Introduction
Prostate cancer (PCa) is the most common malignant
cancer in men and the second leading cause of cancer deaths
(1). As in other tumor entities,
curative therapy such as prostatectomy is limited to the
organ-localized stage of PCa. PCa is initially responsive to
hormonal therapy; however, in most cases, it becomes
androgen-independent, evolving into more aggressive androgen
refractory disease. At present, the treatment modality for patients
with hormonal refractory PCa is chemotherapy (2), and this treatment is currently
associated with significant side-effects and a reduced quality of
life. Therefore, there is a need to develop novel approaches for
this malignancy.
Chalcones are organic compounds that have an enone
moiety between two aromatic rings. The development of these
structures as potential drugs with biological properties has led to
the discovery that these substances have antimalarial,
anti-inflammatory and antitumor activities (3–5).
Targeting apoptosis is a good strategy for cancer prevention and
treatment, since many anticancer drugs induce apoptosis in cancer
cells (6). Apoptosis occurs after
a cascade of cell signalling that regulates proapoptotic and
anti-apoptotic proteins by two major pathways: the mitochondrial
and the death receptor pathways (7). The antitumor activity of chalcones is
mostly related to modulation of the expression of proapoptotic and
anti-apoptotic Bcl-2 family members and subsequent activation of
the mitochondrial apoptotic pathway (8).
Several studies reported a role for arachidonic acid
(AA) metabolism in many biological processes including cell
proliferation, apoptosis and differentiation in many cancer types.
Once released from phospholipid membranes, AA is converted into
various prostanoids by cyclooxygenases (COX) (9). Two isoforms of COX, COX-1 and COX-2,
have been identified: COX-1 has been purported to be a constitutive
enzyme expressed in different tissues and maintains the
physiological level of prostaglandins while modulating diverse cell
processes such as cell proliferation, angiogenesis and platelet
aggregation whereas COX-2 has been considered as inducible by
different agents like proinflammatory cytokines, hormones and tumor
promoters (10). COX-2 is
overexpressed in some cancers, including PCa (11). Currently many compounds identified
from plants were reported to inhibit cancer cell proliferation and
COX-2 expression (12).
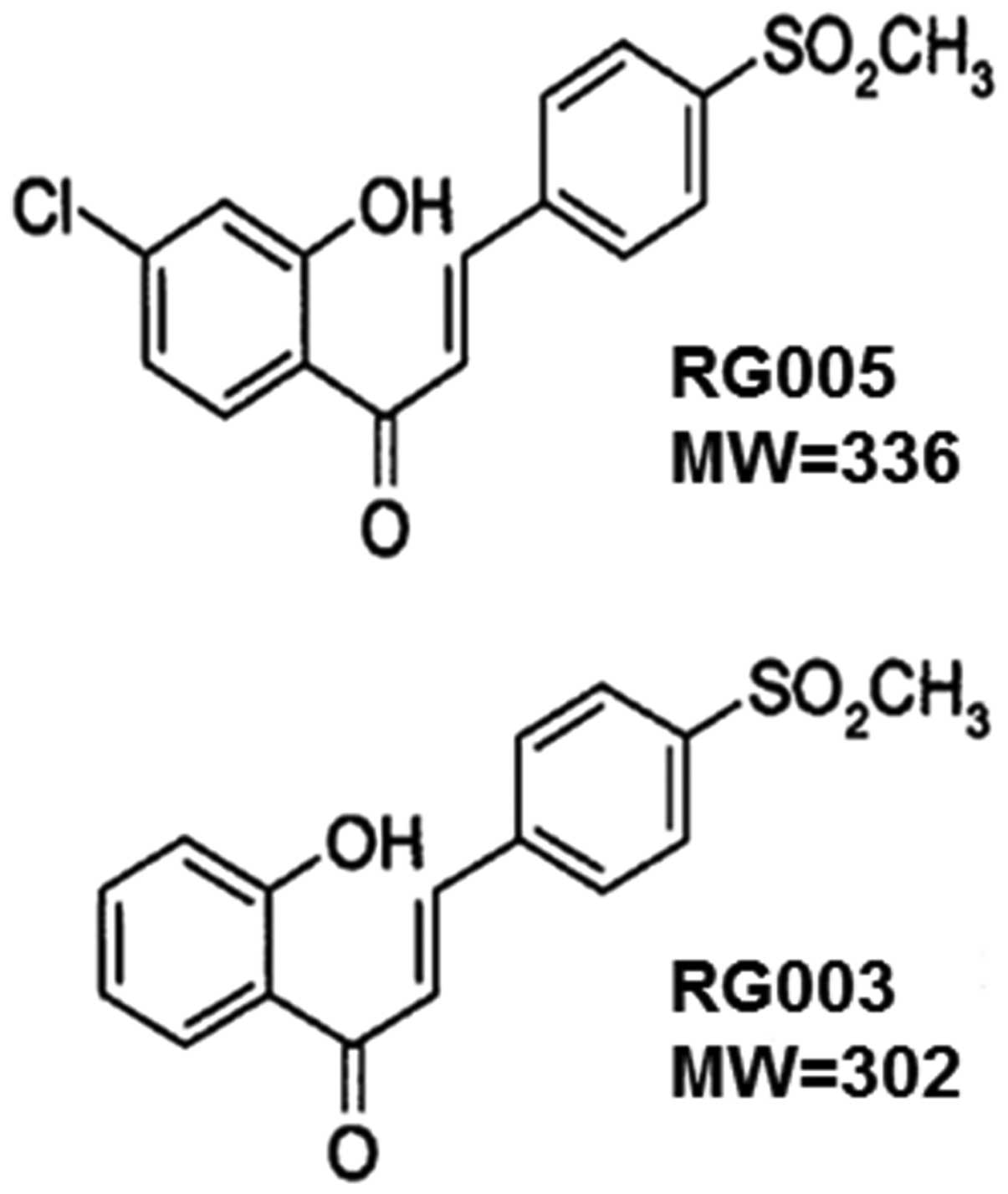
In the present study, apoptotic mechanism of RG003
(2′-hydroxy-4-methylsulfonylchalcone) and RG005
(4′-chloro-2′-hydroxy-4-methylsulfonylchalcone) (Fig. 1) in hormone-independent prostate
carcinoma cells was investigated in association with COX-2
expression. Then, to understand the mechanisms implicated in the
effect of RG003 and RG005, we studied intracellular signalling
pathways.
