Introduction
Primary sclerosing cholangitis (PSC), a chronic
inflammatory disease, is characterized by fibrous thickening of the
bile duct walls and causes multiple stenoses of the intra- and
extra-hepatic bile ducts. Cholangiocarcinoma (CCA) occurs with high
frequency (5–13%) in PSC (1–4). CCA
is a prognostic factor for PSC. However, the underlying mechanism
of carcinogenesis is not well understood.
There are many gastrointestinal cancers that develop
because of underlying chronic inflammation such as that found in
PSC. These include gastric cancers caused by chronic gastritis due
to Helicobacter pylori infection, hepatocellular carcinoma
caused by chronic hepatitis due to hepatitis B virus or hepatitis C
virus infection, colorectal cancer due to inflammatory bowel
disease, and esophageal adenocarcinoma due to Barrett’s esophagus.
The expression of cyclooxygenase-2 (COX-2) and microsomal
prostaglandin E synthase-1 (mPGES-1) is induced by inflammation,
and studies suggest that these enzymes are involved in the
development of these carcinomas (5–11).
COX-2 and mPGES-1 are both involved in the arachidonate cascade:
COX-2 converts arachidonic acid to prostaglandin H2
(PGH2) and mPGES-1 converts PGH2 to PGE2. Thus,
PGE2 is elevated because of increased COX-2 and mPGES-1
expression, and it plays an important role in carcinogenesis by
promoting cell proliferation, angiogenesis, cell infiltration and
inhibiting apoptosis (12). CCA
arising in association with PSC (PSC-associated CCA) also develops
because of underlying chronic inflammation, which implies the
involvement of COX-2 and mPGES-1 in cholangiocarcinogenesis.
However, the mechanism has not yet been investigated.
To elucidate the carcinogenic mechanisms associated
with PSC, this study investigated COX-2 and mPGES-1 expression in
PSC-associated CCA tissues, CCA unrelated to PSC (sporadic CCA)
tissues, non-neoplastic bile duct epithelial cells (BDECs) in PSC,
and non-neoplastic BDECs in sporadic CCA.
Materials and methods
Tissue samples
All tissue samples in this study were obtained from
Hiroshima University Hospital patients by surgical resection or
biopsy. PSC tissue samples were obtained from 15 patients. Seven of
these patients had PSC-associated CCA, of whom 2 had intrahepatic
CCA and 5 had extrahepatic CCA. The histological type was
well-differentiated tubular adenocarcinoma for all patients. The
control group was comprised of 15 sporadic CCA patients, of whom 7
had intrahepatic CCA and 8 had extrahepatic CCA (6 had perihilar
extrahepatic bile duct tumors and two had distal extrahepatic bile
duct tumors). Histologically, they were well-differentiated tubular
adenocarcinomas (6 patients), moderately differentiated tubular
adenocarcinomas (6 patients), and poorly differentiated tubular
adenocarcinoma (3 patients).
Sections fixed in 10% buffered formalin for 24 h
were used for immunohistochemical staining. CCA tissues were frozen
rapidly in liquid nitrogen and stored at −80°C before being used
for reverse transcription-polymerase chain reaction (RT-PCR). This
study was approved by the Hiroshima University Hospital ethics
committee.
Immunohistochemistry and evaluation
The streptavidin-biotin method was used for
immunohistochemical staining of COX-2 and mPGES-1. Four-micron
thick paraffin-embedded sections were first deparaffinized and
soaked for 30 min in 3% hydrogen peroxide solution to inhibit
endogenous peroxidase activity. They were then soaked in Epitope
Retrieval Solution, pH 9.0, at 95°C in a hot water bath for 40 min
(Novocastra Laboratories Ltd., Newcastle upon Tyne, UK). After
being washed in phosphate-buffered saline (PBS) (pH 7.4), the
sections were reacted in 10% normal goat serum at room temperature
for 10 min in order to block non-specific antibody responses. The
sections were then incubated with primary antibodies overnight at
4°C. The primary antibodies were as follows: polyclonal rabbit
anti-COX-2 antibodies (Immuno-Biological Laboratories Co. Ltd,
Gunma, Japan) at a dilution of 1:200 and polyclonal rabbit
anti-mPGES-1 antibodies (Cayman Chemical, Ann Arbor, MI, USA) at a
dilution of 1:500. For the control, normal rabbit IgG (Santa Cruz
Biotechnology, Dallas, TX, USA) was used instead of the primary
antibodies. After washing in PBS, the sections were incubated with
biotin-labeled secondary antibodies at room temperature for 30 min.
The sections were then washed in PBS and incubated with
peroxidase-labeled streptavidin at room temperature for 30 min.
They were visualized using a 3 3′-diaminobenzidine
tetrahydrochloride substrate (Dako Japan, Kyoto, Japan) and
counterstained using Mayer’s hematoxylin.
Ki-67 immunostaining was performed using Ventana
BenchMark Ultra (Ventana Medical Systems, Tucson, AZ, USA)
automatic staining device and rabbit anti-Ki-67 antibodies (Ki-67)
as the primary antibody.
