Introduction
Oral squamous cell carcinoma (OSCC) is a common type
of malignant tumor in the world. New cases of oral cancer occur at
around 275,000 patients per year, OSCC cases comprise approximately
>90% of diagnosed patients with oral cancer (1). Although conventional treatments of
oral cancer, including surgery, radiation and chemotherapy, have
well advanced to date, the five-year survival rate remains <50%
(2). Hence, discovery and
development of effective chemotherapeutic agents for OSCC might
result in improved survival rate of OSCC patients.
Natural products as sources of new drugs have been
explored and expanded in anticancer drug development for the past
several decades. In fact, 74.8% of all the anticancer drugs have
been discovered and semi-modified from natural sources, for
example, 20 small molecules were approved in 2010 (3). Among the plant-derived products,
honokiol (HK) is the most attractive natural compound since it has
been widely used to treat several diseases, including stroke,
anxiety, fever and ischemic heart disease, by Chinese (houpo) and
Japanese (saiboku-to) as a traditional herbal medicine (4). HK is a polyphenolic compound
containing physiologically active small molecules, it is isolated
from the cones, bark and leaves of Magnolia species (Magnolia
officinalis or grandiflora) (4,5).
Many researchers have paid attention to biological
effects of HK in various cancer cells because it has many
remarkable pharmacological abilities, such as anti-inflammatory,
anti-thrombotic, anti-arrhythmic, anti-platelet and anti-oxidative
effects, without appreciable toxicity (6–9).
Several studies on the effect of HK demonstrated its anticancer
activity in various cancer cell lines and tumor models (10–14).
HK was first proposed as a potent chemotherapy candidate for cancer
therapy due to its antitumor activity against xenografted tumors in
mice (15). In that study, HK
treatment resulted in inhibition of tumor growth rate up to 50%. In
addition, it was found to induce apoptotic cell death in B cell
chronic lympocytic leukemia cell lines through a caspase-dependent
pathway. Furthermore, combinatorial treatments of a low dose HK
with chemotherapeutic agents such as fludarabine, cladribine and
chlorambucil, enhanced the cytotoxic effect (10). HK has also been shown to inhibit
the NF-κB signaling pathway via decreasing the TNF-α-induced NF-κB
activation, IKK activity, IκBα phosphorylation and IκBα degradation
in endothelial cells, monocytes, breast cancer and cervical cancer
(16–18). Consequently, HK has received
attention as a potent anticancer drug due to its ability in the
regulation of multiple signal transductions in various cell
lines.
Although the anticancer effects of HK have been well
demonstrated against numerous cancer cell lines and models, little
is known about the effect of HK on oral squamous cell carcinoma
(OSCC). To characterize the effect of HK on OSCC, this study
specifically examined the anticancer effect of HK on cell viability
against two oral squamous cell carcinoma cell lines, HN-22 and
HSC-4, and identified the regulated proteins by HK treatment in
these cells. Interestingly, an important gene regulating protein
specificity protein 1 (Sp1) in cell proliferation, cell cycle
progression and oncogenesis was significantly regulated when cells
were treated with HK (19).
Subsequently, we explored whether downstream proteins of Sp1 and
key apoptotic proteins could be affected in their expression toward
apoptotic cell death through alteration of Sp1 expression by HK
treatment. Our results provide insight for the chemotherapeutic
efficacy of HK in oral squamous cells.
Materials and methods
Cell culture and reagents
The human oral squamous cancer cells, HN-22 and
HSC-4, were generously provided by Dr Sung-Dae Cho (Chonbuk
National University, Jeonju, Korea) and cultured in Hyclone
Dulbecco’s modified Eagle’s medium (DMEM; Thermo Scientific, Logan,
UT) containing 10% heat-inactivated fetal bovine serum and 100 U/ml
each of penicillin and streptomycin (Thermo Scientific) at 37°C
with 5% CO2 in humidified air. HK was purchased from
Sigma-Aldrich (St. Louis, MO).
