Introduction
Hepatocellular carcinoma (HCC) is the most common
primary liver cancer in adults, and is the third leading cause of
cancer mortality worldwide (1).
Although the highest incidence rates of HCC occur in Asia and
Africa, HCC incidence frequency is increasing in developed
countries, including the USA and those of Western Europe (2,3).
Although there are several treatment options such as surgery,
radiation and chemotherapy, most patients suffering from advanced
HCC are candidates for palliative treatments only (4). Therefore, novel therapeutic
approaches for HCC are required for more effective treatment of
this malignancy.
Tumor necrosis factor-related apoptosis-inducing
ligand (TRAIL/Apo2L), a member of the tumor necrosis factor (TNF)
superfamily, is known to induce apoptosis in various cancer cells,
with minimal toxicity to normal cells (5). Due to this selective
apoptosis-inducing activity, TRAIL has received great attention as
a promising candidate for cancer therapeutics. However, recent
studies have demonstrated that many types of malignant cells,
including hepatocellular carcinoma cells, are resistant to the
apoptotic effect mediated by TRAIL (6). Because TRAIL alone is not sufficient
to induce cancer cell death in these resistant cells, recent
studies have aimed at overcoming the resistance of cancer cells to
TRAIL by combination of sensitizing agents with TRAIL. The
combination of chemotherapeutic agents, such as genotoxic drugs,
small molecule-inhibitors and natural products, with TRAIL has been
shown to be successful in the enhancement of susceptibility to
TRAIL-induced cell death in various TRAIL-resistant tumor cells
(7,8).
Polyphenolic compounds, a large group of
phytochemicals, have been reported to possess anticancer and
chemopreventive properties (9,10).
Numerous studies have demonstrated that TRAIL, in combination with
polyphenols, effectively synergizes TRAIL-induced apoptotic cell
death in various malignant cells (11,12).
Here, we examined the potential role of caffeic acid
phenethyl ester (CAPE), a polyphenolic compound in honeybee
propolis, as a sensitizing agent for restoring the susceptibility
of SK-Hep1 cells to TRAIL, and demonstrated the underlying
mechanisms involved in this enhancement of susceptibility.
Materials and methods
Materials
Caffeic acid phenethyl ester (CAPE), MTT (3-(4,5-
dimethylthiazolyl-2-yl)-2,5-diphenyl-tetrazolium bromide), and
3,3′-dihexyloxacarbocyanine iodide (DiOC6) were
purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Soluble
recombinant human TRAIL/Apo2 ligand was purchased from PeproTech
(Rocky Hill, NJ, USA). Caspase inhibitors were obtained from Santa
Cruz Biotechnology (Santa Cruz, CA, USA). Human recombinant DR4/Fc
and DR5/Fc chimera protein were purchased from R&D Systems
(Minneapolis, MN, USA). All the antibodies for western blot
analysis and MAPK inhibitors were purchased from Cell Signaling
(Beverly, MA, USA). Dulbecco’s modified Eagle’s medium (DMEM),
fetal bovine serum (FBS), Dulbecco’s phosphate-buffered saline
(DPBS), trypsin-EDTA and penicillin/streptomycin were purchased
from WelGENE (Daegu, Korea).
Cell culture
Human hepatocellular carcinoma SK-Hep1 cells were
obtained from the Korean Cell Line Bank (Seoul, Korea). Cells were
cultured at 37°C in a humidified condition of 5% CO2 and
maintained in DMEM supplemented with 10% FBS and
penicillin/streptomycin.
Cell viability assay
MTT assay was used to determine cell viability.
Cells were seeded in 96-well plate, incubated for 24 h and treated
as described in individual experiments. After incubation for 24 h,
MTT solution was added to each well for 4 h and the resulting
formazan product was dissolved in dimethyl sulfoxide (DMSO). The
absorbance was measured at 570 nm with a microplate reader (EL800,
Bio-Tek Instrument Inc., Winooski, VT, USA) and the cell viability
(%) was calculated.
Flow cytometric analysis for
mitochondrial membrane potential (MMP)
To analyze loss of MMP, treated cells were harvested
and incubated with DiOC6 (40 nM) at 37°C for 30 min in
the dark. Fluorescence intensity was measured by flow cytometry
(FACSCanto II Flow Cytometer, BD Biosciences, USA).
Detection of apoptosis by flow
cytometry
Apoptotic cells were quantified by Annexin
V/propidium iodide (PI) staining assay. Cells were seeded in 6-well
plates for 24 h and treated as described in individual experiments.
After 24 h, cells were harvested and resuspended in binding buffer.
Then, cells were incubated with Annexin V-FITC and PI for 15 min at
room temperature in the dark. Apoptotic cell death was evaluated by
flow cytometry and the population of Annexin
V+/PI− was considered as apoptotic cells.
