Introduction
Telomeres are important DNA-protein structures that
cap the ends of chromosomes with TTAGGG repeats (1). This is essential to maintain genomic
integrity and stability by protecting chromosome ends from DNA
damage response (2,3). Telomerase activation is responsible
for maintaining the length of telomere and is regarded as a marker
for human malignancies. Telomerase is a ribonucleoprotein complex
including two subunits: the human telomerase RNA (hTR) and the
human telomerase reverse transcriptase (hTERT). hTERT is the
protein subunit and catalyze the process of the synthesis of the
telomeric DNA (4,5). It permits cancer cells to compensate
the progressive loss of telomere during cell division and thus
plays a critical role in cell immortality. In most normal human
somatic cells, telomerase activity is at low level or undetectable.
However, the increased telomerase activity (TA) is found in 90%
human cancer cells (6,7). Therefore, inhibition of hTERT could
be a good antitumor strategy, which was successfully used to reduce
cancer cell growth (8,9).
Human cervical cancer is a prevalent cancer
worldwide. The treatment outcome for human cervical cancer is poor,
despite improved understanding of its pathogenesis. The main reason
is recurrence after radiation that induces repopulation in cancer
cells. SiHa is a squamous cell carcinoma cell line established from
fragments of a primary tissue sample obtained after surgery from a
Japanese patient.
Ionizing radiation (IR) is an important local
therapeutic way that induces DNA damage and double-stranded breaks
and is used in at least 50% of all cancer patients (10). Radiation-induced cell death is
usually attributed to DNA damage, which induces cell apoptosis. A
major factor in the failure of radiotherapy is cellular
radioresistance. Telomerase can heal chromosomes or chromatid
breaks produced by this damage. Thus, telomerase is a novel
hallmark of cellular radiosensitivity and it is possible to
downregulate telomerase to enhance radiosensitivity in human cancer
cells.
Materials and methods
Cell culture
Human cervical cancer SiHa cells (Research Center of
The 2nd Affiliated Hospital of Harbin Medical University) were
maintained in Dulbecco’s minimum essential medium (DMEM,
Invitrogen) supplemented with 10% fetal calf serum (Gibco),
penicillin (100 U/ml) and streptomycin (100 μg/ml)and
incubated at 37°C in a humid environment containing 95% air/5%
CO2.
Construction of hTERT-siRNAs and
transfection
hTERT-siRNA (1–4) and
siRNA-NC (negative control) were constructed by Genepharm (Table I). The presence of siRNA sequences
were confirmed by DNA sequencing. Transfection was performed when
the cells were 80–90% confluent using 5 μl SiRNA-Mate
(Genepharm, Shanghai, China) and 100 pmol SiRNA (Genepharm),
according to the manufacturer’s recommendations. The
SiRNA-Mate-SiRNA complex was allowed to incubate with the cells for
4–6 h before removal and incubating with fresh culture medium
supplemented with antibiotics. Transfection efficiency was
calculated 6 h after the transfection by the percentage of green
fluorescent protein (GFP) expressing cells with an LSM 510 META
(Carl Zeiss).
 | Table I.Sequences of siRNAs used. |
Table I.
Sequences of siRNAs used.