Cell viability assay
Cell viability was measured using the CellTiter 96™
AQueous assay kit (Promega, Madison, WI) according to the
manufacturer’s protocol. Both HN-22 and HSC-4 cells were seeded on
a 96-well microtiter plate (HN-22, 2×103 cells/well and
HSC-4, 3×103 cells/well) and then cells were treated
with different doses of 0, 2.5, 5 or 10 μg/ml HK. Cell
viability was measured by adding dehydrogenase enzyme substrate
(MTS,
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium)
and electron coupling reagent (PMS, phenazinemethosulfate) and then
plates were incubated at 37°C in 5% CO2 for 2 h after 24
h post-treatment of HK. The absorbance was measured at 490 nm using
GloMax-Multi Microplate Multimode Reader (Promega). Percentages of
cell viabilities of HK treated cells were normalized to that of
untreated cells.
Sp1 knockdown using siRNA
The endogenous Sp1 knockdown was induced via the
transient transfection of siRNA. Knockdown of Sp1 was performed
using a pool of four duplexes targeting Sp1 (TARGETplus SMARTpool
siRNA, Thermo Scientific Dharmacon, Lafayette, CO). HN-22 and HSC-4
cells were seeded in 96-well plates and 100-mm culture dishes, and
Sp1 targeting siRNA or non-targeting controls (Dharmacon) at a 50
nM were introduced using the DharmaFECT2 transfection reagent.
After transfection, cells were subjected to MTS assay and western
blot analysis.
Terminal deoxynucleotidyl
transferase-mediated dUTP nick end labeling assay
The apoptotic events were visualized by terminal
deoxynucleotidyltransferase UTP nick end labeling (TUNEL) assay
using an in situ cell death detection kit (Roche, Mannheim,
Germany). Cells were prepared as described previously in 6-well
plates with coverslips. After HK administration, a TUNEL assay was
performed with an In Situ Cell Death Detection kit according to the
manufacturer’s manual. In brief, cells were fixed and permeabilized
with cytofix/cytoperm solution (BD Biosciences, San Diego, CA) for
30 min. The fixed and permeabilized cells were then treated with
the TUNEL reaction mixture and incubated in a humidified dark
chamber at 37°C for 1 h. The samples were washed with PBS and then
the stained cells were observed under a FluoView confocal laser
microscope.
Immunocytochemistry
The cells were seeded over each sterilized glass
coverslips on 6-well tissue culture plates for 24 h and incubated
with HK for 48 h. The cells were fixed and permeabilized with
cytofix/cytoperm solution for 30 min. For expression of Sp1, the
cells were blocked with 1% BSA and then incubated with monoclonal
Sp1 antibody at 4°C overnight. After washing with PBS containing
0.05% Tween-20 (PBST) solution, the Sp1 antibody was reacted with a
Jackson 488-conjugated anti-mouse secondary antibody at room
temperature for 1 h and mounted with Mountin solution-Vectashield
mounting medium for fluorescence with DAPI (Vector Laboratories
Inc., Burlingame, CA) onto the cells. The cells were visualized
using a FluoView confocal laser microscope.
Western blot analysis
The total cell lysate were prepared using PRO-PREP™
protein Extraction Solution (iNtRON Biotechnology, Seoul, Korea)
containing 1 μg/ml aprotinin, 1 μg/ml leupeptin and 1
μM PMSF. Equal amounts of total protein were separated on 10
or 15% v/v SDS-PAGE and then transferred onto
polyvinylidenedifluoride (PVDF) membranes. After blocking with 5%
non-fat dried milk in PBST containing 0.1% Tween-20, membranes were
immunoblotted with specific primary antibodies against Sp1 (1C6),
p21 (F-5), p27 (C-19), cyclin D1 (M-20) (Santa Cruz Biotechnology,
Santa Cruz, CA), PARP (BD Biosciences), Mcl-1, survivin,
cleaved-caspase-3 (Cell Signaling, Danvers, MA) and β-actin (AC-74)
(Sigma-Aldrich) overnight at 4°C. After washing with PBST, the
membranes were incubated with horseradish-peroxidaseconjugated
anti-mouse IgG or anti-rabbit IgG (Santa Cruz Biotechnology) and
chemiluminescence signals were enhanced by Pierce ECL Western
Blotting Substrate (Thermo Scientific, Rockford, IL).
Statistical analysis
Statistical significance was assessed using a
Student’s t-test. A p-value of <0.05 compared with the
non-treated cool was considered statistically significant.
Results
Honokiol inhibits cell viability and
induces apoptosis of OSCCs
Previously, it has been reported that HK inhibits
cell proliferation and tumor growth of various cell lines derived
from different cancers (15,20,21).