Analysis of death receptors on the cell
surface
Surface expression of DR4 and DR5 was analyzed by
indirect staining with primary mouse anti-DR4 and DR5 followed by
phycoerythrin (PE)-conjugated goat anti-mouse IgG1 (Santa Cruz
Biotechnology). As a negative control, cells were incubated with a
normal mouse IgG1 antibody in the same conditions (Santa Cruz
Biotechnology). Death receptor expression was analyzed by flow
cytometry.
Western blot analysis
Cells treated as described in individual experiments
were lysed with RIPA buffer (50 mM Tris-HCl, pH 8.0, with 150 mM
NaCl, 0.1% NP-40, 0.5% sodium deoxycholate and 0.1% SDS). Equal
amount of protein was resolved by 10% SDS-PAGE and then transferred
to polyvinylidene difluoride (PVDF) membranes. The blots were
blocked with 5% non-fat dry milk for 2 h at room temperature and
incubated overnight at 4°C with appropriate primary antibodies.
Horseradish peroxidase-conjugated anti-rabbit or anti-mouse
antibodies were used as secondary antibodies. Protein signals were
visualized with enhanced chemiluminescence (ECL) solution and
quantified by Multi Gauge software (Fuji Photo Film, Japan).
Statistical analysis
Values are presented as the mean ± SD. Statistical
significance was evaluated by using one-way analysis of variance
(ANOVA) followed by Tukey’s test. P-value of <0.05 was regarded
statistically significant.
Results
Combined CAPE and TRAIL treatments
promote TRAIL- induced apoptosis in SK-Hep1 cells
In order to investigate whether CAPE sensitizes
SK-Hep1 cells to TRAIL-induced cell death, we treated cells with
TRAIL, in the presence or absence of CAPE for 24 h at various
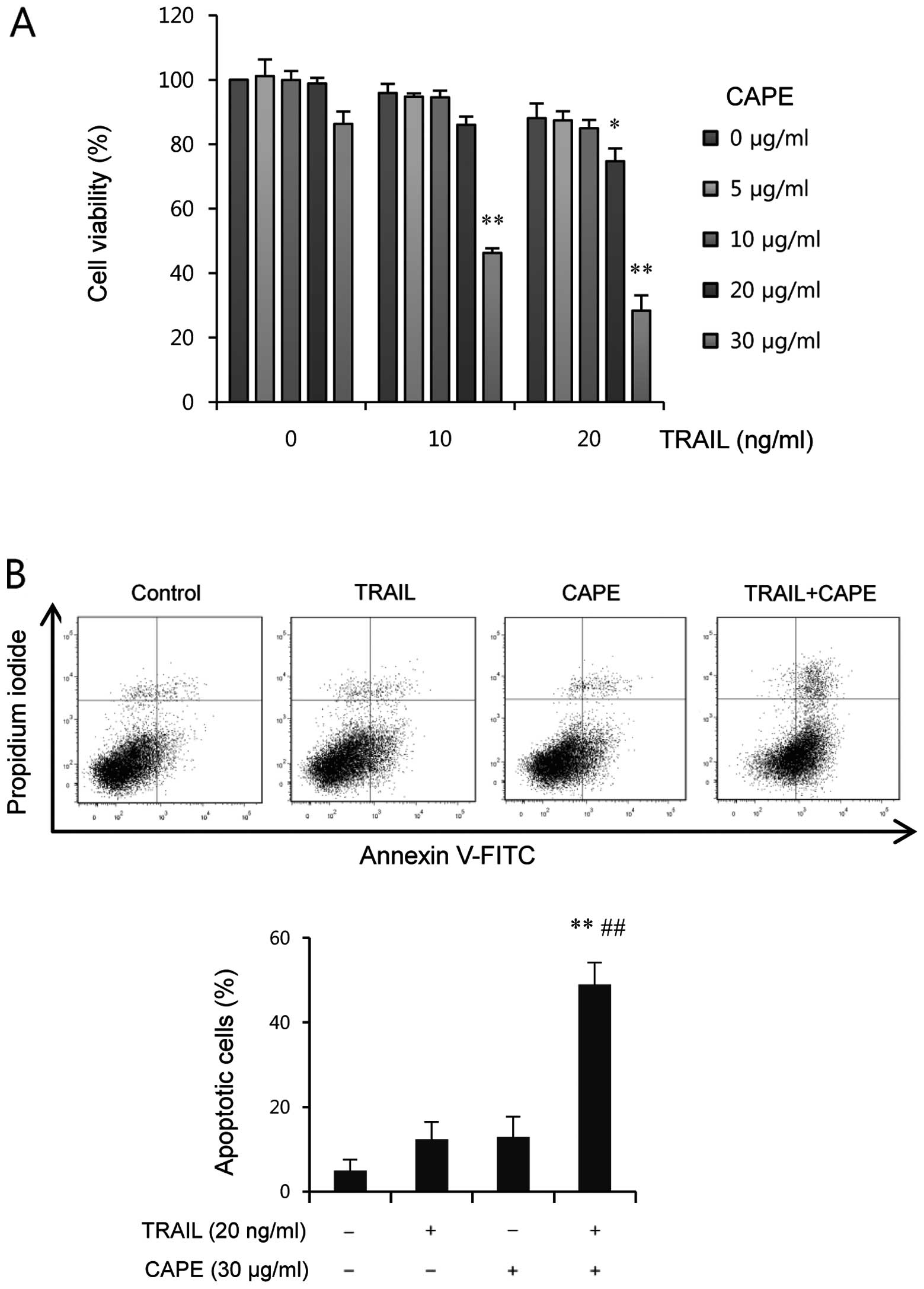
concentrations. As shown in Fig.
