Introduction
The abnormal activation of Wnt/Wingless signaling
pathway has been confirmed to be related to tumorigenesis in many
tumor types (1,2). When the Wnt signal is weak, β-catenin
is incorporated in a destruction complex that contains glycogen
synthase kinase 3 (GSK3), adenomatous polyposis coli (APC), axin,
and casein kinase I (CKI), which results in phosphorylation of
β-catenin; phosphorylated β-catenin is then degraded by a
ubiquitin-mediated proteasomal pathway (3). However, in human cancers, the
dissociation of β-catenin from this destruction complex results in
the accumulation of β-catenin in the cytoplasm and nucleus, then
activates the target genes of Wnt pathway, such as cyclin D1 and
c-myc (1,2,4).
Axin, a key member of the destruction complex, can be recruited to
the plasma membrane by low-density lipoprotein receptor-related
protein (LRP) 5/6 co-receptors, which is facilitated by
dishevelled. This translocation will induce axin dephosphorylation
by protein phosphatase 1 (PP1), resulting in its degradation
(5–7).
Disabled-2 (Dab2) is a member of the
Mammalia/Drosophila disabled gene family (8), and contains 2 isoforms (p67 and p96)
(9). It is a widely expressed
endocytic adapter protein, and a regulator of some
receptor-mediated signaling pathways (2,10–12).
Dab2 can stabilize axin by preventing its interaction with LRP5/6
co-receptors and PP1, or promote LRP6 internalization through
clathrin, and serves as an inhibitor of the Wnt pathway (5,13,14).
Abnormal expression of Dab2 plays an important role in cell
differentiation and in the origin and development of cancers
(15–18). However, the expression level of
Dab2 and its significance for the development of lung cancers
remains unclear.
In this study, we examined the relationship among
Dab2 promoter methylation status, reduced expression of
Dab2, and clinicopathological characteristics in lung cancers. In
addition, we regulated the methylation status or expression level
of Dab2 in lung cancer cells in order to investigate the mechanisms
of Dab2 in the regulation of proliferation and invasiveness.
Materials and methods
Patients and specimens
A total of 100 paired fresh samples of primary lung
cancer and corresponding normal lung tissues were selected randomly
from patients diagnosed with lung cancer who underwent surgery at
The First Affiliated Hospital of China Medical University between
2010 and 2012. The age of patients ranged from 37 to 82 years, and
the mean age was 60 years (58 men and 42 women). The details of
tumors were listed in Table I.
Tumors were classified according to the system of the World Health
Organization (2004), and the TNM classification scheme of the
International Union Against Cancer. The study was conducted
according to the regulations of the institutional review boards at
China Medical University. Fresh tissue samples were stored at −70°C
immediately following resection.
 | Table I.Correlations between Dab2 methylation
and lung cancer clinicopathological factors. |
Table I.
Correlations between Dab2 methylation
and lung cancer clinicopathological factors.
| Clinicopathological
factors | n | Methylation of Dab2
| χ2
value | P-value |
|---|
| Positive | Negative |
|---|
| Gender | | | | 0.002 | 0.962 |
| Male | 58 | 54 | 4 | | |
| Female | 42 | 39 | 3 | | |
| Age | | | | 0.227 | 0.248 |
| <60 | 64 | 61 | 3 | | |
| ≥60 | 36 | 32 | 4 | | |
| Histologic
type | | | | 7.658 | 0.022 |
|
Adenocarcinoma | 53 | 52 | 1 | | |
| Squamous cell
carcinoma | 38 | 32 | 6 | | |
| Small cell lung
cancer | 9 | 9 | 0 | | |
|
Differentiation | | | | 9.703 | 0.009 |
| Well | 24 | 19 | 5 | | |
| Moderate | 50 | 48 | 2 | | |
| Poor | 26 | 26 | 0 | | |
| Lymphatic
metastasis | | | | 7.527 | 0.019 |
| Yes | 50 | 50 | 0 | | |
| No | 50 | 43 | 7 | | |
| TNM stage | | | | 9.933 | 0.007 |
| I | 51 | 44 | 7 | | |
| II | 32 | 32 | 0 | | |
| III | 17 | 17 | 0 | | |
Cell lines
A549, H157, H1299 and H460 cell lines were obtained
from the ATCC (Manassas, VA, USA). LTEP-a-2 (hereafter referred to
as LTE), SPC and LK2 cell lines were obtained from the Cell Bank of
the Chinese Academy (Shanghai, China). The BE1 cell line was kindly
provided by Professor J. Zheng (Medical College of Beijing
University, China) (19–21). A549 and LK2 cells were grown in
Dulbecco’s modified Eagle’s medium (DMEM); other cells were
cultured in RPMI-1640, supplemented with 10% fetal bovine serum
(FBS) at 37°C in a 5% CO2 humidified atmosphere.
