Introduction
Ovarian cancer is one of the most lethal
malignancies of the female reproductive system with an overall 50%
mortality rate. The high mortality rate of ovarian cancer is
largely due to occult metastases within the peritoneal cavity and
the advanced stage at detection. More than 90% of ovarian cancers
are thought to arise from ovarian surface epithelium (OSE).
Epidemiologic studies indicate that gonadotrophin is likely to
increase the risk of ovarian cancer, especially in women at the
post-menopause stage or under certain pathological conditions.
Thus, one emerging theory proposes that the development of ovarian
epithelium cancer (OEC) involves gonadotropins. Elevated
gonadotropins, follicle stimulating hormone (FSH) and luteinizing
hormone (LH) promote OSE cell survival and rapid growth and
facilitate entry into the carcinogenesis process. During these
processes, molecular anti-apoptotic events are important for tumor
establishment. Our previous study confirmed that FSH inhibits
ovarian cancer apoptosis by upregulating survivin and
downregulating the programmed cell death gene 6 (PDCD6) and death
receptor 5 (DR5) (1). However, the
actual role of FSH in OEC apoptosis is not yet fully
understood.
OCT4, a member of the POU family of transcription
factors, plays an important role in the maintenance of pluripotency
and proliferation in embryonic stem cells. Indeed, it has been
detected in a broad spectrum of cancers, including non-small lung
cancer, hepatoma, breast cancer and bladder cancer (2–7).
Moreover, as a stem marker, OCT4 is also expressed in cancer stem
cells (CSC) or cancer stem cell-like cells (CSCLC), a minor
population in tumor cells with specific features, such as
self-renewal and reproducible tumor phenotype (8). A recent study identified that OCT4
and Nanog, another stem cell marker, are present in high-grade lung
adenocarinoma (LAC) and can be used as markers of poor prognosis.
The overexpression of OCT4 and Nanog in LAC was shown to increase
the percentage of CD133-expressing subpopulation, sphere formation
and induce CSC-like properties, implying that increasing OCT4 may,
at least partly, enhance the CSCLC population by activating some
stem cell pathways (9). However,
unlike the majority of cells within the tumor, CSCs or CSCLCs are
resistant to chemotherapy, which may be attributed to their
anti-apoptotic properties. By small interfering RNA (siRNA)
knockdown of OCT4, a previous study documented that the apoptosis
of CSCLCs is mediated through the OCT4-TCL1-AKT pathway (10). In addition, hormones, such as
estrogen and progesterone can augment mammary stem cells, which
profoundly influence breast cancer risk (11,12).
However, whether FSH-inhibited apoptosis well-defined by our
previous study (1) is associated
with increased OCT4 expression and activated stem cell signal
pathway has not been clearly illustrated.
Ovarian CSCs or CSCLCs have been defined and
isolated from ovarian cancer cell lines or peritoneal fluid from
ovarian cancer patients using different markers, including CD133,
CD44, CD117, Myd88 and ABCG2 (8,13–19).
Among these markers, the CD44+CD117+
immunophenotype holds promise as a defining characteristic of
ovarian CSCs or CSCLCs, since the isolated cells exhibit stem cell
functionality. As these cells possessing stem cell properties also
express OCT4, it is reasonable to hypothesize that this molecule
may play a role in expansion of ovarian CSCLCs and activation of
stem cell pathway.
Therefore, our objective in this study was to
examine the relationship among FSH treatment, OCT4 expression and
apoptosis. We detected OCT4 expression in a range of ovarian cancer
tissue types and cell lines. The effects of FSH administration on
OCT4 expression, apoptosis and expansion of the
CD4+CD117+-expressing subpopulation in cancer
cells were also investigated. Finally, the involvement of the
FSH-OCT4-AKT-survivin pathway in apoptosis inhibition of ovarian
cancer cells was explored.
Materials and methods
Clinical sample selection, tissue
handling and pathologic analysis
All evaluated ovarian tissue samples were derived
from the Department of Pathology at the First People’s Hospital
(Shanghai Jiao Tong University, Shanghai, China). A total of 159
ovarian tissues specimens were studied. The formaldehyde-fixed and
paraffin-embedded tissue specimens from 30 cases of benign ovarian
cystadenomas, 30 cases of borderline tumors and 99 cases of ovarian
carcinomas were collected between January 2003 and December 2010.
Patients with a known history of hormone replacement, of prior
radiation or chemotherapy were excluded. Pathological diagnoses of
the above ovarian lesions were made by two gynecological
pathologists based on the World Health Organization
classification.
OCT4 immunohistochemical staining and
evaluation
Immunohistochemical analysis for OCT4 protein
expression was performed as described previously (20). Briefly, OCT4 expression was
detected using a rabbit polyclonal anti-human OCT4 IgG (ab19857;
Abcam, Cambridge, UK). The sections were incubated with anti-OCT4
(1:200 dilution) in a moisture chamber for 2 h followed by a 45-min
incubation with biotinylated secondary antibody. A section of
spermatogonia was included in every experiment as a positive
control. A rabbit IgG not against OCT4 was used as a negative
control. The percentage of positively stained cells and the
intensity of the staining in these slides were assessed in a
blinded manner. OCT4 reactivity was graded on a score ranging from
0 to 12 based on the product of staining intensity (0 to 3) and
percentage (≤5% scored 0, 6–25% scored 1, 26–50% scored 2, 51–75%
scored 3, and >75% scored 4) of the cells stained. The staining
intensity was graded for both on the following scale: 0, no
staining; 1, weak staining; 2, moderate staining; and 3, intense
staining. Individual IHC score for each case was used for
statistical analysis. All IHC slides were reviewed independently by
two investigators.