| siRNA | Sequences of
siRNA | Target site on
hTERT |
|---|
| 1 | S (5′→3′):
CCGAAGAAGCCACCUCUUUTT | hTERT-homo-984 |
| A (5′→3′):
AAAGAGGUGGCUUCUUCGGTT | |
| 2 | S (5′→3′):
GCUCGUGGAGACCAUCUUUTT | hTERT-homo-1135 |
| A (5′→3′):
AAAGAUGGUCUCCACGAGCTT | |
| 3 | S (5′→3′):
GGAAGAGUGUCUGGAGCAATT | hTERT-homo-1788 |
| A (5′→3′):
UUGCUCCAGACACUCUUCCTT | |
| 4 | S (5′→3′):
GCACCAACAUCUACAAGAUTT | hTERT-homo-3049 |
| A (5′→3′):
AUCUUGUAGAUGUUGGUGCTT | |
| NC | S (5′→3′):
UUCUCCGAACGUGUCACGUTT | |
| A (5′→3′):
ACGUGACACGUUCGGAGAATT | |
Real-time PCR analysis
Total RNA was extracted from SiHa cells with TRIzol
reagent (Invitrogen, USA) following the protocol instructed by the
manufacturer and quantified. cDNA and real-time PCR reaction system
was prepared with real-time PCR Universal reagent (Genepharm)
according to standard protocols. Primer sets and probes for hTERT
were: forward, 5′-GGCGACATGGAGAACAAGC-3′; reverse,
5′-CAAGAAATCATCCACCAAACG-3′; the predicted band was 75 bp. For
HGAPDH: forward, 5′-CATGAGAAGTAT GACAACAGCCT-3′; reverse,
5′-AGTCCTTCCACGATACC AAAGT-3′ (113 bp). The cycling program was
95°C for 3 min, 95°C for 30 sec, 62°C for 40 sec (40 cycles). The
relative expression level of RNA was computed using the
2−ΔΔCt analysis method and HGAPDH was used as an
internal reference. Each experiment was repeated three times.
Western blot analysis
Cells were harvested from the plates on ice. The
proteins (20 μg/lane) were extracted with M-PER Mammalian
Protein Extraction Reagent (Thermo) and separated on an 8%
SDS-polyacrylamide gel. The proteins were transferred to PVDF
membrane (Millipore) and then blocked with 5% milk in Tris-buffered
saline containing 0.05% (v/v) Tween-20 for 1 h at room temperature.
The membranes were incubated overnight with primary antibodies
anti-hTERT (Epipomics 1:1,000) and β-actin (Sigma 1:5,000) and then
washed there times and incubated with secondary antibodies
(HRP-conjugated goat anti-rabbit 1:8,000) for 2 h. The protein
bands were visualized using SuperSignal West Pico Chemiluminent
Substrates (Thermo). The analysis of band intensity was performed
with Gel-Pro analyzer. Each experiment was repeated three
times.
Cell proliferation
Cell growth was calculated with CCK-8 assay (Dojindo
Kumanmoto). Cells (5,000) were plated in 96-well and transfection
with siRNAs. Three wells were selected every 24–168 h. CCK-8 (10
μl) was added to each well and incubated for 2 h. The
absorbance of samples was measured at 450 nm. Each experiment was
repeated three times.
Flow cytometry analysis
Analysis of samples was performed with Cytomics™
FC500 (Beckman). Cells were harvested with trypsinization and fixed
with −20°C, 70% ethanol and stored an 4°C overnight. RNaseA (150
μl) and propidium iodide (PI) (100 μl) were added in
the resuspended fixed cells and the cell cycle was analyzed.
Apoptosis was assessed with Annexin V/PI (Mbchem M3031). Cells were
washed and resuspended in 400 μl binding buffer (Mbchem
M3036) and 5 μl Annexin V-FITC, followed by incubation for 5
min at room temperature in the dark (11). After that, flow cytometry was used
to detect cell apoptotic rate. Each experiment was repeated three
times.
Clonogenic assay and irradiation
The cells were planted in 60-mm dishes for ∼12 h in
complete medium until attached, then cells were radiated with
different doses of 6-MV X-ray (0, 2, 4, 6 and 8 Gy) at room
temperature. X-ray was generated by a 23EX accelerator (Elekta) and
the dose efficiency was 400 cGy/min. The medium was changed with a
fresh one 24 h later and incubated at 37°C in 95% air/5%
CO2 for 14 days. The cells were stained with Giemsa and
counted to determine the survival fraction of each group. Colonies
with >50 cells were counted. Each experiment was repeated three
times. Standard radiation survival curve was constructed and the
parameters D0, Dq as well as α and β were
calculated with the multitarget-single hit model and
linear-quadratic model. D0 means the dose required to
reduce the fraction of surviving cells to 37% of its previous
value. Dq means the repair capacity of the cells after
radiation.
