Introduction
Metastasis is a major cause of gastric cancer
mortality. Metastases begin when cancer cells detach from the
original tumor tissue and become circulating tumor cells (CTCs).
The CTCs are highly resistant to anticancer drugs and possess the
potential to implant in distant organs (1), making the treatment of metastatic
gastric cancer very difficult and ineffective. Increasing
chemo-sensitivity of the CTCs prior to, during, or after a surgical
procedure would be an effective therapeutic strategy. However, the
CTCs from body circulation are difficult to isolate and the lack of
the knowledge regarding the mechanisms governing the
chemo-resistance of CTCs have greatly hindered therapy.
Increasing evidence suggests that gastric cancer is
a process in which the persistence of the tumor relies on a small
population of tumor-initiating cells, the cancer stem cells (CSCs)
(2,3). CSCs are capable of self-renewal and
thereby possess the ability for unlimited proliferation. CSC theory
proposes that majority of the cancer cells in the cancer tissue do
not possess the potential of metastasis to generate new tumors.
Thus, only a very small portion of the cancer cells (i.e., the
CSCs) are tumorigenic and have the capability to migrate and
invade. In contrast to the majority of cancer cells, CSCs persist
in tumors as a distinct subpopulation and possess the properties of
stem cells such as self-renewal capability, undifferentiated
status, expression of stem cell markers and chemo-resistance
(4–6). The gastrointestinal cancer stem cells
have been isolated from both cancer tissues and cell lines, using
colon cancer stem cell marker CD133 and gastric cancer stem cell
marker CD44 (2,5,6).
Since CTCs are the metastatic cancer cells prior to invasion and
plantation to distant tissues, these cells exhibit similar
characteristics as CSCs. Indeed, gastric cancer cells cultured in a
suspended spheroid phenotype in vitro were highly resistant
to anticancer drugs (3,7). As non-adherent cells, CTCs lose the
expression of the differentiation marker E-cadherin and showed an
upregulation of CD44 (3). Thus,
examining the role of CTCs or CSCs is a critical point in the
therapeutic strategy of the disease. Chemotherapy directed towards
the CTCs or CSCs by enhancing their chemotherapy sensitivity would
be such an approach.
Cancer cells interact with the microenvironment via
complex autocrine and paracrine mechanisms. Disrupting these
mechanisms can induce aberrant cell proliferation, adhesion,
function and migration that promote malignant behavior. Adipose
tissue, like bone marrow, contains stromal cells called ADSCs
(adipose-derived stromal cells). ADSCs are considered a true
endocrine tissue since adipose lineage cells display a strong
secretory activity. ADSCs have been shown to interfere with the
proliferation of tumor cells by altering cell cycle progression
(8,9). However, the identification of the
mechanisms involved in stromal and cancer cell interactions,
particularly phenotypic changes and chemo-sensitivity of the
gastric cancer cells caused by ADSC has not been established.
The effects of ADSCs on cancer cells have been
studied previously. For instance, when breast cancer cells obtained
in vivo are co-injected with ADSCs, initial tumor growth and
metastasis were observed (10–13).
Crude SVF (stromal vascular fraction) instead of ADSCs were used.
SVF fraction is a heterogeneous mixture containing many different
cell subsets including native ADSCs, mature endothelial and
hematopoietic cells [the latter representing a large portion of
this fraction (≤20%)]. The functional properties of SVF are
discrepant and may explain why the ability of ADSCs to support or
suppress tumor cell proliferation is unclear. Nevertheless, the
functional properties of ADSCs are multi-potent, providing
functional cell support and modulating immuno-inflammatory
functions (14).
To study the interactions between ADSC and cancer
stem cells, we used purified human ADSC instead of SVF in this
study. We hypothesize that a potential attachment surface may be
able to adhere the gastric CTCs in vivo and therefore
promote chemo-sensitivity of the cells to anticancer drugs. We have
successfully isolated viable ADSCs in our lab using fluorescent
activated cell sorting by CD34+, CD73+ and
CD105+ and demonstrated that these type of cells have
functional self-renewal and transdifferentiation potential.
In this investigation, we demonstrated that
introduction of an appropriate attachment surface significantly
promoted chemo-sensitivity of the non-adherent CD44+
gastric cancer cells and that the human adipose stem cells may
function as a ‘living vehicle surface” for such a purpose in
vivo.
Materials and methods
Cell culture
Human gastric carcinoma AGS cells were maintained in
F-12K medium [Catalog No. 30-2004, American Type Culture Collection
(ATCC), Manassas, VA, USA] with addition of 10% non-heat
inactivated fetal bovine serum (FBS). Human gastric carcinoma
Hs746T cells were maintained in Dulbacco’s modified Eagle’s medium
(DMEM) (Catalog No. 30-2002, ATCC) with addition of 10% FBS.