Materials and methods
Cell lines, cell culture, treatment and
light microscopy
The PC-3 and DU145 cell lines were purchased from
the American Type Culture Collection (LGC Standards, Middlesex,
UK). Cells were, respectively, seeded at 1.3×106 and
2×106 cells/well in 60-cm2 tissue culture
dishes and grown in RPMI-1640 medium supplemented with 10% fetal
calf serum (FCS), 100 U/ml penicillin and 100 μg/ml
streptomycin (all from Gibco-BRL, Cergy-Pontoise, France). Cultures
were maintained in a humidified atmosphere with 5% CO2
at 37°C. Cells were grown for 24 h in culture medium prior to
exposure or not to RG003 (2′-hydroxy-4-methylsulfonylchalcone) and
RG005 (4′-chloro-2′-hydroxy-4-methylsulfonylchalcone), compounds
synthesized by our research team. A stock solution of
10−2 M RG003 and RG005 was prepared in dimethyl
sulfoxide (DMSO), and diluted in culture medium to give the
appropriate final concentration. The same amount of vehicle
(percentage of DMSO did not exceed 0.20%) was added to control
cells. Cell viability was determined by the trypan blue dye
exclusion method. For light microscopy, after treatment, cultured
cells were examined under phase-contrast microscopy (magnification,
×200), and pictures were taken with an image acquisition system
(Nikon, Champigny sur Marne, France).
Cell proliferation assay and viability
assessment
Measurement of cell proliferation was determined
using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) assay. PC-3 and DU145 cells were cultured and plated,
respectively, at 5.6×103 and 8.5×103
cells/well in 10% FCS medium in 96-well culture plates and grown 24
h before treatment or not (time 0) with 5–40 μM RG003 and
RG005 for 24–72 h. MTT tests were carried out daily as previously
described (13) and experiments
were performed in three independent assays.
PC-3 and DU145 cells were grown for 24 h then
treated with RG003 and RG005 at 15 and 20 μM. After 24 and
48 h RG003 and RG005 treatment, cells were trypsinized and
resuspended in complete medium. Each sample was mixed with trypan
blue solution (0.14% in HBSS). Colored (non-viable) and
dye-excluding (viable) cells were counted on a Malassez
hemocytometer. Controls were done with the same final DMSO
concentration in the medium as samples.
Mitochondrial membrane potential
(Δψm)
Δψm was estimated using
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazole
carbocyanide iodide (JC-1, Molecular Probes). JC-1 is a fluorescent
compound that exists as a monomer at low concentrations. At higher
concentrations, it forms aggregates. Fluorescence of the JC-1
monomers is green, whereas that of aggregates is red. Mitochondria
with intact membrane potential concentrate JC-1 into aggregates,
which fluoresces red, whereas de-energized mitochondria cannot
concentrate it and are stained green (13,14).
PC-3 and DU145 cells were grown for 24 h before
treatment with 15 and 20 μM of RG003 and RG005,
respectively, for 24 and 48 h. Control cells were grown in medium
containing the same amount of vehicle as treated cells. Then cells
were incubated in 1 ml of medium containing JC-1 (1 μg/ml)
for 30 min at 37°C and pictures were taken with a fluorescence
microscope.
Protein expression
After 15 μM RG003 and RG005 treatment, PC-3
cells were washed and lysed in RIPA lysis buffer (50 mM HEPES pH
7.5, 150 mM NaCl, 1% deoxycholate, 1% NP-40, 0.1% SDS, 20
μg/ml aprotinin) containing protease inhibitors (Complete™
Mini, Roche Diagnostics, Meylan, France). Briefly, as previously
described (15), proteins (10–100
μg) were separated by electrophoresis on SDS-polyacrylamide
gels, transferred to PVDF membranes (Amersham Pharmacia Biotech,
Saclay, France) and probed with respective human antibodies against
Bax, caspase-8, caspase-9 and phospho-Akt (Ser 473) (Cell
Signalling Technology, Ozyme, France), Bcl-2 and poly-ADP-ribose
polymerase (PARP) (Santa Cruz Biotechnology, Tebu-Bio, Le Perray en
Yvelines, France), and COX-2 (Cayman Chemical, Bertin Pharma,
Montigny-le-Bretonneux, France). After incubation with secondary
antibodies (Dako France SAS., Trappes, France), blots were
developed using the ECL Plus Western Blotting Detection System
(Amersham Pharmacia Biotech) and G:BOX system (Syngene, Ozyme,
Saint Quentin en Yvelines, France). Membranes were then reblotted
with anti-β-actin (Sigma Aldrich, Saint Quentin Fallavier, France)
used as a loading control.
Caspase-3 activity
Caspase-3 activity was assayed using
Quantikine® human active caspase-3 (R&D Systems) as
previously described (16). PC-3
cells were treated or not with 15 μM of RG003 and RG005 for
24 and 48 h, and then incubated with 10 μM biotin-ZVKD-fmk
inhibitor for 1 h at 37°C. Caspase-3 activity was measured in
accordance with the manufacturer’s protocol (R&D Systems).
Briefly, cells were harvested, washed in PBS and resuspended in
extraction buffer containing protease inhibitors. Standards and
sample extracts containing covalently linked active
caspase-3-ZVKD-biotin were added to a microplate pre-coated with
monoclonal antibody specific for caspase-3. Then, streptavidin
conjugated to horseradish peroxidase was added to the wells. The
amount of active caspase-3 was quantified by colorimetry at 450 nm
after addition of HRP substrate.
Apoptosis quantification: DNA
fragmentation
PC-3 cells were seeded at 1.3×106 cells
in 60-cm2 tissue culture dishes and then treated or not
with 15 μM of RG003 and RG005 for 24 and 48 h. Apoptosis was
quantified on pooled cells (floating and adherent) using ‘cell
death’ enzyme-linked immunosorbent assay (ELISA) (Cell Death
Detection ELISAPLUS, Roche Diagnostics). Cytosol
extracts were obtained according to the manufacturer’s protocol and
apoptosis was measured as previously described (17).
Subcellular protein fractionation
PC-3 cells were incubated alone or with 15 μM
of RG003 and RG005 for 24 and 48 h. Cytosolic and nuclear fractions
were obtained using the Subcellular Protein Fractionation Kit
according to the manufacturer’s protocol (Thermo Fischer
Scientific, Rockford, IL, USA) as previously described (13).