DNA extraction and methylation-specific
PCR (MSP) analysis
Genomic DNA was isolated from tissue samples or
cells with a tissue/cell DNA extraction reagent kit (Bioteke,
Beijing, China) according to the manufacturer’s protocol. Bisulfite
conversion of DNA was performed with the EZ DNA Methylation kit
(Zymo Research, Beijing, China) according to the manufacturer’s
instructions. The primers of the nested PCR were as follows:
forward, AAAGGTAGTTTTTTGT TTAAAGGG; reverse, TAAACTTAATAACTCCCCCTCA
(product length: 367 bp). First, bisulfite-treated DNA was
amplified for 30 cycles: 95°C for 5 min, followed by cycling at
95°C for 30 sec, 52°C for 30 sec, and 72°C for 45 sec, with a final
extension step at 72°C for 10 min. Next, the nested PCR products
were diluted 100 times, and amplified for 45 cycles with MSP
primers: 95°C for 5 min, followed by cycling at 95°C for 30 sec,
60°C for 30 sec, and 72°C for 30 sec, with a final extension step
at 72°C for 10 min. The primers of MSP were as follows: methylated
forward, GGATTTGTGAAACGA AGTTC; methylated reverse,
CACCAACTAAAAACGATCG (product length, 168 bp); un-methylated
forward, GGATTTG TGAAATGAAGTTT; un-methylated reverse, CACCAACTA
AAAACAATCA (product length, 168 bp). Finally, the MSP products were
electrophoresized on 2% agarose gels containing ethidium bromide
and analyzed using a Bio-Imaging system (UVP, Upland, CA, USA).
Protein extraction and western blot
analysis
We randomly selected 50 paired lung cancer and
corresponding normal lung tissues, in which methylation status had
been examined previously, and extracted nuclear and cytoplasmic
proteins separately using the Nuclear and Cytoplasmic Protein
Extraction kit (Bioteke) according to the manufacturer’s protocol.
The quantified proteins were separated by electrophoresis on 10%
SDS-PAGE and transferred onto polyvinylidene difluoride membranes.
The membranes were subsequently blocked with 5% skim-milk for 2 h
and incubated overnight at 4°C with anti-Dab2 (1:500, Santa Cruz
Biotechnology Inc., Santa Cruz, CA, USA), anti-β-catenin (1:500, BD
Transduction Laboratories, KY, USA), anti-β-actin (1:500, BD
Transduction Laboratories), and anti-histone 3.1 (1:400, Signalway
Antibody, College Park, MD, USA) antibodies. The membranes were
then incubated with appropriate secondary antibodies at 37°C for 2
h. The protein bands were detected using an enhanced
chemiluminescence system (ECL Plus, Bio-Rad Biosciences, Hercules,
CA, USA). The relative expression quantity was scored as the ratio
of β-catenin or Dab2 protein intensity to β-actin or histone H3.1
staining intensity.
Demethylation assay
We performed a demethylation treatment in A549, LTE
and H1299 cells, which showed complete methylation of Dab2
in the MSP examination. Cells were seeded in 6-well plates and
allowed to confluence for 24 h, then treated with
5-Aza-2-deoxycytidine (5-Aza-dC) at a concentration of 5 μM
for 72 h. The medium was changed every day. Cells cultured in the
routine medium without 5-Aza-dC served as negative controls.
Dab2 gene transfection and siRNA
knockdown assay
The Dab2 expression vector pRK5-Dab2 was kindly
provided by Professor P.H. Howe (The Lerner Research Institute,
Cleveland Clinic Foundation, Cleveland, OH, USA) (5,12).
The A549, LTE and H1299 cells were transfected with pRK5-Dab2 using
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer’s instructions. The cells transfected with empty
vectors and un-transfected cells served as negative controls. Dab2
siRNA, control siRNA and siRNA reagent system were purchased from
Santa Cruz Biotechnology Inc. The siRNA interference of Dab2 was
performed according to the manufacturer’s instructions.
