Introduction
Gastric cancer is the fourth most common cancer and
the second leading cause of cancer-related death (1). Peritoneal dissemination is one of the
terminal features of gastric cancer leading to death. There is
currently no standard treatment for peritoneal dissemination, as
neither surgery nor chemotherapy exert beneficial effects on
survival (2). Therefore,
investigating the molecular mechanisms underlying peritoneal
dissemination is required in order to improve the clinical outcomes
of gastric cancer.
Peritoneal dissemination is currently believed to
develop via a direct seeding mechanism. The seeding theory is
composed of several sequential steps, including cancer invasion in
the gastric wall, detachment of cancer cells from the primary
tumor, attachment to the distant peritoneum, invasion into the
subperitoneal space and proliferation with vascular neogenesis
(3). However, most proposed
theories remain speculative and are seldom based on adequate
evidence. The intraperitoneal injection (i.p.) of cultured gastric
cancer cells into the peritoneal cavity in nude mice is currently
used as a principle model to mimic the development of peritoneal
dissemination (4). This model
actually disregards the initial steps of the seeding theory,
including cancer invasion in the gastric wall and detachment of
cancer cells from the primary tumor. Therefore, it is difficult to
conclude that the i.p. model accurately reflects the
characteristics of peritoneal dissemination originating from
primary gastric cancer.
On the other hand, three scirrhous gastric cancer
cell lines (44As3, 58As1 and 58As9) have been established by
repeating orthotopic implantation (o.i.) in nude mice (5). When these cells are implanted into
the stomach wall in nude mice, dissemination to the greater omentum
and mesentery and the formation of bloody ascites are frequently
(90–100%) observed (5). Therefore,
the o.i. model using these cells is a powerful tool for analyzing
the mechanisms underlying the spread of peritoneal dissemination
via natural metastatic routes. Recently, one study examined this
natural metastasis model and reported the essential role of
miR-516a-3p in the development of peritoneal dissemination
(6).
The transcription factor hypoxia-inducible factor
(HIF)-1α is stabilized under hypoxic conditions and plays an
essential role in oxygen homeostasis (7). Various genes that regulate energy
metabolism, angiogenesis, apoptosis and cell survival have been
identified to be HIF-1α targets (8–10).
Furthermore, previous reports have revealed that hypoxia and HIF-1α
over-expression increase tumor aggressiveness and chemoresistance
in various cancers, including gastric cancer (11–24).
Recent studies have shown that each step of cancer metastasis, from
the initial epithelial-mesenchymal transition to the final step of
organotropic colonization, is potentially regulated by hypoxia,
suggesting that HIF-1α plays a role as a master regulator in cancer
metastasis (24). Although
numerous in vitro experiments have addressed the importance
of HIF-1α in cancer metastasis, in vivo studies have not
been well documented.
In the present study, we newly established knockdown
(KD) cells using scirrhous gastric cancer 58As9 cells in which the
HIF-1α expression was stably knocked down via siRNA transfection.
Then, we investigated the metastatic potential for peritoneal
dissemination in the KD cells and control transfectant (SC) cells
using orthotopic implantation (o.i.) and intraperitoneal injection
(i.p.) in nude mice. Finally, the presence of tumor angiogenesis in
the primary stomach tumors and the disseminated mesenteric nodules
was compared between the KD and SC mice. The goal of this study was
to clarify whether the HIF-1α expression is required for the
development of peritoneal dissemination and to elucidate whether
this type of metastasis can be achieved via a direct seeding
mechanism.
Materials and methods
Cell culture and exposure to hypoxia
The gastric cancer cell line 58As9 (5) was kindly provided by Dr K. Yanagihara
(National Cancer Research Institute, Tokyo, Japan). The cells were
cultured in RPMI-1640 medium (Sigma-Aldrich, Inc., St. Louis, MO,
USA) and maintained under conditions of either normoxia (20%
O2 and 5% CO2 in air) or hypoxia (1%
O2, 5% CO2 and 94% N2).
HIF-1α RNA interference
The pBAsi-hU6 Pur DNA plasmid vector (Takara
Biotechnology, Shiga, Japan) was used to construct a HIF-1α siRNA
plasmid by inserting an siRNA-coding sequence under the U6
promoter. The sequences of siRNA targeting HIF-1α and control
scrambled siRNA were designed as follows: HIF-1α (5′-CCA CAT TCA
CGT ATA TGA T-3′) and scrambled (5′-TCT TAA TCG CGT ATA AGG C-3′).
The 58As9 cells were transfected using a MicroPorator-mini (MP-100)
(Digital Bio Technology, Seoul, Korea) according to the
manufacturer's instructions. To obtain KD cells and control SC
cells with stable transfection of the above sequences, the cells
were selected using puromycin (Sigma) at a concentration of 3.0
μg/ml, then maintained in complete medium supplemented with
puromycin.
Western blot analysis
Whole cell lysates obtained from the cultured cells
were prepared using a lysis buffer and protease inhibitor cocktail
mix (Roche, Mannheim, Germany). For the western blot analysis, the
samples were dissolved in NuPage™ LDS sample buffer (Invitrogen
Corp., Carlsbad, CA, USA) and 1 M dithiothreitol, then heated for 5
min at 95°C. Aliquots containing 30 μg of protein were
subjected to 4–12% Bis-Tris Gel (Invitrogen) and
electrophoretically transferred onto an Amersham™ Hybond™-ECL
membrane (GE Healthcare, Buckinghamshire, UK) in a transfer buffer.