Cell lines and culture
Ovarian cancer cell lines Hey, OVCAR-3, ES-2,
HO8910, HO8910PM and A2780 were obtained from the American Type
Culture Collection (Manassas, VA, USA) and cultured based on the
guidelines of the repository. These cells were maintained in
DMEM/F12 supplemented with 10% fetal bovine serum (FBS). The MCV152
and Moody cell lines kindly provided by Dr Wenxin Zheng (Arizona
University, Tucson, AZ, USA) (21,22).
SKOV3 and SKOV3-tax cells were purchased from the Beijing Union
Medical College (Beijing, China) and were cultured in DMEM
supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml
streptomycin, sodium pyruvate and L-glutamine.
Immunoblot analysis
Immunoblot analysis was performed as described
previously (20). Briefly, ovarian
cancer cells were treated with FSH at indicated concentrations and
for indicated periods of time. The treated cells were lysed with
RIPA buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% NP-40, 0.5%
dexycholic acid, sodium, 0.1% SDS). Lysates were loaded on a 10%
SDS-PAGE, transferred to polyvinylidene fluoride (PVDF) membranes
and blocked in 5% non-fat milk in 10 mM Tris, pH 7.5, 100 mM NaCl
and 0.1% (w/v) Tween-20 for 2 h. Proteins of interest were
incubated with corresponding primary antibodies overnight at 4°C.
Anti-β-actin or anti-GAPDH mouse monoclonal antibody (Lab Vision)
was diluted at 1:1,000 for sample loading control. After washing
three times with washing buffer (0.1% Tween-20 in TBS) and
incubated with the appropriate secondary antibody at room
temperature for 1 h, the membranes were washed and the bands
visualized by enhanced chemiluminescence.
Immunocytochemistry assay
Hey cells were plated in 6-well plates with
coverslips for 24 h. The cells were then starved for 24 h in
serum-free media and treated with 50 mIU/ml FSH for another 48 h.
After washing with PBS three times, the cells were fixed for 10 min
with formaldehyde and then permeabilized in 0.1% Triton X-100 in
PBS for 5 min. Subsequently, the cells were incubated with an
anti-human OCT4 primary antibody (diluted 1:100) at 37°C for 1 h,
followed by rinsing three times in PBS for 5 min each time. After
incubation with a FITC-labeled secondary antibody for another hour
at room temperature, the cells were photographed.
Total RNA extraction and RT-PCR
analysis
Total RNA was isolated according to the protocol of
the RNeasy Micro Kit (Qiagen, Frankfurt, Germany), and 2 μg
RNA was used for reverse transcription. The cDNA was then subjected
to PCR amplification with primers list in Table I using the following conditions:
initial denaturation at 95°C for 5 min, followed by 35 cycles of
denaturation at 94°C for 30 sec, annealing at temperature listed in
Table I for 30 sec, and extension
at 72°C for 30 sec. The amplification products were detected on a
1% agarose gel with ethidium bromide staining. Relative mRNA levels
of target genes were normalized to GAPDH which served as a
loading control.
 | Table I.Sequences of primers used for
amplification of target genes. |
Table I.
Sequences of primers used for
amplification of target genes.
| Gene | Primer sequence
(5′→3′) | Annealing temp.
(°C) |
|---|
| OCT4 | F:
GTACTCCTCGGTCCCTTTCC | 58 |
| R:
CAAAAACCCTGGCACAAACT |
| Sox2 | F:
GGAGCTTTGCAGGAAGTTTG | 60 |
| R:
GGAAAGTTGGGATCGAACAA |
| Nanog | F:
TTGGAGCCTAATCAGCGAGGT | 58 |
| R:
GCCTCCCAATCCCAAACAATA |
| Notch | F:
CAACATCCAGGACAACATGG | 60 |
| R:
GGACTTGCCCAGGTCATCTA |
| GAPDH | F:
AACGGATTTGGTCGTATTG | 56 |
| R:
GGAAGATGGTGATGGGATT |
Apoptosis assay
Cells undergoing various treatments were harvested,
fixed in 70% ethanol overnight at 4°C and stained with 50 mg/ml
propidium iodide. The Annexin V-FITC Apoptosis Detection kit was
used to identify apoptotic and viable cells following the
manufacturer’s instructions. The populations of early apoptotic
cells were detected by flow cytometry.
Isolation of ovarian CSCLCs
To evaluate the effect of FSH on ovarian CSCLC
expansion, flow cytometry was used to determine the changes in
populations of CSCLCs. Seven ovarian cancer cell lines were treated
with 50 mIU/ml FSH for 48 h, then the treated and untreated cells
were collected and double labeled with antibodies against CD44
(conjugated with FITC) and CD117 (conjugated with phycoerythrin)
(BD Systems, Minneapolis, MN, USA). Labeled cells were detected
with a FACSCalibur (Becton-Dickinson Immunocytometry Systems, San
Jose, CA, USA).
Transient transfection of OCT4 siRNA and
hormone treatment
Hey and OVCAR-3 cells were plated in 6-cm dishes at
a density of 2×104 cells/ml. After 24 h of culture, the
medium was replaced by Opti-MEM (Invitrogen, Carlsbad, CA, USA) in
the absence of antibiotics and cultured for another 24 h. siRNA
corresponding to the OCT4 gene was designed and synthesized by
Dharmacon (Thermo Scientific), and the sequences of SMARTpool siRNA
against OCT4 included: 5′-GCGAUCAAGCAGCGACUAU-3′,
5′-UCCCAUGCAUUCAAACUGA-3′, 5′-GCACUGUACU CCUCGGUCC-3′,
5′-CGAGAAGGAUGUGGUCCGA-3′. These siRNA were transiently transfected
into the cells with DharmaFECT transfection reagents (Thermo
Scientific) according to the manufacturer’s instructions. After
incubation for another 24 h, a portion of the cells was collected
for RNA extraction and semi-quantitative RT-PCR analysis to
determine the degree of gene silencing and detect the effect of
knocking down OCT4 on Sox2, Notch and Nanog
gene expressions in the absence or presence of FSH. The other
portion of the treated cells was used to investigate the effect of
OCT4 depletion on downstream proteins by western blot analysis in
the absence or presence of FSH.