1A, treatment with CAPE or TRAIL alone resulted in a limited
inhibition of cell viability (<20%) at 24 h. In contrast, cell
viability was markedly reduced by combined treatment when cells
were treated with a fixed concentration of TRAIL and varying
concentrations of CAPE, and vice versa. The CAPE concentration at
30 μg/ml was selected for further study because it
effectively induced a significant reduction of cell viability in
the combined treatment with TRAIL, with only a slight decrease in
the percentage of viable cells. Next, we examined whether the
combined effect of CAPE and TRAIL on cell viability is due to
increased apoptosis. As shown in Fig.
1B, co-treatment of CAPE and TRAIL significantly enhanced the
amount of apoptotic cells (%), whereas treatment with CAPE or TRAIL
alone induced only a slight increase of apoptosis, compared to
untreated group. These findings suggest that the combined treatment
of CAPE and TRAIL effectively induces apoptotic cell death in
SK-Hep1 cells.
Combined CAPE and TRAIL treatment
potentiates the activation of caspase-mediated death signal
Next, we examined whether caspases are activated by
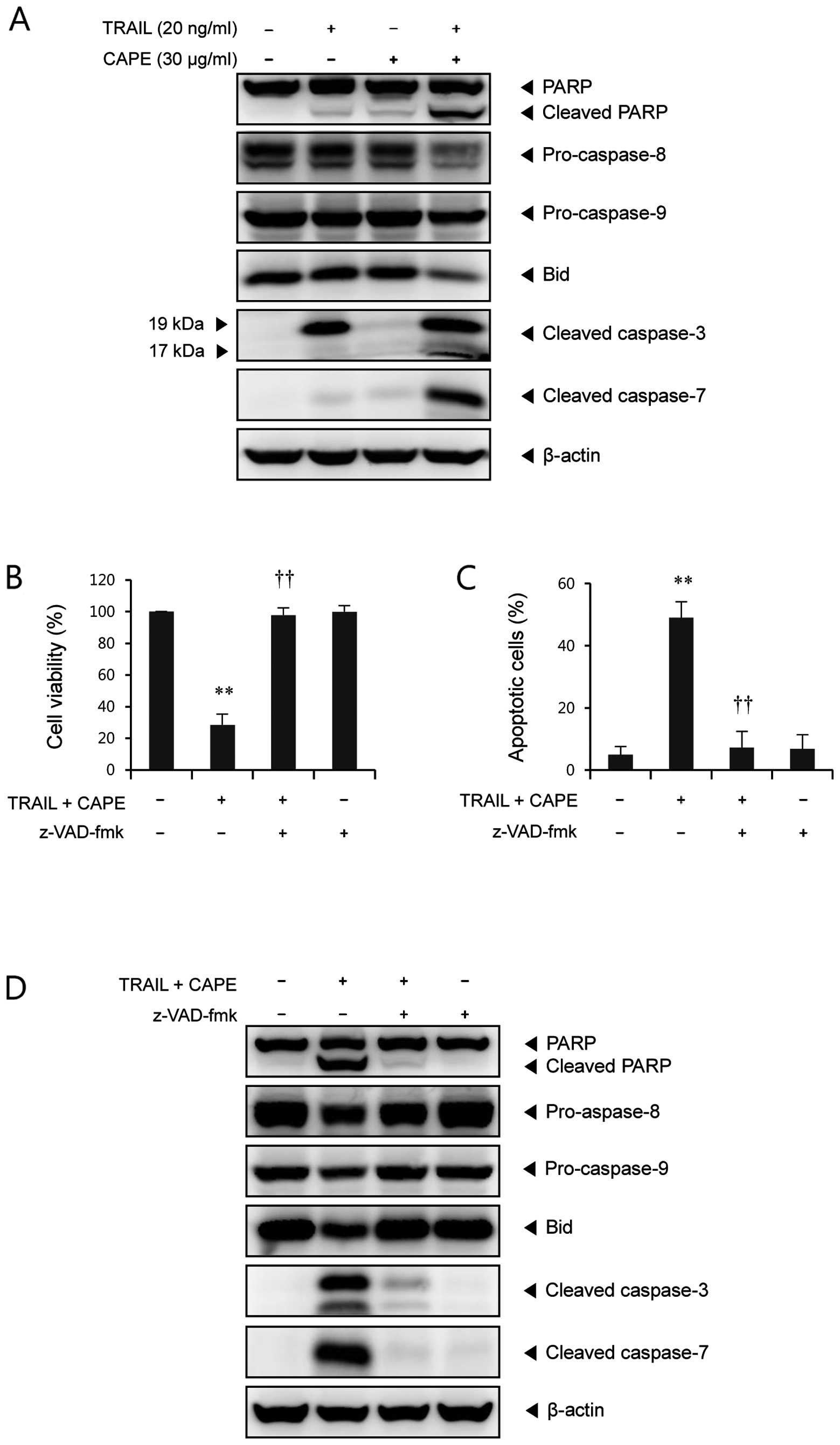
combination of CAPE and TRAIL. As shown in Fig. 2A, CAPE alone did not induce any
significant changes of caspases. In cells treated with TRAIL alone,
caspase-3 was partially cleaved to the intermediate inactive
fragment p19, whereas combined treatment with CAPE and TRAIL
induced further cleavage into p17, the fully active subunit of
caspase-3. Also, cleaved caspase-7 (20 kDa), as the active form of
caspase-7, was significantly increased through the combination of
CAPE and TRAIL, but not through the administration of either agent
alone. We found that PARP, a well known substrate for caspase-3 and
caspase-7, was remarkably cleaved by the combined treatment. It is
well known that the activation of effector caspases, like caspase-3
and -7 is regulated by initiator caspases, such as caspase-8 and
caspase-9. As shown in Fig. 2A,
treatment with a combination of CAPE and TRAIL markedly decreased
the levels of precursor protein of caspase-8 and caspase-9 compared
to either agent alone, indicating the significant activation of
these caspases. In certain cells, BH3-interacting domain death
agonist (Bid) cleaved into tBid (truncated Bid) by caspase-8,
which, in turn, induces mitochondrial damage (13). We found that full-length Bid was
