Introduction
Key knowledge is still missing for the successful
cure of aggressive neuroblastoma (NB), representing one of the most
deadly pediatric malignancies (1–4).
Neuroblastoma is a small round cell tumor of childhood and is
considered to arise from dedifferentiation of primordial neural
crest cells that populate the sympathetic trunks and the adrenal
medulla (reviewed in ref. 5).
During this process, an aberrant response to microenvironment cues
may play an important role in modulating the tumor phenotype, and
hence also lead to the variable clinical presentations of NB in
patients. The clinical presentation spans from a benign type with
the ability to spontaneously regress to a variant with a high rate
of recurrence, metastatic spread and a high frequency of
therapy-resistance. Current consensus supports the importance of a
strong interplay with the surrounding tissue promoting tumor growth
and spread (6). Thus, pre-clinical
studies of childhood NB would for increased relevance benefit from
in vivo models better matching the embryonic neoplastic
niche in early development from which this tumor is believed to
originate.
Pluripotent stem cells (PSC) are defined by their
ability to differentiate into any of the three germ layers and
ectopic injections into mice repeatedly produce what is described
as experimental teratoma (7,8).
Although generically referred to as a tumor, others and we have
previously reported evidence that pluripotent stem cell-derived
teratoma (PSCT) from PSC with normal karyotype can be described as
a failed embryonic process including increasingly chaotic embryonic
tissues and an emerging organoid development (7,8–11).
Despite lacking a developmental axis this process can show striking
similarities to the events in the human embryo, including and often
dominated by components of early neural development (9,10).
When compared to human embryos at diagnosed gestation stages, we
observed minor kinetic deviations in the appearances of several
organoid structures in PSCT developed from the well-studied human
embryonic stem cell line HS181 (10). However, more advanced organoid
structures are rare (7–10) and a prolonged immaturity of some
neural components is a consistent finding also in benign PSCT
induced by PSC with normal karyotype (reviewed in ref. 8). More specifically, such immature
neural areas display a strong resemblance with primitive
neuroectodermal tumors and a similar histology when appearing in
patient samples is considered potentially malignant.
The stem cell induced teratoma environment has
earlier been suggested as an experimental platform for studying
human tumor cell lines of a variety of origins (ovarian, prostate,
lung, glioblastoma, breast, colorectal cancer) (12–14).
Similarly, we studied growth from a malignant melanoma cell line in
human experimental teratoma and identified a de-differentiated
tumor phenotype not present in xenografts from the same cell line
(15), possibly indicating an
adaptation of the tumor cells to the embryonic
microenvironment.
To generate new insights and testing a principle of
closer developmental match between the injected tumor and the PSCT
microenvironment, we conducted experiments to explore the PSCT
milieu specifically for in vivo support of tumors of
embryonic origin. Proliferative tumor cells from the injections of
three aggressive NB cell lines; IMR-32, Kelly and SK-N-BE(2) could
readily be identified and growth was found exclusively integrated
into areas of loose mesenchyme, presenting tumors with histological
resemblance to clinical NB.
Materials and methods
Ethical permission
This study was performed in full accordance with
permission for experiments using human embryonic stem cells, from
the Local Ethics Committee at Karolinska Institute (114/00), and
for animal experimentation from the regional ethics committee
(Stockholm Northern Animal Review Board; Dnr S172-03 and
N105/07).
Cell lines
The human embryonic stem cell lines HS181 (16) and H9 (17), both of 46:XX karyotype, were
maintained in culture as previously described (18). The human male NB cell lines IMR-32,
Kelly and SK-N-BE(2) were obtained from ATCC (Manassas, VA, USA).
For genetic profile see Table I.
All tumor cell lines were cultured in Iscove’s modified Dulbecco’s
medium (IMDM) supplied with 10% fetal bovine serum (FBS) and 1%
penicillin/streptomycin (all from Invitrogen, Carlsbad, CA, USA),
at 37°C, 6.8% CO2, with high humidity.
 | Table IOverview of tumor cell lines and the
experimental setup. |
Table I
Overview of tumor cell lines and the
experimental setup.
| | | Number of injections
and frequency of tumor takes |
|---|
| | |
|
|---|
| Tumor cell line | Origin | Genetic profile | Xenografts | PSCT |
|---|
| IMR-32 | Abdominal tumor
mass | MYCN
amplification
1p deletion | 3/3 | 8/8 |
| Kelly | Brain metastasis | MYCN
amplification
17q amplified
11q deletion | 4/4 | 2/2 |
| SK-N-BE(2) | Bone marrow
metastasis | MYCN
amplification
1p deletion | 2/2 | 3/3 |
Animals and generation of PSCT
SCID/Beige (C.B.-17/GbmsTac-scid-bgDF N7; M&B,
Ry, Denmark), male mice, 6–8 weeks old, were used. PSCT were
generated by injection of 104–105 HS181 under
the testicular capsule of mice, as previously described (8,9).