Plasmid construction and
transfection
The OCT4 ORF was inserted into the
EcoRI-BamHI site of pIRES2-EGFP to generate the
pIRES2-EGFP-OCT4 plasmid. The integrity of the cDNA was confirmed
by sequencing (data not shown). To investigate the effect of OCT4
overexpression on Sox2, Notch and Nanog genes,
4 μg pIRES2-EGFP-OCT4 and 4 μg empty vector were
transfected into Hey and OVCAR-3 cells using Lipofectamine 2000
(Invitrogen) according to the instructions provided by the
manufacturer. The changes in Sox2, Notch and
Nanog gene transcripts were determined by RT-PCR.
GAPDH served as a loading control.
Production of lentiviral particles
In order to prepare lentiviral particles expressing
the OCT4 gene, HEK-293T cells were transfected with
pLenti-OCT4 (OCT4 ORF was constructed into modified
pLOV.CMV.eGFP.EF1a.PuroR which was removed the eGFP gene) plus
lentiviral packaging vectors. Briefly, the cells were seeded in a
6-well plate at a concentration of 1.0×106 cells per
well. After 24 h, the culture medium was aspirated and replaced
with Opti-MEM (Invitrogen). Subsequently, 2 μg pLenti-OCT4
and the Mission Lentiviral Packaging mix (Sigma-Aldrich, St. Louis,
MO, USA) were transfected into cells by Lipofectamine 2000
(Invitrogen) according to the manufacturer’s instructions. The
following day, the culture was replaced with complete medium (DMEM
with 10% FBS). After another 48 h, the culture supernatants
containing the lentiviral particles (Lenti-OCT4) were harvested for
use.
Establishment of OCT4-overexpressing
ovarian cancer stable cell lines
To establish the OCT4-overexpressing ovarian cancer
cells, Hey cells were plated in 6-well plates with
2.0×105 cells per well and transduced with the
Lenti-OCT4 lentiviral particles. On the third day after
transduction, puromycin (Sigma-Aldrich) was added into the culture
medium to a final concentration of 2 μg/ml. During the
selection period, the drug was kept at the same concentration at
each replacement of culture medium. Approximately 2 weeks was
required for the live cells to be eliminated in the mock
transduction group. After that, the selected cultures were expanded
and cryopreserved. The OCT4 mRNA and protein expressions were
detected by PCR and western blot analysis, respectively.
Statistical analyses
The statistical significance of the differences in
the immunohistochemical staining in ovarian tissues was calculated
using the χ2 test. The differences of OCT4 expression in
ovarian cancer tissues grouped by age, grade, stage, lymph node
metastasis and menopause status was investigated using the
χ2 test. A two-sided test with P<0.05 was considered
statistically significant. All statistical analysis was performed
using SPSS 11.0 (SPSS Inc., Chicago, IL, USA) or Prism 5.0
(GraphPad Software).
Results
Ovarian carcinoma overexpress OCT4
protein
Ovarian tissue samples from 159 patients were used
in this study, and the expression of OCT4 was analyzed using
immunohistochemical (IHC) staining. As shown in Table II, 2 of 30 cases (6.7%) of benign
cystadenomas had high levels of OCT4 protein, whereas 14 of 30
cases (46.7%) of borderline tumors and 65 of 99 cases of carcinoma
(65.7%) showed high expression of OCT4 protein. Significantly
increased OCT4 was observed in borderline tumors when compared with
benign cystadenomas. Representative images are shown in Fig. 1A. Low expression of OCT4 in nuclei
could be seen in benign cystadenomas (Fig. 1Ab), whereas positive staining of
OCT4 localized in nuclei in borderline tumors and in cytoplasm in
carcinomas (Fig. 1Ac and Ad).
Spermatogonia and OEC samples cultured with IgG not against OCT4
served as a negative control (Fig.
1Aa). Further study revealed different expression profiles of
OCT4 in different OEC pathological types (Fig. 1C). Low levels of OCT4 protein were
detected in 8 of the 11 clear cell carcinomas (72.7%); however,
high levels of OCT4 protein were examined in 5 of the 8
endometrioid adenocarcinoma (62.5%), 4 of the 6 mucinous
cystadenocarcinoma (66.7%) and 53 of the 74 (71.6%) serous
cystadenocarcinoma cases (Table
III and Fig. 1Cc–f). Oct4
expression was scored as high or low for each case according to the
criteria described in Materials and methods, and the data are
summarized in Fig. 1B and D. The
correlations between OCT4 expression and clinical pathological
factors in serous cystadenocarcinoma were further investigated. We
found that OCT4 expression was significantly associated with
histological grade (P=0.008) but not with age (P=0.611), clinical
stage (P=0.954), lymph node metastasis (P=0.402) or menopause
(P=1.0) (Table IV). These results
suggested OCT4 may play a role in epithelial ovarian cancer.
 | Table II.Expression of OCT4 in distinct tumor
tissue types. |
Table II.
Expression of OCT4 in distinct tumor
tissue types.
| Tissue type | Total | High level | Low level | % | P-value |
|---|
| Cystadenomas | 30 | 2 | 28 | 6.7 | <0.0001 |
| Borderline
tumor | 30 | 14 | 16 | 46.7 | |
| Carcinomas | 99 | 65 | 34 | 65.7 | |
 | Table III.Expression of OCT4 in different OEC
pathological types. |
Table III.