significantly decreased by combined treatment with CAPE and TRAIL,
but not by either agent alone (Fig.
2A).
Next, to investigate the role of caspases in
combination-induced apoptosis of SK-Hep1 cells, a broad caspase
inhibitor, z-VAD-fmk, was used. As shown in Fig. 2B, pretreatment with z-VAD-fmk
completely blocked the enhanced cytotoxicity induced by the
combination of CAPE and TRAIL. Annexin V/PI staining assay also
showed that z-VAD-fmk rescued the increase of apoptotic cell death
by the combination of CAPE and TRAIL (Fig. 2C). We found that z-VAD-fmk almost
completely abolished the combination treatment-induced PARP
cleavage, caspase activation, and Bid cleavage (Fig. 2D). Taken together, these results
indicate that combination treatment-induced apoptosis is mediated
through a caspase-dependent pathway.
Both extrinsic and intrinsic apoptotic
pathways are important for apoptosis in response to combined
treatment with CAPE and TRAIL
We next used various caspase inhibitors to examine
the respective roles of caspases in CAPE and TRAIL
combination-induced apoptosis. As shown in Fig. 2E, the CAPE-triggered enhancement of
TRAIL-induced apoptosis was significantly blocked by z-DEVD-fmk
(caspase-3/-7 inhibitor), z-IETD-fmk (a caspase-8 inhibitor), and
z-LEHD-fmk (a caspase-9 inhibitor), indicating that CAPE enhances
TRAIL-induced apoptosis through activation of caspase-3, -7, -8 and
-9.
Disruption of mitochondrial membrane potential
(ΔΨm) is important for the release of apoptotic factors
such as cytochrome c and apoptosis-inducing factor (AIF),
leading to the induction of the caspase cascade. To further examine
the role of the intrinsic death pathway, we measured ΔΨm
by staining cells with DiOC6 fluorescence, which labels
mitochondria. As shown in Fig. 2F,
CAPE or TRAIL alone slightly induced loss of ΔΨm. In
contrast, combination of CAPE with TRAIL led to the significant
depolarization of the mitochondrial membrane, confirming the
involvement of the mitochondria-dependent intrinsic apoptosis
pathway. Moreover, z-IETD-fmk, a caspase-8 specific inhibitor,
significantly rescued the alteration of ΔΨm by combined
treatment, suggesting the important role of caspase-8 as an
upstream regulator of mitochondria-mediated apoptosis. These
results indicate that both intrinsic and extrinsic apoptotic
pathways are involved in combined treatment-induced apoptosis.
