs the PBOXs
induced a dose-dependent increase in cell death
(sub-G1), G2M cell cycle arrest and
polyploidy (DNA content >4N) in both cell lines (Fig. 1C). However, the sub-G1
population induced by PBOX exposure did not exceed 20%. These data
imply an underlying inherent resistance of these cells to the
PBOXs.
PBOX-induced protective autophagy in
adenocarcinoma cells
Given that autophagy has been associated with
resistance to various chemotherapeutics (31) the presence of autophagy in the
adherent surviving population of cells following PBOX exposure was
next examined. As determined by flow cytometry of acridine orange
stained cells, the PBOXs increased the strength of the red
fluorescence which is directly proportional to the acidity of the
cells in a dose dependent manner (Fig.
2A). The intensity of the fluorescence increased over time
indicating the development of AVOs after a prolonged exposure to
the PBOXs (Fig. 2A). AVOs were
also detected by microscopic analysis of acridine orange stained
CT-26 cells following a 48 h exposure to the PBOXs (data not
shown). AVOs continued to be detected in the adherent population of
cells even after 7 days exposure to the PBOXs further implying a
cytoprotective role of autophagy in these cells following prolonged
exposure to the PBOXs (data not shown). Numerous studies have
demonstrated a dependence of the acidification of cellular
organelles on the vacuolar H+ ATPase using the specific
inhibitor bafilomycin-A1 (BAF-A1). In agreement with these findings
the addition of BAF-A1 prior to acridine orange staining
significantly inhibited the formation of AVOs confirming that
BAF-A1 can inhibit PBOX-induced late stage autophagy in colon
cancer cells (Fig. 2B and C).
LC3, microtubule-associated protein 1 light chain 3,
exists as three isoforms (A–C). LC3B-I has been shown to covalently
conjugate to phosphatidylethanolamine to form LC3B-II during the
formation of autophagosomes. To date, LC3B-II is the only reliable
marker to monitor autophagy. LC3B-II is specifically localized to