Statistical analysis
All numerical experimental data were expressed as
means ± SD and statistical analysis of results were performed using
ANOVA. D0, Dq, α and β were calculated using
Graphpad Prime 5.0 in clonogenic assay. All P-values are based on
two-sided hypothesis testing, P<0.05 is considered statistically
significant.
Results
Inhibition of hTERT expression
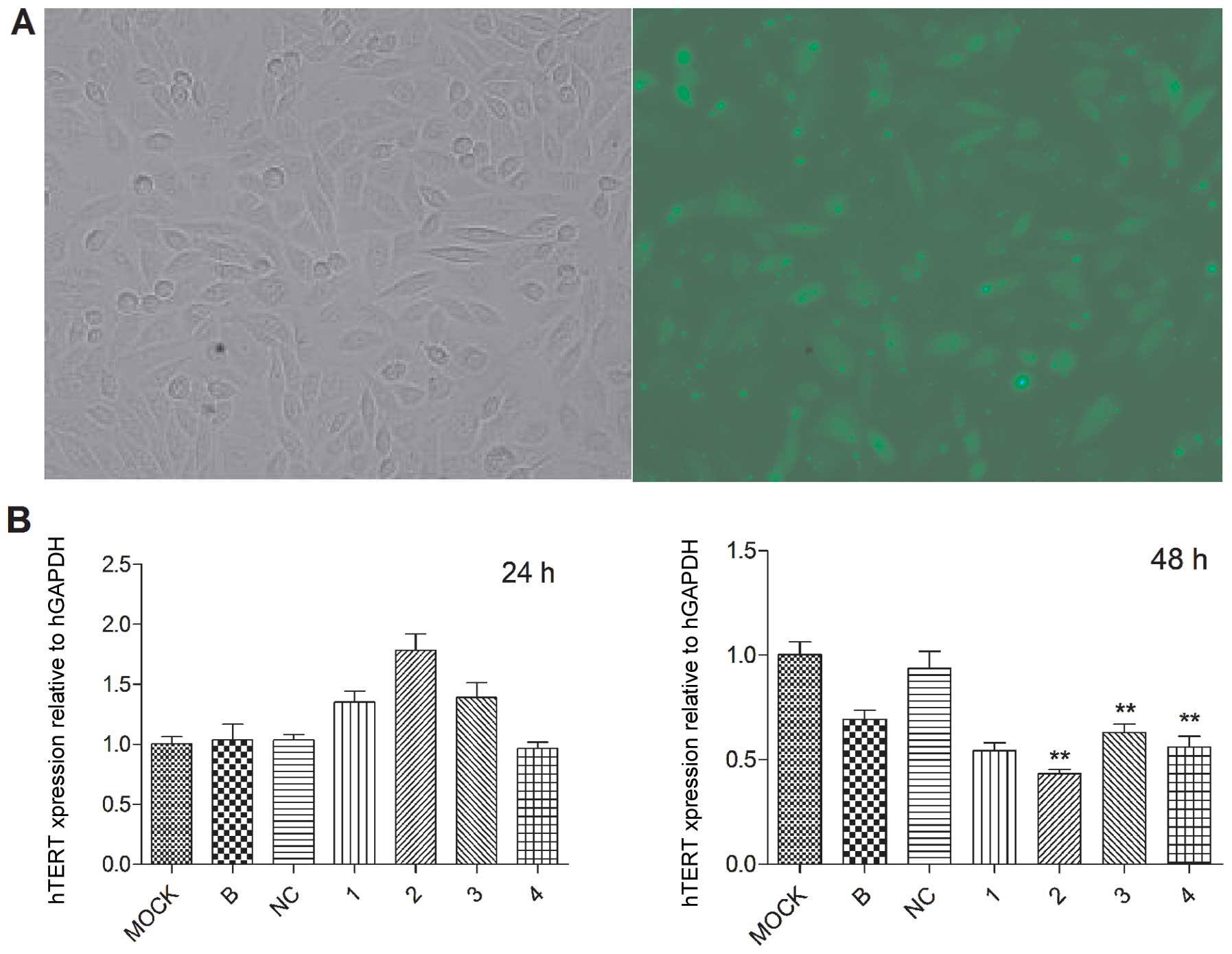
SiHa cells were transfected with siRNAs under
optimal conditions. The percentage of cells expressing GFP 6 h
after the transfection was 69.8±3.0% (Fig. 1A). hTERT mRNA was not reduced 24 h
after transfection, but markedly reduced 48 h after transfection.