Electromobility shift assay (EMSA)
EMSA experiments were performed using DIG Gel Shift
kit (Roche Diagnostics) (18).
Briefly, nuclear extracts were prepared from PC-3 cells treated or
not with 15 μM of RG003 and RG005 for 24 and 48 h. NF-κB
binding reactions were carried out with 10 μg nuclear
proteins incubated with digoxigenin (DIG) labeled NF-κB probe
according to the manufacturer’s protocol. The samples were loaded
on a 5% native polyacrylamide gel in Tris-Borate-EDTA buffer. After
transfer to nylon membranes and incubation with anti-DIG antibody
conjugated with alkaline phosphatase, gel mobility shift was
visualized by incubation with CSPD® chemiluminescence
reagent and G:BOX system (Syngene, Ozyme, Saint Quentin en
Yvelines, France). Quantification of each band was performed by
densitometry analysis software in respect of band intensity and
band area. Results are expressed relative to controls in arbitrary
units.
Statistical analysis
Data are expressed as the arithmetic means ±
standard deviation (SD) of separate experiments. The statistical
significance of results obtained from in vitro studies was
evaluated by the two tailed unpaired Student’s t-test, with
p<0.05 being considered as significant.
Results
Chalcone effect on PC-3 and DU145 cell
proliferation and morphological modifications
Cells were cultured in 10% FCS-medium with or
without chalcones (5–40 μM) for 24–72 h and cell
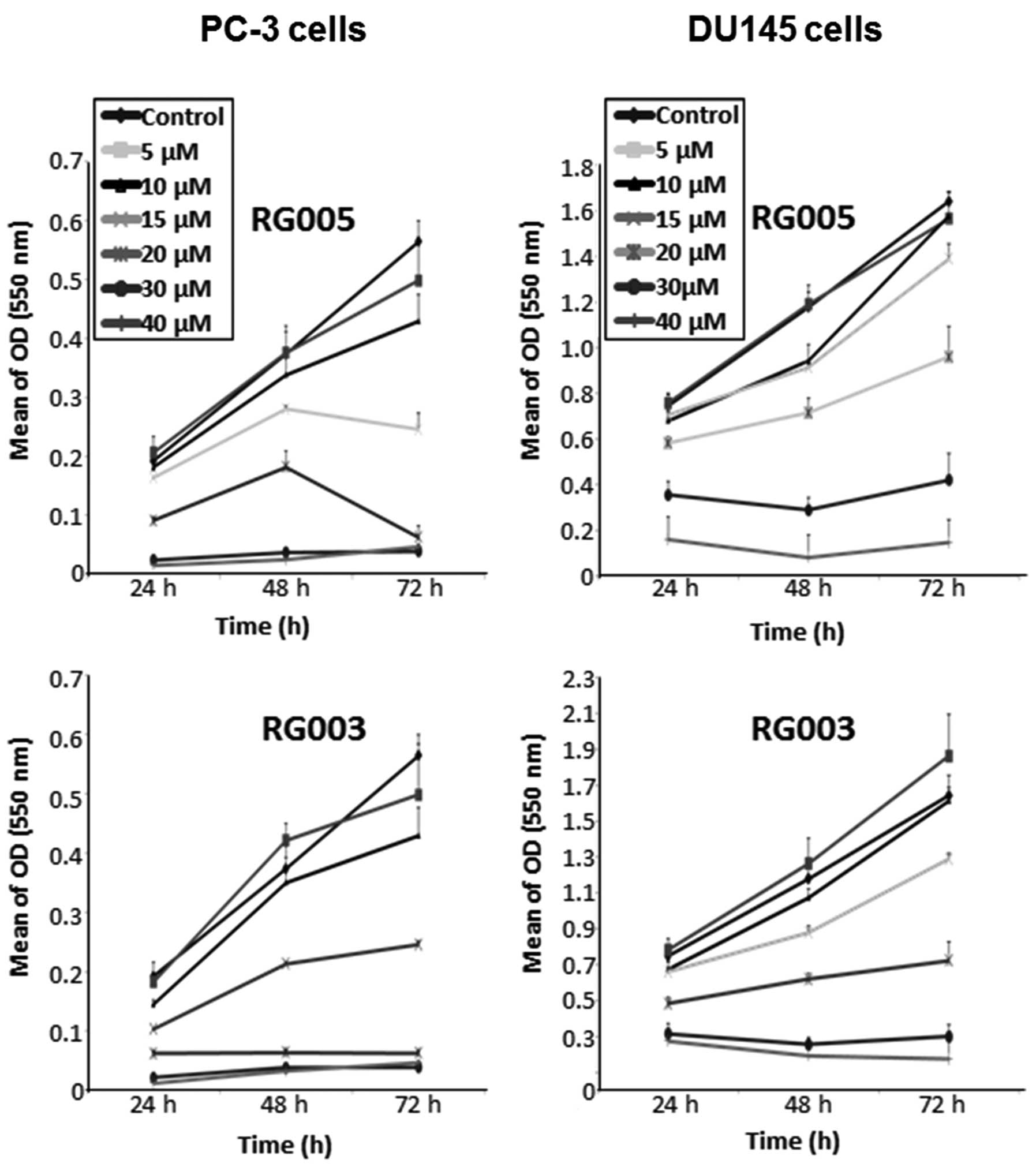
proliferation was evaluated by the MTT test (Fig. 2). Under our experimental
conditions, a decrease in proliferation was observed as early as 24
h after chalcone treatment in a dose- and time-dependent manner. To
confirm these results, relative cell viability was assessed by
trypan blue dye exclusion with, respectively, 15 and 20 μM
chalcone treatment for PC-3 and DU145 cells (Fig. 3A). Trypan blue dye proved the
antiproliferative effect of the two chalcones by decreasing
percentage of living cells with significative dominant effect for
RG003 treatment in both cell lines (33% of living cells versus 55%
for RG003 and RG005, respectively, for PC-3 cells after 48-h
treatment, p<0.05; 17% versus 32% for RG003 and RG005,
respectively, for DU145 cells after 48-h treatment, p<0.05). For
following experiments, we used both compounds at 15 and 20
μM for PC-3 and DU145 cells, respectively.