autophagic structures throughout the autophagic process from
phagophore to lysosomal degradation (32). The time-dependent increase in the
levels of LC3B-II suggests an increase in the number of
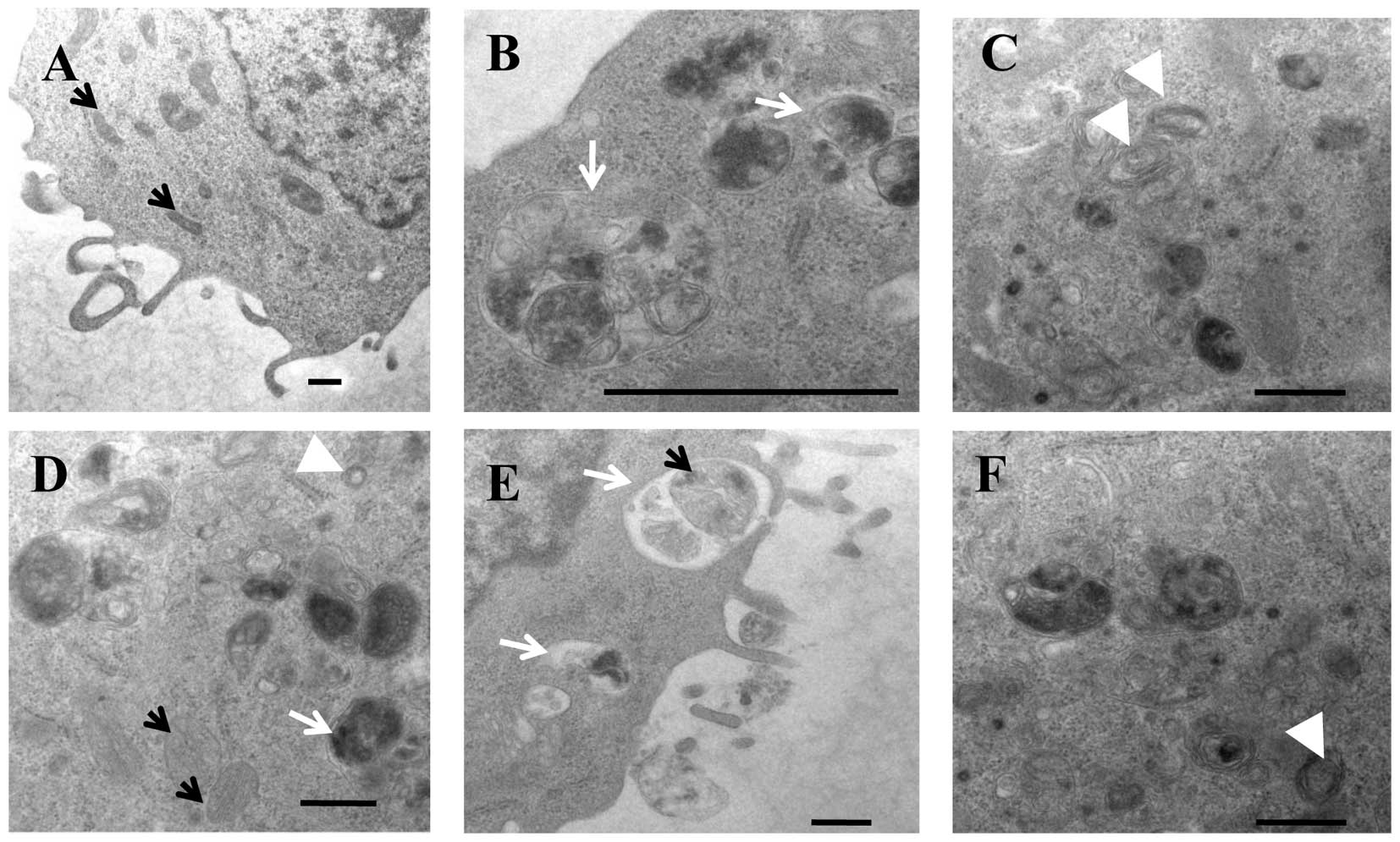
autophagosomes in response to PBOXs (Fig. 2D). PBOX-15 was identified as both
the most potent antiproliferative PBOX and most effective
autophagic initiator in colon cancer cells and was therefore
selected for electron microscopy, the gold standard method for
determination of autophagy. Prolonged exposure of PBOX-15 increased
the formation of autophagosomes and autolysosomes in the surviving
adherent population of colon cancer cells (Fig. 3). PBOX-15 induced autolysosomes
contained a diverse range of sequestered material which varied from
lamellar (including mitochondria derived) to dense granular
structures.
The effect of autophagy inhibition on
PBOX-induced cell death
We have conclusively demonstrated autophagy in the
surviving population of colon cancer cells exposed to PBOXs
(Figs. 2 and 3). Ultrastructural analysis clearly
demonstrated an accumulation of structures associated with
autophagy, such as autophagosomes/multivesicular bodies and
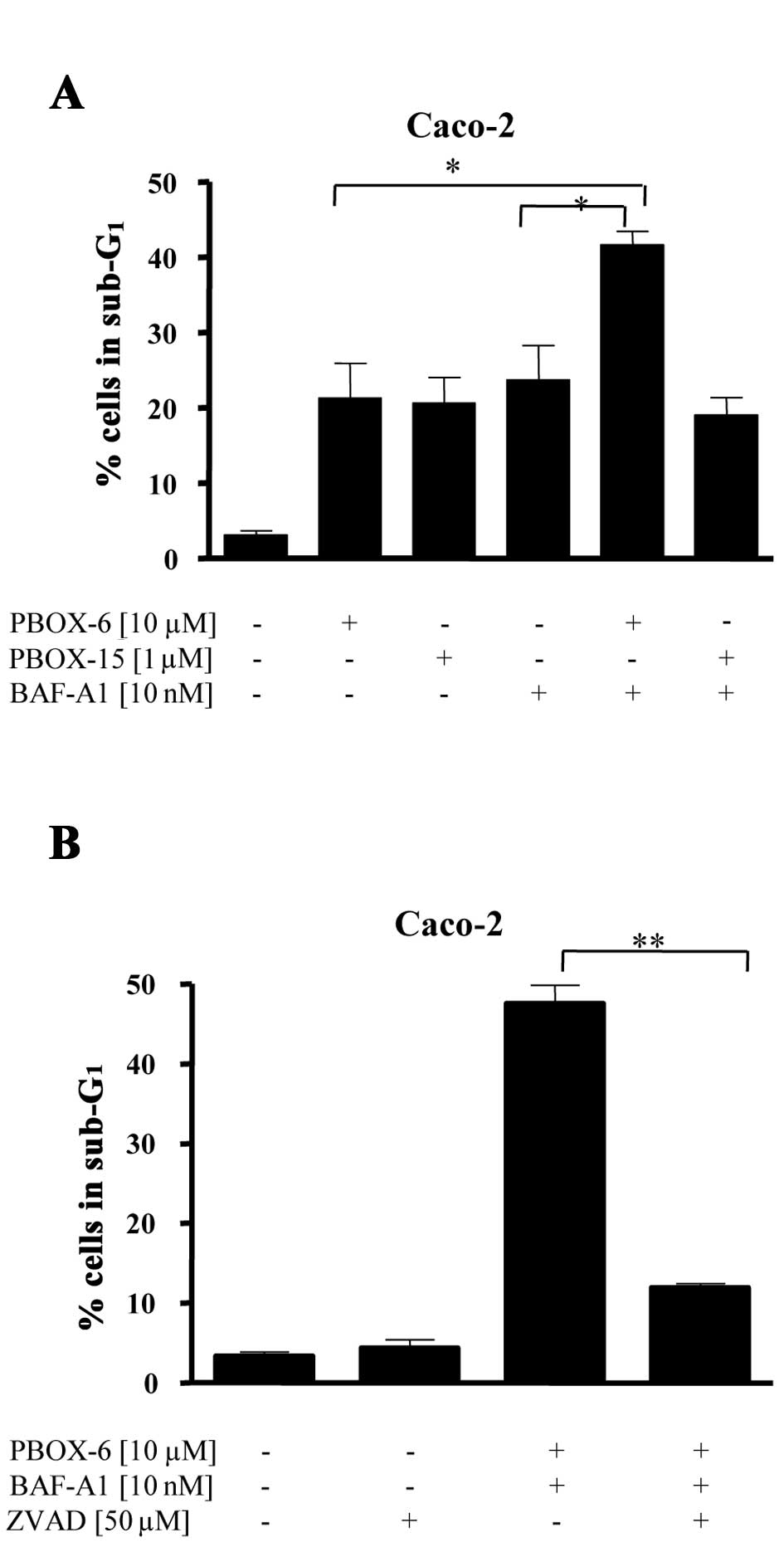
autophagolysosomes, in PBOX treated cells. We also demonstrated
(Fig. 2B) that BAF-A1 effectively
inhibited the late state of autophagy induced by the PBOXs. We next
sought to determine whether inhibition of autophagy by BAF-A1 can
augment PBOX induced cell death. As shown in Fig. 4A inhibition of late stage autophagy
by BAF-A1 enhanced PBOX-6 but not PBOX-15-induced cell death. The
increase in cell death due to the combined stimuli of PBOX-6 and
BAF-A1 was significantly inhibited by the pan-specific caspase
inhibitor Z-VAD suggesting a caspase- dependent pathway (Fig. 4B).
BAF-A1 synergistically enhances
PBOX-6-induced apoptotic cell death, which associates with a
downregulation of the anti-apoptotic protein Mcl-1
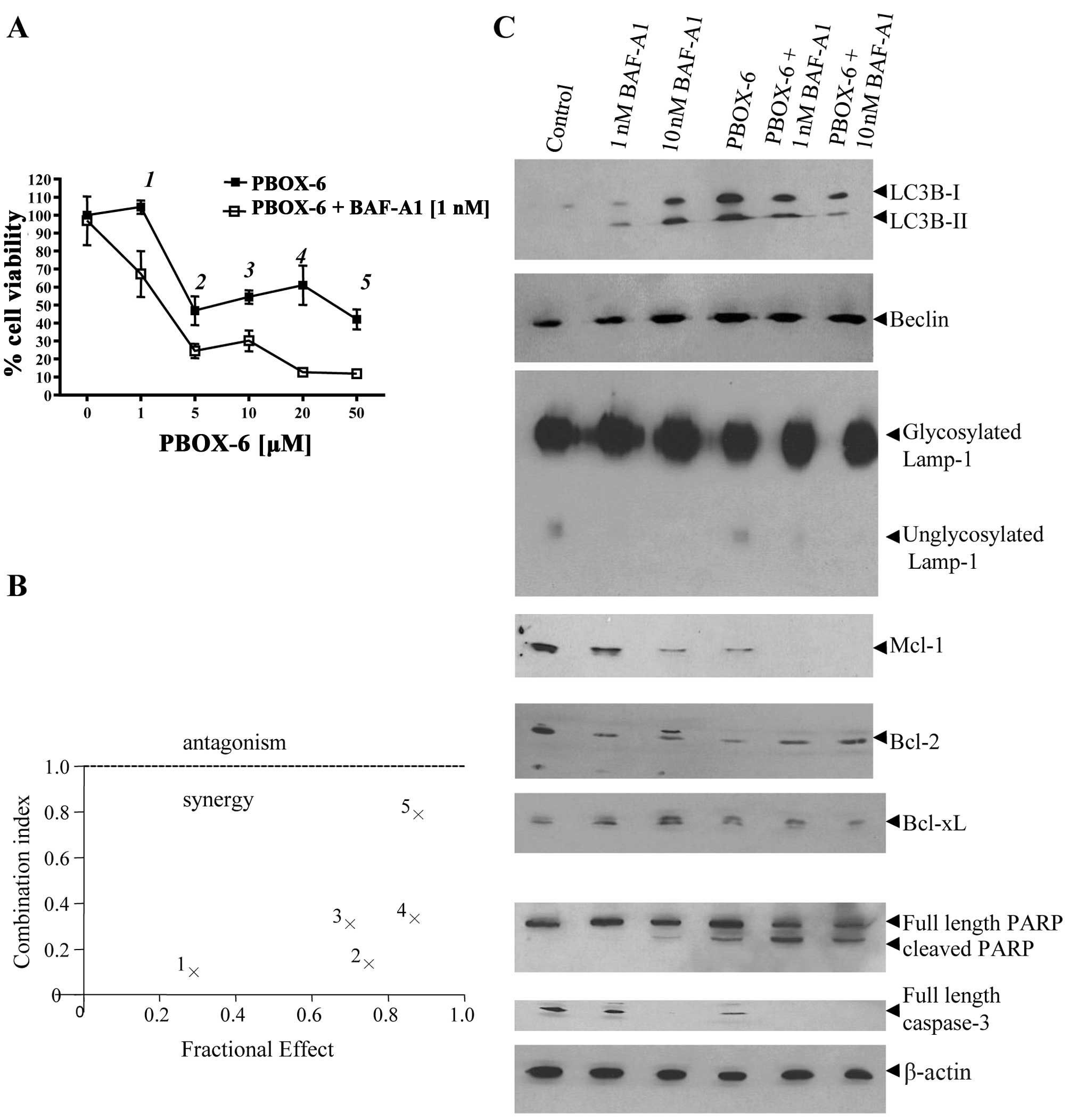
Interactions between PBOX-6 and BAF-A1 were further