Therefore, we examined whether HK could effectively suppress the
cell proliferative capability of the OSCCs, HN-22 and HSC-4. To
determine the cell viability, we employed MTS assay after HK
treatment with different concentrations (2.5, 5, 7.5 or 10
μg/ml) and different time-points (24 or 48 h) into HN-22 or
HSC-4. The IC50 of HK for 48 h treatment in the HN-22
and HSC-4 cells was calculated to be approximately 7.1 and 8.0
μg/ml (Fig. 1B). The cell
viability of HN-22 was, respectively, 95.4±2.3, 79.7±2.0, 44.7±7.1
and 26.3±2.3% at 2.5, 5, 7.5 and 10 μg/ml of HK compared
with the untreated control cells when viability was calculated at
48 h post-treatment. In the case of HSC-4, viability was 97.7±0.5,
94.2±2.7, 56.0±4.8 and 25.2±0.9 at 2.5, 5, 7.5 and 10 μg/ml,
respectively, of HK compared to that of the untreated control cells
at 48 h post-treatment. Next, we confirmed the induction of
apoptosis by HK treatment. TUNEL assay was performed to visualize
the cells undergoing apoptosis to detect DNA fragmentation that
resulted from the apoptotic signaling cascade. As shown in Fig. 1C, TUNEL positive cells were
markedly increased in high-dose treated (10 μg/ml) cells in
both HN-22 and HSC-4 in comparison to the untreated or low-dose
treated cells. These data show that HK treatment effectively
inhibited cell growth and led to apoptotic cell death in OSCCs.
Sp1 protein level is decreased by
honokiol
The transcription factor of Sp1 highly overexpressed
in various cancer-derived cell lines including human glioblastoma,
lung and pancreatic cancers etc., and has regulated transcriptional
activity on differentiation, growth and oncogenesis genes (e.g.
cyclins, c-myc and p53) through modulation of target gene
promoter (19,22–25).
If the expression level of Sp1 protein could be effectively
modulated by a chemotherapeutic agent, then the agent can be a
potent candidate for an anticancer drug through suppression of
tumor progression. Thus, to determine whether Sp1 protein levels
were reduced by HK under the same conditions as MTS assays, two
OSCC cell lines (HN-22 and HSC-4) were treated with different doses
of HK at 0, 2.5, 5 and 10 μg/ml for 48 h. The Sp1 levels
were dramatically decreased in the treated cells with maximum
88.1±9.4% compared to the HN-22 untreated group and 73.2±12.2% of
the HSC-4 untreated group (Fig. 2A and
B). Consistent with these observations, immunocytochemical
results also showed a decreased level of Sp1 positive cells in a
dose-dependent manner in HN-22 and HSC-4 (Fig. 2E). To address the cellular effect
of down-regulated Sp1 by HK, we examined the alterations of
apoptotic-related protein, PARP, by western blot analysis along
with Sp1 expression. When the expression levels of Sp1 and PARP
were monitored for 48 h with 12-h intervals, Sp1 levels were
dramatically diminished as time passed and full-length PARP also
showed the parallel changes with Sp1 under HK treated conditions
(Fig. 2C and D). These results
collectively suggest that down-regulation of Sp1 by HK treatment
could lead to apoptotic cell death.
Suppression of Sp1 expression leads to
apoptotic cell death
It has been reported that transcriptional activity
of Sp1 has an important role in oncogenesis. Indeed, many
cancer-derived cells showed enforced expression levels of Sp1 in
comparison to normal cells. Moreover, the level of Sp1 was shown to
affect the fate of cancer cells through modulating the genes
involved in cell cycle progression, growth and apoptosis (24,25).
Our previous study reported that Sp1 down-regulated cancer cells
were shown to decrease the proliferation rate (26,27).