CD44+ gastric carcinoma cells or human adipose stem
cells (ADSCs) were maintained in modified StemPro medium
(Invitrogen Co., Carlsbad, CA, USA) (1X DMEM/F-12/GlutaMax, 1X
StemPro Growth Supplement, 1.8% BSA, 8 ng/ml FGF, 10 ng/ml Nodal,
10 ng/ml Noggin and 0.1 mM 2-mercaptoethanol) in an Ultra Low
Attachment Surface (ULAS) flask (polystyrene coated with neutral
charged, hydrophilic hydrogel) (Corning Inc.).
Magnetic cell separation
CD44+ gastric cancer cells were labeled
with anti-CD44 antibody (Cell Signaling), followed by incubation
with magnetic-beads conjugated goat anti-mouse secondary antibody
(New England BioLab) and separation of the labeled cells from the
unlabeled cell population using a Magnetic Separation Pack (New
England BioLab).
Isolation of ADSCs
Human (h) ADSCs were isolated from adipose tissue
obtained from 12 female donors during abdominal surgery. At the
time of the surgical procedure, adipose tissues were collected into
sterile containers and transported to the tissue culture
laboratory. Tissues were incubated in a solution containing 33%
penicillin/streptomycin and fungi-zone for 30 min at 4°C to inhibit
bacterial growth. Tissues were then washed with PBS and digested
with collagenase (1%) + 0.05% dispase for 2–3 h at 37°C in a
shaking water bath. Collagenase was neutralized with growth media
containing 10% FBS and single cells isolated by filtering the
suspension through a 70-μm nylon mesh strainer (Falcon).
Human adipose cells were pelleted by centrifugation at 1,500 rpm.
RBCs were lysed with 160 mM ammonium chloride (Sigma) and ADSCs
pelleted as above. Cells were expanded for 3 passages
(corresponding to ∼3 population doublings per passage) in growth
media (DMEM, 10% FBS, 1% penicillin/ streptomycin, 4 mM
L-glutamine, 1 mM sodium pyruvate) before undergoing further
studies. Cultured adipose cells were harvested and subjected to
fluorescence-activated cell sorting (FACS) to characterize cell
phenotype through CD34+, CD73+,
CD105+. A member of the authorized study personnel
obtained patient consent and HIPAA authorization; these approved,
signed forms will be maintained in the Department of Surgery,
Division of Plastic Surgery. For the experiments, cells of the
second and third passage were used.
Morphology
Olympus CKX41SF microscope connected with Olympus
DP-12 camera (Olympus Co., Japan) were used to analyze and record
the morphology of the cells.
Determination of cell viability
The number of viable cells was determined using
Vi-CellTM XR Cell Viability Analyzer (Beckman Coulter,
Inc., Fullerton, CA, USA). In brief, the non-adherent
CD44+ gastric cancer cells and their adherent parental
counterpart gastric cancer cells were treated with 5-FU at the dose
and time indicated in the figures and figure legends. The
non-adherent CD44+ gastric cancer cells were resuspended
in cold PBS buffer. The attached parental gastric cancer cells were
trypsinized and resuspended in PBS buffer. Viable cells were
counted using the instrument.
Cell lysis preparation and western blot
analysis
Western blot analyses were performed as previously
described (15,16). Cells were treated and then total
cell lysates and membrane proteins were extracted. For total cell
lysates, the cells were lysed in lysis buffer. The cell membrane
lysates were prepared using Mem-PER Mammalian Membrane Protein
Extraction Reagent kit (Thermo Scientific, Rockford, IL, USA). The
protein concentration was determined using the Bio-Rad assay system
(Bio-Rad, Hercules, CA, USA). Anti-human thymidylate synthase (TS)
antibody was purchased from Zymed Laboratories, Inc. (Carlsbad, CA,
USA). Anti-human survivin antibody was purchased from R&D
Systems, Inc. (Minneapolis, MN, USA). Anti-human E-cadherin and
anti-human β-catenin antibody were purchased from Abcam Inc.
(Cambridge, MA, USA). Horseradish peroxidaseconjugated anti-rabbit,
anti-mouse, or anti-goat IgG was used as the secondary antibody and
the protein bands were detected using the Fujifilm LAS-3000 system
(Fujifilm Life Science, Stamford, CT, USA). The plasma membrane
marker α2-integrin was used as a control for equal extraction of
cell membrane protein. β-actin was used as internal controls to
evaluate the uniformity of total cell lysate protein loading.
Antibodies against α2-integrin or β-actin were purchased from
Abcam.
Immunoprecipitations
Cell lysates containing 200 μg membrane
protein were immunoprecipitated by 3 μg of monoclonal
antibody against E-cadherin. The complex was then pulled down by
Sepharose-conjugated protein G beads (Thermo Fisher Scientific) at
4°C with gentle tumbling overnight. Immunoprecipitates were washed
4 times, eluted and then analyzed by western blotting with
antibodies against E-cadherin and β-catenin.
Integrin assays
Cell surface integrins were identified using the
CHEMICON® Alpha/Beta Integrin-Mediated Cell Adhesion
Array kit (Chemicon International, Inc., Billerica, MA, USA).