CAPE potentiates TRAIL-induced apoptosis
through DR4 and DR5 upregulation
It is well known that TRAIL interacts with its
receptors, DR4 and DR5, to trigger apoptotic cell death (14). To investigate whether enhanced
apoptosis by combination treatment is associated with this
signaling pathway, we examined the effect of CAPE on the surface
expression of DR4 and DR5. We found that CAPE treatment upregulated
both DR4 and DR5 expression (Fig.
3A). Next, to determine the role of these death receptors in
the enhancement of TRAIL-induced apoptosis by CAPE, we used DR4/Fc
and DR5/Fc chimera antibodies. As shown in Fig. 3B, both DR4 and DR5 blocking by
specific chimera protein significantly suppressed the combination
treatment-induced apoptosis. Furthermore, combination
treatment-induced PARP cleavage, activation of caspases, and Bid
cleavage were markedly attenuated by DR4/Fc and DR5/Fc chimera
proteins (Fig. 3C). These results
indicate that the upregulation of DR4 and DR5 is essential for the
sensitizing effect of CAPE on TRAIL-mediated apoptosis.
Treatment with CAPE alone or together
with TRAIL effectively activates p38 MAPK but suppresses JNK
phosphorylation
We investigated whether the mitogen-activated
protein kinase (MAPK) signaling pathway is affected by the
combination effect of CAPE and TRAIL on SK-Hep1 cells. As shown in
Fig. 4A, p38 was significantly
activated after 16 h of CAPE treatment, whereas JNK activation was
strongly suppressed after 16 h of treatment. However, CAPE had no
significant effect on ERK activation. Combination of CAPE and TRAIL
showed similar trends in relation to their effect on MAPK
phosphorylation (Fig. 4B).
Interestingly, the activation of p38 and the inhibition of JNK were
further enhanced by combination treatment, as compared to treatment
with CAPE alone. The level of ERK was not changed by either single
agents or by combined treatment of CAPE and TRAIL.
p38 MAPK pathway is involved in the
upregulation of DR4 and DR5 and subsequent apoptosis
To investigate the possible role of p38 on
combination treatment-induced apoptosis, cells were pretreated with
SB203580, a specific inhibitor of p38, and apoptosis was evaluated
by Annexin V/PI double staining assay. As shown in Fig. 5A, pretreatment with SB203580
significantly blocked the combination-induced apoptosis, suggesting
the involvement of p38 in combination-mediated apoptosis.
Furthermore, we also examined whether inhibition of p38 attenuates
the upregulation of DR4 and DR5 by CAPE treatment. As shown in
Fig. 5B, CAPE resulted in a
significant upregulation of DR4 and DR5, and pretreatment of
SB203580 suppressed both DR4 and DR5 expression. These results
indicate the important role of the p38 MAPK pathway on DR4 and DR5
upregulation, and on the subsequent induction of apoptosis.
JNK inhibition upregulates DR4 and DR5
and enhances TRAIL-induced apoptosis
JNK activation has been previously reported to
contribute to TRAIL resistance in HCC cells (6). To assess the effect of JNK inhibition
on TRAIL-induced apoptosis, a JNK specific inhibitor, SP600125, was
used. As shown in Fig. 5C,
combination of TRAIL with SP600125, as in the case of the
combination of TRAIL with CAPE, significantly enhanced
TRAIL-induced apoptosis. We also evaluated the effect of JNK
inhibition on DR4 and DR5 surface expression. As shown in Fig. 5D, SP600125 effectively upregulated
DR4 and DR5 surface expression. Taken together, these results
suggest that the CAPE-induced inhibition of JNK may contribute to
the sensitizing effect of CAPE on TRAIL-resistant SK-Hep1
cells.
Discussion
CAPE is an active phenolic component of honeybee
propolis, and has been reported to possess a broad spectrum of
biological activities, including anticancer and apoptosis inducing
properties (15,16). Propolis, a resin-like mixture
produced by honeybees, is a concentrated source of polyphenolic
compounds (17). Previous reports
have shown the synergistic induction of TRAIL-induced cancer cell
death by combination of TRAIL with propolis extracts or
polyphenolic compounds isolated from propolis (18,19).
The molecular mechanisms to overcome TRAIL-resistance by
combination with these polyphenols in propolis have reported for
artepillin C, chrysin, apigenin, and naringenin (20–23).
However, the underlying mechanisms by which CAPE sensitizes
TRAIL-resistant malignant cells have not yet been fully elucidated.