Therefore, we investigated to clearly evaluate whether Sp1
expression level has an effect on cell viability and apoptosis in
HN-22 and HSC-4 cells. To determine the cellular effect by Sp1, we
transiently transfected the Sp1 specific targeting siRNA (siSp1)
into HN-22 and HSC-4 and then monitored cell viabilities at
different transfection time points (24, 48, 72 and 96 h). As
expected, siSp1 transfected HN-22 and HSC-4 showed reduced
viabilities compared to siCon transfected HN-22 and HSC-4 cells
(Fig. 3A and B). Apoptosis
inducing protein levels were also significantly changed in Sp1
knocked down cells by siSp1. The full-length PARP decreased
according to the Sp1 expression level, whereas cleaved-caspase-3
increased (Fig. 3C and D). Our
results demonstrate that the level of Sp1 expression plays an
important role in the physiological progression of OSCCs (HN-22 and
HSC-4).
HK treatment shows the same effects of
Sp1 in suppressed conditions
To determine the regulatory role of HK, we focused
on the expression levels of the Sp1 downstream targets and
pro-apoptotic proteins. We found that cell cycle arrest proteins,
such as p27 and p21, were markedly enhanced in a dose-dependent
manner by HK, whereas cell proliferation and survival associated
proteins, such as cyclin D1, Mcl-1 and survivin, were decreased by
HK treatment (Fig. 4A and B).
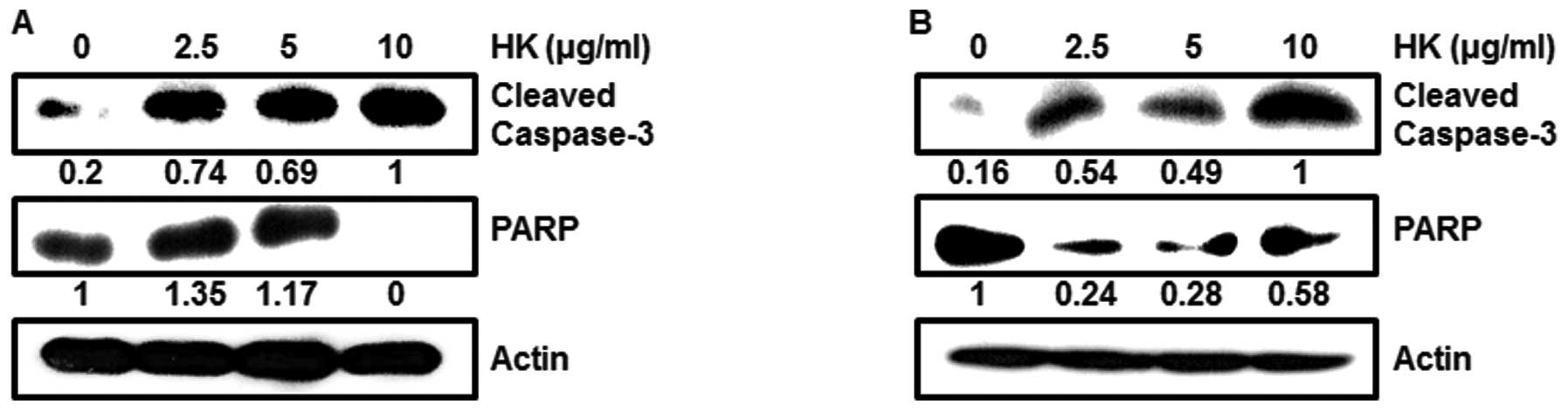
Moreover, when we tested pro-apoptotic protein levels at different
doses, HK dose-dependently caused activation of caspase-3 and PARP
in OSCCs (Fig. 5A and B).
Discussion
HK is a physiologically activated natural product,
which has been widely used in China and Japan as a traditional
herbal medicine for treatment of stroke, fever, anxiety and nervous
disturbance (5) (Fig. 1A). It was reported that HK has
multi-functional roles in cellular processes (4). Several studies have reported that HK
has an antitumor effect on various cancer derived cells, including
B-CLL, prostate and hepatoma cell lines (10–13).
Despite numerous studies on cancer cells, anti-cancer activities of
HK on OSCCs are not well understood.
In this study, we extensively explored the apoptotic
effects of HK in OSCCs, since oral cancer is one of serious
diseases in many parts of the world. Oral cancer, which is the
cancer of the oral and pharyngeal cavities, is ranked as the sixth
most commonly occurring cancer in the world. Oral squamous cell
carcinomas (OSCCs) account for over 90% of oral cancers (1). Despite several clinical approaches
including surgical resection, radiation therapy, chemotherapy or
their combinations, OSCCs still have lower survival rates as well
as being the most aggressive malignant tumor type. Thus,
efficacious drugs are highly required for OSCCs treatment. In HK
treatment of the two OSCCs, HN-22 and HSC-4, at different times and
concentrations (Fig. 1B), TUNEL
positive cells were increased in a dose-dependent manner (Fig. 1C).