Luciferase reporter assays
The cells were seeded in 24-well plates and grown to
70–80% confluence. Cells were transfected with 0.7 μg of
luciferase reporter pTOPFLASH/pFOPFLASH plasmid and 0.1 μg
of Renilla luciferase reporter control plasmid per well, using
Lipofectamine™ 2000 (Invitrogen Life Technologies, Frederick, MD,
USA), as previously described (15–17).
T cell factor (TCF) transcriptional activation activity was
measured using the luciferase reporter pTOPFLASH/pFOPFLASH plasmids
as previously reported (16).
Invasion assay
The invasion assay was performed by using 24-well BD
Biocoated Matrigel invasion chambers with 8-μm
polycarbonated filters (BD Biosciences, Bedford, MA, USA) (18). In brief, the cells were seeded on
Matrigel invasion chamber at 105 cells per well.
Invasive cells that penetrated through matrigel and migrated to the
underside of the membrane were counted under microscopic vision
after fixation with 4% formaldehyde in PBS. The average cell number
of triplicate wells was determined.
Anchorage-independent growth assay
The soft agar assay testing the
anchorage-independent growth in vitro was performed
(19). Five thousand gastric
cancer cells, or 5,000 ADSCs, or a mix of the two (5,000 gastric
cancer cells + 5,000 ADSCs) were resuspended with 0.6 ml of 0.3%
agarose gel (Invitrogen) in StemPro medium at the absence or
presence of 30 μM 5-FU. The cell-agar mixture was
immediately seeded into 24-well plates coated with 0.6% agar in
StemPro medium at the absence or presence of 30 μM 5-FU. The
cultures were maintained in a 37°C, 5% CO2 incubator for
2–4 weeks and the cell colonies were scored under microscopic
vision. The average colony number of triplicate wells was
determined.
Results
The CD44+ gastric cancer cells
exhibit non-adhesion phenotype
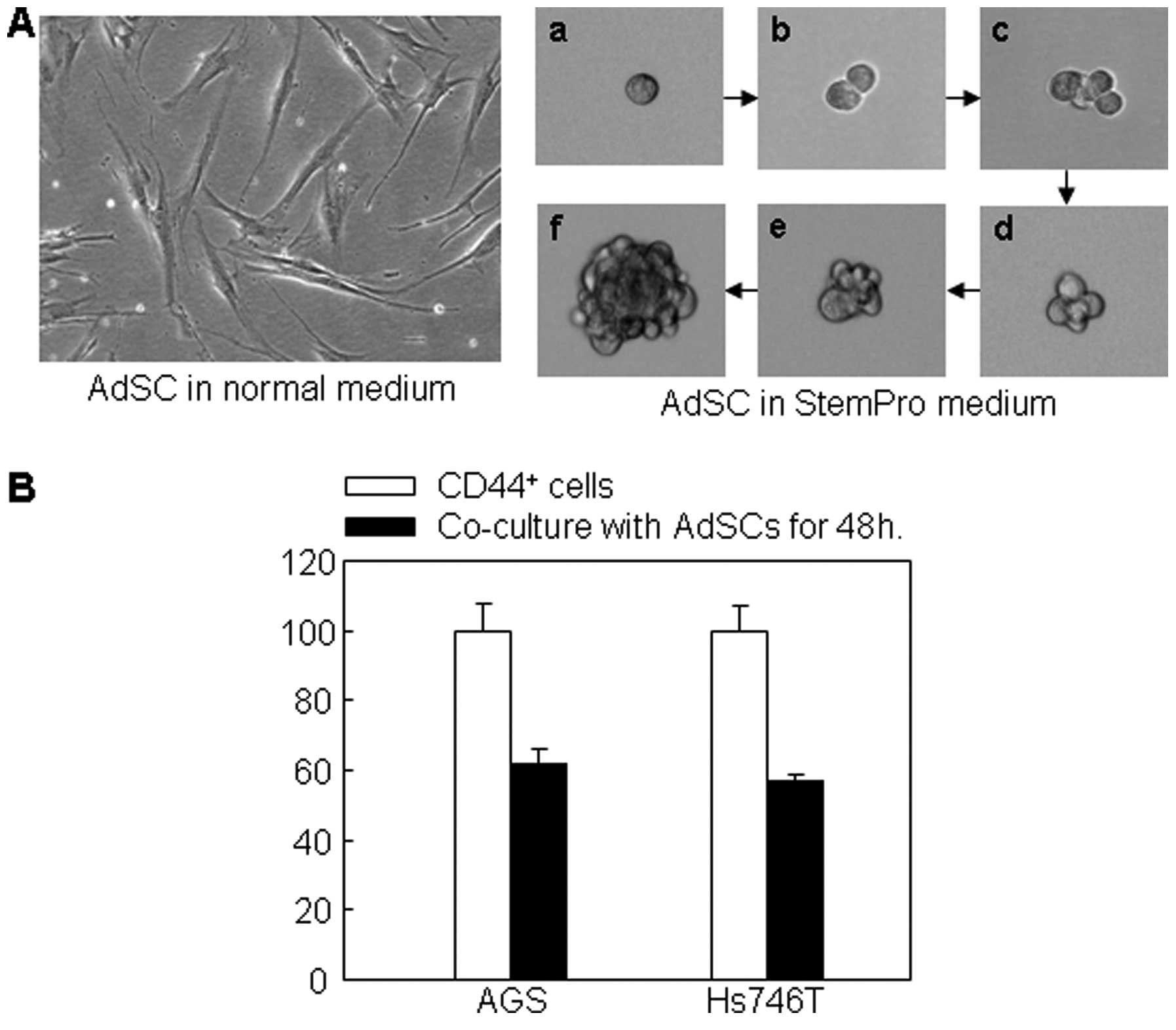
As it is shown in Fig.