After blocking with 5% skim milk for 30 min, the membrane was
incubated with primary antibodies overnight at 4°C. The primary
antibodies used for the western blot analyses were anti-HIF-1α
(1:1000, Epitomics, Burlingame, CA, USA) and anti-β-actin
(1:10,000, Sigma) antibodies. Following incubation with the
corresponding secondary antibodies, the signals were developed
using the Amersham™ ECL Plus Western Blotting Detection System (GE
Healthcare).
Cell viability assay
The cell viability was analyzed using an MTT assay
with a Cell-Titer 96™ non-radioactive cell proliferation assay kit
(Promega, Madison, WI, USA). In brief, 5×103 cells per
well were seeded in triplicate onto 96-well plates and incubated
under normoxia and hypoxia at 37°C in a humidified atmosphere.
After 24 h, the number of viable cells was measured in triplicate
every day for three days. Cell viability curves were then
constructed by calculating the mean values of the optical density
measurements at 590 nm using a 96-well plate reader (Immuno-mini
NJ2300, Nalge Nunc International K.K., Tokyo, Japan).
In vitro invasion assay
Polycarbonate filters (6.5-mm-diameter) (8-μm
pore size) of Falcon Transwell™ chemotaxis chambers
(Beckton-Dickinson, Franklin Lakes, NJ, USA) were coated with 50
μl (1 mg/ml) of Matrigel biomatrix (Beckton-Dickinson) in
cold RPMI-1640 medium and dried overnight. Suspensions of
1×105 cells in 200 μl of complete RPMI-1640
medium were placed in the upper compartments of the chamber, while
the lower compartments were filled with 800 μl of
conditioned medium obtained from MRC5 fibro-blasts. The culture
units were incubated for 24 h at 37°C under normoxia and hypoxia.
The non-invasive cells on the upper surface of the filters were
then completely removed using a cotton swab. Any viable invasive
cells that had infiltrated onto the lower surface of the filter
were fixed with 70% ethanol and the nuclei were stained with
hematoxylin. Next, the number of invasive cells was counted. The
experiments were performed in triplicate and independently repeated
at least three times.
Animal experiments
The animal experimental protocols were approved by
the Animal Care Committee of Saga University. The mice were
purchased from CLEA Japan (Tokyo, Japan) and maintained under
specific pathogen-free conditions. The animals were provided
sterile food and water and housed in cages. The ambient light was
controlled to provide regular 12-h light-dark cycles.
Orthotopic implantation
Six-week-old female BALB/c nude mice were
anesthetized using intraperitoneal injections of
2.2.2-tribromoethanol (Aldrich Chemical, Milwaukee, WI, USA) at a
dose of 0.28 mg/g body weight. In each mouse, a small median
abdominal incision was made under anesthesia and 2×106
cells in a 50-μl volume of RPMI medium were then inoculated
into the middle wall of the greater curvature of the stomach using
a 30-gauge needle (Nipro, Tokyo, Japan). Each of the KD and SC cell
lines were orthotopically implanted into 15 mice. The mice were
sacrificed 70 days after tumor cell inoculation or when they became
moribund and the degree of peritoneal dissemination was evaluated
by counting the number of tumor nodules on the mesentery. In each
case, the stomach and mesenteric tumors were processed for the
histological examinations.
Intraperitoneal injection method used in
the peritoneal dissemination model
The i.p. method used in the peritoneal dissemination
model was performed according to the procedures described in a
previous report with slight modifications (25). To generate the xenograft model,
cancer cells (2×106) were suspended in 200 μl of
PBS and injected on day 0 into the abdominal cavity. Seven mice per
group were injected with each cell line. All of the mice were
sacrificed on day 21 and the number of disseminated nodules was
counted.
Anchorage-independent cell viability
assay
The anchorage-independent cell viability was
assessed using the MTT method described in the cell viability assay
section. The assay was performed using 96-well plates coated with
Ultra-Low Attachment Surface (Corning, NY, USA) instead of normal
plates without any coating.
Adhesion assay
To quantify the number of tumor cells attached to
the extracellular matrix (ECM), an in vitro adhesion assay
was performed using a CytoSelect 48-well cell adhesion assay (ECM
array, Colorimetric) (Cell Biolabs, Inc., San Diego, CA, USA)
according to the manufacturer's instructions. Regarding the
analysis performed under hypoxic conditions, the cells were
incubated for 24 h at 37°C under hypoxia before the assay was
performed.