PSCT were allowed to progress for 45 days before injected with NB
tumor cells. The variations of germ layer formation in separate
HS181 PSCT formed under the present conditions have been described
previously in more detail and were in all cases minor (9,15).
Injection of NB cells
Cells from the indicated cell lines
(1×106 cells in 20 μl PBS), were injected into
45-day-old PSCT, or as a xenograft, directly under the testis
capsule of mice anesthetized using isofluran (3%). Injections of NB
cells resulted in outgrowth in all cases. For number of injections
and frequency of tumor takes see Table
I. Animals were sacrificed two weeks after NB tumor cell
injection, before reaching the limit of the accepted total size of
the PSCT, including NB tumor growth, according to the ethical
permit for the recipient animal.
Tissue preparation
Tumors were harvested after two weeks, fixed in 4%
paraformaldehyde at 4°C 24 hours and dehydrated through a graded
series of alcohol to xylene, embedded in paraffin, then serially
sectioned into 5-μm-thick sections and stained using standard
hematoxylin and eosin (H&E) staining for basic histological
orientation. For each NB cell line, two xenografts and two PSCT
with injected NB tumor were subjected to detailed screening of
10–30 sections each.
Verification of NB tumor growth
Presence of NB tumor cells was verified using
genetic identification as described previously (15). Human Y- and X-chromosomes was
detected using mixed probes (CEP XY; Vysis Inc, Downers Grove, IL,
USA) against the human X- and Y-chromosomes, thus positively
separating human from mouse cells in xenografts, as well as
separating IMR-32 cells (XX) from PSCT cells (XY), as previously
described (15). For the
identification of Kelly and SK-N-BE(2), both lacking stable
Y-chromosome centromeres, the identification of their genetic MYCN
amplification was performed using the probes LSI N-MYC, 2p24 (Vysis
Inc).
Histological analysis
Areas of identified tumor growth were subjected to
immunohistochemistry (IHC) using criterion for histological
analysis of NB cell differentiation as those used in daily clinical
practice and in other studies (19,20).
Dilutions and conditions for the specific antibodies
and secondary detection methods are presented in Table II. The IHC results were verified
using positive control slides from tissues known to express the
antigen of interest. Negative controls were performed by omitting
primary antibody and by using non-specific antibodies as isotype
controls in the same concentration as the primary antibody.
Species-specificity of the human specific antibody for CD31 was
verified internally in each staining, since both xenografts and the
PSCT environment contain numerous vessels of murine origin, which
were all negative.
 | Table IIAntibodies used. |
Table II
Antibodies used.
| Antigen | Source | Dilution | 2nd detection |
|---|
| CD31 | DAKO, M 0823 | 1:20 | DakoCytomation
EnVision+System-HRP |
| Chromogranin | A DAKO, A 0430 | 1:2000 | Bond Polymer Refine
detection kit, Leica |
| Cripto-1 | Rockland,
600-401-99337 | 1:400 | 4+ Biotinylated
Goat Anti-Rabbit-IgG, Biocare Medical |
| E-cadherin | Abcam, ab1416 | 1:50 | Vectastain
Universal Elite ABC |
| HIF2α | Novus Biologicals,
NB100-132 | 1:150 | DakoCytomation
EnVision+System-HRP |
| Ki67 | Abcam, ab833 | 1:50 | Vectastain
Universal Elite ABC |
| Lefty | Santa Cruz,
SC-7408 | 1:25 | 4+ Biotinylated
Mouse Anti-Goat-IgG, Biocare Medical |
| Nodal | Life Span
BioScience, LS-B3955 | 1:100 | 4+ Biotinylated
Mouse Anti-Goat-IgG, Biocare Medical |
| Synaptophysin | Novocastra/Leica,
NCL-L-Synap-299 | 1:100 | DakoCytomation
EnVision+System-HRP |
Image analysis
Imaging was performed using a Zeiss Axiovert 200M
microscope and Openlab 5.0 software. Images were adjusted for auto
levels in Photoshop.
Results
Histopathology of NB cell lines in
xenografts and PSCT
For all three NB cell lines the injections of
106 cells yielded detectable tumor growth in all
animals. The number of injections and frequency of tumor takes are
summarized in Table I.