COX-2 and mPGES-1 expression in CCA tissues and
non-neoplastic BDECs was evaluated using a method reported
previously (13). The staining
intensity for each section was scored 0–3 as follows: 0, negative
staining; 1, weakly positive staining; 2, moderately positive
staining; and 3, strongly positive staining. The maximum intensity
of staining and the most extensive intensity level of positive
cells were evaluated separately. The ‘extent of distribution’ of
positive cells for each section was scored 0–3 as follows: 0,
negative; 1, 1–33%; 2, 34–66%; and 3, 67–100%. The total score of
these three parameters was then used to evaluate each section. The
median score for each histological category was used to perform a
statistical comparison of COX-2 and mPGES-1 immunoreactivity.
Ki-67 was used to calculate the proportion of
positive cells in CCA tissues and non-neoplastic BDECs. In total,
500 cells were counted in areas with many positive cells, and the
percentage of positive cells relative to the total cell count was
expressed as the Ki-67 labeling index (LI).
For non-neoplastic BDECs, large BDECs, comprising
those from the extrahepatic bile duct to the second branches of the
left and right hepatic ducts, and small BDECs, comprising those
from septal and interlobular bile ducts, were evaluated separately.
The non-neoplastic BDECs of sporadic CCA patients were used as
controls for comparison with those from PSC patients.
Immunostaining of cancer tissue was possible for all
patients. Immunostaining of non-neoplastic large BDECs was possible
for 11 PSC and 14 control patients. Immunostaining of
non-neoplastic small BDECs was possible for 9 PSC and 14 control
patients.
RT-PCR
Total RNA was extracted and purified from frozen
tumor sections using the RNeasy mini kit (Qiagen, Germantown, MA,
USA). RT-PCR was performed in two steps. First, total RNA was
converted to cDNA using the PrimeScript RT-PCR Kit (Takara Bio
Inc., Shiga, Japan). A 20 μl reaction mixture containing 200
ng total RNA was prepared and subjected to reverse transcription at