Expression of OCT4 in different OEC
pathological types.
| Pathological
type | Total | Expression
| % | P-value |
|---|
| High | Low |
|---|
| Clear cell
carcinoma | 11 | 3 | 8 | 27.3 | 0.039 |
| Endometrioid
adenocarcinoma | 8 | 5 | 3 | 62.5 | |
| Mucinous
cystadenocarcinoma | 6 | 4 | 2 | 66.7 | |
| Serous
cystadenocarcinoma | 74 | 53 | 21 | 71.6 | |
 | Table IV.Correlations of OCT4 expression and
major clinical pathologic factors in serous cystadenocarcinoma
(n=74). |
Table IV.
Correlations of OCT4 expression and
major clinical pathologic factors in serous cystadenocarcinoma
(n=74).
| Factors | No. of
patients | OCT4 expression
| P-value |
|---|
| High | Low |
|---|
| Age (year) | | | | 0.611 |
| ≥55 | 38 | 26 | 12 | |
| <55 | 36 | 27 | 9 | |
| Histological
grade | | | | 0.008 |
| Low (I) | 26 | 13 | 13 | |
| Intermediate
(II) | 24 | 19 | 5 | |
| High (III) | 24 | 21 | 3 | |
| Clinical stage | | | | 0.954 |
| I | 8 | 6 | 2 | |
| II | 15 | 11 | 4 | |
| III | 51 | 36 | 15 | |
| Lymph node
metastasis | | | | 0.402 |
| No | 31 | 24 | 7 | |
| Yes | 31 | 20 | 11 | |
| Menopause | | | | 1.000 |
| Yes | 42 | 30 | 12 | |
| No | 15 | 11 | 4 | |
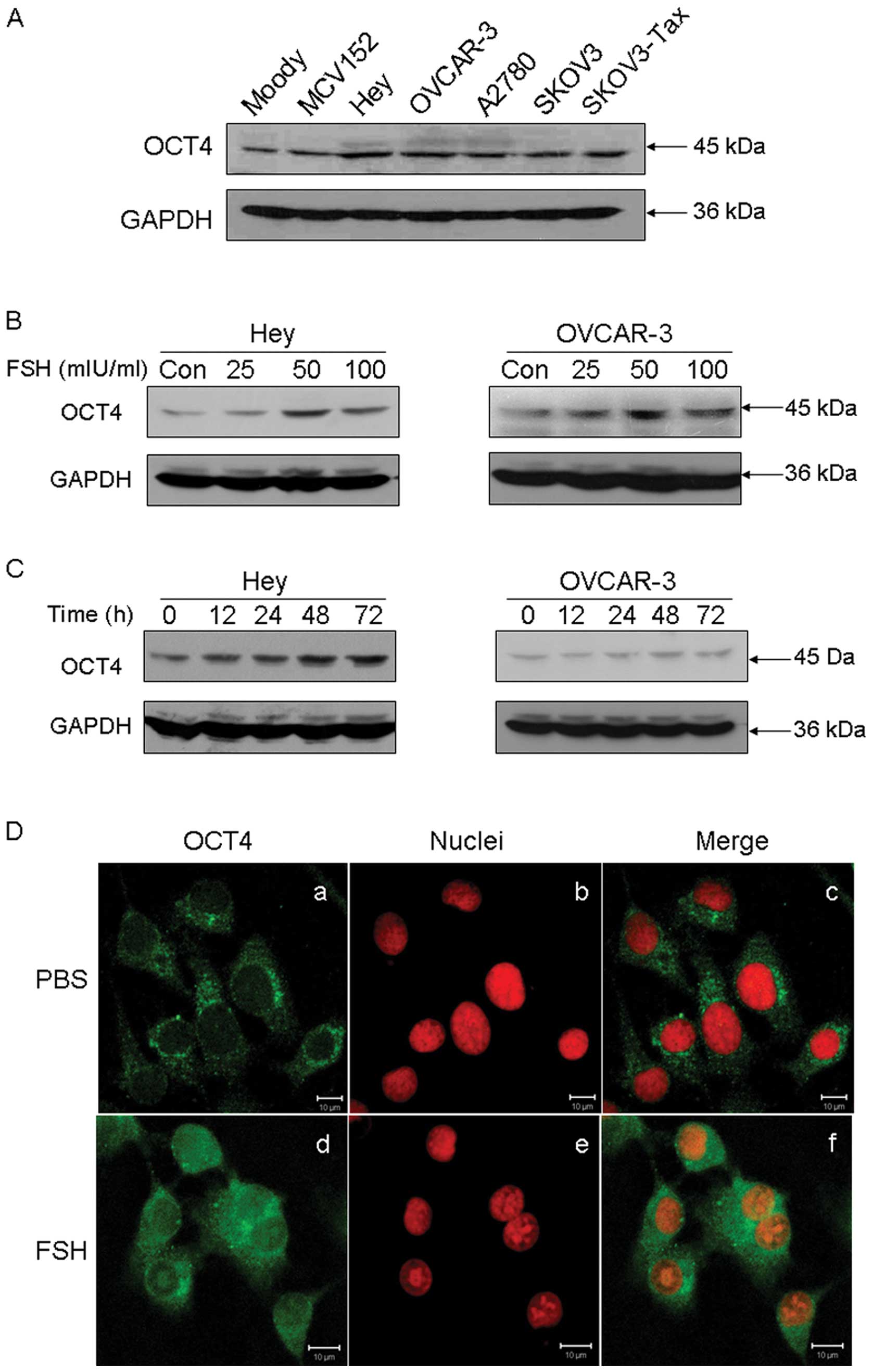
FSH upregulates OCT4 expression
Extensive studies during the last decade have
provided strong evidence that OCT4 is expressed in various cancer
types, including lung cancer, prostate cancer, liver cancer and
cervical cancer (3,4,9,23–25).