Immunohistochemistry
The paraffin-embedded samples were cut into
4-μm-thick sections, then deparaffinized in xylene and
rehydrated in a graded series of ethanol. For antigen retrieval,
the slides were heated in EDTA (a pH of 8.0 for HIF-1α and Ki-67
and a pH of 9.0 for LYVE-1) in a microwave for 6 min or incubated
with proteinase K for 10 min (for PECAM-1) at room temperature. The
following primary antibodies were used: mouse monoclonal anti-human
HIF-1α (1:50, clone HI-67; Novus Biologicals, Littleton, CO, USA),
mouse monoclonal anti-human Ki-67 (1:30, clone MIB-1; Dako
Cytomation, Glostrup, Denmark), rabbit polyclonal anti-mouse LYVE-1
(1:30, 103-PA50AG; RELIA Tech GmbH, Wolfenb üttel, Germany) and
platelet endothelial cell adhesion molecule 1 (PECAM-1) (1:50,
clone MEC13.3; Santa Cruz Biotechnology, Santa Cruz, CA, USA). The
Envision+® System (Dako Cytomation) was used as the
secondary antibody for HIF-1α, Ki-67 and LYVE-1. Biotinylated
rabbit anti-rat (E0468; Dako Cytomation) secondary antibodies were
used for PECAM-1. Immunoreactive proteins were detected using an
avidinbiotin-based peroxidase system or peroxidase streptavidin
(Nichirei Co., Tokyo, Japan). The signals were visualized with
diaminobenzidine tetrahydrochloride (0.02%). The cell nuclei were
counterstained with Mayer's hematoxylin (Merck KGaA, Darmstadt,
Germany). The immunohistochemical expressions of HIF-1α, Ki-67,
PECAM-1 and LYVE-1 were assessed by a certified pathologist (K.
Kai).
Microvessel density assessment
The areas with larger numbers of microvessels in the
tissue sections were marked under microscopy. Using a magnification
of ×400, three optical fields were selected. All single stained
endothelial cells or cell clusters clearly separated from the
adjacent microvessels were considered to be single countable
microvessels. The microvessel density (MVD) was defined as the mean
number of microvessels per optical field (among the three selected
optical fields).
Assessment of angiogenesis and
lymphangiogenesis
The blood vessels were visualized using
immunohistochemical staining of PECAM-1 and assessed according to
the MVD of PECAM-1-positive vessels. Lymphangiogenesis was
evaluated both by counting the number and measuring the diameter of
the LYVE-1-positive vessels in which cancer cells had infiltrated.
The mean number and mean diameter were calculated in 10 of the SC
and seven of the KD stomach tumors.
Total RNA extraction and real-time
quantitative reverse transcription-polymerase chain reaction
Total RNA was extracted from each cell line using an
Isogen® RNA extraction kit (Nippon Gene, Osaka, Japan).
For each cell line, 1 μg of RNA was converted into cDNA
using a ReverTra Ace (Toyobo) reverse transcription reaction kit.
The cDNA was used as a template for the PCR. Real-time RT-PCR was
performed by means of the LightCycler™ instrument system (Roche)
using the Light-Cycler-FastStart DNA Master™ SYBR Green I kit
(Roche). The primers were designed as follows: vascular endothelial
growth factor (VEGF)-A: (5′-GCA GAA TCA TCA CGA AGT GG-3′, 5′-GCA
TGG TGA TGT TGG ACT CC-3′, product length 212 bp), aldolase C
(ALDOC): (5′-AAA TTG GGG TGG AAA ACA CA-3′, 5′-AGA AAA TGA CGC CTC
CAA TG-3′, 102 bp), carbonic anhydrase (CA) 9: (5′-CCG AGC GAC GCA
GCC TTT GA-3′, 5′-GGC TCC AGT CTC GGC TAC CT-3′, 252 bp) and
β-actin: (5′-ACT CTT CCA GCC TTC CTT CC-3′, 5′-GAC AGC ACT GTG TTG
GCG TA-3′, 120 bp). After performing a denaturation step at 95°C
for 3 min, PCR amplification was conducted with 50 cycles of 15 sec
of denaturation at 95°C, 5 sec of annealing at 60°C and 10 sec of
extension at 72°C. The quantitative values were normalized to the
β-actin expression. All experiments were performed in triplicate
and the mean values were calculated.
Statistical analysis
The statistical analysis was performed using the
computer software program SPSS 19.0 for Mac (SPSS, Chicago, IL,
USA). Comparisons between two groups were made using Student's
t-test and the Mann Whitney U test. Survival curves were generated
according to the Kaplan-Meier method and statistical differences
were compared using the log-rank test. P-values of <0.05 were
considered to be statistically significant.
Results
HIF-1α siRNA significantly decreases the
expression of HIF-1α and its target genes
The HIF-1α expression in the SC and KD cells was
validated using a western blot analysis (Fig. 1A). In the SC cells, HIF-1α was
faintly expressed under normoxia, while the HIF-1α expression was
strongly induced by hypoxia. On the other hand, hypoxic induction
of the HIF-1α expression was strongly reduced in the KD cells. To
assess the knockdown effects of HIF-1α, an RT-PCR analysis was
performed on HIF-1α target genes, such as aldolase C (ALDOC),
carbonic anhydrase (CA) 9 and vascular endothelial growth factor
(VEGF)-A. The hypoxic induction of these genes was significantly
reduced in the KD cells compared with that observed in the SC cells
(Fig. 1B).
Effects of HIF-1α knockdown on cell
viability and invasion
The cell viability and invasion activity were
compared between the KD and SC cells. As shown in Fig. 2A, the cell viability did not differ
between the KD and SC cells under normoxia, whereas the cell
viability under hypoxia was significantly decreased in the KD
cells. The invasive ability of the KD cells was strongly decreased
compared with that observed in the SC cells under both normoxia and
hypoxia (Fig. 2B).
Comparison of the KD and SC cells in the
orthotopic implantation model
Orthotopic implantation of SC cells resulted in the
development of massive ascites in the mice ∼20 days after injection
and several mice became moribund (Fig.