Cell proliferation analysis
The A549 cells transfected with Dab2,
interrupted with Dab2 siRNA, or treated with 5-Aza-dC, along with
control cells, were grown in 96-well plates separately at a density
of 2.0×105 cells/ml. Every 24 h, adherent cells were
harvested and analyzed using The CellTiter 96 Aqueous One Solution
cell proliferation assay
[3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide
(MTS)] (Promega, Madison, WI, USA). The absorbance, which is
directly proportional to the number of living cells in culture, was
measured at 490 nm using a microplate reader.
Cell invasion assay
Cell invasive ability was examined using a 24-well
Transwell with 8-μm pore polycarbonate membrane inserts
(Corning Inc., Corning, NY, USA). The A549 cells of each
experimental group and corresponding control groups were seeded on
the upper chamber of an insert coated with Matrigel (Sigma-Aldrich,
Saint Louis, MO, USA) at a density of 5×105 cells/well
in serum-free DMEM medium. The DMEM medium with 10% FBS was added
to the lower chamber (600 μl/well). After 30-h incubation,
the cells remaining on the upper membrane were removed with PBS and
cotton wool, whereas cells that had invaded through the membrane
were fixed with paraformaldehyde and stained with hematoxylin. The
cells were then viewed and counted using an IX71 inverted
microscope (Olympus, Tokyo, Japan).
Statistical analysis
The paired sample t-tests was performed to analyze
the cytoplasmic and nucleic expression level of Dab2 in lung
cancers and the corresponding normal lung tissues. The independent
t-tests was used to evaluate the expression level of Dab2 in lung
cancers between promotor methylated and unmethylated of Dab2
gene. The Pearson’s χ2 test, or likelihood ratio test,
was used to determine relationships between Dab2 promoter
methylation and clinicopathological characteristics of lung cancers
or absent expression of p96-Dab2. The Spearman’s correlation test
was used to examine the correlations between protein expression
levels and Dab2 methylation. Experiments of lung cancer
cells were independently repeated 3 times. P-values <0.05 were
considered statistically significant.
Results
Hypermethylation of Dab2 is common in
lung cancers and correlates with clinicopathological
parameters
In 100 lung cancer tissues, 58 cases (58.0%) showed
complete methylation, and 35 cases (35.0%) showed incomplete
methylation. However, in corresponding normal lung tissues, no case
showed complete methylation, and 35 cases (35.0%) showed incomplete
methylation (Fig. 1A). So, the
methylation rate of Dab2 in lung cancers (93.0%) was significantly
higher than that in corresponding normal lung tissues (35%)
(P<0.001). More importantly, promoter methylation of Dab2 was
correlated with differentiation (P=0.009), lymphatic metastasis
(P=0.019), TNM stage (P=0.007), and histological type (P=0.022),
but not correlated with gender (P=0.962) or age (P=0.248) of the
patients (Table I).
 | Figure 1.Promoter methylation of Dab2 in
lung cancers and normal lung tissues. (A) The methylation rate of
Dab2 in lung cancers was significantly higher than that in
corresponding normal lung tissues. T, lung tumor tissues; N, normal
lung tissues; M, methylated; U, unmethylated. (B) The methylation
status of Dab2 in A549, LTE, H1299, H157, H460, LK2, SPC and
BE1 lung cancer cells. (C) Treatment with 5-Aza-dC eliminated the
methylation status of Dab2 in A549, LTE and H1299 cells. |
Dab2 is localized both in the cytoplasm
and nucleus, and the reduced expression of Dab2 correlates with the
promoter methylation of Dab2 gene
After examination of the methylation status, we
selected 50 paired cases, to detect the cytoplasm and nucleus
expression of Dab2. The expression of p67-Dab2 was observed both in
the cytoplasm and nucleus of lung cancer and normal lung tissues.
However, p96-Dab2 was expressed only in the nuclei of 31 cases
(31/50, 62.0%) of normal lung tissues, and was lost in lung cancer
tissues (Fig. 2A). The cytoplasmic
or nuclear expression of Dab2 in lung cancers was significantly
lower than that in normal lung tissues (for cytoplasmic expression:
0.151±0.109 versus 0.696±0.337, t=−10.836, P<0.001; for nuclear
expression: 0.337±0.181 versus 0.901±0.384; t=−10.726, P<0.001)
(Fig. 2B).