investigated in Caco-2 cells using median dose effect analysis. As
shown in Fig. 5A, BAF-A1
significantly enhanced the antiproliferative effect of PBOX-6 in
human colon cancer cells as determined using the computer software
Calcusyn. A CI value of <1 is indicative of synergy. The effects
of PBOX-6 and BAF-A1 combinations were then analysed in relation to
expression of both regulators of autophagy and apoptosis. As
expected, BAF-A1 alone increased the levels of LC3B-II. It has been
postulated that BAF-A1 can increase the levels of LC3B-II by early
prevention of its degradation in existing autolysosomes and
preventing the fusion of the lysosome with the autophagosome at
later stages and is frequently used to assess the authenticity of
an autophagic flux (33–35). Levels of PBOX-6 induced LC3B-II
where higher in the presence of 1 nM BAF-A1 as compared with BAF-A1
(1 nM) alone indicating an efficient autophagic flux. Levels of
PBOX-6 induced LC3B-II did not increase further in the presence of
10 nM BAF-A1 suggesting that the levels of LC3B-II had become
saturated at this point. Neither drug alone or in combination
influenced the levels of autophagy regulator beclin-1. The effect
of the combinations on the lysosomal system was also assessed by
western blot analysis of the lysosomal protein LAMP-1. BAF-A1 but
not PBOX-6 reduced the levels of unglycosylated LAMP-1 which was
reflected in a marginal increase in the levels of glycosylated
LAMP-1. BAF-A1-induced change in the glycosylation status of LAMP-1
was maintained in the presence of PBOX-6. We next analysed the
effects of the drugs alone and in combination on the Bcl-2 family
of anti-apoptotic proteins. High levels of BAF-A1 (10 nM) that
induced ~20% cell death induced the phosphorylation of Bcl-2 and
Bcl-xL and reduced the levels of Mcl-1. PBOX-6 alone reduced the
levels of Bcl-2 and Mcl-1 and phosphorylated Bcl-xL. BAF-A1 and
PBOX-6 combinations reduced the levels of Mcl-1 as compared to
either drug alone. However, the combined exposure to BAF-A1 and