3A). In contrast, o.i. of KD cells, resulted in moribundity
without ascites formation ∼33 days after injection. Furthermore,
the KD mice demonstrated significantly longer survival times than
the SC mice (Fig. 3B). When the
dead animals were autopsied, the primary stomach tumors were highly
developed in both the SC (15/15:100%) and KD mice (13/15:86.7%)
(Table I). Peritoneal disseminated
nodules with bloody ascites most often developed on the mesentery
(Fig. 3A). The nodules were more
frequently observed in the SC mice (14/15: 93.3%) than in the KD
mice (2/15: 13.3%, P<0.001). (Fig.
3A, Table I). Fig. 3C demonstrates a series of
microscopic analyses using KD and SC stomach tumors. Both the KD
and SC stomach tumors grew beneath the mucosal layer. In the
immunohistochemical examination of HIF-1α, nuclear staining of this
protein was observed in the SC, but not in the KD, tumors. A
western blot analysis also confirmed a higher expression of HIF-1α
in the SC tumors than in the KD tumors (Fig. 3D). In contrast, the percentage of
cells exhibiting Ki-67 staining was almost the same between the KD
and SC tumors (Fig. 3E;
P=0.775).
 | Table I.Incidence of peritoneal dissemination
following orthotopic implantation (o.i.) of SC and KD cells. |
Table I.
Incidence of peritoneal dissemination
following orthotopic implantation (o.i.) of SC and KD cells.
| Cell lines | Survival (days) | Stomach tumor | Ascites | Peritoneal
dissemination |
|---|
| SC | 35±7a (24–47) | 15/15 (100%) | 14/15a (93.3%) | 14/15a (93.3%) |
| KD | 50±15 (33–70) | 13/15 (86.7%) | 3/15 (20%) | 2/15 (13.3%) |
Comparison of the KD and SC cells in the
intraperitoneal injection model
The results of i.p. in KD and SC cells were compared
and with those observed in the o.i. model. Distinct from the o.i.
model, peritoneal dissemination and ascites were frequently
observed in both the KD i.p. and SC i.p. mice (Table II). Notably, the KD i.p. mice
exhibited significantly greater numbers of disseminated nodules of
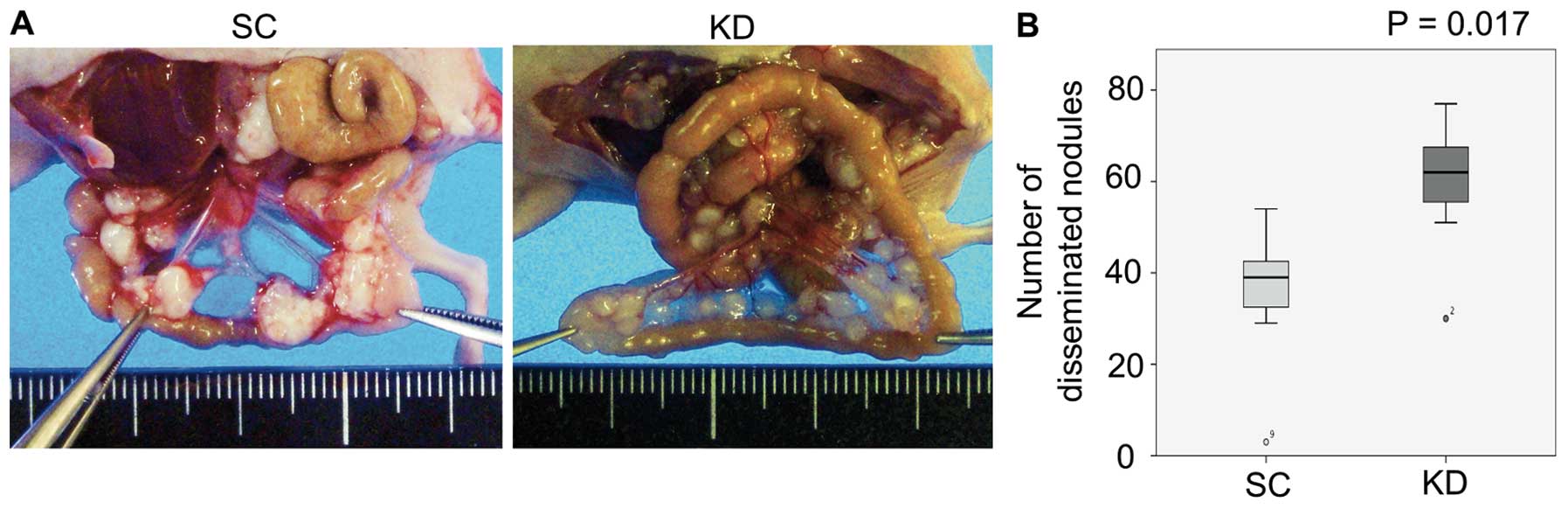
smaller sizes than those observed in the SC i.p. mice (Fig. 4; P=0.017).
 | Table II.Incidence of peritoneal dissemination
following intraperitoneal injection (i.p.) of SC and KD cells. |
Table II.