All NB cell lines presented growth in smaller
nodules as well as larger areas of cohesive tumor growth. A
consistent finding in both xenografts and PSCT was a poorly
differentiated tumor with a variable amount of fibrovascular
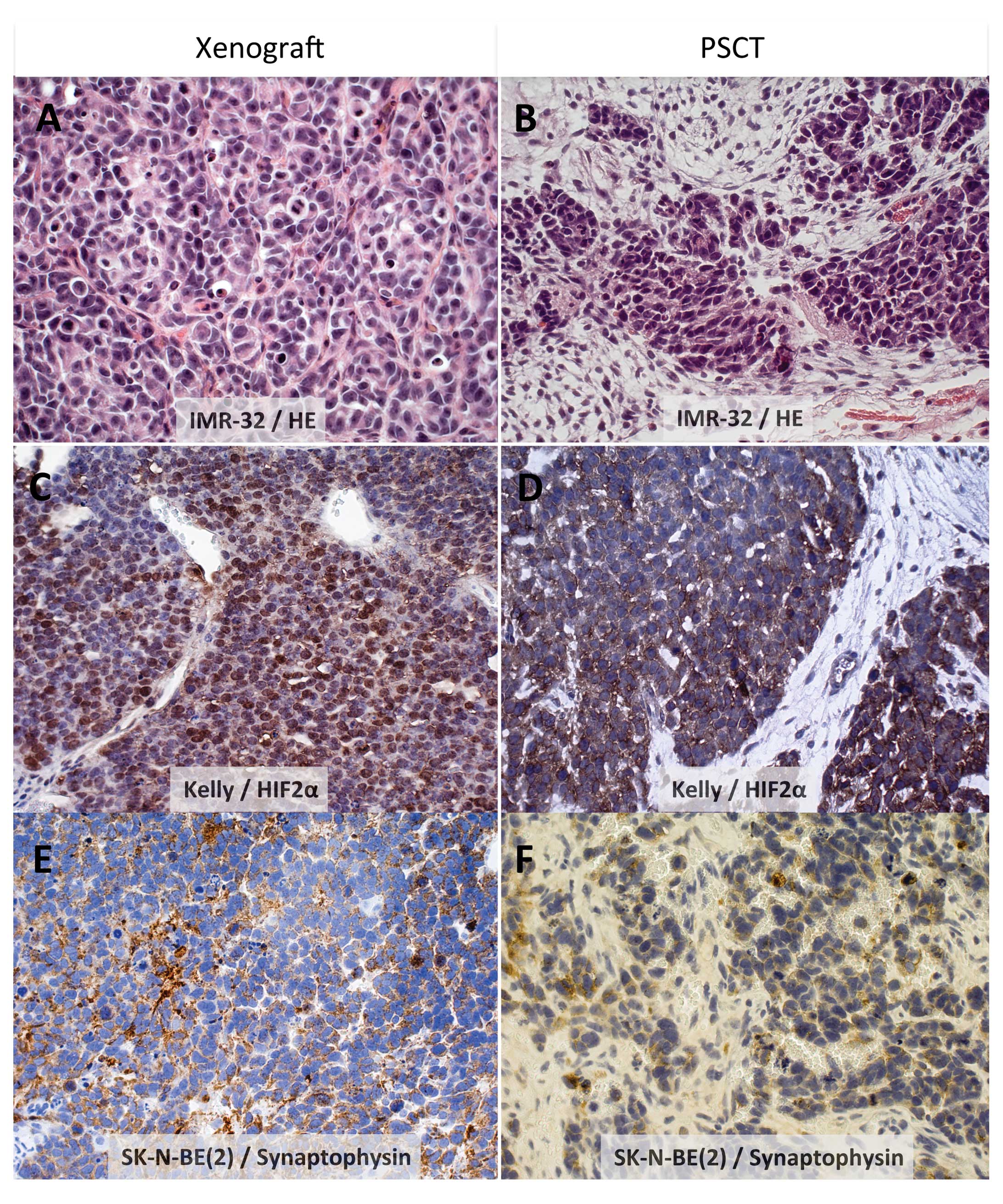
stroma, and no Schwann cell differentiation (Table III and illustrated in Fig. 1A–F for all three NB lines).
Further, presence of a neuroendocrine phenotype as reflected by
positive staining for synaptophysin and chromogranin A was a
consistent finding for all three NB cell lines in both models, as
exemplified by SK-N-BE(2) in Figs.
1E,F and 2D.
 | Table IIIIn vivo growth
characteristics. |
Table III
In vivo growth
characteristics.
| Tumor cell
line | Xenograft (intra
testis) | PSCT |
|---|
| IMR-32 | Poorly
differentiated deliminated growth pattern with a rich fibrovascular
stroma. Polygonal tumor cells with sheets of neurofibrillary
material. | Poorly
differentiated, moderately small, blastic cells with scant
stroma.
No evident neuropil. |
| Kelly | Poorly
differentiated deliminated growth pattern with a fine fibrovascular
stroma. Moderate amount of cytoplasm forming neuropil. | Undifferentiated
stroma-poor appearance. Small nuclei with condensed chromatin and
scant cytoplasm without any sign of differentiation. Band-like
necrotic areas |
| SK-N-BE(2) | Undifferentiated
solid areas as well as infiltrating growth pattern. Pleomorphic
nuclei and a moderate amount of eosinophilic cytoplasm without
fibrillary extensions. | Similar to
xenografts. |
The numbers of mitosis were similar in PSCT and
xenografts; IMR-32 showed a proliferation index of >70% in both
models, while SK-N-BE and Kelly showed a proliferation index of
40–50%.
Tropism of NB outgrowth in the PSCT
microenvironment
For practical reasons the NB cells were injected
into random positions in the PSCT cell mass. Small nodules of tumor
growth were however detected exclusively in areas compatible with
undifferentiated mesenchymal stroma, and never observed in areas
with well differentiated somatic tissue i.e. bone, muscle, gut or
areas of other easily identifiable tissue types.