Direct observation with phase-contrast microscopy
demonstrated that cells treated with compounds showed numerous
morphological differences compared to control cells (Fig. 3B). Indeed, cell shrinkage,
cytoplasm condensation and formation of cytoplasmic filaments
appeared after compound treatment. Furthermore, the pictures of
treated cells confirmed that antiproliferative effect was more
important with RG003 than RG005 treatment.
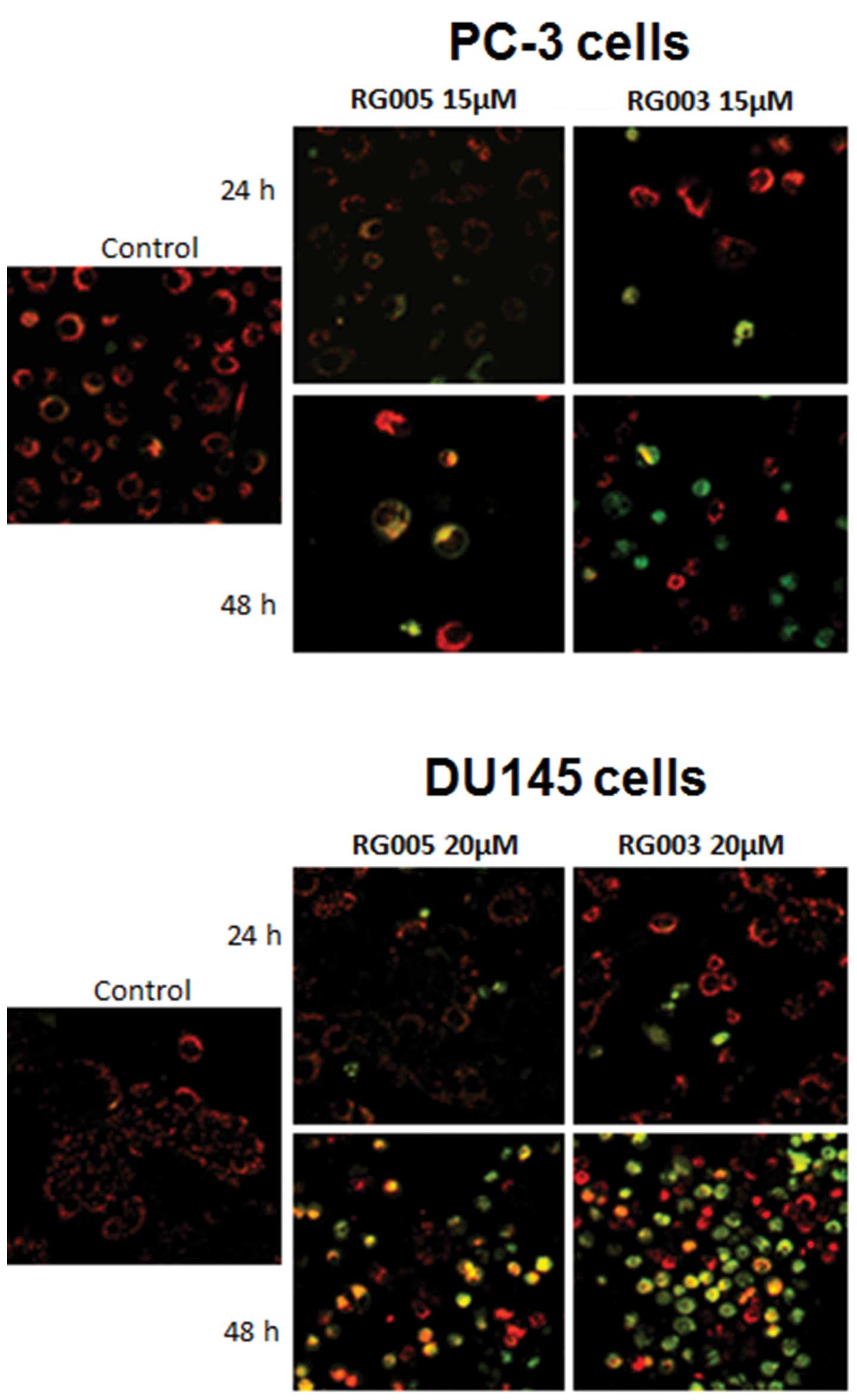
Chalcone induces disruption of Δψm in
PC-3 and DU145 cell lines
To determine potential mechanisms by which chalcone
inhibited the human PC-3 and DU145 cell proliferation, we analyzed
the effect of RG003 and RG005 on Δψm, because alterations in
mitochondrial structure and function have been shown to play a
crucial role at early stages of apoptosis (19).
Δψm was analyzed after 24 and 48-h treatment with
chalcones using JC-1. We found that compounds decreased Δψm in both
PC-3 and DU145 cells in a time-dependent manner (Fig. 4), shown by the incorporation of
JC-1 monomers into mitochondria (green fluorescence), compared with
cytosolic JC-1 aggregate formation at high membrane potentials in
control cells (red fluorescence). Furthermore, this assay showed a
dominant effect of RG003 on early intrinsic apoptosis compared to
RG005.
Chalcones induce apoptosis with
activation of intrinsic pathway
The process of apoptosis is tightly regulated by a
large variety of proteins that promote or block cell death at
different stages of apoptosis. The regulatory role of Bcl-2 family
proteins is now well established. The Bax/Bcl-2 ratio, which is a
critical determinant of apoptosis (20), was determined after western blot
analysis. Apoptotic ratio (Bax/Bcl-2) obtained with western blot
analysis indicated that after 24 h 15 μM RG003 treatment
increased the apoptotic ratio (1.5-fold versus control,
*p<0.05) whereas RG005 treatment had no effect on
PC-3 cells (Fig. 5A). After 48-h
treatment, RG003 and RG005 both increased the Bax/Bcl-2 ratio (2.4-
and 1.6-fold, respectively, versus control, **p<0.01
and *p<0.05) and our results showed again a dominant
effect of RG003 treatment. In fact, the increase in the Bax/Bcl-2
ratio seems to be mainly due to a decline in Bcl-2 expression
during RG003 and RG005 chalcone treatment.