1A–a), both the AGS cells and the Hs746T cells demonstrated a
monolayer flattened growth pattern when cultured in normal 10%
FBS/DMED/F-12 medium and attached to the surface of cell culture
flasks. However, the CD44+ gastric cancer cells growing
in StemPro medium exhibited a non-adherent, spheroid phenotype
(Fig. 1A–b). Of interest, the
CD44+ stem-like cells lost their original morphology and
acquired similar spheroid phenotype (compare Fig. 1A–a and -b). Furthermore, the
non-adherent CD44+ gastric cancer cells were able to
re-attach to ECM material vitronectin (Fig. 1A–c), fibronectin (Fig. 1A–d), or laminin (Fig. 1A–e) coated surface and regained the
adhesion phenotype.
The non-adherent CD44+ gastric
cancer cells possess cancer stem cell properties
The non-adherent CD44+ gastric cancer
cells were almost completely resistant to the chemotherapeutic drug
fluorouracil (5-FU) at a dose as high as 300 μM.
Alternatively, 5-FU killed more than 80% of the adherent cells in
monolayer attachment growth (Fig.
1B). However, the cells regained drug sensitivity when they
became attached to the ECM coated surface (Fig. 1B and C). Upon further examination
of the cancer stem cell-like CD44+ gastric cancer cells,
the CD44+ non-adherent cancer cells had increased
potential of invasion (Fig. 1D)
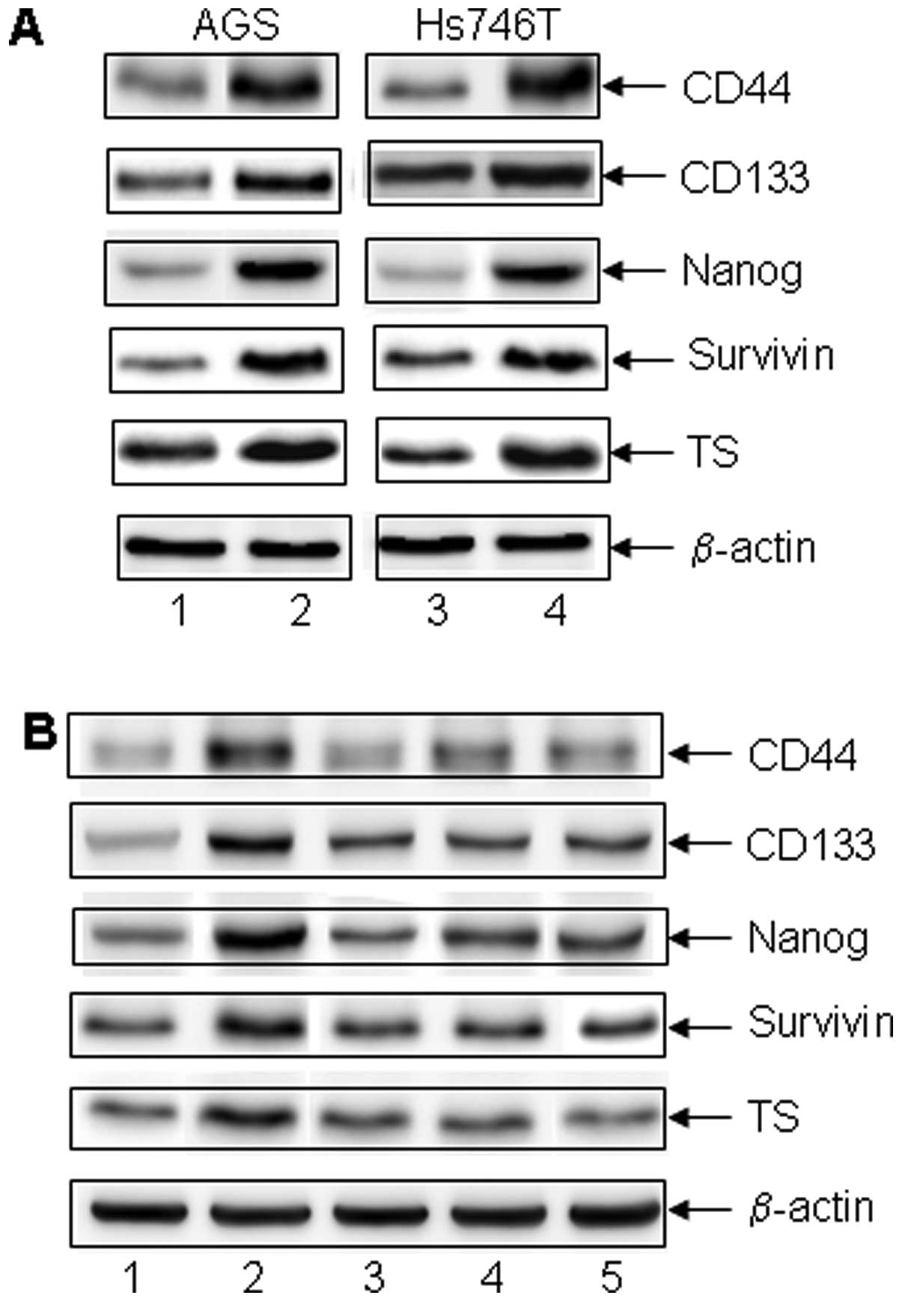
and anchorage-independent colony formation (Fig. 1E). Stem cell markers and molecules
involving in chemo-resistance in the CD44+ non-adherent
cancer cells were also upregulated (gastrointestinal cancer stem
cell markers CD44, CD133, embryo stem cell marker Nanog,
anti-apoptotic protein survivin and the DNA de novo synthase
TS (Fig. 2A). The increase in the
expression of these molecules was reversed when the cells were
attached to ECM material coated surface. Thus, CD44+
non-adherent cancer cells exhibited cancer stem cell properties
including chemo-resistance, high-grade malignancy and the
expression of stem cell markers.
Alteration of integrin and Wnt signaling
pathways in the CD44+ non-adherent cancer cells
To further investigate the mechanisms underlying the
stem cell-like characteristics of the CD44+ non-adherent
cancer cells, we examined the integrin expression pattern between
the CD44+ non-adherent cancer cells and the parental
adherent cells. Integrin α2/ β2 is significantly upregulated in
CD44+ non-adherent cancer cells. To determine the