In the current study, the expression of OCT4 was determined by
western blot analysis in OET cells. As shown in Fig. 2A, OCT4 was found to be
overexpressed in ovarian cancer cells, compared with the normal
Moody ovarian cell line and the SV40-transformed benign ovarian
MCV152 cell line.
FSH is a confirmed risk factor for OEC. In order to
evaluate the relationship between FSH and OCT4 overexpression in
ovarian cancer tissues, the effect of FSH on OCT4 expression was
studied. As shown in Fig. 2B, FSH
stimulation resulted in a significant increase in OCT4 protein in a
dose-dependent manner in both Hey and OVCAR-3 cell lines. The most
significant upregulation of OCT4 was observed when cells were
exposed to 50 mIU/ml FSH for 48 h. Moreover, FSH was found to
enhance OCT4 expression with a time-dependent manner (Fig. 2C). Subcellular analysis by
immunofluorescence staining confirmed that FSH stimulation
increased OCT4 protein expression in both the cytoplasm and nucleus
in Hey cells (Fig. 2D).
FSH inhibits apoptosis in OEC cells
The overexpression of OCT4 protein in ovarian cancer
tissue implies that it is involved in OET development. Indeed, it
was previously confirmed that depletion of OCT4 in lung cancer
cells would result in CSCLC apoptosis (10). Although we showed positive
regulation of OCT4 expression by FSH treatment, whether FSH has a
direct effect on ovarian cancer cell apoptosis requires further
exploration. We found that treatment with the chemotherapeutic
drugs cisplatin or paclitacel at various concentrations could
induce early apoptosis in a dose-dependent manner; however, in the
presence of FSH, the induced apoptosis rates were partly abrogated
in Hey (Fig. 3) cells. We thus
concluded that FSH is involved in apoptosis inhibition in ovarian
cancer cells.
Apoptosis induced by knockdown of OCT4 is
attenuated by FSH
In order to determine whether OCT4 plays a role in
FSH-induced inhibition of apoptosis, OCT4 was knocked down by
siRNA. As shown in Fig. 4A and B,
OCT4 mRNA and protein levels were potently reduced after depletion
of OCT4. Moreover, knocking down OCT4 resulted in a significantly
increased early apoptosis rate in Hey and OVCAR-3 cells. However,
in the presence of FSH (50 mIU/ml), this early apoptosis rate
induced by OCT4 knockdown was decreased in both cell lines,
although marked changes were not observed in OVCAR-3 cells
(Fig. 4C).
FSH induces ovarian CSCLC expansion
Although OCT4, Sox2, Nanog and
Notch are all important stem cell markers, the relationships
between them are not clear. Here we studied changes in other CSC
markers while modulating OCT4 expression. As indicated in Fig. 5A, depletion of OCT4 using siRNA
resulted in reduction of Notch, Sox2 and Nanog
mRNA. FSH induced Notch, Sox2 and Nanog
expression were abolished by knocking down OCT4. Similar patterns
were obtained in both Hey and OVCAR-3 cells. By contrast,
upregulated Sox2, Nanog and Notch mRNA were
detected after transient transfection with the pIRES2-EGFP-OCT4
plasmid, whereas transfection with empty vector had no effect on
the expression of these genes (Fig.
5B). Similar to the effect of pIRES2-EGFP-OCT4 transfection in
OEC cells, FSH stimulation also potently enhanced the expression of
these CSC markers (Fig. 5B). These
data suggest that FSH may play a role in regulating CSC marker
expression via OCT4 mediated stem signal pathway. Although these
markers positively responded to FSH treatment, whether FSH
increases the population of CSCLCs remained to be clarified. To
investigate the changes in a broad range of ovarian CSCLCs, we used
Hey, OVCAR-3, A2780, ES-2, SKOV3, HO8910 and HO8910PM cell line to
detect the population of CD44+CD117+ cells
after 50 mIU/ml FSH stimulation for 48 h. The results indicated
that FSH induced expansion of the CD44+CD117+
cell population, especially in the Hey and ES-2 cell lines
(Fig. 5C and D), which implies
that OCT4 mediated stem signal pathway may involve inhibition of
FSH-induced apoptosis.