Apoptosis induction requires activation of specific
proteins. It is well known that apoptosis is characterized by
chromatin condensation and DNA fragmentation, and is mediated by
the cysteine protease family called caspases. Caspase activation
can be regulated through an extrinsic or intrinsic signalling
pathway. The extrinsic pathway, which involves Fas and tumor
necrosis factor receptor stimulation, activates caspase-8. The
intrinsic pathway, which may be the primary means of activating
apoptotic caspase in mammals, triggers the mitochondrial release of
cytochrome c, which oligomerizes with Apaf-1 and
procaspase-9 to form the apoptosome complex. Activated caspase-9 in
this complex activates caspase-3 which is the major executioner of
apoptosis (21). PARP is one of
the best known caspase substrates and its inactivation by cleavage
is now an apoptosis hallmark. DNA fragmentation occurs
simultaneously with this phenomenon and is now considered as a
major marker of apoptotic cells.
In our study, we showed that intrinsic apoptosis
pathway was implicated in contrast to extrinsic apoptosis pathway.
Indeed, we showed that chalcones induced an activation of caspase-9
but not caspase-8 in PC-3 cells as shown in Fig. 5A and B, respectively. RG005 and
RG003 induced a cleavage of caspase-9 at 24 and 48 h but the
expression of cleaved fragment of caspase-9 after RG003 treatment
is more important than RG005 treatment. Consequently, RG005 induced
only a slight activation of executive caspase-3 activity (+1.2-fold
versus control at 24 and 48 h, p<0.05). On the contrary,
caspase-3 activity was greater after RG003 treatment (+1.4-and
+1.6-fold at 24 and 48 h, respectively, versus control, p<0.05)
(Fig. 6A). These observations were
directly correlated with PARP cleavage because western blot
analysis detected the native form of PARP but not a significant
cleaved fragment in RG005 treated PC-3 cells. In contrast, cleaved
fragment of PARP was strongly expressed starting at 24-h RG003
treatment and maintained after 48-h treatment (Fig. 6B).
DNA fragmentation, considered as a major marker of
apoptotic cells, was observed in PC-3 cells after chalcone
treatment. Quantitative determination of cytoplasmic
histone-associated-DNA-fragments (mono and oligonucleosomes) was
performed by ELISA in our study. Results showed that DNA
fragmentation was induced in PC-3 cells after 24-h RG005 and RG003
treatment (+2.3- and +2.8-fold, respectively, versus control,
p<0.05) whereas DNA fragmentation was markedly enhanced after
48-h RG003 treatment compared to RG005 tretament (+6.5-and
+3.5-fold, respectively, versus control, p<0.05) (Fig. 6C).
In summary, even if both chalcones induced apoptosis
of PC-3 cells, a dominant effect of RG003 treatment was observed
resulting in activation of the intrinsic pathway with disruption of
Δψm, caspase-9 and caspase-3 activation, PARP cleavage and DNA
fragmentation.
Downregulation of survival pathways after
chalcone treatment in PC-3 cells
Nuclear factor-κB (NF-κB) is a ubiquitous
transcription factor that has been shown to promote cell survival
by initiating the transcription of genes involved in cell
proliferation or encoding anti-apoptotic proteins (22). Akt promotes cell survival by
phosphorylating substrates that decrease the activity of
pro-apoptotic proteins or increase the activity of anti-apoptotic
proteins (23). We analyzed the
effect of RG005 and RG003 on two survival pathways: Akt and NF-κB.
Western blot analysis showed that both chalcone markedly inhibited
Akt phosphorylation in PC-3 cells (Fig. 7A). Since NF-κB activation is
critical for apoptosis resistance, we examined the effect of RG005
and RG003 on nuclear activation of NF-κB. Our results showed that
chalcone treatment inhibited NF-κB activation. Furthermore, this
inhibition was stronger with RG003 treatment (Fig. 7B).
Effect of RG005 and RG003 on COX-2
expression in PC-3 cells
It is well known that COX-2 expression is correlated
with the activities of intracellular signalling proteins such as
NF-κB (24). Furthermore, we
showed recently that COX-2 positively regulated Akt signalling and
enhanced survival of cancer cells exposed to anticancer agents
(17). Numerous studies have shown
that COX-2 expression prevents apoptosis in cancer cells,
especially in colon (25) and
prostate cancer (26,27). Here, we showed that both chalcones
reduced significantly expression of COX-2 by 24-h treatment in PC-3
cells. RG003 was the more effective in decreasing COX-2 expression
(Fig. 8).
Discussion
In light of the reported chemopreventive and
chemosensitive effects of chalcones on various tumor cells and
animal models, we postulated that our new methylsulfonyl chalcones
may mediate their effects through apoptosis induction with
suppression of cell survival pathways. Here, we observed that RG003
and RG005 could indeed suppress Akt/NF-κB/COX-2 activation and
exert significant anti-proliferative and apoptotic effects in
androgen-independent PCa cells. We also clearly demonstrated that
RG003 and RG005 induce apoptosis through mitochondrial pathway in
human PCa PC-3 cells.
PCa is very uncommon in men younger than 45 years,
but becomes more common with advancing age (28). Many of the risk factors for
prostate cancer are more prevalent in the developed world,
including longer life expectancy, alcohol/tobacco intake, and diets
high in red meat (29).
Interestingly, the American Dietetic Association and Dieticians of
Canada report a decreased risk of developing prostate cancer for
those following a vegetarian diet (30). However, the specific causes of
prostate cancer still remain unknown (31).
Researchers have established a few PCa cell lines to
investigate the mechanism involved in the progression of PCa.
Androgen-independent PC-3 and DU145 cells are commonly used.
Although previous studies have shown that chalcones can inhibit the
cell proliferation in these human prostate cancer cells including
PC-3 (32–34) and DU145 (33,35)
cells, our study is the first report on the specific examination of
intrinsic apoptosis and Akt/NF-κB/COX-2 pathways in human PCa cells
upon synthetic chalcones exposure.
Apoptosis is characterized by chromatin condensation
and DNA fragmentation, and is mediated by caspases (36). Mitochondria are involved in a
variety of key events, including release of caspase activators,
changes in electron transport, loss of mitochondrial membrane
potential (Δψm), and participation of both pro- and anti-apoptotic
Bcl-2 family proteins (37).