possible involvement of Wnt signaling in maintaining the cancer
stem cell-like phenotype of the CD44+ non-adherent
cancer cells, we investigated the complex formation of
E-cdherin/β-catenin (a cell differentiation marker located in the
cell membrane) in the cell models. Our results demonstrate that the
expression of E-cadherin was significantly downregulated in
CD44+ cells compared to their parental cells (Fig. 3A), suggesting the undifferentiated
state of the CD44+ cells. The complex formation of
E-cadherin and β-catenin in the plasmid membrane was also
downregulated in CD44+ cells (Fig. 3A and B). Accordingly, β-catenin
migrated into nuclei where it formed a complex with T cell factor 4
(Tcf-4), initiating Tcf-4 transcriptional activation indicated by
the luciferase reporter pTOPFLASH/pFOPFLASH activity (Fig. 3C).
ADSCs promotes chemo-sensitivity of the
CD44+ non-adherent cancer cells
Interestingly, the CD44+ non-adherent
cancer cells re-acquired chemo-sensitivity when they were attached
to ECM material coated surface. However, in the in vivo
environment, no such attachment surface can be introduced into the
circulation to adhere the CTCs. Therefore, we explored ADSCs for
such a purpose. We found that ADSCs possessed two distinct
phenotypes, i.e., the monolayer flattened attachment pattern and
the non-adherent, three-dimensional, spheroid pattern (Fig. 4A). Cloning of a single ADSC using
limit delusion method showed that a single ADSC was able to divide
and proliferate to a full clone (Fig.
4A-a-f). Co-culture of ADSCs with CD44+ non-adherent
cancer cells resulted in significant downregulation of the markers
of cancer cell proliferation. Since the co-culture device only
allowed the communication between the two cell culture media, but
not between the cells, the results suggest that molecules in the
medium produced by the ADSCs had inhibitory effects on the gastric
cancer cells. As expected, ADSCs as non-malignant cells did not
form colonies in soft agar gel whereas CD44+ cancer
cells did (Fig. 4C). When the two
cells were mixed, the cancer cells formed less colonies (Fig. 4D). In the presence of 5-FU on the
soft agar gel, the colony formation by the cancer cells in the cell
mix group was significantly reduced to almost zero compared to the
group of cancer cells alone (Fig.
4D). These results suggest that ADSCs promoted
chemo-sensitivity of the CD44+ cancer cells.