42°C for 30 min. The mixture was then incubated at 95°C for 5 min
to deactivate reverse transcriptase and then cooled to 4°C.
The Applied Biosystems 7900HT Fast Real-time PCR
System (Applied Biosystems, Foster City, CA, USA) was used to
perform RT-PCR. Each PCR reaction mixture contained 1 μl
cDNA template, 10 μl Power SYBR-Green PCR Master mix
(Applied Biosystems), 8 pmol primer set, and water to make a total
volume of 20 μl. First denaturation was performed at 95°C
for 10 min and then 50-cycle PCR (95°C for 10 min, 50°C for 1 min)
was conducted. Primer sequences were as follows: COX-2 sense,
5′-TTCAAATGAGATTGTGGAAAATTGCT-3′; antisense,
5′-GATCATCTCTGCCTGAGTATCTT-3′; mPGES-1 sense,
5′-ACCAGACCATGGGCCAAGAG-3′; antisense 5′-GGCCCACCACAATCTGGAA-3′.
Amplification results were analyzed using sequence detection system
2.4.1 software (Applied Biosystems). mRNA expression levels were
corrected using glyceraldehyde 3-phosphate dehydrogenase as a
housekeeping gene and expressed as arbitrary units.
Statistical analysis
Statistical analysis was performed using JMP 9.0.0
(SAS, Cary, NC, USA). All results are expressed as means ± SEM.
Continuous variables between the two groups were compared using the
Mann-Whitney U test. P<0.05 was considered statistically
significant.
Results
Immunohistochemical analysis of
COX-2
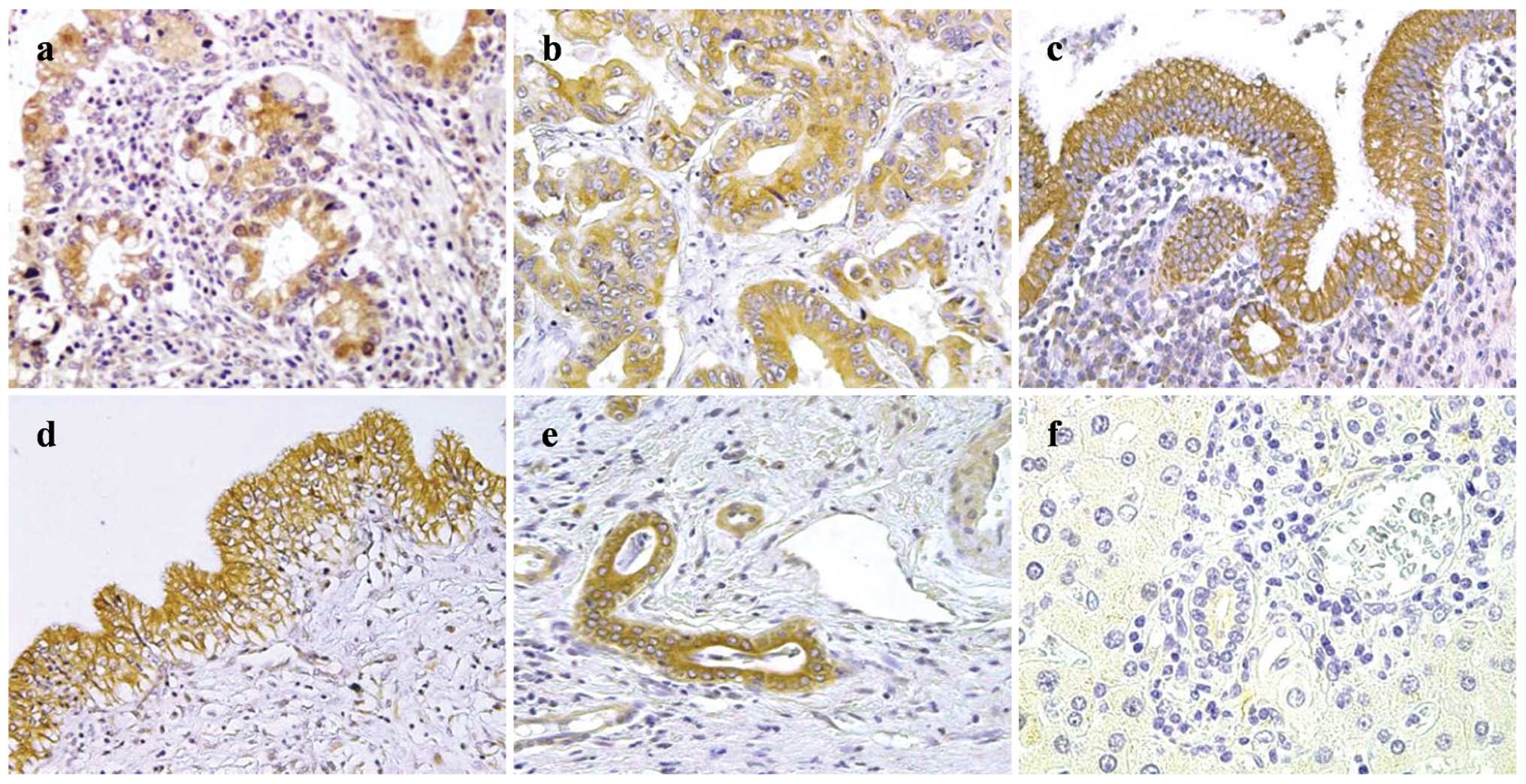
Examples of COX-2 staining are shown in Fig. 1. In PSC-associated CCA tissues
(Fig. 1a), COX-2 expression was
strong in all of the patients. In the sporadic CCA tissues
(Fig. 1b), COX-2 expression was
observed for 7/15 patients (47%). In the non-neoplastic large
BDECs, COX-2 expression was strong for all PSC patients (Fig. 1c), whereas the expression was
observed in 12 of 15 control patients (80%; Fig. 1d), and immunoreactivity was lower
than that in the PSC patients. In the non-neoplastic small BDECs,
COX-2 expression was moderate to strong for all PSC patients
(Fig. 1e), whereas the expression
was negative for 12 of 14 control patients (86%; Fig. 1f). COX-2 expression scores for the
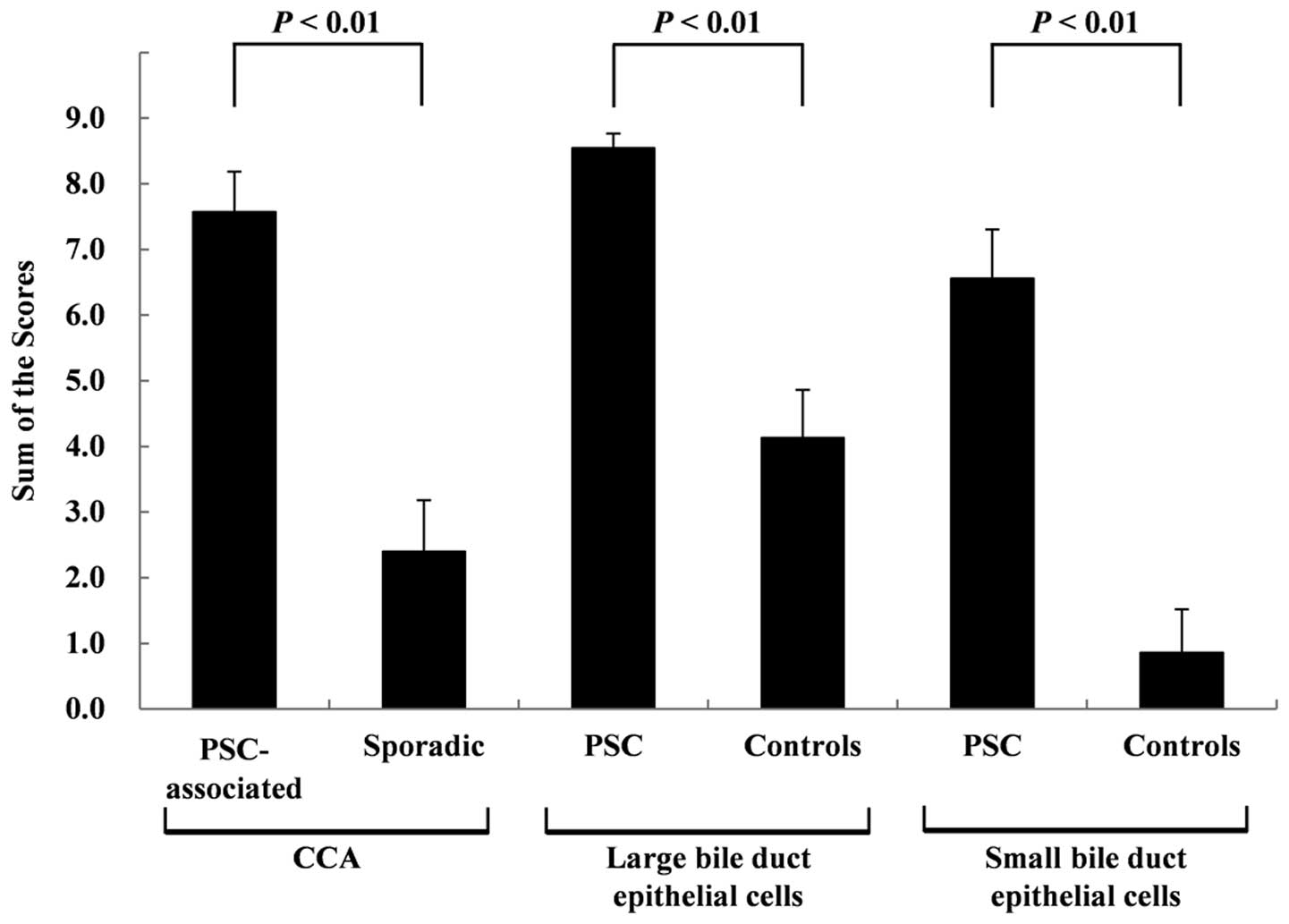