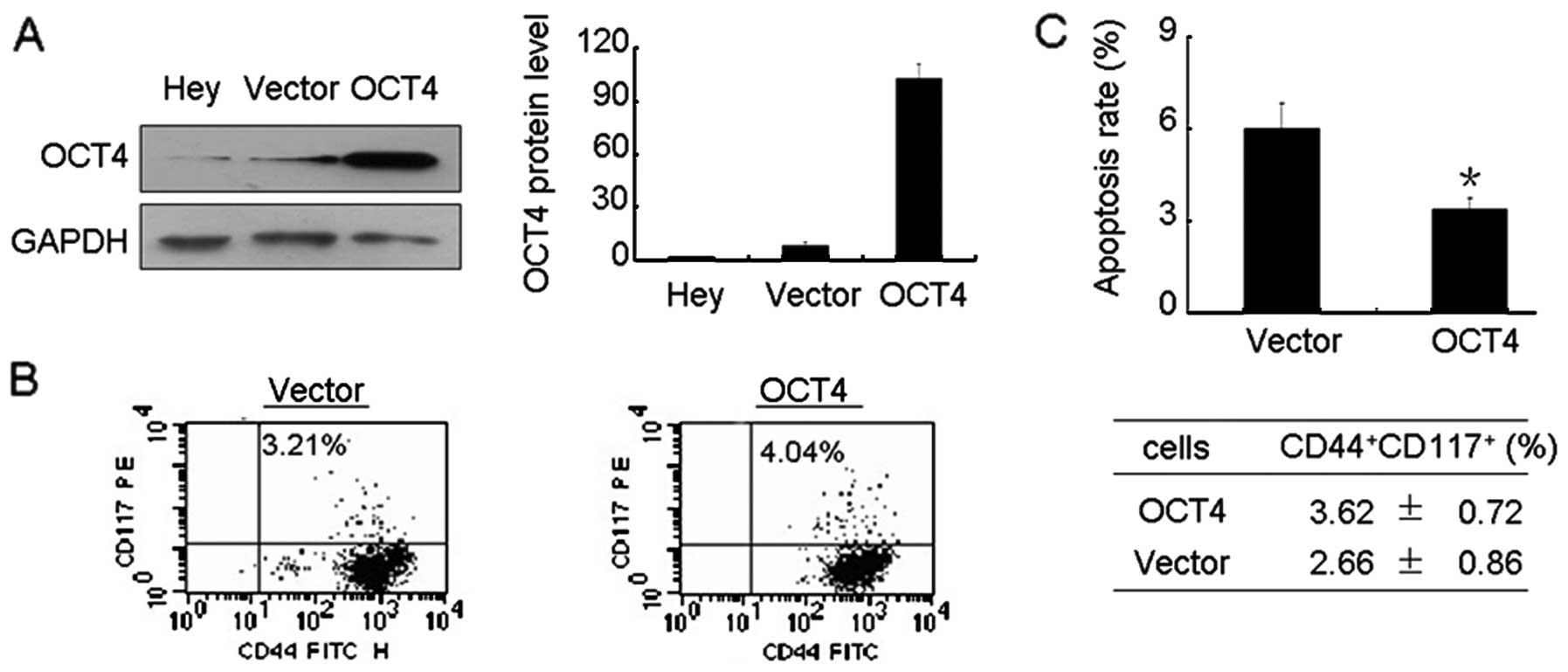
Stable transfection of OCT4 increases
CSCLCs and inhibits apoptosis
To further investigate the role of FSH-elevated OCT4
in ovarian cancer apoptosis, we stably overexpressed OCT4 in Hey
cells as described in Materials and methods. As shown in Fig. 6A, a high level of OCT4 protein was
observed in OCT4 transfected Hey cells. Moreover, elevated OCT4
resulted in an increased level of CD44+CD117+
cells (Fig. 6B). Further study
found that elevated OCT4 significantly reduced the apoptosis rate
compared with Hey cells transfected with empty plasmid (Fig. 6C).
OCT4-AKT-survivin pathway is involved in
FSH mediated inhibition of apoptosis
Our previous work confirmed that survivin regulates
apoptosis in ovarian cancer and endometrial cancer cells. Moreover,
the OCT4-AKT-ABCG2 and OCT4-TCL1-AKT signaling pathways were
demonstrated to participate in chemo-resistance and apoptosis
inhibition. Therefore, we investigated the relationships between
OCT4, AKT and survivin. As shown in Fig. 7A, depletion of OCT4 resulted in
reduction of p-AKT and survivin, and also attenuated FSH-induced
p-AKT and survivin protein levels. Furthermore, chemical inhibition
of AKT using LY294002 abolished the FSH mediated elevation of
survivin. These data indicated that FSH induces ovarian cancer
apoptosis through the OCT4-AKT-survivin signaling pathway.
Discussion
Epithelial ovarian cancer is a common gynecological
cancer worldwide, especially in postmenopausal women or individuals
who have received treatment to induce ovulation (26–28).
Prior studies have confirmed that the enriched hormonal environment
of the ovary influences the development of OEC (27,29).
Elevated gonadotropins in postmenopausal women, especially FSH,
have been hypothesized to contribute to the incidence of OEC.
Indeed, there are considerable lines of evidence showing that FSH
promotes ovarian cancer cell proliferation and invasion (30–32).
Our recent study implicated an anti-apoptotic effect of FSH in OEC
development (1). It has also been
demonstrated that chemoresistant hepatocellular carcinoma cells are
enriched for CSCs and that the OCT4-AKT-ABCG2 pathway acts on CSCs
to promote cell proliferation through inhibition of apoptosis
(25). Therefore, whether the
apoptosis inhibition in ovarian cancer cells result from
FSH-induced stem cell related signal pathway remains obscure.
In the current study, we investigated OCT4 as a
potential stem cell and CSC marker for OEC. OCT4 protein expression
was measured in one hundred fifty-nine cases of different types of
ovarian lesions. The results of IHC staining indicated that OCT4
expression was higher in ovarian carcinomas and borderline tumors,
while it was marginally expressed in benign cystadenomas (Fig. 1A). Similar expression patterns were
also observed in a normal ovarian epithelial cell line and ovarian
cancer cells (Fig. 2A). Moreover,
we found a statistically significant association of OCT4 expression
with ovarian carcinomas of different pathological types. A
relatively low level of OCT4 expression was found in clear cell
carcinoma, while a large percent of serous carcinoma had a higher
level of OCT4 when compared with endometrioid adenocarcinoma and
mucinous cystadenocarcinoma, all of which showed positive staining
for OCT4 (Fig. 1C). This finding
is consistent with previous studies showing that a high level of
OCT4 is usually present in poor prognosis patients (24,33).
To our knowledge, this is the first large-scale study of OCT4
expression in ovarian lesions.