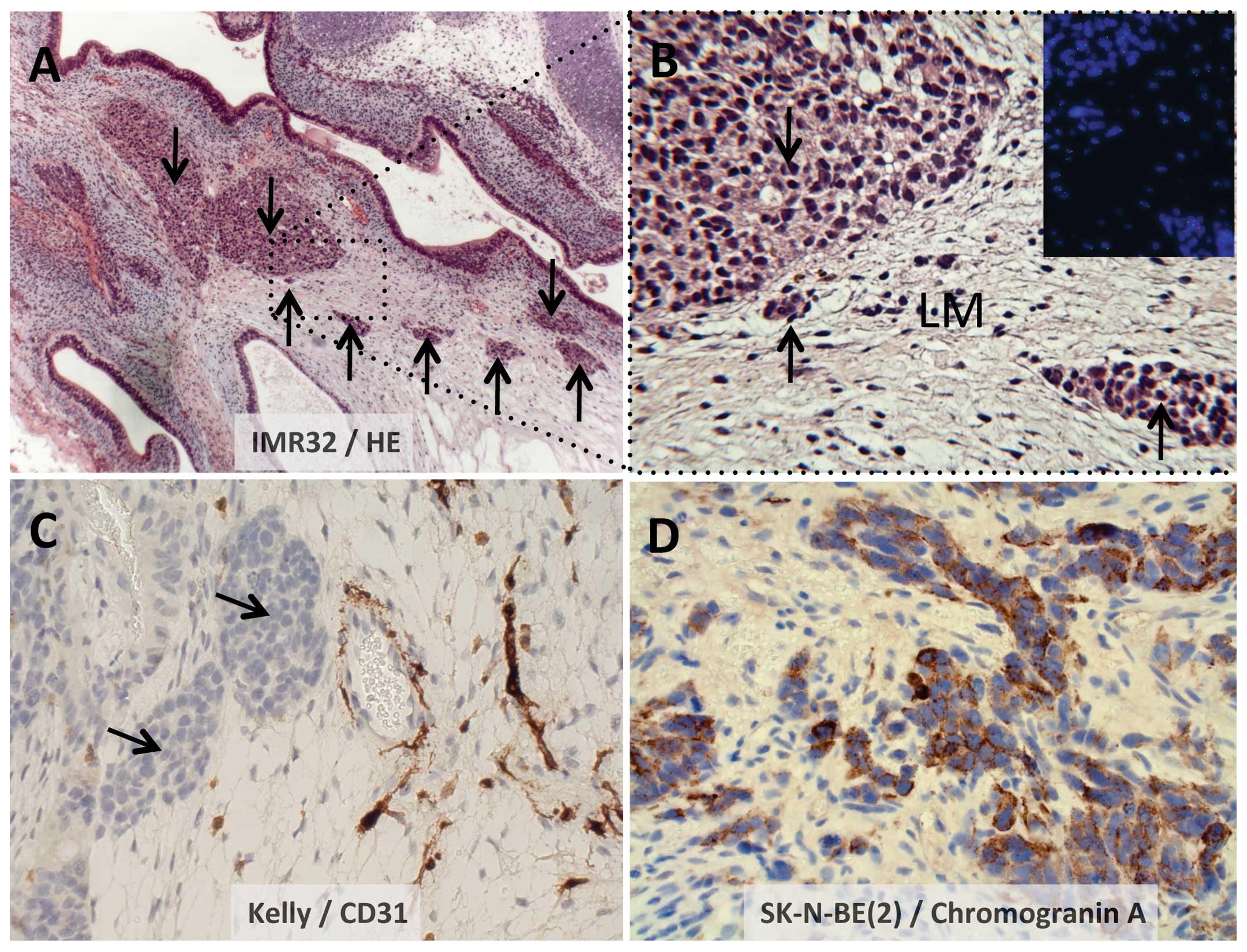
Fig. 2A illustrates
IMR-32 nodular growth (arrows) located in a loose mesenchyme
environment, with the dotted boxed area illustrated at a higher
magnification (x40) in Fig. 2B.
Fig. 2C illustrates the nodular
growth of Kelly, here located next to vessels staining for human
CD31. Similarly, Fig. 2D
illustrates small groups of SK-N-BE(2) tumor cells, here stained
for Chromogranin A, embedded in loose mesenchyme.