CCA tissues and non-neoplastic BDECs are shown in Fig. 2. The scores for the PSC-associated
CCA tissues were significantly higher (P<0.01) than those for
the sporadic CCA tissues at 7.57±0.61 and 2.40±0.78, respectively.
The scores for the non-neoplastic large BDECs in the PSC patients
were significantly higher (P<0.01) than those in the control
patients at 8.55±0.22 and 4.13±0.73, respectively. The scores for
the non-neoplastic small BDECs in the PSC patients were
significantly higher (P<0.01) than those for the control
patients at 6.56±0.75 and 0.86±0.66, respectively. No significant
differences were observed between the scores for the non-neoplastic
BDECs in the PSC patients with CCA and PSC patients without CCA
(data not shown).
Immunohistochemical analysis of
mPGES-1
Examples of mPGES-1 staining are shown in Fig. 3. mPGES-1 expression in the
PSC-associated CCA tissues (Fig.
3a) was moderate to strong for the majority of patients.
Expression was observed in sporadic CCA tissues (Fig. 3b) in the majority of patients, but
immunoreactivity in the sporadic CCA tissues was lower than that in
the PSC-associated CCA tissues. mPGES-1 expression was moderate to
strong in the non-neoplastic large BDECs of the majority of PSC
tissues (Fig. 3c) and control
(Fig. 3d) patients. Moderate or
higher expression was observed in the non-neoplastic small BDECs of
8 of 9 PSC patients (89%; Fig.
3e), whereas the expression was negative in the non-neoplastic
small BDECs of 6 of 13 control patients (43%; Fig. 3f). The scores for mPGES-1
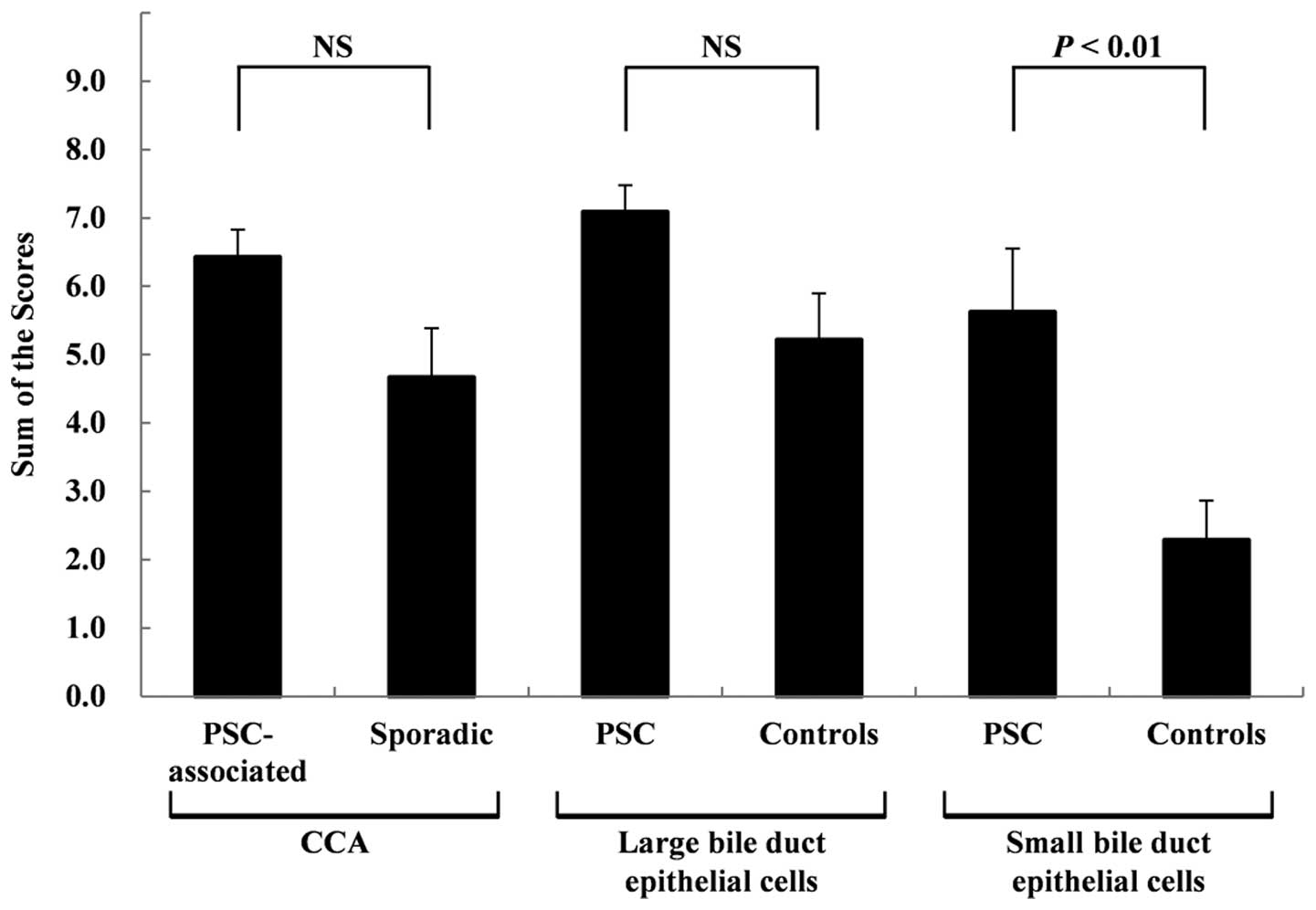
expression are shown in Fig. 4. No
significant differences were observed between the scores for the
PSC-associated and sporadic CCA tissues at 6.43±0.40 and 4.67±0.72,
respectively. No significant differences were observed between the
scores for the non-neoplastic large BDECs in the PSC and control
patients (7.09±0.39 and 5.21±0.68, respectively). However, a trend
of stronger expression was observed in the non-neoplastic large
BDECs of the PSC patients. The scores for the non-neoplastic small