To further investigate the relationship between
elevated OCT4 expression and the development of OEC, determination
of the effect of FSH, as a high risk factor for OEC incidence, on
OCT4 expression was required. Our results clearly indicated that
FSH markedly enhanced OCT4 expression in both the cytoplasm and
nucleus (Fig. 2B–D). This result
prompted us to further investigate the role of FSH-induced OCT4 in
OEC development. In previous reports, depletion of OCT4 was shown
to result in apoptosis and cell growth arrest (10,34),
whereas OCT4 overexpression enhances chemotherapy resistance in
liver cancer (25). In this study,
we found that FSH attenuated ovarian cancer cell apoptosis induced
by chemo-therapeutic drugs (Fig.
3). Further investigation revealed that FSH abolished depletion
of OCT4 induced-apoptosis (Fig.
4). These data suggest that OCT4 participates in FSH-induced
apoptosis inhibition. However, the effects of changes of OCT4 on
the expression and proportionality of other CSCs or CSCLCs are not
clear. Chiou et al reported that co-expression of OCT4 and
Nanog, another stem cell marker, in lung adenocarcinomas can
increase the proportion of CD133-expressing subpopulation, sphere
formation and enhance drug resistance (9). In ovarian cancer, Zhang et al
identified CD44+CD117+ cells as ovarian
CSC-like cells (8). In the current
findings, it was confirmed that the cells stably transfected with
OCT4 had a slightly increased level of the
CD44+CD117+ subpopulation (Fig. 6B) and markedly induced inhibition
of cellular apoptosis (Fig. 6C),
implying that stem signal pathway may involve apoptosis inhibition.
Since FSH upregulated OCT4 expression, the elevated OCT4 may
mediate FSH-induced apoptosis inhibition. As expected, most ovarian
cancer cell lines showed an increase in the
CD44+CD117+ subpopulation (Fig. 5C and D) after FSH treatment. It was
observed that FSH upregulated Notch, Sox2 and
Nanog mRNA, and this effect was blocked by knocking down
OCT4. Thus, FSH-induced stem signal related signal pathway may be
another mechanism of FSH related apoptosis inhibition in ovarian
cancer cells.
Previous studies showed that the OCT4-TCL1-AKT and
OCT4-AKT-ABCG2 signaling pathways are involved in CSC
proliferation, chemoresistance and apoptosis (10,25).
As we have found in prior studies that survivin participates in
inhibition of apoptosis in various cancer cells (1,20,35,36),
we investigated the relationship between OCT4 and survivin. Our
data clearly showed that knockdown of OCT4 decreased AKT activation
and reduced survivin expression. FSH-induced activation of AKT and
elevated survivin were abolished by depletion of OCT4 (Fig. 7A), while blockage of AKT signaling
inhibited FSH-induced survivin (Fig.
7B). This finding indicates that FSH inhibits apoptosis through
the OCT4-AKT-survivin signal pathway in OEC cells.
In conclusion, we showed that OCT4 may play a role
in OEC development as it was overexpressed in human ovarian
carcinomas compared with benign cystadenomas. OCT4 expression was
also associated with the tumor grading, with higher levels
indicating poor prognosis. We further found that elevated OCT4
contributes to FSH-induced apoptosis inhibition through
OCT4-AKT-survivin signal pathway. Our findings provide novel
insights into FSH inhibition of ovarian cancer apoptosis.
Abbreviations:
|
CSCLC
|
cancer stem cell-like cell;
|
|
CSC
|
cancer stem cells;
|
|
FSH
|
follicle stimulating hormone;
|
|
OET
|
ovarian epithelial tumor;
|
|
OEC
|
ovarian epithelial cancer
|
Acknowledgements
The project was supported by grants
from the National Natural Science Foundation of China (NSFC nos.
81020108027, 30872755, 81172478 and 81001155), supported in part by
the grant (no. 2009028) from Shanghai Municipal Health Bureau, the
grant (no. 10JC1413100) from Shanghai Science and Technology
Committee. Additional support was provided by the MD Anderson SPORE
in Ovarian Cancer NCI P50 CA83639, the National Foundation for
Cancer Research, the Zarrow Foundation and Stuart and Gaye Lynn
Zarrow.
References
|
1.
|
Huang Y, Jin H, Liu Y, et al: FSH inhibits
ovarian cancer cell apoptosis by up-regulating survivin and
down-regulating PDCD6 and DR5. Endocr Relat Cancer. 18:13–26. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Gu G, Yuan J, Wills M and Kasper S:
Prostate cancer cells with stem cell characteristics reconstitute
the original human tumor in vivo. Cancer Res. 67:4807–4815. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Karoubi G, Gugger M, Schmid R and Dutly A:
OCT4 expression in human non-small cell lung cancer: implications
for therapeutic intervention. Interact Cardiovasc Thorac Surg.
8:393–397. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Atlasi Y, Mowla SJ, Ziaee SA and Bahrami
AR: OCT-4, an embryonic stem cell marker, is highly expressed in
bladder cancer. Int J Cancer. 120:1598–1602. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Ezeh UI, Turek PJ, Reijo RA and Clark AT:
Human embryonic stem cell genes OCT4, NANOG, STELLAR, and GDF3 are
expressed in both seminoma and breast carcinoma. Cancer.
104:2255–2265. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Shin S, Mitalipova M, Noggle S, et al:
Long-term proliferation of human embryonic stem cell-derived
neuroepithelial cells using defined adherent culture conditions.
Stem Cells. 24:125–138. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Tai MH, Chang CC, Kiupel M, Webster JD,
Olson LK and Trosko JE: Oct4 expression in adult human stem cells:
evidence in support of the stem cell theory of carcinogenesis.
Carcinogenesis. 26:495–502. 2005.PubMed/NCBI
|
|
8.
|
Zhang S, Balch C, Chan MW, et al:
Identification and characterization of ovarian cancer-initiating
cells from primary human tumors. Cancer Res. 68:4311–4320. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Chiou SH, Wang ML, Chou YT, et al:
Coexpression of Oct4 and Nanog enhances malignancy in lung
adenocarcinoma by inducing cancer stem cell-like properties and
epithelial-mesenchymal transdifferentiation. Cancer Res.