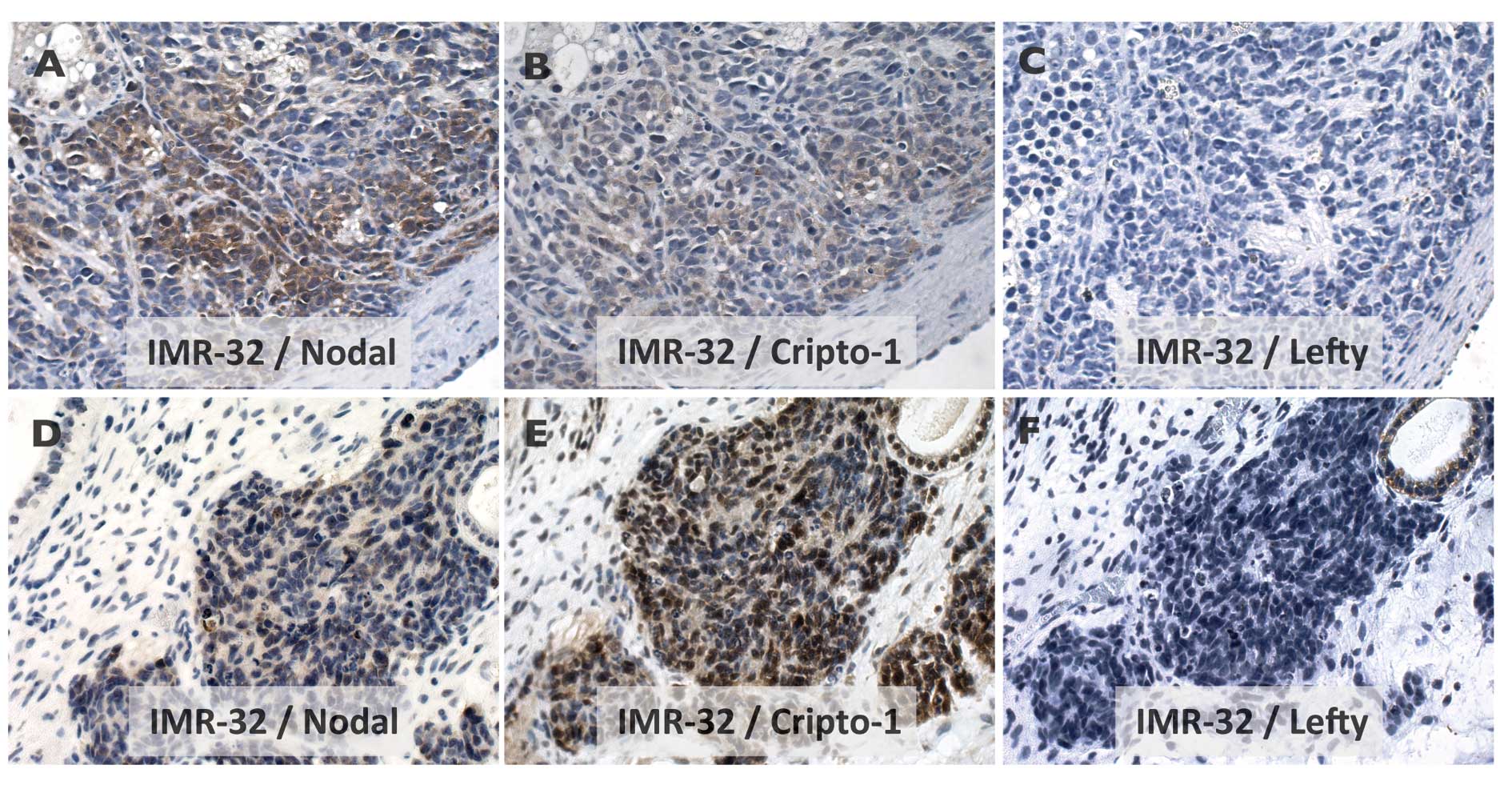
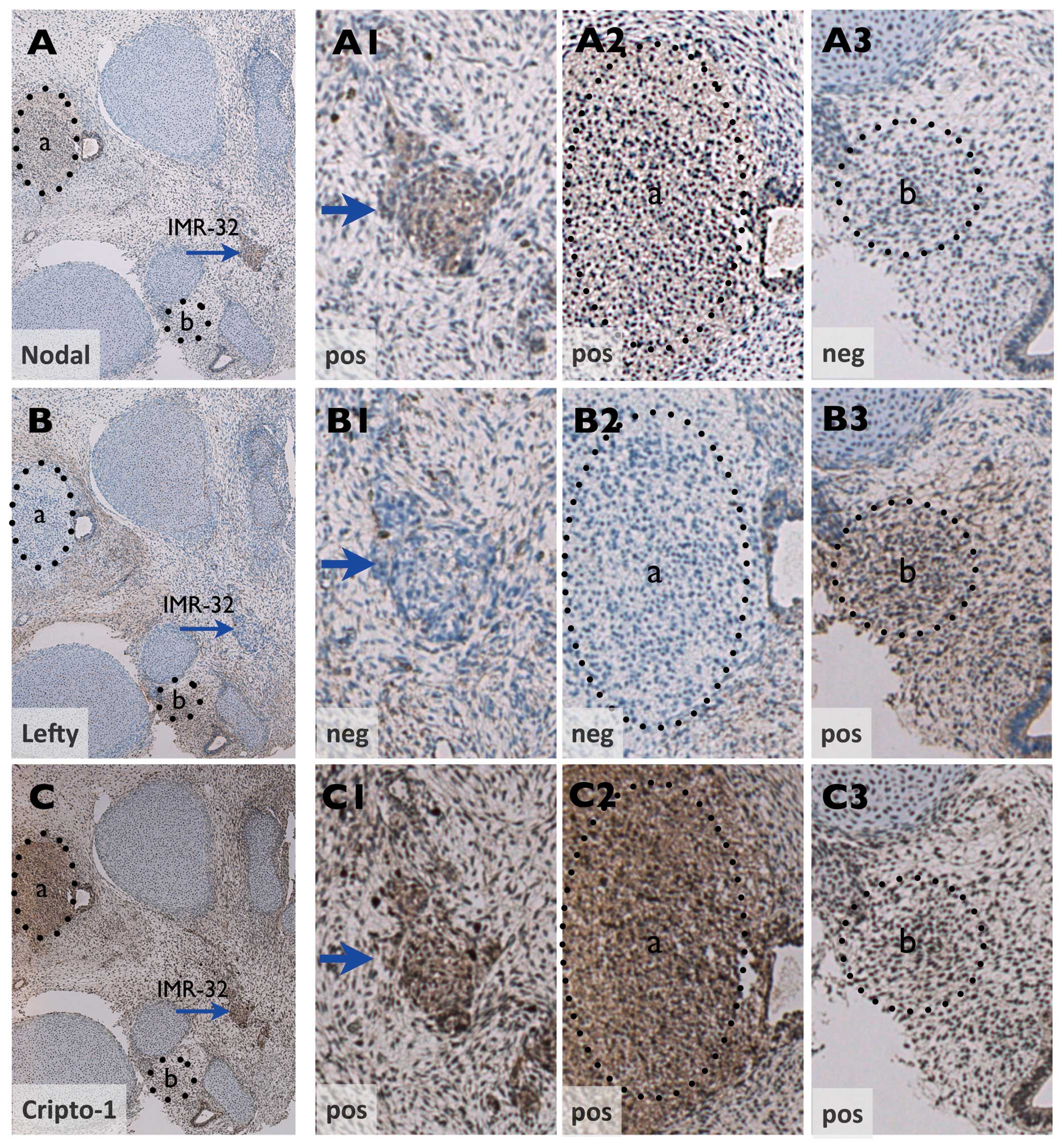
Nodal-signaling pathway
Expression of Nodal and Cripto-1 but not Lefty was
demonstrated for IMR-32 growth in xenografts (Fig. 3A–C) as well as in the PSCT
microenvironment (Figs. 3D–F and
4A–C). Notably, in the PSCT
environment, non-tumor areas of Cripto-1 positive condensing
mesenchyme with similar histology as NB tumor growth could be shown
to exhibit a reciprocal Nodal/Lefty expression pattern (compare
Fig. 4A2 and A3 with B2 and
B3).
Discussion
A comparable histology was observed for the three NB
cell lines in the PSCT microenvironment or when growing as
xenografts. However, the PSCT environment offered additional
information and a conspicuous finding following injections into
PSCT was that the smaller nodules of tumor growth, presumably
representing the initial manifestation of NB, showed for all three
NB cell lines a strict tissue preference between the components of
the embryonic process in the stem cell induced mature teratoma.
Initial growth was observed exclusively in areas compatible with
embryonic loose mesenchymal stroma. There was no evidence for NB
cells occurring in areas compatible with bone, muscle, gut or areas
of other identifiable tissue types.
An upfront explanation regarding the mechanism
behind this tropism could be the ‘open’ nature of the loose
mesenchyme, i.e. more easily giving space to new cells compared to
other more dense tissues. In this context, a recent report that
rigidity of the extracellular matrix may provide a physical cue
that can modulate NB differentiation, reduce NB proliferation and