Alterations in mitochondrial structure and function have been shown
to play a crucial role in caspase-9-dependent apoptosis (38). Caspase-9 cleaves and activates
caspase-3, the executioner caspase, which cleaves PARP and
activates endonucleases leading to DNA fragmentation (38). To analyze the effect of RG003 and
RG005 on induction of apoptosis in PCa cells, apoptosis was
evaluated on pooled cell fractions (floating and adherent).
Mitochondria have, apart from their function in respiration, an
important role in the apoptotic-signalling pathway. It is well
known that the modification of Δψm depends on the nature of the
stimulus and the cell system and the collapse of Δψm is an early
step in the apoptotic cascade (39). We showed that RG003 and RG005
decreased Δψm in both PC-3 and DU145 cells in a time-dependent
manner.
Moreover, the Bax/Bcl-2 ratio, which is a critical
determinant of apoptosis (20),
was determined after western blot analysis. In our study, the
increase in the Bax/Bcl-2 ratio seems to be mainly due to a decline
in Bcl-2 expression during RG003 and RG005 chalcone treatment, with
a dominant effect of RG003 treatment. Since Bcl-2 protects human
prostate cancer cells from the induction of apoptosis (40), its downregulation could contribute
to the capability of RG003 and RG005 to induce apoptosis in PCa
PC-3 cells.
Caspase-3 is a key executioner of apoptosis, its
activation is mediated by the initiator caspases such as caspase-8
and caspase-9 (41). We showed
that the intrinsic apoptosis pathway was implicated but not the
extrinsic apoptosis pathway. We showed that chalcones induced
activation of caspase-9 but not caspase-8 in PC-3 cells.
Consequently, RG005 induced only a slight activation of executive
caspase-3 activity compared to RG003 treatment. These observations
were directly correlated with PARP cleavage because western blot
analysis detected the native form of PARP but not the significant
cleaved fragment in RG005 treated PC-3 cells. In contrast, cleaved
fragment of PARP was strongly detected with RG003 treatment. PARP
is a nuclear enzyme involved in the repair of DNA damage (42). Moreover, it is known that PARP is a
substrate for caspases such as caspase-3 and is typically cleaved
and inactivated during the apoptotic process (43). DNA fragmentation occurs
simultaneously with this phenomenon and is considered as a major
marker of apoptotic cells. Apoptosis quantification was performed
by ELISA, and results showed that DNA fragmentation was induced in
PCa PC-3 cells by 24-h chalcone treatment. In our study, chalcone
treatment in PCa PC-3 cells induces a disruption of Δψm, caspase-9
and -3 activation, PARP cleavage (only after RG003 treatment) and
DNA fragmentation. Furthermore, we demonstrated a dominant effect
of RG003 treatment versus RG005 one.
Among the cell signalling pathways that promote cell
survival, Akt is one of the most important (44). It has been previously reported that
the level of Akt activation is drastically enhanced in
androgen-independent PC-3 cells as compared with the
androgen-dependent cells (45).
Activated Akt can also exert anti-apoptotic effects, positively
regulate NF-κB transcription, modulate angiogenesis, promote tumor
invasion/metastasis and antagonize cell cycle arrest (46). Hence, in the present report, we
investigated the effects of RG003 and RG005 on Akt pathway in
androgen-independent PCa PC-3 cells. We found that chalcones
inhibited the expression of phospho-Akt (Ser 473) in PC-3 cells.
Akt is also reported to modulate the NF-κB transcription factor
through the phosphorylation of p65 to enhance the transcriptional
activity of NF-κB (47). NF-κB
activation is also known to regulate the expression of various cell
survival, proliferative, metastatic and angiogenic gene products
(48). Our results showed that
RG003 and RG005 treatment inhibited NF-κB activation. In regard to
these results, it is clear that the simultaneous inhibition of Akt
and NF-κB signalling can significantly contribute to the anticancer
effects of RG003 and RG005 in PCa PC-3 cells.
Recent studies on the relationship between the
arachidonic acid (AA) cascade and carcinogenesis revealed novel
molecular targets for cancer treatment (49). It has been demonstrated that the
metabolism of AA, a polyunsatured fatty acid, by either the COX or
lipoxygenase (LOX) pathway, generates a host of proinflammatory
metabolites known to modulate diverse physiological and
pathological responses such as angiogenesis, apoptosis and
hyperproliferation (50,51). COX-2 has been demonstrated to play
an important role in apoptosis resistance and carcinogenesis,
particularly in colon carcinogenesis (25,52).
Previous studies from our laboratory reported a role for COX-2 in
resistance to apoptosis in colorectal and prostate cancer cells
(53,54). Here, we demonstrated that RG003 and
RG005 reduced COX-2 expression in PCa PC-3 cells. We demonstrated
that NF-κB activation was inhibited after chalcone treatment. Its
inhibition was correlated with reduction of COX-2 expression and
induction of apoptosis. NF-κB activation signalling pathway was
reported to regulate COX-2 expression and to promote cell survival
(24).
Our results clearly indicate for the first time that
RG003 and RG005 exert their potent anti-proliferative and
pro-apoptotic effects through the modulation of Akt/NF-κB/COX-2
signal transduction pathways in PCa PC-3 cells and do not act
specifically on any one cellular signalling cascade. It is obvious
that RG003 and RG005 are not active against a specific signalling
cascade but they can interfere with a multitude of targets in PCa
PC-3 cells. This is quite relevant to the changing paradigm in
cancer therapy, as increasing evidence indicates that the
mono-targeted drugs, once called smart drugs, have not had a
significant impact on cancer treatment and the use of
multi-targeted drugs has become increasingly accepted, as it is
obvious that cancer is caused by dysregulation of multiple pathways
(55).
Our results show that RG003 has a dominant effect
compared to RG005 treatment on apoptosis of PCa cells. The only
structural difference is the presence of one chlorine atom in the A
cycle at 4′ position for RG005. To understand the difference of
their biological activities, two major characteristics could be
implicated: spatial conformation and redox reactivity. Both
characteristics can be examined by molecular modeling as previously
used by our team (56).
Furthermore, the significance of our in vitro study between
RG003 and RG005 effects in PCa cells is very encouraging suggesting
the relevance of testing these compounds in xenograft animal
models.
Acknowledgements
The authors are grateful to Claire
Carrion for help in performing mitochondrial membrane potential
assays. This research was supported by grants from the French
Ministry of Education and Research and from the Lebanese National
Council for Scientific Research (CNRS-L, doctoral scholarship to
B.I.).