PBOX-6 did not yield any further decrease in the levels of Bcl-2 or
Bcl-xL as compared to either drug alone. Finally, BAF-A1 and PBOX-6
combinations increased the extent of PARP cleavage confirming
enhanced levels of apoptosis as compared to single drug exposure.
Collectively, our results demonstrate that the inhibition of
autophagy synergistically sensitises colon cancer cells to
PBOX-6.
Discussion
The PBOXs are a novel class of microtubule targeting
agents (MTAs) which are structurally distinct from MTAs currently
used within the clinic. The PBOXs have demonstrated promising
anticancer activity in ex vivo patient samples and in
vivo tumour models (5,8,9,36).
However, as with all MTAs a significant proportion of cells remain
viable following prolonged exposure to the PBOXs. For this reason
alternative approaches that combine the PBOXs with a second agent
exhibiting a different mechanism of action is required to further
improve the therapeutic potential of the PBOXs. We have previously
published preclinical data demonstrating successful combinations of
the PBOXs with the tyrosine kinase inhibitor, imatinib mesylate
(37,38), the cyclin dependent kinase
inhibitor, flavopiridol (39,40)
and also with the tumour necrosis factor-related apoptosis-inducing
ligand (36). Furthermore, we have
demonstrated potential clinical benefits in combining the PBOXs
with radiotherapy for the treatment of prostate cancer (41).
In this study, we report novel findings
demonstrating that the tubulin-targeting PBOX-6 induces both
apoptosis and autophagy in Caco-2 cells, highlighting autophagy as
a novel therapeutic target which could potentially augment PBOX
induced cell death. Activation of autophagy has long been
recognised as a cellular response to stress, in particular nutrient
deprivation. Recent data suggest autophagy is induced as a
pro-survival strategy to many anticancer therapies. The
cytoprotective role of autophagy to chemotherapeutics has generated
immense interest in the potential to target autophagy as an
adjuvant therapy.