The hTERT expression level was decreased by siRNA#1-4 to siRNA#1
54.33±6.51%, siRNA#2 43.33±3.51% siRNA#3 63.00±7.00% siRNA#4
56.00±9.00%, compared with the control group of mock (Fig. 1B). The protein expression amount in
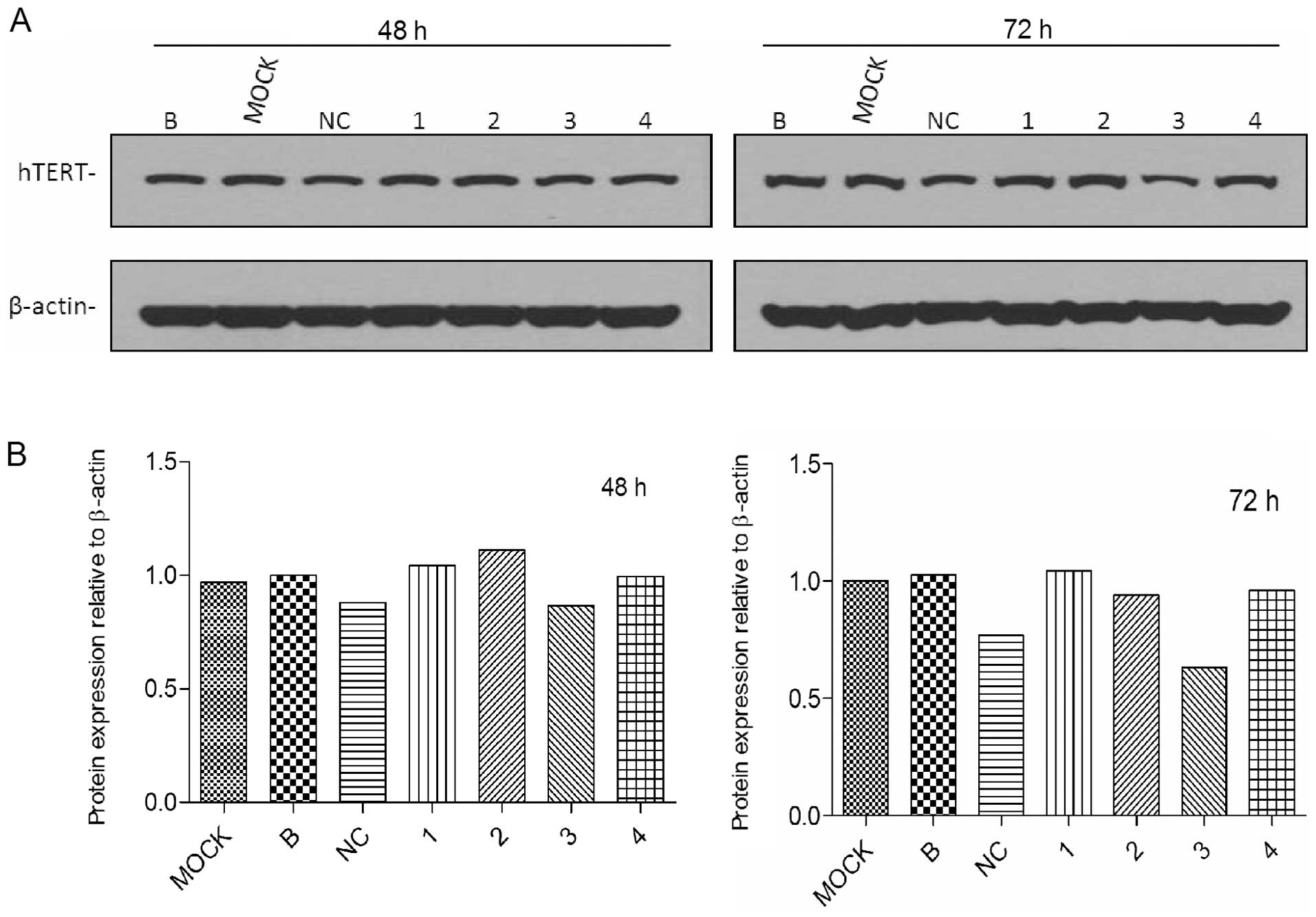
each of the groups was shown in western blot analysis 48 and 72 h
after transfection (Fig. 2A).
hTERT protein was reduced by >40% in cells after siRNA#3
transfection (Fig. 2B). Other
siRNAs also silenced protein expression, but less significantly. In
the experiments, no variability was observed in the expression of
housekeeping genes (HGAPDH and actin), thus, the RNAi was
target-specific. Therefore, we chose siRNA#3 in the following
experiments.
Reduced proliferation in SiHa cells after
hTERT knockdown
The effects of transient siRNA#3 on proliferation of
SiHa cells were calculated by CCK-8 assay at 24, 48, 72, 96 and 120
h. As shown in Fig. 3, siRNA#3
reduced the number of viable SiHa cells significantly, compared
with the control of NC. The results showed that downregulation of
hTERT resulted in inhibition of SiHa cell proliferation.
The effect of hTERT gene RNAi on cell
cycle and apoptosis
We evaluated the cellular effects of hTERT knockdown
in SiHa cells. As shown in the cell population in the Q2 quadrant
(Fig. 4A), after 48 h of siRNA#3
treatment, the early apoptosis rate of SiHa increased to
10.50±0.20% (P=0.0006), compared with control group of NC
(5.80±0.10%). But the late apoptosis rate of siRNA#3 (3.23±0.31%)