Incidence of peritoneal dissemination
following intraperitoneal injection (i.p.) of SC and KD cells.
| Cell lines | Ascites | Peritoneal
dissemination |
|---|
| SC | 5/7 (71.4%) | 7/7 (100%) |
| KD | 6/7 (85.7%) | 7/7 (100%) |
Anchorage-independent cell viability and
adhesion to the extracellular matrix
In vitro anchorage-independent cell viability
assays and adhesion assays were performed to investigate why the KD
cells developed greater numbers of peritoneal nodules than the SC
cells in the i.p. model. The results showed that the
anchorage-independent cell viability was higher in the KD cells
under both normoxia and hypoxia (Fig.
5A). In the adhesion assay, the attachment value to fibronectin
was significantly higher in the KD cells than in the SC cells under
normoxic conditions (Fig. 5B;
P=0.003). However, the attachment values to collagen I, IV and
laminin under hypoxia were significantly higher in the KD cells
than in the SC cells (Fig. 5B;
P<0.001, P=0.003, P=0.001, respectively).
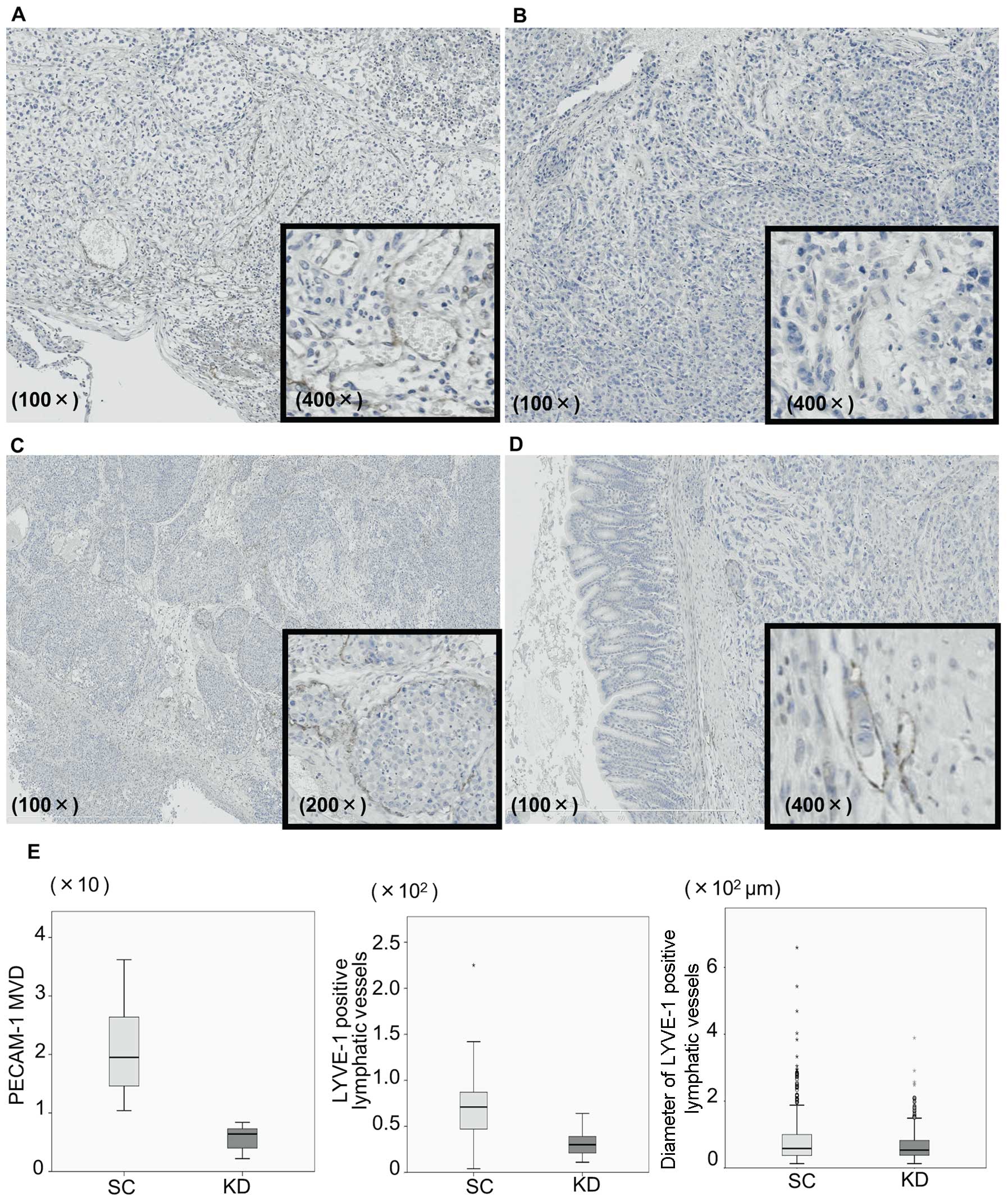
Immunohistochemistry for LYVE-1 and
PECAM-1
Tumor angiogenesis was assessed not only in the
implanted stomach tumors, but also in the disseminated nodules.