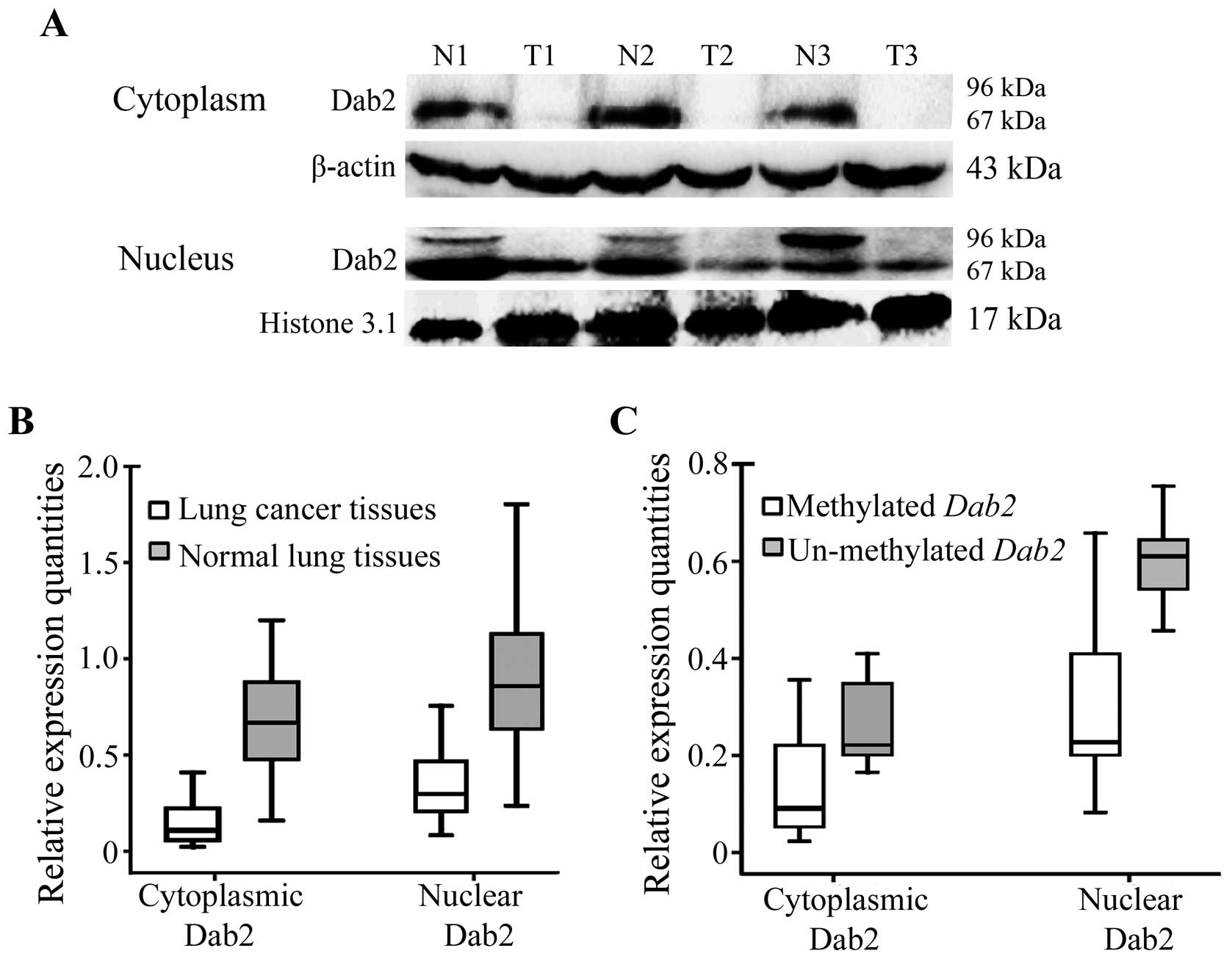 | Figure 2.Nuclear and cytoplasmic expression of
Dab2 in lung cancers and corresponding normal lung tissues. (A) The
expression of p67-Dab2 was reduced in both the cytoplasm and the
nucleus of lung cancer tissues compared to that in corresponding
normal lung tissues. P96-Dab2 was expressed only in the nucleus of
normal lung tissues, but lost in lung cancer tissues. β-actin and
histone 3.1 served as internal controls in the cytoplasm and
nucleus, respectively. T1, T2 and T3: lung tumor tissues; N1, N2
and N3: corresponding normal lung tissues. (B) The relative
expression quantity of Dab2 in lung cancers (cytoplasmic
expression, 0.151±0.109; nuclear expression, 0.337±0.181) was
significantly less than that in corresponding normal lung tissues
(cytoplasmic expression, 0.696±0.337; nuclear expression,
0.901±0.384) (P<0.001). (C) The relative expression quantity of
Dab2 in lung cancers with Dab2 promoter methylation
(cytoplasmic expression, 0.136±0.103; nuclear expression,
0.301±0.158) was much less than that in corresponding normal lung
tissues (cytoplasmic expression, 0.261±0.095; nuclear expression,
0.603±0.101) (P<0.001). |
Moreover, cytoplasmic expression levels of Dab2 in
lung cancer cases with Dab2 promoter methylation
(0.136±0.103) were significantly lower than that in lung cancer
cases without Dab2 promoter methylation (0.261±0.095;
t=−2.992, P=0.021). The nuclear expression of Dab2 also showed
similar results (0.301±0.158 versus 0.603±0.101; t=−4.532,
P<0.001) (Fig. 2C). The
Spearman’s correlation tests confirmed that Dab2 promoter
methylation was negatively correlated with Dab2 expression levels
in the cytoplasm (correlation coefficient, −0.258, P=0.009) and in
the nucleus (correlation coefficient, −0.298, P=0.003) in lung
cancer tissues. The loss of p96-Dab2 in corresponding normal lung
tissues also correlated with the promoter methylation status of
Dab2 (χ2=12.063, P=0.001). However, Dab2
expression levels did not correlate with clinicopathological
parameters of patients (data not shown).