MYCN expression (21), is here of
particular interest.
The ability of tumors to invade host tissues is
dependent on signals mediated by several routes that normally
enable important physiological functions, such as morphogenesis and
angiogenesis. Notably, the mesenchyme of the early embryo is
composed of a matrix of reticular fibers and myxoid ground
substance and includes undifferentiated spindle or star shaped
mesenchymal cells but also cells from other germ layers, e.g.
ectodermal neural crest cells. The loose mesenchyme in PSCT is thus
best understood as an embryonic tissue in which all connective
tissue cell types may occur. The three main components of loose
myxoid ground; glycosaminoglycans, proteoglycans and glycoproteins,
are known components in cell-cell interactions and are here ideally
situated for trophic factors. Thus, it can also be speculated from
the presented findings that embryonic loose mesenchyme may supply
developmental cues that attracted or promoted the integration of NB
tumor cells in the present study.
Whether the explicit tropism identified for the
three NB lines in the present study can be of a more general
importance for NB, or specific for the tumors used, remains to be
determined using a larger panel of NB tumor lines.
Another typical finding was that the NB cells were
regularly found in the immediate proximity of vessels. A homing to
the perivascular niche is in line with the notion that NB could be
considered a stem cell disease of the sympathetic nervous system
(5) and that the perivascular
niche supports stem cell self-renewal capacity and an
undifferentiated state of neural tumor cells (22).