Immunohistochemical staining of PECAM-1 as a blood vessel marker
and LYVE-1 as a lymphatic vessel marker was assessed in the stomach
tumors injected with KD or SC cells (Fig. 6). Vessels with a PECAM-1-positive
expression were frequently observed at the tumor periphery in the
SC stomach tumors (Fig. 6A), while
such vessels were observed less frequently in the KD tumors and
their tube formation was more immature (Fig. 6B). The mean MVD of the
PECAM-1-positive vessels in the SC tumors was significantly higher
than that observed in the KD tumors (20.2±8.0 vs 5.6±2.4, P=0.001,
Fig. 6E). LYVE-1 was also
expressed at the tumor periphery in the SC tumors (Fig. 6C). Furthermore, vascular invasion,
in which cancer cells invaded and grew inside the vessels, was
frequently observed in the SC tumors. These aspects of lymphatic
vessels were also found in the KD tumors; however, the tube
formation appeared to be immature compared with that observed in
the SC tumors (Fig. 6D). Indeed,
the number as well as the diameter of the LYVE-1-positive vessels
in the KD tumors were significantly smaller than those of the
vessels observed in the SC tumors (Fig. 6E; P=0.043 and P=0.04). The presence
of tumor angiogenesis in the disseminated mesenteric nodules was
further assessed (Fig. 7). In the
nodules obtained from the SC o.i. mice, PECAM-1- and
LYVE-1-positive vessels with cancer invasion were observed in the
intratumoral regions (Fig. 7A and
D). In sharp contrast, vessels lacking tumor invasion had
formed at the tumor periphery in the nodules obtained from the SC
i.p. and KD i.p. mice (Fig. 7B, C, E
and F).
Discussion
The present study primarily aimed to investigate
whether HIF-1α is involved in the development of peritoneal
dissemination using nude mouse models. First, stable transfectant
KD cells lacking an HIF-1α expression and control SC cells were
established from parental 58As9 cells. A series of in vitro
analyses confirmed the effective knockdown of HIF-1α and the
subsequent suppression of HIF-1α target genes in the KD cells. A
functional analysis revealed a significant reduction in the
viability and invasive ability of the KD cells under hypoxia. In
the o.i. model using KD and SC cells, primary tumors were
frequently observed in the stomach wall in both types of mice, with
no significant differences in the incidence of tumors. Furthermore,
the degree of cell proliferation, as estimated based on Ki-67
immunostaining, did not differ between the tumors. With respect to
the incidence of peritoneal dissemination and ascites production,
significant differences were observed between the KD and SC o.i.
mice. These results indicate that the loss of the HIF-1α expression
did not affect cell proliferation in the primary tumors, but rather
strongly inhibited peritoneal dissemination and yielded worse
survival outcomes in the SC o.i. mice.
In addition to the o.i. model, we further employed
an i.p. model using these cells and analyzed the presence of
disseminated nodules on the mesentery. The results demonstrated
that peritoneal dissemination, as well as ascites production,
frequently occurs in both KD i.p. and SC i.p. mice. Of interest, a
greater number of disseminated nodules grew on the mesentery in the
KD i.p. mice compared with that observed in the SC i.p. mice,
although the nodules were smaller in size. We previously reported
similar findings showing that the inactivation of HIF-1α in the
gastric cancer cells MKN45 and MKN74 induces the formation of a
greater number of mesenteric nodules in the i.p. model (25). Additionally, in an in vitro
analysis, higher resistance against anoikis was observed in the KD
cells under both normoxic and hypoxic conditions. Regarding the
adhesion ability of cells to components of the extracellular
matrix, such as laminin and collagen I and IV, a stronger activity
was observed in the KD cells than in the SC cells under hypoxia.
Laminin and collagen I and IV are major components of the
mesothelial basement membrane and play key roles in the initial
adhesion of cancer cells to the peritoneum (26,27).
These results indicate that the HIF-1α expression may play an
unfavorable role not only in the survival of free cancer cells in
the peritoneal cavity, but also in the attachment of cells to the
ECM of the mesentery. These in vitro data also support the
in vivo findings that greater number of disseminated nodules
formed in the KD i.p. mice. Based on the clear difference in the
incidence of peritoneal dissemination between the o.i. and i.p.
models, an alternative mechanism distinct from the seeding theory
that acts on the development of peritoneal dissemination
originating from primary tumors may exist.
Finally, the present study addressed the effects of
HIF-1α knockdown on tumor angiogenesis in o.i. and i.p. mice. The
number as well as morphological features of the blood and lymphatic
vessels in the tumors were immunohistochemically evaluated
according to the PECAM-1 and LYVE-1 expression. In the stomach
tumors formed by o.i., PECAM-1-positive endothelial cells grew at
the periphery of the tumors derived from both the KD and SC cells.
However, the MVD of the blood vessels was significantly higher in
the SC tumors than in the KD tumors. These data strongly indicate
that knockdown of the HIF-1α expression attenuates angiogenesis
within implanted tumors. Stoeltzing et al reported similar
findings showing that suppression of HIF-1α by the dominant
negative form impairs angiogenesis and vessel maturation in stomach
tumors. The authors concluded that inhibition of the HIF-1α
activity reduces VEGF secretion from cancer cells and leads to
suppression of proangiogenic microenvironments in tumors (28). In the present study, hypoxic
induction of the VEGF-A gene was suppressed by HIF-1α knockdown.