References
|
1.
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar
|
|
2.
|
Dreicer R: Current status of cytotoxic
chemotherapy in patients with metastatic prostate cancer. Urol
Oncol. 26:426–429. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Alcaraz MJ, Vicente AM, Araico A,
Dominguez JN, Terencio MC and Ferrándiz ML: Role of nuclear
factor-kappaB and heme oxygenase-1 in the mechanism of action of an
anti-inflammatory chalcone derivative in RAW 264.7 cells. Br J
Pharmacol. 142:1191–1199. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Domínguez JN, León C, Rodrigues J, Gamboa
de Domínguez N, Gut J and Rosenthal PJ: Synthesis and evaluation of
new anti-malarial phenylurenyl chalcone derivatives. J Med Chem.
48:3654–3658. 2005.PubMed/NCBI
|
|
5.
|
Liu X and Go ML: Antiproliferative
activity of chalcones with basic functionalities. Bioorg Med Chem.
15:7021–7034. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Aggarwal BB and Shishodia S: Molecular
targets of dietary agents for prevention and therapy of cancer.
Biochem Pharmacol. 71:1397–1421. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Hu W and Kavanagh JJ: Anticancer therapy
targeting the apoptotic pathway. Lancet Oncol. 4:721–729. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Shen KH, Chang JK, Hsu YL and Kuo PL:
Chalcone arrests cell cycle progression and induces apoptosis
through induction of mitochondrial pathway and inhibition of
nuclear factor kappa B signalling in human bladder cancer cells.
Basic Clin Pharmacol Toxicol. 101:254–261. 2007. View Article : Google Scholar
|
|
9.
|
Smith WL, DeWitt DL and Garavito RM:
Cyclooxygenases: structural, cellular, and molecular biology. Annu
Rev Biochem. 69:145–182. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Xie WL, Chipman JG, Robertson DL, Erikson
RL and Simmons DL: Expression of a mitogen-responsive gene encoding
prostaglandin synthase is regulated by mRNA splicing. Proc Natl
Acad Sci USA. 88:2692–2696. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Gupta S, Srivastava M, Ahmad N, Bostwick
DG and Mukhtar H: Over-expression of cyclooxygenase-2 in human
prostate adenocarcinoma. Prostate. 42:73–78. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Wenzel U, Kuntz S, Brendel MD and Daniel
H: Dietary flavone is a potent apoptosis inducer in human colon
carcinoma cells. Cancer Res. 60:3823–3831. 2000.PubMed/NCBI
|
|
13.
|
Corbiere C, Liagre B, Terro F and
Beneytout JL: Induction of antiproliferative effect by diosgenin
through activation of p53, release of apoptosis-inducing factor
(AIF) and modulation of caspase-3 activity in different human
cancer cells. Cell Res. 14:188–196. 2004. View Article : Google Scholar
|
|
14.
|
Smiley ST, Reers M, Mottola-Hartshorn C,
et al: Intracellular heterogeneity in mitochondrial membrane
potentials revealed by a J-aggregate-forming lipophilic cation
JC-1. Proc Natl Acad Sci USA. 88:3671–3675. 1991. View Article : Google Scholar
|
|
15.
|
Lepage C, Liagre B, Cook-Moreau J, Pinon A
and Beneytout JL: Cyclooxygenase-2 and 5-lipoxygenase pathways in
diosgenin-induced apoptosis in HT-29 and HCT-116 colon cancer
cells. Int J Oncol. 36:1183–1191. 2010.PubMed/NCBI
|
|
16.
|
Leger DY, Liagre B and Beneytout JL: Role
of MAPKs and NF-κB in diosgenin-induced megakaryocytic
differentiation and subsequent apoptosis in HEL cells. Int J Oncol.
28:201–207. 2006.
|
|
17.
|
Bertrand J, Liagre B, Ghezali L, Beneytout
JL and Leger DY: Cyclooxygenase-2 positively regulates Akt
signalling and enhances survival of erythroleukemia cells exposed
to anti-cancer agents. Apoptosis. 18:836–850. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Ghezali L, Leger DY, Limami Y, Cook-Moreau
J, Beneytout JL and Liagre B: Cyclopamine and jervine induce COX-2
overexpression in human erythroleukemia cells but only cyclopamine
has a pro-apoptotic effect. Exp Cell Res. 319:1043–1053. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Green DR and Reed JC: Mitochondria and
apoptosis. Science. 281:1309–1312. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Oltvai ZN, Milliman CL and Korsmeyer SJ:
Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that
accelerates programmed cell death. Cell. 74:609–619. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
MacKenzie SH, Schipper JL and Clark AC:
The potential for caspases in drug discovery. Curr Opin Drug Discov
Dev. 13:568–576. 2010.PubMed/NCBI
|
|
22.
|
Liu YQ, Hu XY, Lu T, Cheng YN, Young CY,
Yuan HQ and Lou HX: Retigeric acid B exhibits antitumor activity
through suppression of nuclear factor-κB signalling in prostate
cancer cells in vitro and in vivo. PLoS One.
7:e380002012.PubMed/NCBI
|
|
23.
|
Datta SR, Dudek H, Tao X, Masters S, Fu H,
Gotoh Y and Greenberg ME: Akt phosphorylation of BAD couples
survival signals to the cell-intrinsic death machinery. Cell.
91:231–241. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Giri K and Aggarwal BB: Constitutive
activation of NF-kappaB causes resistance to apoptosis in human
cutaneous T cell lymphoma HuT-78 cells. Autocrine role of tumor
necrosis factor and reactive oxygen intermediates. J Biol Chem.
273:14008–14014. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Wang D and Dubois RN: The role of COX-2 in
intestinal inflammation and colorectal cancer. Oncogene.
29:781–788. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Kirschenbaum A, Liu X, Yao S and Levine
AC: The role of cyclooxygenase-2 in prostate cancer. Urology.
58:127–131. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Lee KS, Lee HJ, Ahn KS, et al:
Cyclooxygenase-2/prostaglandin E2 pathway mediates icariside II
induced apoptosis in human PC-3 prostate cancer cells. Cancer Lett.