The result of autophagy manipulation is dependent on
the combined influence of several factors including cell type,
autophagy initiator, the combined stimuli to the autophagy
initiator and selected combination. Given the varied and complex
outcome of autophagy manipulation it is not yet fully understood
how to effectively modulate autophagy to enhance the therapeutic
efficacy of individual anticancer therapies. Furthermore, as
autophagy functions as regulator of homeostasis the use of
autophagy inhibitors as an adjunctive therapy in organs in which
autophagy plays a homeostatic role may yield undesirable
side-effects (42). Hence given
the infancy of autophagy manipulation as a novel anticancer
strategy there is an urgent need to accumulate preclinical data on
a broad spectrum of chemotherapeutics in various cell types and in
in vivo models.
Herein we present novel significant findings
demonstrating that inhibition of late stage autophagy by BAF-A1
enhanced PBOX-6 but not PBOX-15-induced apoptotic death. Recent
studies demonstrated unique autophagy properties endowed by a C,
D-spirolactone analogue of paclitaxel not shared with the parent
compound highlighting the complexity of autophagy stimulation
(43). Inhibition of the early
stages of autophagy by 3-methyladenine did not augment PBOX-induced
cell death (data not shown). Similarly, other studies have
demonstrated therapeutic benefits in inhibiting late stage but not
early stage autophagy in combination with anticancer agents
(44). These results accentuate
the varied and complex outcome of autophagy manipulation warranting
continued research into autophagy manipulation as an adjuvant
therapy.
In concurrence with independent research carried out
in different colon cancer derived cells (24) we found that inhibition of autophagy
with BAF-A1 induced apoptotic cell death which was associated with
cleavage of caspase-3 and PARP. In this report we present novel
findings demonstrating that BAF-A1-induced apoptosis was associated
with changes to the Bcl-2 family of anti-apoptotic proteins; namely
phosphorylation of Bcl-2 and Bcl-xL and downregulation of Mcl-1.
These findings compliment other studies demonstrating that BAF-A1
can overcome Bcl-xL mediated chemoresistance in small cell lung
carcinoma cells by restoration of a caspase-independent apoptotic
pathway (45). Similarly, in the
mouse insulinoma M1N6 cell line, BAF-A1 reduced Bcl-2 expression
coincided with a reduction in cell viability (46). Other autophagy inhibitors 3-MA and
chloroquine induced caspase-dependent apoptosis which was
associated with the downregulation of Mcl-1 (47). It was also postulated that
interactions between Bcl-2 family members and ATG12 may constitute
an important point between autophagy and apoptosis in response to
specific signals (48). Also,
Bcl-2L11/BIM was recently identified as a novel molecular link
between autophagy and apoptosis (49). Taken together these findings
suggest that apoptosis induced following the inhibition of
autophagy associates with changes in the expression of members of
the Bcl-2 family of anti-apoptotic proteins. PBOX-6 induced
apoptosis was also associated with a downregulation of Mcl-1.
Furthermore, co-treatment with BAF-A1 enhanced PBOX-6 induced
apoptosis which associated with a further decline in Mcl-1 protein
levels. These findings provide a rationale for combining agents
which target Mcl-1 in combination with autophagy inhibitors for the
treatment of human cancer.
We also report a novel finding demonstrating that
BAF-A1 reduced the levels of unglycosylated LAMP-1. LAMP proteins
are required for the mobility of lysosomes and their fusion with
phagosomes (50). Glycosylation of
LAMP-1 is important for the stability of LAMP-1 in the lysosomal
membrane (51). Inhibition of
Abl-kinase signaling by imatinib was shown to alter LAMP-1
glycosylation in A549 lung carcinoma cell line (52). Furthermore, following imatinib
exposure the increase in the glycosylated form of LAMP-1 associated
with decreased autophagy (52).
Here we show that inhibition of autophagy by BAF-A1 decreases the
amount of the unstable unglycosylated LAMP-1 which coincided with a
marginal increase in the glycosylated stable form of LAMP-1.
Together these findings suggest that inhibition of autophagy
associates with changes in the glycosylation status of LAMP-1.
In summary, we have demonstrated novel findings
showing that the PBOXs induce apoptosis and autophagy in colon
cancer cells. Blocking autophagy in the late stages represents a
novel strategy to augment PBOX-6-induced lethality in these cells.
Autophagy plays a cytoprotective role in response to PBOX therapy
therefore further studies are warranted to delineate what
components of the autophagic pathway exhibit a cytoprotective
function to further enhance the inherent chemotherapeutic
properties of the PBOXs.
Acknowledgements
We would like to thank the Health Research Board
Ireland for funding the project.
References
|
1
|
Campiani G, Nacci V, Fiorini I, De
Filippis MP, Garofalo A, Ciani SM, Greco G, Novellino E, Williams
DC, Zisterer DM, et al: Synthesis, biological activity, and SARs of
pyrrolobenzoxazepine derivatives, a new class of specific
‘peripheral-type’ benzodiazepine receptor ligands. J Med Chem.