Therefore, the axis from HIF-1α to VEGF-A may play an important
role in the angiogenesis of SC-derived tumors. We further assessed
the contribution of HIF-1α to lymphangiogenesis using
immunostaining of the lymphatic marker LYVE-1 in the SC and KD
stomach tumors. The results showed that lymphatic vessels
containing cancer cells frequently develop at the tumor periphery
in both SC and KD tumors. However, the number as well as the
diameter of the areas of lymphatic invasion were significantly
greater in the SC tumors than in the KD tumors. These results
indicate that the HIF-1α expression accelerates lymphangiogenesis
and lymphatic invasion in gastric tumors. Albrecht and Christofori
recently reported that tumor cells under hypoxia secrete
lymphangiogenic factors, such as VEGF-C, VEGF-D, PDGF-BB,
Angiopoietin 1, Angiopoietin 2 and PlGF, all of which contribute to
tumor lymphangiogenesis (29). We
analyzed the hypoxic induction of VEGF-C and -D in an RT-PCR
analysis; however, no expression of these factors was detected in
the parental 58As9 cells (data not shown). Other secretory factors
that accelerate lymphatic angiogenesis and lymphatic invasion may
be induced by HIF-1α in SC tumors. Taken together, the results
suggest that HIF-1α activates neovascularization of blood as well
as lymphatic vessels and increases the intravasation of cancer
cells within gastric tumors. These results further prompted us to
hypothesize that the peritoneal dissemination observed in SC o.i.
mice develops through an activated vascular network induced by
HIF-1α. To assess this speculation, the presence of vascular
formation in mesenteric nodules was investigated using SC i.p., KD
i.p. and SC o.i. mice. Consequently, blood and lymphatic vessels
without vascular invasion were observed at the tumor periphery in
the nodules obtained from the SC i.p. and KD i.p mice. In contrast,
vascular invasion, particularly in lymphatic vessels, was
frequently observed in the intratumoral regions of the mesenteric
nodules in the SC o.i. mice. This notable difference indicates that
disseminated nodules in SC o.i. mice may be formed via the
extravasation of cancer cells. Moreover, cancer cells present on
the mesentery may be primarily interconnected to stomach tumors
through vascular networks. Silvermann reported that the
subperitoneal space consists of fatty tissue, blood vessels,
lymphatics and lymph nodes enveloped by a serosal lining. This
space provides a complex interconnecting network that is an
important conduit for tumor cells within the peritoneal cavity
(30). This report strongly
supports our rationale that peritoneal dissemination is a type of
distant metastasis that travels through a transvessel route whereby
HIF-1α initiates tumor angiogenesis within the primary tumor.
In conclusion, the present study demonstrated for
the first time that HIF-1α is a crucial factor involved in the
development of peritoneal dissemination via the natural metastatic
route in scirrhous gastric cancer. Our report also provides a
possible mechanism, breaking through the conventional concept that
HIF-1α activates tumor angiogenesis, showing that HIF-1α may
increase metastasis to the peritoneum via vascular networks.
However, in order to elucidate this novel mechanism, it must be
clarified whether inhibition of vascular formation leads to the
suppression of peritoneal dissemination in the 58As9 o.i. model. In
the future, the clinical use of HIF-1α inhibitors is expected to
suppress peritoneal dissemination of scirrhous gastric cancer by
impairing tumor angiogenesis.
Acknowledgements
We would like to thank Mr. F. Mutoh
for his valuable contributions to the immunohistochemical
studies.
References
|
1.
|
Garcia M, Jemal A, Ward EM, Center MM, Hao
Y, Siegel RL and Thun MJ: Global Cancer Facts and Figures 2007.
American Cancer Society; Atlanta, GA: 2007
|
|
2.
|
Chu DZ, Lang NP, Thompson C, Osteen PK and
Westbrook KC: Peritoneal carcinomatosis in non gynecologic
malignancy. A prospective study of prognostic factors. Cancer.
63:364–367. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Yonemura Y, Kawamura T, Bandou E,
Tsukiyama G, Endou Y and Miura M: The natural history of free
cancer cells in the peritoneal cavity. Advances in Peritoneal
Surface Oncology. Gonzalez-Moreno S: Springer; Berlin: pp. 11–23.
2007, View Article : Google Scholar
|
|
4.
|
Kotanagi H, Saito Y, Shiozawa N and Koyama
K: Establishment of a human cancer cell line with high potential
for peritoneal dissemination. J Gastroenterol. 30:437–438. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Yanagihara K, Takigahira M, Tanaka H, et
al: Development and biological analysis of peritoneal metastasis
mouse models for human scirrhous stomach cancer. Cancer Sci.
96:323–332. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Takei Y, Takigahira M, Mihara K, Tarumi Y
and Yanagihara K: The metastasis-associated microRNA miR-516a-3p is
a novel therapeutic target for inhibiting peritoneal dissemination
of human scirrhous gastric cancer. Cancer Res. 71:1442–1453. 2010.
View Article : Google Scholar
|
|
7.
|
Wang GL and Semenza GL: General
involvement of hypoxiainducible factor 1 in transcriptional
response to hypoxia. Proc Natl Acad Sci USA. 90:4304–4308. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Semenza GL: HIF-1 and tumor progression:
pathophysiology and therapeutics. Trends Mol Med. 8:S62–7. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar
|
|
10.
|
Kitajima Y and Miyazaki K: The critical
impact of HIF-1α on gastric cancer biology. Cancers. 5:15–26.
2013.
|
|
11.
|
Sumiyoshi Y, Kakeji Y, Egashira A,
Mizokami K, Orita H and Maehara Y: Overexpression of
hypoxia-inducible factor 1α and p53 is a marker for an unfavorable
prognosis in gastric cancer. Clin Cancer Res. 12:5112–5117.