did not increase compared to the control group of NC (P>0.05).
The rate of dead cells (2.97±0.55%) were slightly decreased
compared to NC group (Fig. 4B).
The necrotic cells did show slight decrease after siRNA#3
treatment, thus indicating that the knockdown of hTERT caused early
apoptosis instead of necrosis in SiHa cells.
The effect of siRNA#3 on the cell cycle of SiHa
cells was assessed and each test was repeated three times (Fig. 5A). The proportion of cells in S
phase was significantly increased to 21.88±2.06% by siRNA#3
compared to control of NC, 14.01±2.64% (P<0.05). The proportion
of cells in G1 and G2-M was slightly decreased to 47.29±1.21 and
30.82±1.33%, compared to 52.17±1.63% (G1), 33.82±3.09% (G2+M) for
NC-treated controls (P>0.05). The knockdown of hTERT in SiHa
cells led to cell cycle arrest in S phase (Fig. 5B).
siRNA#3 enhances radiosensitivity in SiHa
cells
The observed survival fractions of two groups were
used to form the survival curve with multitarget-single hit model
and linear-quadratic model. Then we calculated D0,
Dq, α and β in two groups with Graphpad Prime 5.0. The
results (multitarget-single hit model) were D0=1.53 Gy
Dq=0.77 Gy for siRNA#3 and D0=2.19 Gy
Dq=1.31 Gy for the control of B (Fig. 6A). The results of α and β
calculated with linear-quadratic model were α= 0.45, β=0.03 for
siRNA#3 and α=0.26, β=0.02 for the control of B (Fig. 6B). All results showed SiHa cells
treated with siRNA#3 were more radiosensitive than SiHa cells.