The cellular origin is not exactly known but NB is
assumed to originate from neural crest precursor cells that
specifically differentiate into the sympathoadrenal lineage. In
this context, it is of interest that the timing of NB tumor cell
injections used in this study, day 45 of the developing embryonic
process in PSCT, overlaps with the presence in PSCT of neural
components compatible with gestation stages immediately preceding
the positioning of adrenal sympatical progenitors in embryonic
mesenchyme (neural crest development; E25–35) (3,19).
Thus, we tentatively hypothesize that day 45 HS181 PSCT may provide
a timely embryonic niche for in vivo studies on NB.
Some further indirect support for this hypothesis
comes from the findings with the embryonic protein Nodal, member of
the TGF-β superfamily. Nodal acts as a morphogen in human early
development, balanced by its antagonist Lefty, but is not expressed
by most normal adult tissues (23–25).
Dysregulated expression has however been detected in several
malignancies and expression of Nodal has been linked with
invasiveness and metastasis; as reported for melanoma, glioma, and
cancers of breast endometrium and prostate (25–28).
In a previous study using a melanoma cell line we detected Nodal
positive tumor cells invading surrounding mesenchymal stroma in the
PSCT model (15). We therefore
wanted to know whether expression of Nodal could be similarly
identified for NB. For this we selected IMR-32 based on its
observed infiltrative growth into the surrounding stroma. Our
results demonstrated that aggressive IMR-32 tumor growth indeed
showed expression of both Nodal and its co-receptor Cripto-1, but
not the antagonist Lefty. Intriguingly, the reciprocal Nodal/Lefty
expression of IMR-32 in PSCT mirrored that of nearby Cripto-1
positive areas of condensing mesenchyme. This indicates that the
expression of Nodal/Lefty in IMR-32 concurred with Nodal regulation
in PSCT early embryonic patterning (24).
In conclusion, aiming for the principle of a closer
developmental match between the tumor and the microenvironment this
study demonstrates the feasibility of using the embryonic PSCT
microenvironment for modeling in vivo growth of childhood
neuroblastoma. The exclusive integration of NB cells into embryonic
loose mesecnhymal stroma added histology with close recapitulation
of NB native presentation in patients. This finding, together with
the advantage of a species identity of surrounding stroma, suggests
clear benefits for the PSCT model compared to xenotransplantation
and the PSCT microenvironment to be an important well-needed
complement for pre-clinical studies of NB, facilitating clinical
translation. Our continued studies will involve expanding the NB
model from cell lines to clinical tumor material from young NB
patients. Considering the extremely poor prognosis of malevolent
NB, development of new clinically relevant models is of utmost
importance.
Acknowledgements
This work was supported by the Swedish Childhood
Cancer Foundation (08/092), the Cancer Research Funds of
Radiumhemmet (09 1551 and 09 4271), Little Heroes Pediatric Cancer
Research Foundation, Petrus and Augusta Hedlund’s stiftelse and
Karolinska Institutet. S. Jamil and R. Ali were supported by
stipends from the Higher Education Commission of Pakistan.
References
|
1
|
Brodeur GM and Maris JM: Neuroblastoma.
Principles and Practice of Pediatric Oncology. Pizzo PA and Poplack
DG: 5th edition. J.B. Lippincott & Co; Philadelphia, PA: pp.
933–970. 2006
|
|
2
|
Maris JM, Hogarty MD, Bagatell R and Cohn
SL: Neuroblastoma. Lancet. 369:2106–2120. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brodeur GM: Neuroblastoma: biological
insights into a clinical enigma. Nat Rev Cancer. 3:203–216. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Johnsen JI, Kogner P, Albihn A and
Henriksson MA: Embryonal neural tumours and cell death. Apoptosis.
14:424–438. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mohlin SA, Wigerup C and Påhlman S:
Neuroblastoma aggressiveness in relation to sympathetic neuronal
differentiation stage. Semin Cancer Biol. 21:276–282. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Blum B and Benvenisty N: The
tumorigenicity of human embryonic stem cells. Adv Cancer Res.
100:133–158. 2008. View Article : Google Scholar
|
|
8
|
Cedervall J, Gertow K, Damjanov I and
Ährlund-Richter L: Characterization of human pluripotent stem
cell-derived teratoma. Human Stem Cell Manual. Loring JF and
Peterson S: 2nd edition. Elsevier Press; Philadelphia, PA: pp.
345–360. 2012, View Article : Google Scholar
|
|
9
|
Gertow K, Wolbank S, Rozell B, Sugars R,
Andäng M, Parish CL, Imreh MP, Wendel M and Ährlund-Richter L:
Organized development from human embryonic stem cells after
injection into immunodeficient mice. Stem Cells Dev. 13:421–435.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gertow K, Cedervall J, Jamil S, Ali R,
Imreh MP, Parish CL, Imreh MP, Wendel M and Ährlund-Richter L:
Early events in xenograft development from the human embryonic stem
cell line HS181 - resemblance with an initial multiple epiblast
formation. PLoS One. 6:e277412011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lensch MW and Ince TA: The terminology of
teratocarcinomas and teratomas. Nat Biotechnol. 25:12112007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tzukerman M, Rosenberg T, Ravel Y, Reiter
I, Coleman R and Skorecki K: An experimental platform for studying
growth and invasiveness of tumor cells within teratomas derived
from human embryonic stem cells. Proc Natl Acad Sci USA.