In the present study, we demonstrated that treatment with CAPE
effectively sensitized SK-Hep1 cells to TRAIL-mediated
apoptosis.
Caspases belong to a family of cysteine proteases
which play a pivotal role in apoptotic cell death. Activation of
the caspase cascade is mediated by two major pathways: one is the
death receptor-mediated (extrinsic) pathway, and the other is the
mitochondria-mediated (intrinsic) pathway (24). Apoptosis by death receptors is
initiated by binding of the death-inducing ligands to its
receptors, leading to the formation of death-inducing signaling
complexes (DISC) and the subsequent activation of caspase-8. Active
caspase-8 initiates the caspase cascade by direct activation of
effector caspases, such as caspase-3 and -7 (25). In the mitochondria-dependent
pathway, intrinsic apoptotic stimuli trigger mitochondria membrane
permeabilization and the subsequent release of cytochrome c,
which activates caspase-9 by forming the apoptosome (26). In some cells, Bid is cleaved to
tBid by caspase-8 and serves as a molecular link between the
extrinsic and intrinsic pathways by inducing release of
mitochondrial proteins (13). In
our results, the combination of CAPE and TRAIL enhanced the
activation of effector caspases (caspase-3, -7) and their substrate
PARP cleavage. We also observed the activation of caspase-9 as well
as the activation of capsase-8 and Bid cleavage in
combination-treated cells. Furthermore, such activation of
apoptosis-related proteins by combination treatment was strongly
inhibited by the pan-caspase inhibitor, z-VAD-fmk, suggesting the
critical role of caspases in combination-treated cells. Moreover,
pretreatment with z-IETD-fmk (caspase-8 inhibitor) and z-LEHD-fmk
(caspase-9 inhibitor) resulted in a significant inhibition of
combination-induced apoptosis, indicating the involvement of
extrinsic and intrinsic apoptotic pathways. Involvement of
mitochondria-mediated apoptosis was also confirmed by the marked
increase of mitochondrial membrane depolarization in response to
combination treatment with CAPE and TRAIL.
TRAIL-induced death signal is initiated by the
binding of TRAIL with its two death receptors DR4 (TRAIL-R1) and
DR5 (TRAIL-R2), leading to induction of the extrinsic apoptosis
pathway (27). Therefore,
targeting of DR4 and DR5 in TRAIL-resistant cells seems to be a
useful strategy to sensitize cancer cells to TRAIL-induced
apoptosis. Previous reports have shown that a combination of agents
which upregulate DR4 and/or DR5 TRAIL receptors induced a
synergistic apoptotic activation in TRAIL-resistant tumor cells
(28,29). In this study, we found that
upregulation of DR4 and DR5 by CAPE was critical for the
CAPE-mediated enhancement of TRAIL-induced apoptosis. Blocking of
TRAIL binding with DR4 and DR5 death receptors by their respective
dominant-negative chimeric proteins markedly suppressed the effect
of CAPE on TRAIL-induced apoptosis. However, DR5/Fc chimera was
more effective in reducing apoptosis than DR4/Fc blocking protein.
In agreement with our results, several reports have shown the
prominent role of DR5 over that of DR4 in TRAIL-mediated apoptosis
(30,31).
We also investigated whether MAPKs are involved in
the CAPE-induced sensitization of SK-Hep1 cells to TRAIL-induced
apoptosis. The MAPK pathways participate in the regulation of a
wide variety of cellular processes, such as growth, proliferation,
differentiation, stress response, survival, and apoptosis (32,33).
In this study, we investigated the roles of p38 and JNK in the
sensitizing effect of CAPE on TRAIL-induced apoptosis in SK-Hep1
cells, and found that p38 and JNK played opposing roles. Our
results showed that the p38 inhibitor, SB203580, significantly
attenuated CAPE-induced DR4 and DR5 expression, and abolished the
combination treatment-induced apoptosis. These results regarding
the upregulation of TRAIL receptors through p38 activation are
consistent with those of previous studies regarding diosgenin
(34), damnacanthal (35) and lupulone (36).
Mucha et al have reported that the frequent
overexpression of JNK in HCC cells contributes to TRAIL-resistance
in these cells (6). They showed
that JNK inhibition by SP600125 greatly sensitized hepatoma cells
to TRAIL-induced apoptosis, without affecting normal cells,
suggesting the novel strategy of targeting JNK for effective
TRAIL-based cancer therapy. In our results, we found that JNK
phosphorylation was significantly reduced by CAPE alone, or in
combination with TRAIL. Consistent with the results of Mucha et
al, combination of SP600125 and TRAIL enhanced TRAIL-mediated
apoptosis in SK-Hep1 cells. Moreover, SP600125 significantly
upregulated the expression of DR4 and DR5, just like CAPE,
indicating that inhibition of JNK by CAPE resulted in the
upregulation of DRs, and the subsequent enhancement of
TRAIL-induced apoptosis in SK-Hep1 cells.