Discussion
RNA interference (RNAi) could knockdown the mRNAs
and protein level of specific genes through post-transcriptional
gene silencing mechanism. This technology is of high efficiency,
specificity and low toxicity and is used in functional genomic
studies and therapeutic gene regulation (12,13).
The methods of antisense nucleotides, ribozymes, dominant-negative
proteins and surviving promoter-driven siRNA have been developed to
inhibit hTERT (14,17). In our study we chose four sites to
target hTERT through siRNAs. All siRNAs could decrease mRNA level,
but only siRNA#3 silenced hTERT in both mRNA and protein level
effectively. It is possible that siRNAs can be potent hTERT
inhibitors without immediate cytotoxicity.
Our results show that downregulation of hTERT
induces a rapid inhibition in proliferation of SiHa cells. These
results are consistent with other reports in different cancer cells
(15–18). The cell cycle analysis shows an
obvious block in the S phase. However, contradictory results have
been reported. Some reaserch shows that the downregulation of hTERT
induces G2 block in breast cancer cells (8), while others induced G1 block
(17). The deficient P53 tumor
suppressor gene is relative to G1 cell cycle arrest (19,20).
Thus, P53 gene may regulate SiHa cells leading to S phase arrest
after hTERT downregulation. According to Luo et al,
knockdown of hTERT induces inhibition of proliferation of SiHa
cells by S phase arrest (15).
Telomerase binding TPP1 at telomere (21) could elongate telomere in rounds of
extension (22) during the S phase
of the cell cycle and may also be related with S phase arrest.
Research has shown there is a close relationship
among telomerase, telomere and radiosensitivity (4,8,17,23–25).
Ram et al (10) showed that
radiation increases telomerase activity specially in cancer cells;
furthermore, it is regulated by post-translational mechanism via
Ras/phosphatidylinositol 3-kinase/Akt pathway. Findings of
Natarajan et al (26) and
Natarajan et al (27)
indicate that the mechanism of low-let γ-radiation inducing
telomerase activity is NF-κB activation. HER-2 positive cells
upregulate telomerase activity in irradiated breast cancer cells
(28). Also, increased telomerase
activity shows greater resistance in skin fibroblast cells
(29). Telomere affects
sensitivity to ionizing radiation, the short telomeres have more
radiosensitivity than long telomeres and telomere length could act
as biomarker of individual chromosome instability upon exposure to
radiation (30,31). Drissi et al (32) have reported that kinetics of the
DNA damage response is changed in cells with short telomere after
ionizing radiation and telomere shortening is related with
chromatin structure changes. However, there are also contrary
reports on the relationship between telomerase and telomere.
Guilleret et al (33)
reported that downregulation of telomerase could induce shortening
telomere. Conversely, Ji et al (34) have reported that telomeres is
unchanged after silencing telomerase. The reason could be that
decreased telomerase was not able to change telomere in a short
time. We need more information on the cellular pathways in
radiation-induced telomerase upregulation, to find targets to
enhance radiosensitivity. The clonogenic assay is the gold standard
to measure the radiosensitivity of cells. In this study, we used
two different models to assess radiosensitivity after silencing
hTERT in SiHa cells. All parameters prove that knockdown of hTERT
was able to enhance radiosensitivity in SiHa cells.
The results of our study add to accumulating
conclusion that telomerase is an important target in regulation of
radio-sensitivity. Downregulation of telomerase can be an important
anticancer therapy in cancer cells. Also, our data suggest that
silencing of hTERT leads to rapid growth inhibition, arresting cell
cycle in S phase and early apoptosis. This may offer a future
gene-based therapy to alter radioresistance in different cancer
cells, and more cancer cells could be destroyed with less severe
side effects in radiotherapy through this technology.