Transcription factor, Sp1 is known to be
ubiquitously expressed and closely associated in various cellular
processes through its anti-tumor activity and regulation of signal
transductions (19).
Interestingly, many different types of cancer cells were reported
to show highly enhanced Sp1 expression levels (24,25,28).
Therefore, numerous studies have investigated whether up-regulated
Sp1 could have an effect on biological processes such as
proliferation, differentiation and oncogenesis (19). As an example to cell cycle
progression, down-regulation of Sp1 level by siSp1, decoy or
ectopic expression of dominant-negative protein induced
G1 phase cell cycle arrest in human glioblastoma, lung,
pancreatic and cervical cancer cells, resulting from alterations of
cycle modulating proteins such as cyclin D1 and p27 (22,23,29,30).
Therefore, Sp1 has been suggested as an ideal target for molecular
therapy against cancer. Our results show that Sp1 was significantly
reduced in the HK treated cells (Fig.
2) and pro-apoptotic proteins, PARP and caspase-3, were also
regulated toward apoptosis (Fig.
5). These cellular effects of HK were similar to the effects
produced by the Sp1 specific inhibitor, mithramycin A which
inhibited the expression and transcriptional regulatory activity of
Sp1 on target genes including c-myc, cyclin D1, Mcl-1 and
survivin (26,27,31).
To evaluate whether HK can change target protein expression levels
and apoptosis related proteins in OSCCs, we monitored the proteins
whose expression was closely associated with cell cycle arrest and
survival, to gain mechanistic insights into the role of anticancer
effect in OSCCs. Moreover, expression levels of transcriptional
regulatory factors of cyclin-dependent kinase inhibitors (CKI), p27
and p21, were studied extensively. Many human cancers frequently
show down-regulation of p27 which is correlated with cancer cell
malignancy (32). Another cdk
inhibitor, p21, is also down-regulated in various human cancers
including colorectal, tonsillar carcinoma, gastric and breast
cancer (33). Both p21 and p27 are
well characterized as negative regulators of cell cycle progression
and their functional roles in G1 phase arrest result
from the interaction of cyclins and cyclin-dependent kinase (CDKs)
complexes (34,35). Therefore, we postulated that if
CDKs are positively regulated in cells by a therapeutic agent,
malignancies of cancer cells could be effectively suppressed via
inhibition of cell cycle arrest. In this study, we found that two
CKIs, p21 and p27, were significantly increased in HK
dose-dependent manner, whereas their upstream regulator Sp1 was
found to be decreased (Fig. 4).
These results suggest that HK was able to negatively regulate Sp1
expression, resulting in down-regulation of p21 and p27.
Another cell cycle involving protein, cyclin D1, was
also regulated by HK treatement. It has been reported that cyclin
D1 is indispensable for cell cycle progression because it promotes
G1/S phase transition via interaction with cyclin
dependent kinases. Thus, its expression level is closely associated
with tumorigenesis and cell maintenance. A study showed that cyclin
D1 was induced by oncogenes including Ras, Src and β-catenin, when
cells were stimulated by oncogenic signals (36–38).
Also, an increased level of cyclin D1 has been frequently observed
in human cancers (39). Therefore,
it is likely that reduction of Sp1 by HK treatment could not induce
transcriptional activation of cyclin D1 on the promoter, resulting
in suppression of neoplastic proliferation of OSCCs.
Unlike the cell cycle arrest proteins such as p21
and p27, we observed that pro-survival proteins were significantly
reduced by HK in OSCCs. It is known that survivin is a member of
inhibitor of apoptosis protein family and its expression level has
been considered to play an important role in oncogenesis (40). Numerous studies have demonstrated
that negative regulation of survivin expression or inhibition of
its cellular function could lead to apoptotic cell death in cancer
cells (40). Recently, a study
reported that the transcription factor Sp1 can regulate
transactivation of survivin via direct binding to the GC-rich
promoter region (41,42). Another study revealed that
anti-apoptotic protein, Mcl-1, is a member of the Bcl-2 family and
is also associated in cancer progression and malignancies (43–45).