BDECs in the PSC patients were significantly higher (P<0.01)
than those in the control patients at 5.63±0.92 and 2.29±0.58,
respectively.
Ki-67 labeling index
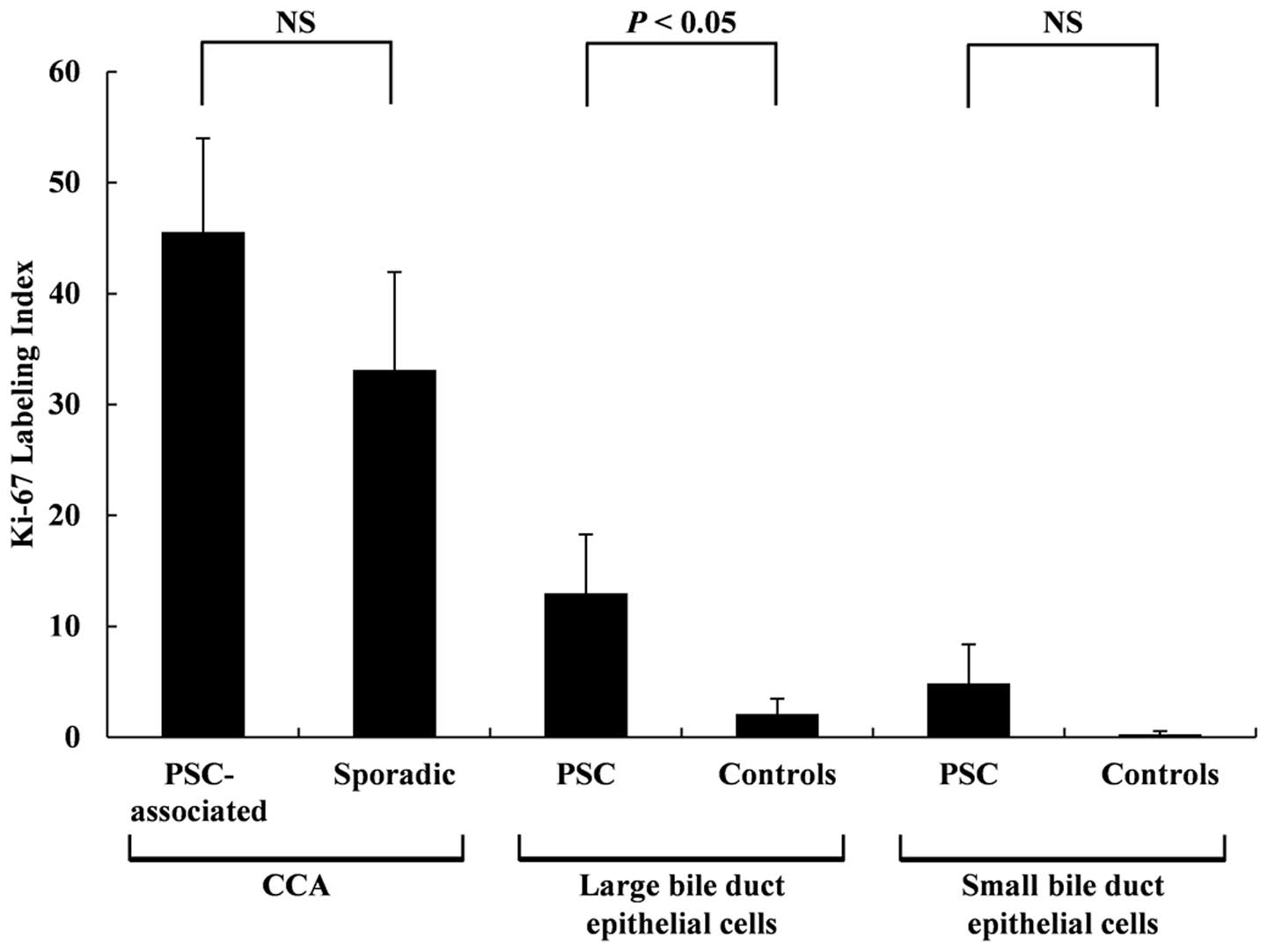
Examples of Ki-67 staining are shown in Fig. 5. The Ki-67 LI results are shown in
Fig. 6. No statistically
significant differences were observed between the Ki-67 LI for the
PSC-associated CCA tissues and the sporadic CCA tissues at 45.6±8.4
and 33.1±8.8, respectively, although a trend of higher Ki-67 LI was
observed for the PSC-associated CCA tissues. The Ki-67 LI for the
non-neoplastic large BDECs in the PSC patients were significantly
higher (P<0.01) than those in the control patients at 13.0±5.3
and 2.1±1.4, respectively. No statistically significant differences
were observed between Ki-67 LI for the non-neoplastic small BDECs
of the PSC and control patients (4.9±3.5 and 0.28±0.28,
respectively), but a trend of higher Ki-67 LI was observed in the
non-neoplastic small BDECs of the PSC.
RT-PCR
COX-2 and mPGES-1 mRNA levels in the CCA tissues
were evaluated using quantitative real-time PCR. This was performed
using the tissues samples of 7 PSC-associated CCA patients and 15
sporadic CCA patients. The COX-2 mRNA levels were significantly
higher (P<0.05) in the PSC-associated CCA tissues than in the
sporadic CCA tissues. In contrast, no significant differences in
mPGES-1 mRNA expression were observed between the two groups
(Fig. 7).
Discussion
This study indicated that COX-2 and mPGES-1
expression was upregulated in both CCA tissues and non-neoplastic
BDECs in PSC patients. COX-2 expression is known to be upregulated
in inflammatory diseases that give rise to cancer (5–11) as
well as in various carcinomas (14–17)
and is believed to be involved in carcinogenesis and tumor
proliferation. High COX-2 expression has been reported in
non-neoplastic BDECs in PSC (18,19).
However, the correlation between COX-2 expression and
carcinogenesis has not yet been investigated in patients. It is
known that COX-2 is highly expressed in sporadic CCA (20–22),
which might suggest its involvement in CCA progression via