39:3435–3450. 1996.PubMed/NCBI
|
|
2
|
Zisterer DM, McGee MM, Campiani G, Ramunno
A, Fattorusso C, Nacci V, Lawler M and Williams DC:
Pyrrolo-1,5-benzoxazepines: a new class of apoptotic agents.
Biochem Soc Trans. 29:704–706. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mulligan JM, Greene LM, Cloonan S, McGee
MM, Onnis V, Campiani G, Fattorusso C, Lawler M, Williams DC and
Zisterer DM: Identification of tubulin as the molecular target of
proapoptotic pyrrolo-1,5-benzoxazepines. Mol Pharmacol. 70:60–70.
2006.PubMed/NCBI
|
|
4
|
McGee MM, Greene LM, Ledwidge S, Campiani
G, Nacci V, Lawler M, Williams DC and Zisterer DM: Selective
induction of apoptosis by the pyrrolo-1,5-benzoxazepine
7-[[dimethylcarbamoyl]oxy]-6-(2-naphthyl)pyrrolo-[2,1-d]
(1,5)-benzoxazepine (PBOX-6) in leukemia cells occurs via the c-Jun
NH2-terminal kinase-dependent phosphorylation and inactivation of
Bcl-2 and Bcl-XL. J Pharmacol Exp Ther. 310:1084–1095.
2004.PubMed/NCBI
|
|
5
|
Greene LM, Fleeton M, Mulligan J, Gowda C,
Sheahan BJ, Atkins GJ, Campiani G, Nacci V, Lawler M, Williams DC,
et al: The pyrrolo-1, 5-benzoxazepine, PBOX-6, inhibits the growth
of breast cancer cells in vitro independent of estrogen
receptor status and inhibits breast tumour growth in vivo.
Oncol Rep. 14:1357–1363. 2005.PubMed/NCBI
|
|
6
|
Greene LM, Campiani G, Lawler M, Williams
DC and Zisterer DM: BubR1 is required for a sustained mitotic
spindle checkpoint arrest in human cancer cells treated with
tubulin-targeting pyrrolo-1,5-benzoxazepines. Mol Pharmacol.
73:419–430. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nathwani SM, Butler S, Fayne D, McGovern
NN, Sarkadi B, Meegan MJ, Lloyd DG, Campiani G, Lawler M, Williams
DC, et al: Novel microtubule-targeting agents,
pyrrolo-1,5-benzoxazepines, induce apoptosis in
multi-drug-resistant cancer cells. Cancer Chemother Pharmacol.
66:585–596. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McElligott AM, Maginn EN, Greene LM,
McGuckin S, Hayat A, Browne PV, Butini S, Campiani G, Catherwood
MA, Vandenberghe E, et al: The novel tubulin-targeting agent
pyrrolo-1,5-benzoxazepine-15 induces apoptosis in poor prognostic
subgroups of chronic lymphocytic leukemia. Cancer Res.
69:8366–8375. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bright SA, McElligott AM, O’Connell JW,
O’Connor L, Carroll P, Campiani G, Deininger MW, Conneally E,
Lawler M, Williams DC, et al: Novel pyrrolo-1,5-benzoxazepine
compounds display significant activity against resistant chronic
myeloid leukaemia cells in vitro, in ex vivo patient samples and in
vivo. Br J Cancer. 102:1474–1482. 2010. View Article : Google Scholar
|
|
10
|
Nathwani SM, Butler S, Meegan MJ, Campiani
G, Lawler M, Williams DC and Zisterer DM: Dual targeting of tumour
cells and host endothelial cells by novel microtubule-targeting
agents, pyrrolo-1,5-benzoxazepines. Cancer Chemother Pharmacol.
65:289–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sato K, Tsuchihara K, Fujii S, Sugiyama M,
Goya T, Atomi Y, Ueno T, Ochiai A and Esumi H: Autophagy is
activated in colorectal cancer cells and contributes to the
tolerance to nutrient deprivation. Cancer Res. 67:9677–9684. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu YC, Wang XJ, Yu L, Chan FK, Cheng AS,
Yu J, Sung JJ, Wu WK and Cho CH: Hydrogen sulfide lowers
proliferation and induces protective autophagy in colon epithelial
cells. PLoS One. 7:e375722012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li J, Hou N, Faried A, Tsutsumi S and
Kuwano H: Inhibition of autophagy augments 5-fluorouracil
chemotherapy in human colon cancer in vitro and in vivo model. Eur
J Cancer. 46:1900–1909. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li DD, Sun T, Wu XQ, Chen SP, Deng R,
Jiang S, Feng GK, Pan JX, Zhang XS, Zeng YX, et al: The inhibition
of autophagy sensitises colon cancer cells with wild-type p53 but
not mutant p53 to topotecan treatment. PLoS One. 7:e450582012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Paillas S, Causse A, Marzi L, de Medina P,
Poirot M, Denis V, Vezzio-Vie N, Espert L, Arzouk H, Coquelle A, et
al: MAPK14/p38alpha confers irinotecan resistance to TP53-defective
cells by inducing survival autophagy. Autophagy. 8:1098–1112. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ravikumar B, Sarkar S, Davies JE, Futter
M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M,
Korolchuk VI, Lichtenberg M, Luo S, et al: Regulation of mammalian
autophagy in physiology and pathophysiology. Physiol Rev.