The down-regulation of Mcl-1 and survivin promotes apoptosis in
cancer cells (46–48). Based on these reports, modulation
of survivin and Mcl-1 could effectively suppress oncogenesis in
vivo and in vitro, suggesting the potential use of these
proteins in cancer treatment as a potential therapeutic target
gene. To further confirm whether HK could modulate anti-apoptotic
protein expressions toward apoptosis, we monitored alterations of
Mcl-1 and survivin when cells were treated with different doses.
Mcl-1, survivin and cell cycle regulatory proteins were greatly
reduced by HK treatment in a dose-dependent manner (Fig. 4). Therefore, HK can positively
regulate p27 and p21, and negatively regulate cyclin D1, Mcl-1 and
survivin in OSCCs, resulting in activation of a caspase-dependent
apoptosis pathway through activated caspase-3 and PARP (Fig. 5).
In this study, we investigated the cancer
chemoprevention effect of HK on OSCCs. Our results indicate that HK
has cell growth inhibitory activity and induces apoptosis in OSCCs
through inhibition of Sp1 expression, followed by transcriptional
regulation of the cell cycle regulating and anti-apoptotic
proteins. Taken together, HK might be a promising therapeutic agent
in the treatment of oral cancers. However, molecular mechanisms and
clinical studies for HK are necessary to elucidate its unexpected
potential toxicity and its clinical applications.
Abbreviations:
|
MTS
|
3-(4,5-dimethylthiazol–2-yl)-5-(3-carboxyme
thoxyphenyl)-2-(4-sulfophenyl)-2H–tetrazolium;
|
|
PMS
|
phenazine methosulfate;
|
|
DAPI
|
4′-6-diamidino-2-phenylindole;
|
|
TUNEL
|
terminal deoxynucleotidyltransferase
UTP nick end labeling
|
Acknowledgements
This research was supported by Basic
Science Research program through the National Research Foundation
Korea (NRF) Funded by the Ministry of Education, Science and
Technology (2011-0008463 and 2010-0021532) and the Cooperative
Research Program for Agriculture Science and Technology Development
(PJ007963), Rural Development Administration, Republic of
Korea.
References
|
1.
|
Hamada T, Wakamatsu T, Miyahara M, et al:
MUC4: a novel prognostic factor of oral squamous cell carcinoma.
Int J Cancer. 130:1768–1776. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Warnakulasuriya S: Global epidemiology of
oral and oropharyngeal cancer. Oral Oncol. 45:309–316. 2009.
View Article : Google Scholar
|
|
3.
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs over the 30 years from 1981 to 2010. J Nat
Prod. 75:311–335. 2012.PubMed/NCBI
|
|
4.
|
Fried LE and Arbiser JL: Honokiol, a
multifunctional anti-angiogenic and antitumor agent. Antioxid Redox
Signal. 11:1139–1148. 2009. View Article : Google Scholar
|
|
5.
|
Fujita M, Itokawa H and Sashida Y: Studies
on the components of Magnolia obovata Thunb. 3. Occurrence
of magnolol and honokiol in M. obovata and other allied
plants. Yakugaku Zasshi. 93:429–434. 1973.(In Japanese).
|
|
6.
|
Teng CM, Chen CC, Ko FN, et al: Two
antiplatelet agents from Magnolia officinalis. Thromb Res.
50:757–765. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Liou KT, Lin SM, Huang SS, Chih CL and
Tsai SK: Honokiol ameliorates cerebral infarction from
ischemia-reperfusion injury in rats. Planta Med. 69:130–134. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Lo YC, Teng CM, Chen CF, Chen CC and Hong
CY: Magnolol and honokiol isolated from Magnolia officinalis
protect rat heart mitochondria against lipid peroxidation. Biochem
Pharmacol. 47:549–553. 1994.
|
|
9.
|
Kuribara H, Kishi E, Hattori N, Yuzurihara
M and Maruyama Y: Application of the elevated plus-maze test in
mice for evaluation of the content of honokiol in water extracts of
magnolia. Phytother Res. 13:593–596. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Battle TE, Arbiser J and Frank DA: The
natural product honokiol induces caspase-dependent apoptosis in
B-cell chronic lymphocytic leukemia (B-CLL) cells. Blood.