In summary, our study showed that CAPE effectively
augmented TRAIL-induced apoptosis in SK-Hep1 cells through
caspase-dependent extrinsic and intrinsic apoptotic pathways. The
upregulation of death receptors by CAPE treatment was involved in
p38 activation and JNK inhibition. Collectively, our results
demonstrated a novel function of CAPE in TRAIL-induced apoptosis in
HCC cells.
Acknowledgements
This work was supported by Basic
Science Research Program through the National Research Foundation
(NRF) funded by the Korea government (MEST) (2010-0010538) and the
NRF grant funded by the Korea government (2011-0030699).
References
|
1.
|
Schutte K, Bornschein J and Malfertheiner
P: Hepatocellular carcinoma-epidemiological trends and risk
factors. Dig Dis. 27:80–92. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
McGlynn KA, Tsao L, Hsing AW, Devesa SS
and Fraumeni JF Jr: International trends and patterns of primary
liver cancer. Int J Cancer. 94:290–296. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Harnois DM: Hepatitis C virus infection
and the rising incidence of hepatocellular carcinoma. Mayo Clin
Proc. 87:7–8. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Paul SB, Manjunatha YC and Acharya SK:
Palliative treatment in advanced hepatocellular carcinoma: has it
made any difference? Trop Gastroenterol. 30:125–134.
2009.PubMed/NCBI
|
|
5.
|
Ashkenazi A, Pai RC, Fong S, et al: Safety
and antitumor activity of recombinant soluble Apo2 ligand. J Clin
Invest. 104:155–162. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Mucha SR, Rizzani A, Gerbes AL, et al: JNK
inhibition sensitises hepatocellular carcinoma cells but not normal
hepatocytes to the TNF-related apoptosis-inducing ligand. Gut.
58:688–698. 2009. View Article : Google Scholar
|
|
7.
|
Voelkel-Johnson C: TRAIL-mediated
signaling in prostate, bladder and renal cancer. Nat Rev Urol.
8:417–427. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Hellwig CT and Rehm M: TRAIL signaling and
synergy mechanisms used in TRAIL-based combination therapies. Mol
Cancer Ther. 11:3–13. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Spagnuolo C, Russo M, Bilotto S, Tedesco
I, Laratta B and Russo GL: Dietary polyphenols in cancer
prevention: the example of the flavonoid quercetin in leukemia. Ann
NY Acad Sci. 1259:95–103. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Stagos D, Amoutzias GD, Matakos A, Spyrou
A, Tsatsakis AM and Kouretas D: Chemoprevention of liver cancer by
plant polyphenols. Food Chem Toxicol. 50:2155–2170. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Siddiqui IA, Malik A, Adhami VM, et al:
Green tea polyphenol EGCG sensitizes human prostate carcinoma LNCaP
cells to TRAIL-mediated apoptosis and synergistically inhibits
biomarkers associated with angiogenesis and metastasis. Oncogene.
27:2055–2063. 2008. View Article : Google Scholar
|
|
12.
|
Jacquemin G, Shirley S and Micheau O:
Combining naturally occurring polyphenols with TNF-related
apoptosis-inducing ligand: a promising approach to kill resistant
cancer cells? Cell Mol Life Sci. 67:3115–3130. 2010. View Article : Google Scholar
|
|
13.
|
Luo X, Budihardjo I, Zou H, Slaughter C
and Wang X: Bid, a Bcl2 interacting protein, mediates cytochrome c
release from mitochondria in response to activation of cell surface
death receptors. Cell. 94:481–490. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Wang S and El-Deiry WS: TRAIL and
apoptosis induction by TNF-family death receptors. Oncogene.
22:8628–8633. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Grunberger D, Banerjee R, Eisinger K, et
al: Preferential cytotoxicity on tumor cells by caffeic acid
phenethyl ester isolated from propolis. Cell Mol Life Sci.
44:230–232. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Ozturk G, Ginis Z, Akyol S, Erden G, Gurel
A and Akyol O: The anticancer mechanism of caffeic acid phenethyl
ester (CAPE): review of melanomas, lung and prostate cancers. Eur
Rev Med Pharmacol Sci. 16:2064–2068. 2012.PubMed/NCBI
|
|
17.
|
Khalil ML: Biological activity of bee
propolis in health and disease. Asian Pac J Cancer Prev. 7:22–31.
2006.PubMed/NCBI
|
|
18.
|
Szliszka E, Czuba ZP, Domino M, Mazur B,
Zydowicz G and Krol W: Ethanolic extract of propolis (EEP) enhances
the apoptosis-inducing potential of TRAIL in cancer cells.