90:1383–1435. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Han W, Sun J, Feng L, Wang K, Li D, Pan Q,
Chen Y, Jin W, Wang X, Pan H, et al: Autophagy inhibition enhances
daunorubicin-induced apoptosis in K562 cells. PLoS One.
6:e284912011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shen S, Kepp O and Kroemer G: The end of
autophagic cell death? Autophagy. 8:1–3. 2012. View Article : Google Scholar
|
|
19
|
Greene LM, O’Boyle NM, Nolan DP, Meegan MJ
and Zisterer DM: The vascular targeting agent Combretastatin-A4
directly induces autophagy in adenocarcinoma-derived colon cancer
cells. Biochem Pharmacol. 84:612–624. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cook KL, Shajahan AN and Clarke R:
Autophagy and endocrine resistance in breast cancer. Expert Rev
Anticancer Ther. 11:1283–1294. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zois CE and Koukourakis MI:
Radiation-induced autophagy in normal and cancer cells: towards
novel cytoprotection and radio-sensitization policies? Autophagy.
5:442–450. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Clarke PG and Puyal J: Autophagic cell
death exists. Autophagy. 8:867–869. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Boya P, Gonzalez-Polo RA, Casares N,
Perfettini JL, Dessen P, Larochette N, Metivier D, Meley D,
Souquere S, Yoshimori T, et al: Inhibition of macroautophagy
triggers apoptosis. Mol Cell Biol. 25:1025–1040. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu YC, Wu WK, Li Y, Yu L, Li ZJ, Wong CC,
Li HT, Sung JJ and Cho CH: Inhibition of macroautophagy by
bafilomycin A1 lowers proliferation and induces apoptosis in colon
cancer cells. Biochem Biophys Res Commun. 382:451–456. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bing L, Dejuan K, Yang L, Nan L, Mengzi H,
Shumei M and Xiaodong L: Autophagy inhibition plays the synergetic
killing roles with radiation in the multi-drug resistant SKVCR
ovarian cancer cells. Radiat Oncol. 7:2132012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu S, Cao L, Yu Y, Yang L, Yang M, Liu K,
Huang J, Kang R, Livesey KM and Tang D: Inhibiting autophagy
potentiates the anticancer activity of IFNα in chronic myeloid
leukemia cells. Autophagy. 9:317–327. 2012.PubMed/NCBI
|
|
27
|
Swampillai AL, Salomoni P and Short SC:
The role of autophagy in clinical practice. Clin Oncol (R Coll
Radiol). 24:387–395. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Garcia-Echeverria C and Sellers WR: Drug
discovery approaches targeting the PI3K/Akt pathway in cancer.
Oncogene. 27:5511–5526. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cerniglia GJ, Karar J, Tyagi S,
Christofidou-Solomidou M, Rengan R, Koumenis C and Maity A:
Inhibition of autophagy as a strategy to augment radiosensitization
by the dual phosphatidylinositol 3-kinase/mammalian target of
rapamycin inhibitor NVP-BEZ235. Mol Pharmacol. 82:1230–1240. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang ZJ, Chee CE, Huang S and Sinicrope
FA: The role of autophagy in cancer: therapeutic implications. Mol
Cancer Ther. 10:1533–1541. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maycotte P and Thorburn A: Autophagy and
cancer therapy. Cancer Biol Ther. 11:127–137. 2011. View Article : Google Scholar
|
|
32
|
Nakatogawa H, Suzuki K, Kamada Y and
Ohsumi Y: Dynamics and diversity in autophagy mechanisms: lessons
from yeast. Nat Rev Mol Cell Biol. 10:458–467. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yamamoto A, Tagawa Y, Yoshimori T,
Moriyama Y, Masaki R and Tashiro Y: Bafilomycin A1 prevents
maturation of autophagic vacuoles by inhibiting fusion between
autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E
cells. Cell Struct Funct. 23:33–42. 1998. View Article : Google Scholar
|
|
34
|
Jahreiss L, Menzies FM and Rubinsztein DC:
The itinerary of autophagosomes: from peripheral formation to
kiss-and-run fusion with lysosomes. Traffic. 9:574–587. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Klionsky DJ, Elazar Z, Seglen PO and
Rubinsztein DC: Does bafilomycin A1 block the fusion of
autophagosomes with lysosomes? Autophagy. 4:849–950. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Maginn EN, Browne PV, Hayden P,
Vandenberghe E, MacDonagh B, Evans P, Goodyer M, Tewari P, Campiani
G, Butini S, et al: PBOX-15, a novel microtubule targeting agent,
induces apoptosis, upregulates death receptors, and potentiates
TRAIL-mediated apoptosis in multiple myeloma cells. Br J Cancer.