106:690–697. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Shigemura K, Arbiser JL, Sun SY, et al:
Honokiol, a natural plant product, inhibits the bone metastatic
growth of human prostate cancer cells. Cancer. 109:1279–1289. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Garcia A, Zheng Y, Zhao C, et al: Honokiol
suppresses survival signals mediated by Ras-dependent phospholipase
D activity in human cancer cells. Clin Cancer Res. 14:4267–4274.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Deng J, Qian Y, Geng L, et al: Involvement
of p38 mitogen-activated protein kinase pathway in honokiol-induced
apoptosis in a human hepatoma cell line (hepG2). Liver Int.
28:1458–1464. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Li Z, Liu Y, Zhao X, et al: Honokiol, a
natural therapeutic candidate, induces apoptosis and inhibits
angiogenesis of ovarian tumor cells. Eur J Obstet Gynecol Reprod
Biol. 140:95–102. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Bai X, Cerimele F, Ushio-Fukai M, et al:
Honokiol, a small molecular weight natural product, inhibits
angiogenesis in vitro and tumor growth in vivo. J Biol Chem.
278:35501–35507. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Sheu ML, Chiang CK, Tsai KS, et al:
Inhibition of NADPH oxidase-related oxidative stress-triggered
signaling by honokiol suppresses high glucose-induced human
endothelial cell apoptosis. Free Radic Biol Med. 44:2043–2050.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Lee J, Jung E, Park J, et al:
Anti-inflammatory effects of magnolol and honokiol are mediated
through inhibition of the downstream pathway of MEKK-1 in NF-kappaB
activation signaling. Planta Med. 71:338–343. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Tse AK, Wan CK, Shen XL, Yang M and Fong
WF: Honokiol inhibits TNF-alpha-stimulated NF-kappaB activation and
NF-kappaB-regulated gene expression through suppression of IKK
activation. Biochem Pharmacol. 70:1443–1457. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Li L and Davie JR: The role of Sp1 and Sp3
in normal and cancer cell biology. Ann Anat. 192:275–283. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Hirano T, Gotoh M and Oka K: Natural
flavonoids and lignans are potent cytostatic agents against human
leukemic HL-60 cells. Life Sci. 55:1061–1069. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Yang SE, Hsieh MT, Tsai TH and Hsu SL:
Down-modulation of Bcl-XL, release of cytochrome c and sequential
activation of caspases during honokiol-induced apoptosis in human
squamous lung cancer CH27 cells. Biochem Pharmacol. 63:1641–1651.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Abdelrahim M, Smith R III, Burghardt R and
Safe S: Role of Sp proteins in regulation of vascular endothelial
growth factor expression and proliferation of pancreatic cancer
cells. Cancer Res. 64:6740–6749. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Ishibashi H, Nakagawa K, Onimaru M, et al:
Sp1 decoy transfected to carcinoma cells suppresses the expression
of vascular endothelial growth factor, transforming growth factor
beta1, and tissue factor and also cell growth and invasion
activities. Cancer Res. 60:6531–6536. 2000.
|
|
24.
|
Kong LM, Liao CG, Fei F, Guo X, Xing JL
and Chen ZN: Transcription factor Sp1 regulates expression of
cancer-associated molecule CD147 in human lung cancer. Cancer Sci.
101:1463–1470. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Chuang JY, Wu CH, Lai MD, Chang WC and
Hung JJ: Overexpression of Sp1 leads to p53-dependent apoptosis in
cancer cells. Int J Cancer. 125:2066–2076. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Choi ES, Shim JH, Jung JY, et al:
Apoptotic effect of tolfenamic acid in androgen
receptor-independent prostate cancer cell and xenograft tumor
through specificity protein 1. Cancer Sci. 102:742–748. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Shim JH, Shin JA, Jung JY, et al:
Chemopreventive effect of tolfenamic acid on KB human cervical
cancer cells and tumor xenograft by downregulating specificity
protein 1. Eur J Cancer Prev. 20:102–111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Davie JR, He S, Li L, et al: Nuclear
organization and chromatin dynamics - Sp1, Sp3 and histone
deacetylases. Adv Enzyme Regul. 48:189–208. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Grinstein E, Jundt F, Weinert I, Wernet P
and Royer HD: Sp1 as G1 cell cycle phase specific transcription
factor in epithelial cells. Oncogene. 21:1485–1492. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Chen F, Zhang F, Rao J and Studzinski GP:
Ectopic expression of truncated Sp1 transcription factor prolongs
the S phase and reduces the growth rate. Anticancer Res.