Discussion
We report a novel CD44+ cancer stem
cell-like cell model prepared from the human gastric cancer AGS and
Hs746T cell lines. CD44+ cells exhibited cancer stem
cell properties including a non-adherent, spheroid phenotype and
high chemo-resistance. More importantly, this is the first study to
demonstrate that the cells regained chemo-sensitivity after they
were re-attached to an extracellular matrix coated surface. Mixed
co-culturing CD44+ cancer stem cell-like cell with ADSCs
from different donors inhibited cancer cell viability,
proliferation and phenotype and promoted chemo-sensitivity of the
CD44+ cancer cells.
To establish our gastric cancer stem cell-like
model, we isolated CD44 positive cells from human gastric carcinoma
cell lines (AGS and Hs746T cells). The isolated-cells were cultured
in a stem-cell culture medium (StemPro) to retain the cells in an
undifferentiated proliferation status. The isolated
CD44+ cancer cells growing in the StemPro exhibited a
suspended, non-adherent, 3-dimentional spherical growth phenotype
compared to their adherent counterparts grown in the regular medium
which showed monolayer attachment to the surface of tissue culture
plate. Remarkably, the non-adherent CD44+ cancer
stem-like cells were highly resistant to 5-FU, a chemotherapeutic
agent used clinically to treat gastrointestinal malignancies. As
shown in Fig. 1B, the non-adherent
cancer cells tolerated 5-FU in a concentration as high as 300
μM. In humans, this would not be achievable at therapeutic
doses of 400 mg/m2/day. We suggest that this may be a
reason why chemotherapeutic drugs fail to kill the circulating
gastric tumor cells in vivo. Moreover, when the non-adherent
CD44+ gastric cancer cells were placed on ECM material
coated surface, the cells quickly reacquired attachment phenotype.
Thus, the re-attached cancer cells re-gained chemo-sensitivity
similar to the adherent cells.
The mechanism of how CD44+ non-adherent
cancer cells remain highly resistant to 5-FU is not clear. Other
investigators have reported that the non-adherent cancer cells
expressed a high level of cancer stem cell markers such as CD44 and
CD133 compared to the adherent cells (4,5,20).
Furthermore, Nanog, a transcription factor functioning in
maintaining embryo stem cells in the undifferentiated state
(21), survivin, an anti-apoptotic
molecule (22,23) and thymidylate synthase (TS), a key
enzyme involved in the de novo synthesis of DNA which
circumvents the efficacy of 5-FU (24,25)
are also significantly upregulated in the CD44+
non-adherent cells. These results suggest that the CD44+
non-adherent cancer cells possess CSC-like properties which cause
drug resistance of these cells. CSCs are defined as a distinct
subpopulation of cancer-initiating cells that constitute a small
percentage of the tumor bulk. It is believed that in the general
cancer cell population, only CSCs possess the stem cell-like
characteristics including undifferentiated status, drug resistance,
tumorigenicity, expression of stem cell markers, self-renewal and
metastasis (26–29).
The CD44+ non-adherent cancer cells in
vitro resembled metastatic gastric CTCs in vivo in that
both cell types survived in an anchorage-independent, non-adherent
manner and both possessed the potential of re-attachment. Like the
CD44+ non-adherent cells that are more resistant than
their adherent counterparts, CTCs are also resistant to anticancer
drugs (1). Converting CTCs from a
non-adherent to an adherent pheno-type may increase the sensitivity
of the cells to anticancer drugs. In the cell culture condition,
cell attachment proteins bind to the negatively charged,
hydrophilic surface of the polystyrene surface of the tissue
culture plates to retain the cells in an attachment state. In
intact tissue, the structural framework formed by fibroblasts and
their synthesized extracellular matrix and collagen provides
surface for cells to attach and grow. Accordingly, we hypothesize
that introduction of a surface coating with the necessary
attachment materials would allow the CTCs to adhere in vivo.
Thus, the adherent cancer cells would be much more sensitive to
anticancer drugs and standard doses of chemotherapeutic agent could
then be used to kill the attached CTCs. Hypothetically, circulating
cells would not be attached to the attachment surface since blood
cells are naturally anchorage-independent and do not possess the
potential of adhesion.
We tested our hypothesis that converting CTCs from a
non-adherent to an adherent phenotype may increase the sensitivity
of the gastric cancer cells to chemotherapeutic agents by providing
the ADSCs as surface vehicles. ADSCs are an active component of ECM
producing tissues. Therefore ADSCs have the potential to adhere to
CD44+ non-adherent cells and trigger the adhesion
signals in the cells (i.e., living vehicle surface). ADSCs are easy
to acquire from the waste of body fat tissues and the cells do not
induce immune rejection reaction from the host. Since isolated
ADSCs no longer possess properties of adipose cells, they would not
accumulate materials that may cause fat embolism. ADSCs are
pluripotent adult stem cells that can be manipulated into the cell
type that is desired for the aforementioned purpose in vivo.
Reports regarding the effects of ADSCs against cancer are mixed.
Both pro- or anti-breast cancer by ADSCs have been recorded
(10–13). The effects of ADSCs on
gastrointestinal cancers are not established. A recent study
reported that ADSCs provoked pancreatic cancer cell death both
in vitro and in vivo (30). ADSCs have also been manipulated to
act as a ligand delivery vehicle for cancer therapy (31). In these prior studies, crude SVF
(stromal vascular fraction) instead of isolated ADSCs were used.