2006.
|
|
12.
|
Koukourakis MI, Giatromanolaki A,
Skarlatos J, et al: Hypoxia inducible factor (HIF-1α and HIF-2α)
expression in early esophageal cancer and response to photodynamic
therapy and radiotherapy. Cancer Res. 61:1830–1832. 2001.
|
|
13.
|
Bos R, van der Groep P, Greijer AE, et al:
Levels of hypoxiainducible factor-1α independently predict
prognosis in patients with lymph node negative breast carcinoma.
Cancer. 97:1573–1581. 2003.
|
|
14.
|
Nakanishi K, Hiroi S, Tominaga S, et al:
Expression of hypoxiainducible factor-1α protein predicts survival
in patients with tranitional cell carcinoma of the upper urinary
tract. Clin Cancer Res. 11:2583–2590. 2005.
|
|
15.
|
Birner P, Schindl M, Obermair A,
Breitenecker G and Oberhuber G: Expression of hypoxia-inducible
factor 1α in epithelial ovarian tumors: its impact on prognosis and
on response to chemotherapy. Clin Cancer Res. 7:1661–1668.
2001.
|
|
16.
|
Nakamura J, Kitajima Y, Kai K, Hashiguchi
K, Hiraki M, Noshiro H and Miyazaki K: HIF-1alpha is an unfavorable
determinant of relapse in gastric cancer patients who underwent
curative surgery followed by adjuvant 5-FU chemotherapy. Int J
Cancer. 127:1158–1171. 2010. View Article : Google Scholar
|
|
17.
|
Ide T, Kitajima Y, Miyoshi A, et al:
Tumor-stromal cell interaction under hypoxia increases the
invasiveness of pancreatic cancer cells through the hepatocyte
growth factor/c-Met pathway. Int J Cancer. 119:2750–2759. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Kitajima Y, Ide T, Ohtsuka T and Miyazaki
K: Induction of hepatocyte growth factor activator gene expression
under hypoxia activates the hepatocyte growth factor/c-Met system
via hypoxia inducible factor-1 in pancreatic cancer. Cancer Sci.
99:1341–1347. 2008. View Article : Google Scholar
|
|
19.
|
Liu L, Sun L, Zhang H, et al:
Hypoxia-mediated up-regulation of MGr1-Ag/37LRP in gastric cancers
occurs via hypoxiainducible-factor 1-dependent mechanism and
contributes to drug resistance. Int J Cancer. 124:1707–1715. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Song IS, Wang AG, Yoon SY, Kim JM, Kim JH,
Lee DS and Kim NS: Regulation of glucose metabolism-related genes
and VEGF by HIF-1α and HIF-1β, but not HIF-2α, in gastric cancer.
Exp Mol Med. 41:51–58. 2009.
|
|
21.
|
Zhang R, Fu H, Chen D, Hua J, Hu Y, Sun K
and Sun X: Subcellular distribution of S100A4 and its
transcriptional regulation under hypoxic conditions in gastric
cancer cell line BGC823. Cancer Sci. 101:1141–1146. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Liu L, Ning X, Sun L, et al:
Hypoxia-inducible factor-1α contributes to hypoxia-induced
chemoresistance in gastric cancer. Cancer Sci. 99:121–128.
2008.
|
|
23.
|
Miyoshi A, Kitajima Y, Ide T, et al:
Hypoxia accelerates cancer invasion of hepatoma cells by
upregulating MMP expression in an HIF-1α-independent manner. Int J
Oncol. 29:1533–1539. 2006.PubMed/NCBI
|
|
24.
|
Xin L and Yibin K: Hypoxia and
hypoxia-inducible factors: master regulators of metastasis. Clin
Cancer Res. 16:5928–5935. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Hiraki M, Kitajima Y, Kai K, Nakamura J,
Hashiguchi K, Noshiro H and Miyazaki K: Knockdown of
hypoxia-inducible factor-1a accelerates peritoneal dissemination
via the upregulation of MMP-1 expression in gastric cancer cell
lines. Exp Ther Med. 4:355–362. 2012.PubMed/NCBI
|
|
26.
|
Liotta LY: Tumor invasion and metastases -
role of the extra-cellular matirix: Rhoads Memorial Award lecture.
Cancer Res. 46:1–7. 1986.PubMed/NCBI
|
|
27.
|
Kawamura T, Endo Y, Yonemura Y, et al:
Significance of integrin alpha2/beta1 in peritoneal dissemination
of a human gastric cancer xenograft model. Int J Oncol. 18:809–815.
2001.PubMed/NCBI
|
|
28.
|
Stoeltzing O, McCarty MF, Wey JS, et al:
Role of hypoxiainducible factor 1alpha in gastric cancer cell
growth, angiogenesis and vessel maturation. J Natl Cancer Inst.
96:946–956. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Albrecht I and Christofori G: Molecular
mechanisms of lymphangiogenesis in development and cancer. Int J
Dev Biol. 55:483–494. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Silverman MP: The subperitoneal space:
mechanisms of tumor spread in the peritoneal cavity, mesentery and
omentum. Cancer Imaging. 4:25–29. 2003. View Article : Google Scholar : PubMed/NCBI
|