20:661–667. 2000.PubMed/NCBI
|
|
31.
|
Blume SW, Snyder RC, Ray R, Thomas S,
Koller CA and Miller DM: Mithramycin inhibits SP1 binding and
selectively inhibits transcriptional activity of the dihydrofolate
reductase gene in vitro and in vivo. J Clin Invest. 88:1613–1621.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Porter PL, Malone KE, Heagerty PJ, et al:
Expression of cell-cycle regulators p27Kip1 and cyclin E, alone and
in combination, correlate with survival in young breast cancer
patients. Nat Med. 3:222–225. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Abbas T and Dutta A: p21 in cancer:
intricate networks and multiple activities. Nat Rev Cancer.
9:400–414. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Sherr CJ and Roberts JM: CDK inhibitors:
positive and negative regulators of G1-phase progression. Genes
Dev. 13:1501–1512. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Murray AW: Recycling the cell cycle:
cyclins revisited. Cell. 116:221–234. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Albanese C, Johnson J, Watanabe G, et al:
Transforming p21ras mutants and c-Ets-2 activate the cyclin D1
promoter through distinguishable regions. J Biol Chem.
270:23589–23597. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Lee RJ, Albanese C, Stenger RJ, et al:
pp60(v-src) induction of cyclin D1 requires collaborative
interactions between the extracellular signal-regulated kinase,
p38, and Jun kinase pathways. A role for cAMP response
element-binding protein and activating transcription factor-2 in
pp60(v-src) signaling in breast cancer cells. J Biol Chem.
274:7341–7350. 1999.
|
|
38.
|
Shtutman M, Zhurinsky J, Simcha I, et al:
The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway.
Proc Natl Acad Sci USA. 96:5522–5527. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Weinstein IB: Relevance of cyclin D1 and
other molecular markers to cancer chemoprevention. J Cell Biochem
(Suppl). 25:23–28. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Li F: Survivin study: what is the next
wave? J Cell Physiol. 197:8–29. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Xu R, Zhang P, Huang J, Ge S, Lu J and
Qian G: Sp1 and Sp3 regulate basal transcription of the survivin
gene. Biochem Biophys Res Commun. 356:286–292. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Chun JY, Hu Y, Pinder E, Wu J, Li F and
Gao AC: Selenium inhibition of survivin expression by preventing
Sp1 binding to its promoter. Mol Cancer Ther. 6:2572–2580. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Yoon JH, Werneburg NW, Higuchi H, et al:
Bile acids inhibit Mcl-1 protein turnover via an epidermal growth
factor receptor/Raf-1-dependent mechanism. Cancer Res.
62:6500–6505. 2002.PubMed/NCBI
|
|
44.
|
Okaro AC, Deery AR, Hutchins RR and
Davidson BR: The expression of antiapoptotic proteins Bcl-2,
Bcl-X(L), and Mcl-1 in benign, dysplastic, and malignant biliary
epithelium. J Clin Pathol. 54:927–932. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Akgul C: Mcl-1 is a potential therapeutic
target in multiple types of cancer. Cell Mol Life Sci.
66:1326–1336. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Andersson Y, Juell S and Fodstad O:
Downregulation of the antiapoptotic MCL-1 protein and apoptosis in
MA-11 breast cancer cells induced by an anti-epidermal growth
factor receptor-pseudomonas exotoxin a immunotoxin. Int J Cancer.
112:475–483. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Chetoui N, Sylla K, Gagnon-Houde JV, et
al: Down-regulation of mcl-1 by small interfering RNA sensitizes
resistant melanoma cells to fas-mediated apoptosis. Mol Cancer Res.
6:42–52. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Wei SH, Dong K, Lin F, et al: Inducing
apoptosis and enhancing chemosensitivity to gemcitabine via RNA
interference targeting Mcl-1 gene in pancreatic carcinoma cell.
Cancer Chemother Pharmacol. 62:1055–1064. 2008. View Article : Google Scholar : PubMed/NCBI
|