Treatment with 5-Aza-dC enhances the
expression of Dab2, and inhibits the expression of β-catenin and
the proliferative and invasive abilities of lung cancer cells
The promoter of Dab2 was methylated in all
lung cancer cell lines used in this study. Complete methylation was
observed in A549, LTE and H1299 cells, whereas incomplete
methylation was observed in BE1, H460, SPC, H157 and LK2 cells
(Fig. 1B). The completely
methylated cells were then treated with 5-Aza-dC for 72 h
separately. The promoter methylation of Dab2 was successfully
eliminated (Fig. 1C), and
expression levels of Dab2 were increased significantly (P<0.05),
whereas β-catenin were significantly reduced in lung cancer cells
(P<0.05) (Fig. 3A).
Furthermore, the invasive cell number of 5-Aza-dC-treated A549
cells (9±2) was lower than that of untreated A549 cells (20±4)
(P<0.05) (Fig. 3B and C). The
growth rate of 5-Aza-dC-treated A549 cells was also reduced
relative to that of untreated A549 cells at the second, third, and
fourth days of detection (P<0.05) (Fig. 3D).
Dab2 overexpression reduces the
expression of β-catenin and inhibits the proliferative and invasive
ability of lung cancer cells
Both the nuclear and cytoplasmic expression of Dab2
was significantly enhanced after Dab2 gene transfection in
A549, LTE and H1299 cells (P<0.05). Whereas, the expression of
β-catenin was reduced (P<0.05) (Fig. 4A). The invasive cell number of A549
cells after Dab2 gene transfection (5±2) was reduced
relative to the vector control A549 (23±4) and untransfected A549
cells (21±4) (P<0.05) (Fig. 5A and
B). The growth rate of Dab2-transfected A549 cells was also
reduced relative to that of vector control A549 and untransfected
A549 cells at the second, third, and fourth days of detection
(P<0.05) (Fig. 5C).