PGE2 production (23–25).
Tsuneoka et al reported that a selective COX-2 inhibitor
suppressed CCA development in hamsters (26), suggesting that COX-2 might be
involved in cholangiocarcinogenesis. Our study demonstrated marked
COX-2 expression in non-neoplastic BDECs and CCA tissues in PSC.
This finding suggests that local PGE2 production might
be elevated in both non-neoplastic BDECs and CCA tissues in PSC.
PGE2 exhibits various effects, including cell
proliferation, angiogenesis, and inhibition of apoptosis, and it
plays an important role in cancer development and progression
(12). Ki-67 immunohistochemical
staining was performed to evaluate the cell proliferative effect of
PGE2. Our findings demonstrated that cell proliferation
was upregulated not only in CCA tissues but also in non-neoplastic
BDECs in PSC. We suggest that in PSC, chronic inflammation could
upregulate expression of COX-2 and mPGES-1, resulting in increased
PGE2 production and promotion of carcinogenesis.
Previous studies have suggested that nitric oxide-induced oxidative
damage to DNA (27) and gene
mutations due to activated cytidine deaminase (28) lead to PSC carcinogenesis. It is
therefore believed that PGE2 acts as a promoter when
BDECs in PSC that have acquired gene mutations form tumors and the
cancer progresses.
This study investigated mPGES-1 expression in PSC.
mPGES-1 is one of three types of PGES. Similar to COX-2, mPGES-1 is
an inducible enzyme and its expression level is increased by
inflammatory irritation (29).
mPGES-1 expression is known to be elevated in various carcinomas
(30–34), and mPGES-1 might be involved in the
carcinogenesis of gastrointestinal cancers that develop because of
underlying chronic inflammation (6,9,11).
Lu et al (35) reported
that mPGES-1 is overexpressed in human CCA tissues and that mPGES-1
promotes CCA development in SCID mice. Our study demonstrated for
the first time that mPGES-1 is highly expressed not only in CCA
tissues but also in the non-neoplastic BDECs in PSC. Cooperation of
COX-2 and mPGES-1 might promote local PGE2
production.