Since the SVF fraction is heterogeneous and contains many cell
subsets including native ADSC, mature endothelial and hematopoietic
cells (the latter representing a large portion of this fraction,
≤20%), the functional properties of SVF are variable. Thus, prior
studies have not been able to demonstrate the ability of ADSCs to
support or suppress tumor cell proliferation when using SVF. In our
studies, we used purified human ADSCs instead of SVF using
fluorescent activated cell sorting by CD34+,
CD73+ and CD105+. Our results suggest that
purified ADSCs suppressed the growth of the non-adherent cancer
stem cell-like CD44+ cells.
We used ADSCs as a living vehicle surface to adhere
CTCs in the circulation. The presence of the ADSCs did promote the
chemo-sensitivity of the cancer stem cell-like CD44+
cells to 5-FU, as indicated by soft agar assay. Application of
ADSCs for such a purpose has not been previously reported.
In conclusion, we have established a gastric cancer
stem cell-like model which can be used as an in vitro tool
for the study of CTCs and CSCs. The non-adherent cancer cell model
may also be applied in high-throughput screening of agents
targeting CSCs or resistant cancer cells. In principle, the
application of automated screening technologies could facilitate
the identification of agents that kill CSCs or resistant cancer
cells. However, the screening depends on the ability to propagate
stable, highly enriched populations of CSCs in vitro, which
is not currently possible for the CSCs of solid tumors (32). The non-adherent cancer cells
possess CSC characteristics of high drug resistance and they are
easy to prepare and maintain. Thus, the non-adherent cancer cell
model may be useful for such purposes.
Our work represents the first study using
non-engineered cells (ADSCs) to treat gastric cancer stem cells in
a non-adherent cancer cell model. This effect of ADSCs on promoting
chemo-sensitivity on gastric cancer stem cell-like CD44+
cells is mediated at least in part by ADSC acting as a living
vehicle surface and cell-cell interaction. We speculate that in
vivo ADSC may modify the microenvironment of the tumor and thus
promote its sensitivity to chemotherapeutic agents and inhibit its
proliferation.
Abbreviations:
|
5-FU
|
5-fluorouracil;
|
|
ADSCs
|
human adipose stem cells;
|
|
CSCs
|
cancer stem cells;
|
|
CTCs
|
circulating tumor cells
|
Acknowledgements
This study was supported by grants
from the American Cancer Society Illinois Division (09-11) and
Memorial Medical Center Foundation to G.L.
References
|
1.
|
Raimondi C, Naso G, Gradilone A, Gianni W,
Cortesi E and Gazzaniga P: Circulating tumor cells in cancer
therapy: are we off target? Curr Cancer Drug Targets. 10:509–518.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Takaishi S, Okumura T, Tu S, et al:
Identification of gastric cancer stem cells using the cell surface
marker CD44. Stem Cells. 27:1006–1020. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Mayer B, Klement G, Kaneko M, Man S, Jothy
S, Rak J and Kerbel RS: Multicellular gastric cancer spheroids
recapitulate growth pattern and differentiation phenotype of human
gastric carcinomas. Gastroenterology. 121:839–852. 2001. View Article : Google Scholar
|
|
4.
|
O’Brien CA, Pollett A, Gallinger S and
Dick JE: A human colon cancer cell capable of initiating tumour
growth in immunodeficient mice. Nature. 445:106–110.
2007.PubMed/NCBI
|
|
5.
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
Biffoni M, Todaro M, Peschle C and De Maria R: Identification and
expansion of human colon-cancer-initiating cells. Nature.
445:111–115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Boman BM and Huang E: Human colon cancer
stem cells: a new paradigm in gastrointestinal oncology. J Clin
Oncol. 26:2828–2838. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Han ME, Jeon TY, Hwang SH, et al: Cancer
spheres from gastric cancer patients provide an ideal model system
for cancer stem cell research. Cell Mol Life Sci. 68:3589–3605.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Vilalta M, Dégano IR, Bagó J, Aguilar E,
Gambhir SS, Rubio N and Blanco J: Human adipose tissue derived
mesenchymal stromal cells as vehicles for tumor bystander effect: a
model based on bioluminescence imaging. Gene Ther. 16:547–557.
2009. View Article : Google Scholar
|
|
9.
|
Lamfersd M, Idema S, van Milligen F, et
al: Homing properties of adipose-derived stem cells to
intracerebral glioma and the effects of adenovirus infection.
Cancer Lett. 274:78–87. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Iyengar P, Combs TP, Shah SJ, et al:
Adipocyte-secreted factors synergistically promote mammary
tumorigenesis through induction of anti-apoptotic transcriptional
programs and protooncogene stabilization. Oncogene. 22:6408–6423.