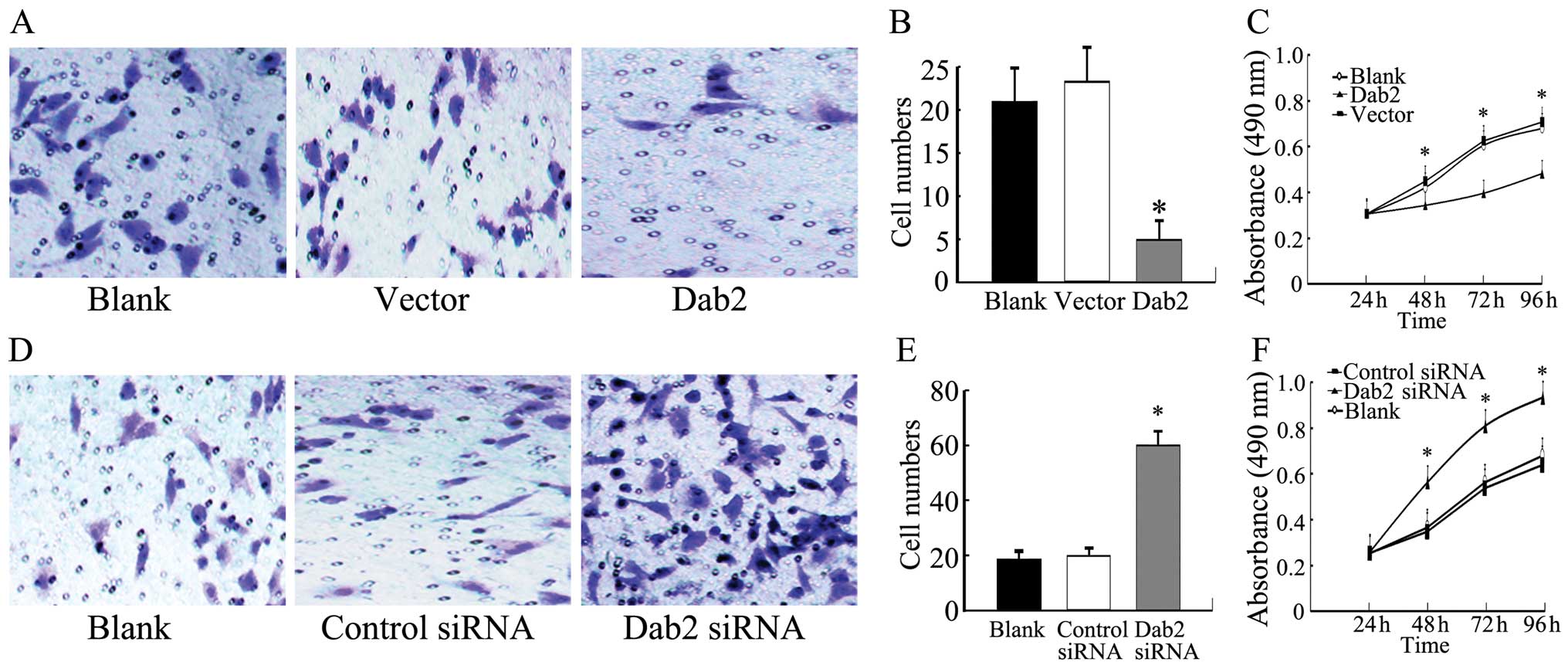 | Figure 5.Invasiveness and proliferation of
A549 cells with Dab2 gene transfection or Dab2 siRNA
interference. (A) Representative microscope fields of filters under
the Matrigel are shown from Dab2-transfected A549 cells,
vector control A549 cells, and blank A549 cells, respectively
(original magnification, ×400). (B) The number of invasive cells in
Dab2-transfected A549 cells was reduced relative to that of
vector control A549 and blank A549 cells (bar, SD;
*P<0.05). (C) The growth curves indicated that the
growth rate of Dab2-transfected A549 cells was reduced
relative to that of vector control A549 and blank A549 cells (bar,
SD; *P<0.05). (D) The representative microscope
fields of filters under the Matrigel are shown from A549 cells with
Dab2 siRNA interference, control siRNA A549 cells, and blank A549
cells, respectively (original magnification, ×400). (E) The number
of invasive cells in A549 cells with Dab2 siRNA interference was
increased relative to the control siRNA A549 and blank A549 cells
(bar, SD; *P<0.05). (F) The growth curves indicated
that the growth rate of A549 cells with Dab2 siRNA interference was
increased relative to the control siRNA A549 and blank A549 cells
(bar, SD; *P<0.05). |
Downregulation of the expression of Dab2
promotes the accumulation of β-catenin and enhances proliferation
and invasiveness of lung cancer cells
After interference with Dab2 siRNA, both the nuclear
and cytoplasmic expression of Dab2 was weak or absent in A549, LTE
and H1299 cells, respectively (P<0.05). The expression of
β-catenin was increased (P<0.05) (Fig. 4B). The invasive cell number of A549
cells with Dab2 siRNA interference (60±5) was increased compared to
the A549 cells treated with control siRNA (20±3) and untreated A549
cells (19±4) (P<0.05) (Fig. 5D and
E). The growth rate of A549 cells with Dab2 siRNA interference
was also increased relative to A549 cells treated with control
siRNA and untreated A549 cells at the second, third, and fourth
days of detection (P<0.05) (Fig.
5F).
Discussion
Dab2 has been shown to be a widely expressed
endocytic adaptor protein (10),
and participates in a variety of physiological processes such as
cell mitosis (22), endothelial
cell differentiation (23),
development of the central nervous system (24), and in the regulation of the
TGF-β/Smad (12), and
Wnt/β-catenin signaling pathways (13). Reduced expression of Dab2 will
result in the activation of Wnt pathway. Furthermore, loss of Dab2
expression may facilitate the establishment of an autocrine TGFβ
signalling loop, and promote TGFβ-stimulated
epithelial-to-mesenchymal transition, and therefore increase the
propensity for metastasis (25).