100:13507–13512. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tzukerman M, Rosenberg T, Reiter I,
Ben-Eliezer S, Denkberg G, Coleman R, Reiter Y and Skorecki K: The
influence of a human embryonic stem cell-derived microenvironment
on targeting of human solid tumor xenografts. Cancer Res.
66:3792–3801. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Katz E, Skorecki K and Tzukerman M:
Niche-dependent tumorigenic capacity of malignant ovarian
ascites-derived cancer cell subpopulations. Clin Cancer Res.
15:70–80. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cedervall J, Jamil S, Prasmickaite L,
Cheng Y, Eskandarpour M, Hansson J, Maelandsmo GM, Ringborg U,
Gulyas M, Suo Z, Kanter L and Ährlund-Richter L: Species-specific
in vivo engraftment of the human BL melanoma cell line results in
an invasive dedifferentiated phenotype not present in xenografts.
Cancer Res. 69:3746–3754. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hovatta O, Mikkola M, Gertow K, Strömberg
AM, Inzunza J, Hreinsson J, Rozell B, Blennow E, Andäng M and
Ährlund-Richter L: A culture system using human foreskin
fibroblasts as feeder cells allows production of human embryonic
stem cells. Hum Reprod. 18:1404–1409. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thomson JA, Itskovitz-Eldor J, Shapiro SS,
Waknitz MA, Swiergiel JJ, Marshall VS and Jones JM: Embryonic stem
cell lines derived from human blastocysts. Science. 282:1145–1147.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Imreh MP, Wolbank S, Unger C, Gertow K,
Aints A, Szeles A, Imreh S, Hovatta O, Fried G, Dilber S and
Ährlund-Richter L: Culture and expansion of the human embryonic
stem cell line HS181, evaluated in a double-color system. Stem
Cells Dev. 13:337–343. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Joshi VV, Cantor AB, Altshuler G, Larkin
EW, Neill JS, Shuster JJ, Holbrook CT, Hayes FA and Castleberry RP:
Recommendations for modification of terminology of neuroblastic
tumor and prognostic significance of Shimada classification. A
clinicopathologic study of 213 cases from the Pediatric Oncology
Group. Cancer. 69:2183–2196. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shimada H, Umehara S, Monobe Y, Hachitanda
Y, Nakagawa A, Goto S, Gerbing RB, Stram DO, Lukens JN and Matthay
KK: International neuroblastoma pathology classification for
prognostic evaluation of patients with peripheral neuroblastic
tumors: a report from the Children’s Cancer Group. Cancer.
92:2451–2461. 2001.
|
|
21
|
Lam WA, Cao L, Umesh V, Keung AJ, Sen S
and Kumar S: Extracellular matrix rigidity modulates neuroblastoma
cell differentiation and N-myc expression. Mol Cancer. 9:1–7.
2010.PubMed/NCBI
|
|
22
|
Calabrese C, Poppleton H, Kocak M, Hogg
TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M,
Frank A, Bayazitov IT, Zakharenko SS, Gajjar A, Davidoff A and
Gilbertson RJ: A perivascular niche for brain tumor stem cells.
Cancer Cell. 11:69–82. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schier AF: Nodal signaling in vertebrate
development. Annu Rev Cell Dev Biol. 19:589–621. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shen MM: Nodal signaling: developmental
roles and regulation. Development. 134:1023–1034. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Postovit LM, Margaryan NV, Seftor EA,
Kirschmann DA, Lipavsky A, Wheaton WW, Abbott DE, Seftor RE and
Hendrix MJ: Human embryonic stem cell microenvironment suppresses
the tumorigenic phenotype of aggressive cancer cells. Proc Natl
Acad Sci USA. 105:4329–4334. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Papageorgiou I, Nicholls PK, Wang F,
Lackmann M, Makanji Y, Salamonsen LA, Robertson DM and Harrison CA:
Expression of nodal signalling components in cycling human
endometrium and in endometrial cancer. Reprod Biol Endocrinol.
7:1222009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee CC, Jan HJ, Lai JH, Ma HI, Hueng DY,
Lee YC, Cheng YY, Liu LW, Wei HW and Lee HM: Nodal promotes growth
and invasion in human gliomas. Oncogene. 29:3110–3123. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lawrence MG, Margaryan NV, Loessner D,
Collins A, Kerr KM, Turner M, Seftor EA, Stephens CR and Lai J; APC
BioResource. Postovit LM, Clements JA and Hendrix MJ: Reactivation
of embryonic nodal signaling is associated with tumor progression
and promotes the growth of prostate cancer cells. Prostate.
71:1198–1209. 2011.PubMed/NCBI
|