Acknowledgements
This study was supported by a grant
from the Natural Science Foundation of Heilongjiang Province
China.
References
|
1.
|
Collins K and Mitchell JR: Telomerase in
the human organism. Oncogene. 21:564–579. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Blackburn EH: Swiching and signaling at
the telomere. Cell. 106:661–673. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
De Lange T: How telomeres solve the
end-protection problem. Science. 326:948–952. 2009.PubMed/NCBI
|
|
4.
|
Satra M, Tsougos I, Papanikolaou V,
Theodorou K, Kappas C and Tsezou A: Correlation between
radiation-induced telomerase activity and human telomerase reverse
transcriptase mRNA expression in HeLa cells. Int J Radiat Biol.
6:401–409. 2009.PubMed/NCBI
|
|
5.
|
Qi DL, Ohhira T, Fujisaki C, et al:
Identification of PITX1 as a TERT suppressor gene located on human
chromosome. Mol Cell Biol. 31:1624–1636. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
He X, Qiao Q, Ge N, Nan J, Shen S, Wang Z,
Yang Y and Bao G: Irradiation-induced telomerase activity and
gastric cancer risk: a case-control analysis in a Chinese Han
population. BMC Cancer. 10:312–321. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Kyo S, Takakura M, Fujiwara T and Inoue M:
Understanding and exploiting hTERT promoter regulation for
diagnosis and treatment of human cancers. Cancer Sci. 99:1528–1538.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Gomez-Millan J, Goldblatt EM, Gryaznov SM,
Mendonca MS and Herbert BS: Specific telomere dysfunction induced
by GRN163L increases radiation sensitivity in breast cancer cells.
Int J Radiat Oncol Biol Phys. 67:897–905. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Liu X, Huang H, Wang J, Wang C, Wang M,
Zhang B and Pan C: Dendrimers-delivered short hairpin RNA targeting
hTERT inhibits oral cancer cell growth in vitro and in vivo.
Biochem Pharmacol. 82:17–23. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Ram R, Uziel O, Eldan O, et al: Ionizing
radiation upregulates telomerase activity in cancer cell lines by
post-translational mechanism via ras/phosphatidylinositol
3-kinase/akt pathway. Clin Cancer Res. 15:914–923. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Liu X, Jiang L, Wang A, Yu J, Shi F and
Zhou X: MicroRNA-138 suppresses invasion and promotes apoptosis in
head and neck squamous cell carcinoma cell lines. Cancer Lett.
28:217–222. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Dykxhoorn DM, Palliser D and Lieberman J:
The silent treatment: siRNAs as small molecule drugs. Gene Ther.
13:541–552. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Gazzaniga P, Gradilone A, Giuliani L, et
al: Expression and prognostic significance of Livin, Survivin and
other apoptosis-related genes in the progression of superficial
bladder cancer. Ann Oncol. 14:85–90. 2003. View Article : Google Scholar
|
|
14.
|
Cech TR: Beginning to understand the end
of the chromosome. Cell. 116:273–279. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Luo Y, Yi Y and Yao Z: Growth arrest in
ovarian cancer cells by hTERT inhibition short-hairpin RNA
targeting human telomerase reverse transcriptase induces immediate
growth inhibition but not necessarily induces apoptosis in ovarian
cancer cells. Cancer Invest. 27:960–970. 2009. View Article : Google Scholar
|
|
16.
|
Zheng JN, Pei DS, Sun FH, et al:
Inhibition of renal cancer cell growth by oncolytic adenovirus
armed short hairpin RNA targeting hTERT gene. Cancer Biol Ther.
8:1–8. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Wang R, Lin F, Wang X, et al: The
therapeutic potential of surviving promoter-driven siRNA on
suppressing tumor growth and enhancing radiosensitivity of human
cervical carcinoma cells via downregulating hTERT gene expression.