Although downregulation of Dab2 has previously been demonstrated in
other cancers (15,17,18,26),
concrete explanations for this observation have yet to be well
addressed.
We, for the first time, showed that Dab2 was
expressed in the cytoplasm and nucleus using immunofluorescence
(27), and western blot analysis,
and its expression was significantly reduced in lung cancers,
especially the p96 isoforms. We further showed that Dab2
overexpression inhibited the accumulation of β-catenin by
Dab2 gene transfection, which conclusively inhibited
proliferation and invasiveness of lung cancer cells. However,
downregulation of the expression of Dab2 by Dab2 siRNA induced
opposite results. We confirmed that reduced expression of Dab2
could induce the abnormal activation of Wnt pathway and promote the
development of lung cancers.
Our study demonstrated that the methylation of
Dab2 is common in lung cancers, similar to the reports in
other tumors (23,28–30).
Furthermore, the methylation of Dab2 was correlated with the
differentiation, lymphatic metastasis, and TNM stage of lung
cancers. Importantly, we found that the methylation of Dab2
was significantly correlated with reduced expression of the Dab2
protein in lung cancers. After treatment with 5-Aza-dC in A549, LTE
and H1299 cells, which show complete methylation of the Dab2
promoter, we found the methylation of the Dab2 promoter was
eliminated, and the expression of Dab2 was restored, which resulted
in downregulation of β-catenin and the inhibition of the
proliferative and invasive abilities of lung cancer cells. These
results therefore demonstrated that the hypermethylation of
Dab2 is a contributing factor in the reduced protein
expression in lung cancers, and is also related to the development
of lung cancers. So, the development of methods that could
eliminate the methylation status of Dab2 or enhance the
expression of Dab2 would offer potential therapeutic treatments for
lung cancers.
In conclusion, the methylation of the gene
Dab2 is common in lung cancers, and is one of the most
important factors responsible for the reduced expression of Dab2.
Furthermore, aberrant hypermethylation and reduced expression of
Dab2 promote the development of lung cancers.
Acknowledgements
This study was supported by the
National Science Foundation of China (Grant No. 81372497 to H.-T.
Xu) and the Program for Liaoning Excellent Talents in University
(Grant No. LJQ2011085 to H.-T. Xu).
References
|
1.
|
Lustig B and Behrens J: The Wnt signaling
pathway and its role in tumor development. J Cancer Res Clin Oncol.
129:199–221. 2003.PubMed/NCBI
|
|
2.
|
Prunier C, Hocevar BA and Howe PH: Wnt
signaling: physiology and pathology. Growth Factors. 22:141–150.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Kimelman D and Xu W: beta-catenin
destruction complex: insights and questions from a structural
perspective. Oncogene. 25:7482–7491. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Jiang Y, Luo W and Howe PH: Dab2
stabilizes Axin and attenuates Wnt/beta-catenin signaling by
preventing protein phosphatase 1 (PP1)-Axin interactions. Oncogene.
28:2999–3007. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
He X, Semenov M, Tamai K and Zeng X: LDL
receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling:
arrows point the way. Development. 131:1663–1677. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Zeng X, Tamai K, Doble B, et al: A
dual-kinase mechanism for Wnt co-receptor phosphorylation and
activation. Nature. 438:873–877. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Gertler FB, Bennett RL, Clark MJ and
Hoffmann FM: Drosophila abl tyrosine kinase in embryonic CNS
axons: a role in axonogenesis is revealed through dosage-sensitive
interactions with disabled. Cell. 58:103–113. 1989. View Article : Google Scholar
|
|
9.