Although weaker than in those of the PSC patients,
COX-2 and mPGES-1 expression was observed in the non-neoplastic
large BDECs of the control patients. This might be explained as
follows. First, the non-neoplastic large BDECs used as controls
were obtained from sporadic CCA patients. Many of these sporadic
CCA patients exhibited cholangitis resulting from bile duct
occlusion. Furthermore, all patients underwent cholangiography and
treatment for obstructive jaundice prior to surgery. The irritation
associated with temporary bile duct inflammation and preoperative
treatment might have affected COX-2 and mPGES-1 expression. There
were differences in the levels of COX-2 and mPGES-1 expression in
the non-neoplastic BDECs of the control patients. COX-2 and mPGES-1
expression is induced by inflammatory stimulation, but different
transcriptional regulatory mechanisms are involved in the
expression of each. While NF-κB, CRE, E-box, and NF-IL6 are
believed to be important in the transcriptional regulation of COX-2
(36), Egr-1 plays a vital role in
the transcriptional regulation of mPGES-1 (37). This difference might have had an
effect on the transcriptional differences observed between the two
enzymes.
In conclusion, COX-2 and mPGES-1 were highly
expressed in PSC-associated CCA tissues and non-neoplastic BDECs in
PSC and were involved in CCA development. However, the sample size
of this study was small. It is therefore necessary to conduct a
study using a larger sample size in order to clarify the
correlations between COX-2/mPGES-1 and CCA carcinogenesis in
PSC.
Acknowledgements
We are deeply grateful to Koji Arihiro
and Cytotechnologist Miyo Oda of the Department of Anatomical
Pathology, Hiroshima University Hospital for their kind support in
performing Ki-67 immunostaining.
References
|
1.
|
Rosen CB, Nagorney DM, Wiesner RH, Coffey
RJ Jr and LaRusso NF: Cholangiocarcinoma complicating primary
sclerosing cholangitis. Ann Surg. 213:21–25. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Bergquist A, Ekbom A, Olsson R, et al:
Hepatic and extrahepatic malignancies in primary sclerosing
cholangitis. J Hepatol. 36:321–327. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Burak K, Angulo P, Pasha TM, Egan K, Petz
J and Lindor KD: Incidence and risk factors for cholangiocarcinoma
in primary sclerosing cholangitis. Am J Gastroenterol. 99:523–526.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Morris-Stiff G, Bhati C, Olliff S, et al:
Cholangiocarcinoma complicating primary sclerosing cholangitis: a
24-year experience. Dig Surg. 25:126–132. 2008.PubMed/NCBI
|
|
5.
|
Sheu BS, Yang HB, Sheu SM, Huang AH and Wu
JJ: Higher gastric cyclooxygenase-2 expression and precancerous
change in Helicobacter pylori-infected relatives of gastric
cancer patients. Clin Cancer Res. 9:5245–5251. 2003.PubMed/NCBI
|
|
6.
|
Takasu S, Tsukamoto T, Cao XY, et al:
Roles of cyclooxygenase-2 and microsomal prostaglandin E synthase-1
expression and beta-catenin activation in gastric carcinogenesis in
N-methyl-N-nitrosourea-treated K19-C2mE transgenic mice. Cancer
Sci. 99:2356–2364. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Giannitrapani L, Inqrao S, Soresi M, et
al: Cycloxygenase-2 expression in chronic liver disease and
hepatocellular carcinoma: an immunohistochemical study. Ann NY Acad
Sci. 1155:293–299. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Takii Y, Abiru S, Fujioka H, et al:
Expression of microsomal prostaglandin E synthase-1 in human
hepatocellular carcinoma. Liver Int. 17:989–996. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Talero E, Sánchez-Fidalgo S, Villegas I,
de la Lastra CA, Illanes M and Motilva V: Role of different
inflammatory and tumor biomarkers in the development of ulcerative
colitis-associated carcinogenesis. Inflamm Bowel Dis. 17:696–710.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Wilson KT, Fu S, Ramanujam KS and Meltzer
SJ: Increased expression of inducible nitric oxide synthase and
cyclooxygenase-2 in Barrett’s esophagus and associated
adenocarcinoma. Cancer Res. 58:2929–2934. 1998.
|
|
11.
|
Jang TJ, Min SK, Bae JD, et al: Expression
of cycloxygenase-2, microsomal prostaglandin E synthase 1, and EP
receptors is increased in rat oesophageal squamous cell dysplasia
and Barrett’s metaplasia induced by duodenal contents reflux. Gut.
53:27–33. 2004.PubMed/NCBI
|
|
12.
|
Wang D and Dubois RN: Eicosanoids and
cancer. Nat Rev Cancer. 10:181–193. 2010. View Article : Google Scholar
|
|
13.
|
Koga H, Sakisaka S, Ohishi M, et al:
Expression of cyclooxygenase-2 in human hepatocellular carcinoma:
relevance to tumor dedifferentiation. Hepatology. 29:688–696. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Wendum D, Masliah J, Trugnan G and Fléjou
JF: Cyclooxygenase-2 and its role in colorectal cancer development.
Virchows Arch. 445:327–333. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Jang TJ: Expression of proteins related to
prostaglandin E2 biosynthesis is increased in human gastric and
during gastric carcinogenesis. Virchows Arch. 445:564–571. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Davies G, Martin LA, Sacks N and Dowsett
M: Cyclooxygenase-2 (COX-2), aromatase and breast cancer: a
possible role for COX-2 inhibitors in breast cancer
chemoprevention. Ann Oncol. 13:669–678. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Ermert L, Dierkes C and Ermert M:
Immunohistochemical expression of cyclooxygenase isoenzymes and
downstream enzymes in human lung tumors. Clin Cancer Res.