Cancer Biol Ther. 6:1295–1301. 2007. View Article : Google Scholar
|
|
18.
|
Hauguel T and Bunz F: Haploinsufficiency
of hTERT leads to telomere dysfunction and radiosensitivity in
human cancer cells. Cancer Biol Ther. 2:679–684. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Emastman A: Cell cycle checkpoints and
their impact on anticancer therapeutic strategies. J Cell Biochem.
91:223–231. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Tomlinson RL, Ziegler TD, Supakorndej T,
et al: Cell cycle-regulated trafficking of human telomerase to
telomeres. Mol Biol Cell. 17:955–965. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Abreu E, Aritonovska E, Reichenbach P, et
al: TIN2-tethered TPP1 recruits human telomerase to telomere in
vivo. Mol Cell Biol. 30:2971–2982. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Zhao Y, Abreu E, Kim J, et al: Progressive
and distributive extention of human telomeres by telomerase under
homeostatic and nonequilibrium conditions. Mol Cell. 42:297–307.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Kurvinen K, Rantanen V, Syrjänen S and
Johansson B: Radiation-induced effects on telomerase in
gynecological cancer cell lines with different radiosensitivity and
repair capacity. Int J Radiat Biol. 82:859–867. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Nedime S, Rikke C, Jesper G, et al:
Ectopically hTERT expressing adult human mesenchymal stem cells are
less radio-sensitive than their telomerase negative counterpart.
Exp Cell Res. 313:1056–1067. 2007. View Article : Google Scholar
|
|
25.
|
Goytisolo FA, Samper E, Martín-Caballero
J, et al: Short telomeres result in organismal hypersensitivity to
ionizing radiation in mammals. J Exp Med. 192:1625–1636. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Natarajan M, Mohan S, Konopinski R, Aotto
R, et al: Induced telomerase activity in primary aortic endothelial
cells by low-let γ-radiation is mediated through NF-κB activation.
Br J Radiol. 81:711–720. 2008.PubMed/NCBI
|
|
27.
|
Natarajan A, Jamunarani V, Rakhesh M, et
al: Curcumin regulates low-linear energy transfer
γ-radiation-induced NF-κB-dependent telomerase activity in human
neuroblastoma cells. Int J Radiat Oncol Biol Phys. 79:1206–1215.
2011.PubMed/NCBI
|
|
28.
|
Papanikolaou V, Iliopoulos D, Dimou I, et
al: The involvement of HER2 and p53 status in the regulation of
telomerase in irradiated breast cancer cells. Int J Oncol.
35:1141–1149. 2009.PubMed/NCBI
|
|
29.
|
Hideaki N: hTERT-immortalized cells useful
for analyzing effects of low-dose-rate radiation on human cells. J
Radiat Res. 49:9–15. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Castella M, Puerto S, Creus A, et al:
Telomere length modulateds human radiation sensitivity in vitro.
Toxicol Lett. 172:29–36. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Weng D, Cunin MC, Song B, et al:
Radiosensitization of mammary carcinoma cells by telomere homolog
oligonucleotide pretreatment. Breast Cancer Res. 12:R712010.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Drissi R, Wu J, Hu Y, et al: Telomere
shorting alters the kinetics of the DNA damage response after
ionizing radiation in human cells. Cancer Prev Res. 4:1973–1981.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Guilleret I and Benhattar J: Demethylation
of the human telomerase catalytic subunit (hTERT) gene promoter
reduced hTERT expression and telomerase activity and shortened
telomeres. Exp Cell Res. 289:326–334. 2003. View Article : Google Scholar
|
|
34.
|
Ji XM, Xie CH, Fang MH, et al: Efficient
inhibition of human telomerase activity by antisense oligonucotides
sensitizes cancer cells to radiotherapy. Acta Pharmacol Sin.
27:1185–1191. 2006. View Article : Google Scholar : PubMed/NCBI
|