|
Kim JA, Bae SH, Choi YJ, Kim KH and Park
SS: Feed-back regulation of disabled-2 (Dab2) p96 isoform for
GATA-4 during differentiation of F9 cells. Biochem Biophys Res
Commun. 421:591–598. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Fu L, Rab A, Tang LP, Rowe SM, Bebok Z and
Collawn JF: Dab2 is a key regulator of endocytosis and
post-endocytic trafficking of the cystic fibrosis transmembrane
conductance regulator. Biochem J. 441:633–643. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Hung WS, Huang CL, Fan JT, Huang DY, Yeh
CF, Cheng JC and Tseng CP: The endocytic adaptor protein Disabled-2
is required for cellular uptake of fibrinogen. Biochim Biophys
Acta. 1823:1778–1788. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Hocevar BA, Smine A, Xu XX and Howe PH:
The adaptor molecule Disabled-2 links the transforming growth
factor beta receptors to the Smad pathway. EMBO J. 20:2789–2801.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Jiang Y, Prunier C and Howe PH: The
inhibitory effects of Disabled-2 (Dab2) on Wnt signaling are
mediated through Axin. Oncogene. 27:1865–1875. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Jiang Y, He X and Howe PH: Disabled-2
(Dab2) inhibits Wnt/beta-catenin signalling by binding LRP6 and
promoting its internalization through clathrin. EMBO J.
31:2336–2349. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Karam JA, Shariat SF, Huang HY, et al:
Decreased DOC-2/DAB2 expression in urothelial carcinoma of the
bladder. Clin Cancer Res. 13:4400–4406. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Yang DH, Smith ER, Cohen C, et al:
Molecular events associated with dysplastic morphologic
transformation and initiation of ovarian tumorigenicity. Cancer.
94:2380–2392. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Kleeff J, Huang Y, Mok SC, Zimmermann A,
Friess H and Büchler MW: Down-regulation of DOC-2 in colorectal
cancer points to its role as a tumor suppressor in this malignancy.
Dis Colon Rectum. 45:1242–1248. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Anupam K, Tusharkant C, Gupta SD and Ranju
R: Loss of disabled-2 expression is an early event in esophageal
squamous tumorigenesis. World J Gastroenterol. 12:6041–6045.
2006.PubMed/NCBI
|
|
19.
|
Chetrit D, Barzilay L, Horn G, Bielik T,
Smorodinsky NI and Ehrlich M: Negative regulation of the endocytic
adaptor disabled-2 (Dab2) in mitosis. J Biol Chem. 286:5392–5403.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Yang DH, Smith ER, Roland IH, et al:
Disabled-2 is essential for endodermal cell positioning and
structure formation during mouse embryogenesis. Dev Biol.
251:27–44. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Zhu W, Zheng J and Fang W: Isolation and
characterization of human lung cancer cell subline with different
metastatic potential. Zhonghua Bing Li Xue Za Zhi. 24:136–138.
1995.(In Chinese).
|
|
22.
|
Liu CR, Ma CS, Ning JY, You JF, Liao SL
and Zheng J: Differential thymosin beta 10 expression levels and
actin filament organization in tumor cell lines with different
metastatic potential. Chin Med J (Engl). 117:213–218.
2004.PubMed/NCBI
|
|
23.
|
Xu HT, Wei Q, Liu Y, et al: Overexpression
of axin down-regulates TCF-4 and inhibits the development of lung
cancer. Ann Surg Oncol. 14:3251–3259. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Cheung KK, Mok SC, Rezaie P and Chan WY:
Dynamic expression of Dab2 in the mouse embryonic central nervous
system. BMC Dev Biol. 8:762008. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Martin JC, Herbert BS and Hocevar BA:
Disabled-2 down-regulation promotes epithelial-to-mesenchymal
transition. Br J Cancer. 103:1716–1723. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Bagadi SA, Prasad CP, Srivastava A,
Prashad R, Gupta SD and Ralhan R: Frequent loss of Dab2 protein and
infrequent promoter hypermethylation in breast cancer. Breast
Cancer Res Treat. 104:277–286. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Xu HT, Yang LH, Li QC, Liu SL, Liu D, Xie
XM and Wang EH: Disabled-2 and Axin are concurrently colocalized
and underexpressed in lung cancers. Hum Pathol. 42:1491–1498. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Tong JH, Ng DC, Chau SL, et al: Putative
tumour-suppressor gene DAB2 is frequently down regulated by
promoter hyper-methylation in nasopharyngeal carcinoma. BMC Cancer.
10:2532010. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Hannigan A, Smith P, Kalna G, et al:
Epigenetic downregulation of human disabled homolog 2 switches
TGF-beta from a tumor suppressor to a tumor promoter. J Clin
Invest. 120:2842–2857. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Yang Y, Zhang Q, Xu F, Chang C and Li X:
Aberrant promoter methylation of Dab2 gene in myelodysplastic
syndrome. Eur J Haematol. 89:469–477. 2012. View Article : Google Scholar : PubMed/NCBI
|



