2003. View Article : Google Scholar
|
|
11.
|
Manabe Y, Toda S, Miyazaki K and Sugihara
H: Mature adipocytes, but not preadipocytes, promote the growth of
breast carcinoma cells in collagen gel matrix culture through
cancer-stromal cell interactions. J Pathol. 201:221–228. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Muehlberg FL, Song YH, Krohn A, et al:
Tissue-resident stem cells promote breast cancer growth and
metastasis. Carcinogenesis. 30:589–597. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Sun B, Roh KH, Park JR, et al: Therapeutic
potential of mesenchymal stromal cells in a mouse breast cancer
metastasis model. Cytotherapy. 11:289–298. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Casteilla L, Planat-Benard V, Laharrague P
and Cousin B: Adipose-derived stromal cells: their identity and
uses in clinical trials, an update. World J Stem Cells. 3:25–33.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Chakrabarty S, Wang H, Canaff L, Hendy GN,
Appelman H and Varani J: Calcium sensing receptor in human colon
carcinoma: interaction with Ca(2+) and 1,25-dihydroxyvitamin D(3).
Cancer Res. 65:493–498. 2005.PubMed/NCBI
|
|
16.
|
Chakrabarty S, Radjendirane V, Appelman H
and Varani J: Extracellular calcium and calcium sensing receptor
function in human colon carcinomas: promotion of E-cadherin
expression and suppression of beta-catenin/TCF activation. Cancer
Res. 63:67–71. 2003.
|
|
17.
|
Zajickova K, Vrbikova J, Canaff L, Pawelek
PD, Goltzman D and Hendy GN: Identification and functional
characterization of a novel mutation in the calcium-sensing
receptor gene in familial hypocalciuric hypercalcemia: modulation
of clinical severity by vitamin D status. J Clin Endocrinol Metab.
92:2616–2623. 2007. View Article : Google Scholar
|
|
18.
|
Albini A, Iwamoto Y, Kleinman HK, Martin
GR, Aaronson SA, Kozlowski JM and McEwan RN: A rapid in vitro assay
for quantitating the invasive potential of tumor cells. Cancer Res.
47:3239–3245. 1987.PubMed/NCBI
|
|
19.
|
Liu G, Bode A, Ma WY, Sang S, Ho CT and
Dong Z: Two novel glycosides from the fruits of Morinda
citrifolia (noni) inhibit AP-1 transactivation and cell
transformation in the mouse epidermal JB6 cell line. Cancer Res.
61:5749–5756. 2001.PubMed/NCBI
|
|
20.
|
Marhaba R, Klingbeil P, Nuebel T,
Nazarenko I, Buechler MW and Zoeller M: CD44 and EpCAM:
cancer-initiating cell markers. Curr Mol Med. 8:784–804. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Chambers I, Colby D, Robertson M, Nichols
J, Lee S, Tweedie S and Smith A: Functional expression cloning of
Nanog, a pluripotency sustaining factor in embryonic stem cells.
Cell. 113:643–655. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Fukuda S and Pelus LM: Survivin, a cancer
target with an emerging role in normal adult tissues. Mol Cancer
Ther. 5:1087–1098. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Zaffaroni N and Daidone MG: Survivin
expression and resistance to anticancer treatments: perspectives
for new therapeutic interventions. Drug Resist Updat. 5:65–72.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Marsh S: Thymidylate synthase
pharmacogenetics. Invest New Drugs. 23:533–537. 2005. View Article : Google Scholar
|
|
25.
|
Rose MG, Farrell MP and Schmitz JC:
Thymidylate synthase: a critical target for cancer chemotherapy.
Clin Colorectal Cancer. 1:220–229. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Li X, Lewis MT, Huang J, et al: Intrinsic
resistance of tumorigenic breast cancer cells to chemotherapy. J
Natl Cancer Inst. 100:672–679. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Vermeulen L, Todaro M, de Sousa Mello F,
et al: Single-cell cloning of colon cancer stem cells reveals a
multi-lineage differentiation capacity. Proc Natl Acad Sci USA.
105:13427–13432. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Odoux C, Fohrer H, Hoppo T, et al: A
stochastic model for cancer stem cell origin in metastatic colon
cancer. Cancer Res. 68:6932–6941. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Cousin B, Ravet E, Poglio S, et al: Adult
stromal cells derived from human adipose tissue provoke pancreatic
cancer cell death both in vitro and in vivo. PLoS One. 4:e62782009.
View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Grisendi G, Bussolari R, Cafarelli L, et
al: Adipose-derived mesenchymal stem cells as stable source of
tumor necrosis factor-related apoptosis-inducing ligand delivery
for cancer therapy. Cancer Res. 70:3718–3729. 2010.
|
|
32.
|
Gupta PB, Onder TT, Jiang G, Tao K,
Kuperwasser C, Weinberg RA and Lander ES: Identification of
selective inhibitors of cancer stem cells by high-throughput
screening. Cell. 138:645–659. 2009. View Article : Google Scholar : PubMed/NCBI
|