9:1604–1610. 2003.PubMed/NCBI
|
|
18.
|
Hayashi N, Yamamoto H, Hiraoka N, et al:
Differential expression of cyclooxygenase-2 (COX-2) in human bile
duct epithelial cells and bile duct neoplasm. Hepatology.
34:638–650. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Endo K, Yoon BI, Pairojkul C, Demetris AJ
and Sirica AE: ERBB-2 overexpression and cyclooxygenase-2
up-regulation in human cholangiocarcinoma and risk conditions.
Hepatology. 36:439–450. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Chariyalersak S, Sirikulchayanonta V,
Mayer D, et al: Aberrant cyclooxygenase isozyme expression in human
intrahepatic cholangiocarcinoma. Gut. 48:80–86. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Wu GS, Wang JH, Liu ZR and Zou SQ:
Expression of cyclooxygenase-1 and -2 in extra-hepatic
cholangiocarcinoma. Hepatobiliary Pancreat Dis Int. 1:429–433.
2002.PubMed/NCBI
|
|
22.
|
Kim HJ, Lee KT, Kim EK, et al: Expression
of cyclooxygenase-2 in cholangiocarcinoma: correlation with
clinicopathological features and prognosis. J Gastroenterol
Hepatol. 19:582–588. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Nzeako UC, Guicciardi ME, Yoon JH, Bronk
SF and Gores GJ: COX-2 inhibits Fas-mediated apoptosis in
cholangiocarcinoma cells. Hepatology. 35:552–559. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Wu T, Han C, Lunz JG 3rd, Michalopoulos G,
Shelhamer JH and Demetris AJ: Involement of 85-kd cytosolic
phospholipase A2 and cyclooxygenase-2 in the proliferation of human
cholangiocarcinoma cells. Hepatology. 36:363–373. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Han C and Wu T: Cyclooxygenase-2 derived
prostaglandin E2 promotes human cholangiocarcinoma cell growth and
invasion through EP1 receptor-mediated activation of the epidermal
growth factor receptor and Akt. J Biol Chem. 280:24053–24063. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Tsuneoka N, Tajima Y, Kitazato A, et al:
Chemopreventative effect of a cyclooxygenase-2-specific inhibitor
(etodolac) on chemically induced biliary carcinogenesis in
hamsters. Carcinogenesis. 29:830–833. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Jaiswal M, LaRusso NF, Shapiro RA, Billar
TR and Gores GJ: Nitric oxide-mediated inhibition of DNA repair
potentiates oxidative DNA damage in cholangiocytes.
Gastroenterology. 120:190–199. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Komori J, Marusawa H, Machimoto T, et al:
Activation-induced cytidine deaminase links bile duct inflammation
to human cholangiocarcinoma. Hepatology. 47:888–896. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Jakobsson PJ, Thorén S, Morgenstern R and
Samuelsson B: Identification of human E synthase: a microsomal,
glutathione-dependent, inducible enzyme, constituting a potential
novel drug target. Proc Natl Acad Sci USA. 96:7220–7225. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Yoshimitsu K, Altorki NK, Golojanin D, et
al: Inducible prostaglandin E synthase is overexpressed in
non-small cell lung cancer. Clin Cancer Res. 7:2669–2674.
2001.PubMed/NCBI
|
|
31.
|
Van Rees BP, Sivula A, Thorén S, et al:
Expression of microsomal prostaglandin E synthase-1 in intestinal
type gastric adenocarcinoma and in gastric cancer cell lines. Int J
Cancer. 107:551–556. 2003.PubMed/NCBI
|
|
32.
|
Yoshimatsu K, Golijanin D, Paty PB, et al:
Inducible prostaglandin E synthase is overexpressed in colorectal
adenomas and cancer. Clin Cancer Res. 7:3971–3976. 2001.PubMed/NCBI
|
|
33.
|
Mehrotra S, Morimiya A, Agarwal B, Konger
R and Badve S: Microsomal prostaglandin E2 synthase-1 in breast
cancer: a potential target for therapy. J Pathol. 208:356–363.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Kawata R, Hyo S, Araki M and Takenaka H:
Expression of cyclooxygenase-2 and microsomal prostaglandin E
synthase-1 in head and neck squamous cell carcinoma. Auris Nasus
Larynx. 37:482–487. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Lu D, Han C and Wu T: Microsomal
prostaglandin E synthase-1 inhibits PTEN and promotes experimental
cholangiocarcinogenesis and tumor progression. Gastroenterology.
140:2084–2094. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Tanabe T and Tohnai N: Cyclooxygenase
isozymes and their gene structures and expression. Rostaglandins
Other Lipid Mediat. 68–69:95–114. 2002.PubMed/NCBI
|
|
37.
|
Subbaramaiah K, Yoshimatsu K, Scheri E, et
al: Microsomal prostaglandin E synthase-1 is overexpressed in
inflammatory bowel disease. Evidence for involvement of
transcription factor Egr-1. J Biol Chem. 279:12647–12658. 2004.
View Article : Google Scholar : PubMed/NCBI
|