104:281–289. 2011. View Article : Google Scholar
|
|
37
|
Greene LM, Kelly L, Onnis V, Campiani G,
Lawler M, Williams DC and Zisterer DM: STI-571 (imatinib mesylate)
enhances the apoptotic efficacy of pyrrolo-1,5-benzoxazepine-6, a
novel microtubule-targeting agent, in both STI-571-sensitive and
-resistant Bcr-Abl-positive human chronic myeloid leukemia cells. J
Pharmacol Exp Ther. 321:288–297. 2007. View Article : Google Scholar
|
|
38
|
Bright SA, Greene LM, Greene TF, Campiani
G, Butini S, Brindisi M, Lawler M, Meegan MJ, Williams DC and
Zisterer DM: The novel pyrrolo-1, 5-benzoxazepine, PBOX-21,
potentiates the apoptotic efficacy of STI571 (imatinib mesylate) in
human chronic myeloid leukaemia cells. Biochem Pharmacol.
77:310–321. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bright SA, Campiani G, Deininger MW,
Lawler M, Williams DC and Zisterer DM: Sequential treatment with
flavopiridol synergistically enhances
pyrrolo-1,5-benzoxazepine-induced apoptosis in human chronic
myeloid leukaemia cells including those resistant to imatinib
treatment. Biochem Pharmacol. 80:31–38. 2010. View Article : Google Scholar
|
|
40
|
Nathwani SM, Cloonan SM, Stronach M,
Campiani G, Lawler M, Williams DC and Zisterer DM: Novel
microtubule-targeting agents, pyrrolo-1,5-benzoxazepines, induce
cell cycle arrest and apoptosis in prostate cancer cells. Oncol
Rep. 24:1499–1507. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Forde JC, Maginn EN, McNamara G, Martin
LM, Campiani G, Williams DC, Zisterer D, McElligott AM, Lawler M,
Lynch TH, et al: Microtubule-targeting-compound PBOX-15
radiosensitizes cancer cells in vitro. Cancer Biol Ther.
11:421–428. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kimura T, Takabatake Y, Takahashi A and
Isaka Y: Chloroquine in cancer therapy: a double-edged sword of
autophagy. Cancer Res. 73:3–7. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Trmcic MV, Matovic RV, Tovilovic GI,
Ristic BZ, Trajkovic VS, Ferjancic ZB and Saicic RN: A novel
C,D-spirolactone analogue of paclitaxel: autophagy instead of
apoptosis as a previously unknown mechanism of cytotoxic action for
taxoids. Org Biomol Chem. 25:4933–4942. 2012. View Article : Google Scholar
|
|
44
|
Kanematsu S, Uehara N, Miki H, Yoshizawa
K, Kawanaka A, Yuri T and Tsubura A: Autophagy inhibition enhances
sulforaphane-induced apoptosis in human breast cancer cells.
Anticancer Res. 30:3381–3390. 2010.PubMed/NCBI
|
|
45
|
Sasazawa Y, Futamura Y, Tashiro E and
Imoto M: Vacuolar H+-ATPase inhibitors overcome
Bcl-xL-mediated chemoresistance through restoration of a
caspase-independent apoptotic pathway. Cancer Sci. 100:1460–1467.
2009.
|
|
46
|
Hettiarachchi KD, Zimmet PZ and Myers MA:
The plecomacrolide vacuolar-ATPase inhibitor bafilomycin, alters
insulin signaling in MIN6 beta-cells. Cell Biol Toxicol.
22:169–181. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pliyev BK and Menshikov M: Differential
effects of the autophagy inhibitors 3-methyladenine and chloroquine
on spontaneous and TNF-alpha-induced neutrophil apoptosis.
Apoptosis. 17:1050–1065. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rubinstein AD, Eisenstein M, Ber Y, Bialik
S and Kimchi A: The autophagy protein Atg12 associates with
antiapoptotic Bcl-2 family members to promote mitochondrial
apoptosis. Mol Cell. 44:698–709. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Luo S and Rubinsztein DC: BCL2L11/BIM: A
novel molecular link between autophagy and apoptosis. Autophagy.
9:104–105. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Huynh KK, Eskelinen EL, Scott CC,
Malevanets A, Saftig P and Grinstein S: LAMP proteins are required
for fusion of lysosomes with phagosomes. EMBO J. 26:313–324. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kundra R and Kornfeld S: Asparagine-linked
oligosaccharides protect Lamp-1 and Lamp-2 from intracellular
proteolysis. J Biol Chem. 274:31039–31046. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yogalingam G and Pendergast AM: Abl
kinases regulate autophagy by promoting the trafficking and
function of lysosomal components. J Biol Chem. 283:35941–35953.
2008. View Article : Google Scholar : PubMed/NCBI
|