Molecules. 14:738–754. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Szliszka E, Czuba ZP, Bronikowska J,
Mertas A, Paradysz A and Krol W: Ethanolic extract of propolis
augments TRAIL-induced apoptotic death in prostate cancer cells.
Evid Based Complement Alternat Med. 2011:5351722011. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Szliszka E, Zydowicz G, Mizgala E and Krol
W: Artepillin C (3,5-diprenyl-4-hydroxycinnamic acid) sensitizes
LNCaP prostate cancer cells to TRAIL-induced apoptosis. Int J
Oncol. 41:818–828. 2012.PubMed/NCBI
|
|
21.
|
Li X, Wang JN, Huang JM, et al: Chrysin
promotes tumor necrosis factor (TNF)-related apoptosis-inducing
ligand (TRAIL) induced apoptosis in human cancer cell lines.
Toxicol In Vitro. 25:630–635. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Kim EY, Yu JS, Yang M and Kim AK:
Sub-toxic dose of apigenin sensitizes HepG2 cells to TRAIL through
ERK-dependent up-regulation of TRAIL receptor DR5. Mol Cells.
35:32–40. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Jin CY, Park C, Hwang HJ, et al:
Naringenin up-regulates the expression of death receptor 5 and
enhances TRAIL-induced apoptosis in human lung cancer A549 cells.
Mol Nutr Food Res. 55:300–309. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Nicholson DW: Caspase structure,
proteolytic substrates, and function during apoptotic cell death.
Cell Death Differ. 6:1028–1042. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Thorburn A: Death receptor-induced cell
killing. Cell Signal. 16:139–144. 2004. View Article : Google Scholar
|
|
26.
|
Kim HE, Du F, Fang M and Wang X: Formation
of apoptosome is initiated by cytochrome c-induced dATP hydrolysis
and subsequent nucleotide exchange on Apaf-1. Proc Natl Acad Sci
USA. 102:17545–17550. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Zhang L and Fang B: Mechanisms of
resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther.
12:228–237. 2004. View Article : Google Scholar
|
|
28.
|
Kauntz H, Bousserouel S, Gosse F and Raul
F: The flavonolignan silibinin potentiates TRAIL-induced apoptosis
in human colon adenocarcinoma and in derived TRAIL-resistant
metastatic cells. Apoptosis. 17:797–809. 2012. View Article : Google Scholar
|
|
29.
|
Prasad S, Yadav VR, Kannappan R and
Aggarwal BB: Ursolic acid, a pentacyclin triterpene, potentiates
TRAIL-induced apoptosis through p53-independent up-regulation of
death receptors: evidence for the role of reactive oxygen species
and JNK. J Biol Chem. 286:5546–5557. 2011. View Article : Google Scholar
|
|
30.
|
Kelley RF, Totpal K, Lindstrom SH, et al:
Receptor-selective mutants of apoptosis-inducing ligand 2/tumor
necrosis factor-related apoptosis-inducing ligand reveal a greater
contribution of death receptor (DR) 5 than DR4 to apoptosis
signaling. J Biol Chem. 280:2205–2212. 2005. View Article : Google Scholar
|
|
31.
|
Gupta SC, Reuter S, Phromnoi K, et al:
Nimbolide sensitizes human colon cancer cells to TRAIL through
reactive oxygen species- and ERK-dependent up-regulation of death
receptors, p53, and Bax. J Biol Chem. 286:1134–1146. 2011.
View Article : Google Scholar
|
|
32.
|
Raman M, Chen W and Cobb MH: Differential
regulation and properties of MAPKs. Oncogene. 26:3100–3112. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Pimienta G and Pascual J: Canonical and
alternative MAPK signaling. Cell Cycle. 6:2628–2632. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Lepage C, Leger DY, Bertrand J, Martin F,
Beneytout JL and Liagre B: Diosgenin induces death receptor-5
through activation of p38 pathway and promotes TRAIL-induced
apoptosis in colon cancer cells. Cancer Lett. 301:193–202. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Lin FL, Hsu JL, Chou CH, Wu WJ, Chang CI
and Liu HJ: Activation of p38 MAPK by damnacanthal mediates
apoptosis in SKHep 1 cells through the DR5/TRAIL and
TNFR1/TNF-alpha and p53 pathways. Eur J Pharmacol. 650:120–129.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Lamy V, Bousserouel S, Gosse F, Minker C,
Lobstein A and Raul F: Lupulone triggers p38 MAPK-controlled
activation of p53 and of the TRAIL receptor apoptotic pathway in
human colon cancer-derived metastatic cells. Oncol Rep. 26:109–114.
2011.PubMed/NCBI
|