280:93–100. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Hankey BF, Feuer EJ, Clegg LX, et al:
Cancer surveillance series: Interpreting trends in prostate
cancer-part I: evidence of the effects of screening in recent
prostate cancer incidence, mortality, and survival rates. J Natl
Cancer Inst. 91:1017–1024. 1999. View Article : Google Scholar
|
|
29.
|
Ganesh B, Saoba SL, Sarade MN and Pinjari
SV: Risk factors for prostate cancer: an hospital-based
case-control study from Mumbai, India. Indian J Urol. 27:345–350.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
American Dietetic Association; Dietitians
of Canada: Position of the American Dietetic Association and
Dietitians of Canada: Vegetarian diets. J Am Diet Assoc.
103:748–765. 2003. View Article : Google Scholar
|
|
31.
|
Hsing AW and Chokkalingam AP: Prostate
cancer epidemiology. Front Biosci. 11:1388–1413. 2006. View Article : Google Scholar
|
|
32.
|
Rodrigues J, Abramjuk C, Vásquez L, et al:
New 4-maleamic acid and 4-maleamide peptidyl chalcones as potential
multi-target drugs for human prostate cancer. Pharm Res.
28:907–919. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Deb Majumdar I, Devanabanda A, Fox B,
Schwartzman J, Cong H, Porco JA Jr and Weber HC: Synthetic
cyclohexenyl chalcone natural products possess cytotoxic activities
against prostate cancer cells and inhibit cysteine cathepsins in
vitro. Biochem Biophys Res Commun. 416:397–402. 2011.
|
|
34.
|
Nagaraju M, Gnana Deepthi E, Ashwini C, et
al: Synthesis and selective cytotoxic activity of novel hybrid
chalcones against prostate cancer cells. Bioorg Med Chem Lett.
22:4314–4317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Jung JI, Lim SS, Choi HJ, et al:
Isoliquiritigenin induces apoptosis by depolarizing mitochondrial
membranes in prostate cancer cells. J Nutr Biochem. 17:689–696.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Zamzami N, Susin SA, Marchetti P, et al:
Mitochondrial control of nuclear apoptosis. J Exp Med.
183:1533–1544. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Green D and Kroemer G: The central
executioners of apoptosis: caspases or mitochondria? Trends Cell
Biol. 8:267–271. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Liu X, Kim CN, Yang J, Jemmerson R and
Wang X: Induction of apoptotic program in cell-free extracts:
requirement for dATP and cytochrome c. Cell. 86:147–157. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Lebedeva IV, Sarkar D, Su ZZ, et al: Bcl-2
and Bcl-x(L) differentially protect human prostate cancer cells
from induction of apoptosis by melanoma differentiation associated
gene-7, mda-7/IL-24. Oncogene. 22:8758–8773. 2003. View Article : Google Scholar
|
|
41.
|
Salvesen GS and Dixit VM: Caspases:
intracellular signalling by proteolysis. Cell. 91:443–446. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
D’Amours D, Desnoyers S, D’Silva I and
Poirier GG: Poly(ADP-ribosyl)ation reactions in the regulation of
nuclear functions. Biochem J. 342:249–268. 1999.
|
|
43.
|
Nicholson DW: Caspase structure,
proteolytic substrates, and function during apoptotic cell death.
Cell Death Differ. 6:1028–1042. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Chen X, Thakkar H, Tyan F, et al:
Constitutively active Akt is an important regulator of TRAIL
sensitivity in prostate cancer. Oncogene. 20:6073–6083. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Murillo H, Huang H, Schmidt LJ, Smith DI
and Tindall DJ: Role of PI3K signalling in survival and progression
of LNCaP prostate cancer cells to the androgen refractory state.
Endocrinology. 142:4795–4805. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Testa JR and Bellacosa A: AKT plays a
central role in tumorigenesis. Proc Natl Acad Sci USA.
98:10983–10985. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Madrid LV, Mayo MW, Reuther JY and Baldwin
AS Jr: Akt stimulates the transactivation potential of the RelA/p65
Subunit of NF-kappa B through utilization of the Ikappa B kinase
and activation of the mitogen-activated protein kinase p38. J Biol
Chem. 276:18934–18940. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Ahn KS, Sethi G and Aggarwal BB: Nuclear
factor-kappa B: from clone to clinic. Curr Mol Med. 7:619–637.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Yang P, Cartwright CA, Li J, et al:
Arachidonic acid metabolism in human prostate cancer. Int J Oncol.
41:1495–1503. 2012.PubMed/NCBI
|
|
50.
|
Narayanan NK, Narayanan BA and Reddy BS: A
combination of docosahexaenoic acid and celecoxib prevents prostate
cancer cell growth in vitro and is associated with
modulation of nuclear factor-κB, and steroid hormone receptors. Int
J Oncol. 26:785–792. 2005.PubMed/NCBI
|
|
51.
|
Sarveswaran S, Gautam SC and Ghosh J:
Wedelolactone, a medicinal plant-derived coumestan, induces
caspase-dependent apoptosis in prostate cancer cells via
downregulation of PKCε without inhibiting Akt. Int J Oncol.
41:2191–2199. 2012.PubMed/NCBI
|
|
52.
|
Patsos HA, Greenhough A, Hicks DJ, et al:
The endogenous cannabinoid, anandamide, induces COX-2-dependent
cell death in apoptosis-resistant colon cancer cells. Int J Oncol.
37:187–193. 2010.PubMed/NCBI
|
|
53.
|
Limami Y, Pinon A, Leger DY, et al: HT-29
colorectal cancer cells undergoing apoptosis overexpress COX-2 to
delay ursolic acid-induced cell death. Biochimie. 93:749–757. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
54.
|
Limami Y, Pinon A, Leger DY, et al: The
P2Y2/Src/p38/COX-2 pathway is involved in the resistance to ursolic
acid-induced apoptosis in colorectal and prostate cancer cells.
Biochimie. 94:1754–1763. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55.
|
Mencher SK and Wang LG: Promiscuous drugs
compared to selective drugs (promiscuity can be a virtue). BMC Clin
Pharmacol. 5:32005. View Article : Google Scholar : PubMed/NCBI
|
|
56.
|
Trouillas P, Corbière C, Liagre B, Duroux
JL and Beneytout JL: Structure-function relationship for saponin
effects on cell cycle arrest and apoptosis in the human 1547
osteosarcoma cells: a molecular modelling approach of natural
molecules structurally close to diosgenin. Bioorg Med Chem.
13:1141–1149. 2005. View Article : Google Scholar
|