70:10433–10444. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Hu T, Liu S, Breiter DR, Wang F, Tang Y
and Sun S: Octamer 4 small interfering RNA results in cancer stem
cell-like cell apoptosis. Cancer Res. 68:6533–6540. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Asselin-Labat ML, Vaillant F, Sheridan JM,
et al: Control of mammary stem cell function by steroid hormone
signalling. Nature. 465:798–802. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Joshi PA, Jackson HW, Beristain AG, et al:
Progesterone induces adult mammary stem cell expansion. Nature.
465:803–807. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Hu L, McArthur C and Jaffe RB: Ovarian
cancer stem-like side-population cells are tumourigenic and
chemoresistant. Br J Cancer. 102:1276–1283. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Baba T, Convery PA, Matsumura N, et al:
Epigenetic regulation of CD133 and tumorigenicity of
CD133+ ovarian cancer cells. Oncogene. 28:209–218. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Ferrandina G, Bonanno G, Pierelli L, et
al: Expression of CD133-1 and CD133-2 in ovarian cancer. Int J
Gynecol Cancer. 18:506–514. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Curley MD, Therrien VA, Cummings CL, et
al: CD133 expression defines a tumor initiating cell population in
primary human ovarian cancer. Stem Cells. 27:2875–2883.
2009.PubMed/NCBI
|
|
17.
|
Alvero AB, Chen R, Fu HH, et al: Molecular
phenotyping of human ovarian cancer stem cells unravels the
mechanisms for repair and chemoresistance. Cell Cycle. 8:158–166.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Dou J, Jiang C, Wang J, et al: Using
ABCG2-molecule-expressing side population cells to identify cancer
stem-like cells in a human ovarian cell line. Cell Biol Int.
35:227–234. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Gao Q, Geng L, Kvalheim G, Gaudernack G
and Suo Z: Identification of cancer stem-like side population cells
in ovarian cancer cell line OVCAR-3. Ultrastruct Pathol.
33:175–181. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Chen X, Zhang Z, Feng Y, et al: Aberrant
survivin expression in endometrial hyperplasia: another mechanism
of progestin resistance. Mod Pathol. 22:699–708. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Ji Q, Liu PI, Chen PK and Aoyama C:
Follicle stimulating hormone-induced growth promotion and gene
expression profiles on ovarian surface epithelial cells. Int J
Cancer. 112:803–814. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Zhang Z, Jia L, Feng Y and Zheng W:
Overexpression of follicle-stimulating hormone receptor facilitates
the development of ovarian epithelial cancer. Cancer Lett.
278:56–64. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Liu D, Zhou P, Zhang L, Wu G, Zheng Y and
He F: Differential expression of Oct4 in HPV-positive and
HPV-negative cervical cancer cells is not regulated by DNA
methyltransferase 3A. Tumour Biol. 32:941–950. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Iki K and Pour PM: Expression of Oct4, a
stem cell marker, in the hamster pancreatic cancer model.
Pancreatology. 6:406–413. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Wang XQ, Ongkeko WM, Chen L, et al:
Octamer 4 (Oct4) mediates chemotherapeutic drug resistance in liver
cancer cells through a potential Oct4-AKT-ATP-binding cassette G2
pathway. Hepatology. 52:528–539. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Venn A, Watson L, Bruinsma F, Giles G and
Healy D: Risk of cancer after use of fertility drugs with in-vitro
fertilisation. Lancet. 354:1586–1590. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Brekelmans CT: Risk factors and risk
reduction of breast and ovarian cancer. Curr Opin Obstet Gynecol.
15:63–68. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Tavani A, Ricci E, La Vecchia C, et al:
Influence of menstrual and reproductive factors on ovarian cancer
risk in women with and without family history of breast or ovarian
cancer. Int J Epidemiol. 29:799–802. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Riman T, Persson I and Nilsson S: Hormonal
aspects of epithelial ovarian cancer: review of epidemiological
evidence. Clin Endocrinol (Oxf). 49:695–707. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Li Y, Ganta S, Cheng C, Craig R, Ganta RR
and Freeman LC: FSH stimulates ovarian cancer cell growth by action
on growth factor variant receptor. Mol Cell Endocrinol. 267:26–37.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Lau MT, Wong AS and Leung PC:
Gonadotropins induce tumor cell migration and invasion by
increasing cyclooxygenases expression and prostaglandin E(2)
production in human ovarian cancer cells. Endocrinology.
151:2985–2993. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Choi JH, Choi KC, Auersperg N and Leung
PC: Gonadotropins activate proteolysis and increase invasion
through protein kinase A and phosphatidylinositol 3-kinase pathways
in human epithelial ovarian cancer cells. Cancer Res. 66:3912–3920.
2006. View Article : Google Scholar
|
|
33.
|
Xu H, Wang W, Li C, et al: WWP2 promotes
degradation of transcription factor OCT4 in human embryonic stem
cells. Cell Res. 19:561–573. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Chen T, Du J and Lu G: Cell growth arrest
and apoptosis induced by Oct4 or Nanog knockdown in mouse embryonic
stem cells: a possible role of Trp53. Mol Biol Rep. 39:1855–1861.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Qian X, Xi X and Li L: Nuclear survivin is
associated with malignant potential in epithelial ovarian
carcinoma. Appl Immunohistochem Mol Morphol. 19:126–132. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Zhang Z, Liao H, Chen X, et al:
Luteinizing hormone upregulates survivin and inhibits apoptosis in
ovarian epithelial tumors. Eur J Obstet Gynecol Reprod Biol.
155:69–74. 2010. View Article : Google Scholar : PubMed/NCBI
|