Introduction
Colorectal cancer (CRC) is a malignant neoplasm
arising from the lining of the large intestine (colon and rectum).
CRC is the third most common malignancy and one of the major causes
of cancer-related death in the United States (1). Colitis-associated cancer (CAC) is the
type of colon cancer which is preceded by clinically detectable
inflammatory bowel disease (IBD), such as Crohn’s disease (CD) or
ulcerative colitis (UC) (2). IBD
results from the inappropriate and ongoing activation of the
mucosal immune system, and this is driven by the presence of normal
luminal flora. As many as 1.4 million persons in the United States
and 2.2 million persons in Europe suffer from these diseases
(3). The incidence of IBD in Korea
has increased significantly over the past few decades. In case of
prevalence for UC in South Korea, it was quadrupled from
7.57/105 individuals in 1997 to 30.9/105
individuals in 2005. Adjusted prevalence rates of CD and UC per
105 individuals were 11.2 and 30.9, respectively
(4). In case of incidence of IBD
in Japan, the number of patient is increased with time. The
age-standardized prevalence of UC in Japan in 2005 was
63.6/105 individuals, and that of CD was
21.2/105 individuals. Incidence rate of UC and CD are
higher than South Korea. The prevalence of inflammatory bowel
diseases is much lower in Asian countries, including Japan and
Korea, than in Western countries, but it is rapidly increasing
(5). Chronic IBD such as UC and CD
cause colitis-associated colon cancer.
Peroxisome proliferator-activated receptors γ
(PPARγ), which belongs to the nuclear receptor superfamily, is a
ligand-activated transcription factor that forms heterodimer with
retinoic X receptor (RXR) and stimulates expression of target
genes. It is expressed in various tissues and cell types, including
those from the pancreas, liver, kidney, adipose tissue and colon
(6). Further, several lines of
evidence indicate that PPARγ plays an important role in regulating
inflammatory responses in the intestine (7). PPARγ and its activators are known as
important modulators having anti-inflammatory properties that can
modulate nuclear factor-κB (NF-κB) activation. Rosiglitazone, PPARγ
activator, was tested in clinical trials and was found to be
effective in the treatment of UC (8). Dietary punicic acid ameliorates
intestinal inflammation by activation of PPARγ in mice (9). Previous studies have provided
evidence that PPARγ can inhibit inflammatory gene expression by
several mechanisms, including competition for a limiting pool of
co-activators, direct interaction with NF-κB, p65 and p50 subunits,
modulation of p38 mitogen-activated protein kinase (MAPK) activity,
and partitioning the co-repressor B cell lymphoma-6 (BCL-6)
(10). So use of PPARγ agonists
could be beneficial in inflammation related diseases such as
IBD.
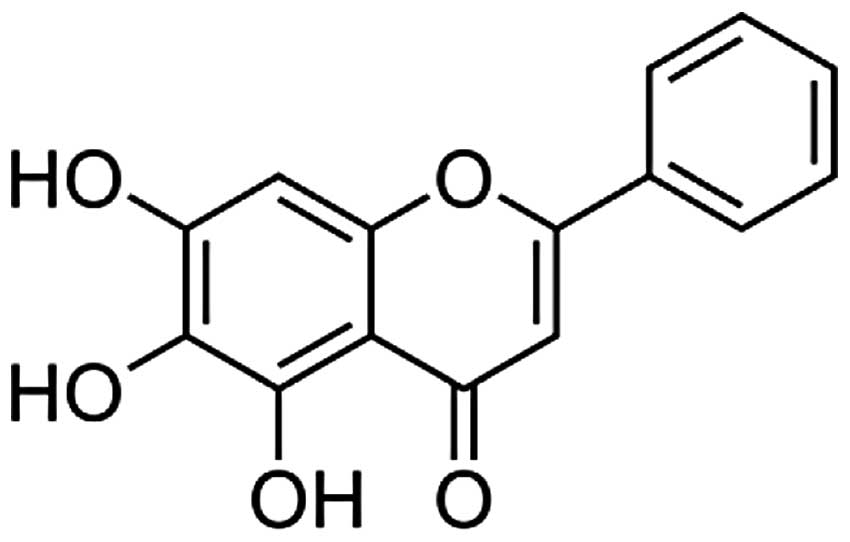
Baicalein (5,6,7-trihydroxyflavone, Fig. 1) one of four major flavonoids found
in Scutellaria baicalensis Georgi, is widely used in Chinese
herbal medicine and has been used in various inflammatory diseases
and ischemia (11). Treatment with
baicalein has been reported to attenuate endothelium intimal
hyperplasia by inhibiting inflammatory signaling pathways involving
extracellular signal-regulated kinase (ERK), Akt and NF-κB
activities in vascular smooth muscle cells (12). Baicalein attenuates the
radiation-induced inflammation process in mouse kidney by
modulation of NF-κB and Forkhead family of transcription factors
(FOXOs) (13). It has been shown
that baicalein has an inhibitory effect on colorectal cancer
(14). Baicalein also enacts
anticancer activity by inhibiting platelet-type 12-lipoxygenase
(12-LOX), which has been shown to regulate growth, metastasis and
angiogenesis in prostate cancer (15). The above accumulating evidence
demonstrates that biacalein possesses potent anticancer and
anti-inflammatory activities.
However, knowledge of the protective role of
baicalein in colon cancer on the expression and regulation of
apoptosis and inflammatory mediators associated with colon cancer
is still unknown. Hence, in the present study we aimed to evaluate
the chemopreventive effects of baicalein on human colon cancer
cells and AOM/DSS-induced colitis-associated colon cancer in mice.
We demonstrate that baicalein is a potent chemopreventive and
anti-inflammatory agent that may act through the activation of
PPARγ to inhibit NF-κB activation in colon cancer.
Materials and methods
Chemicals
Baicalein was purchased from Sigma-Aldrich Co. (St.
Louis, MO). Baicalein was freshly prepared before each experiment
and was solubilized with dimethylsulfoxide (DMSO). The final
concentration of DMSO in the medium was less than 0.1% (vol/vol) in
the treatment range (25–100 μM) and showed no influence on
cell growth (data not shown).
Cell culture and cell viability
assay
The human colorectal cancer HCT116 cells were
cultured in RPMI-1640 (HyClone, Logan, UT) supplemented with 10%
fetal bovine serum (FBS, HyClone), 2 mM glutamine (Sigma-Aldrich),
100 U/ml penicillin (HyClone) and 100 μg/ml streptomycin
(HyClone) at 37°C in a humidified 5% CO2. Cell viability
was determined by MTT assay. For the MTT assay, HCT116 cells were
seeded in a 24-well culture plate at a density of 4×104
cells/well, cultured for 24 h in the growth media and then treated
with or without baicalein for the indicated concentrations. The
cells were incubated with 0.5 mg/ml MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]
(Sigma-Aldrich) at 37°C for 2 h. The formazan granules generated by
the live cells were dissolved in DMSO and the absorbance at 540 nm
was monitored by using a multi-well reader.
Western blot analysis
The cells were treated under the appropriate
conditions, harvested, washed with cold PBS and then lysed in lysis
buffer [40 mM Tris (pH 8.0), 120 mM NaCl, 0.5% NP-40, 0.1 mM sodium
orthovanadate, 2 μg/ml aprotinin, 2 μg/ml leupeptin
and 100 μg/ml phenymethylsulfonyl fluoride (PMSF)]. The
supernatant was collected and protein concentrations were measured
(Pierce, Rockford, IL). Protein extracts were denatured by boiling
at 100°C for 5 min in sample buffer (0.5 M Tris-HCl, pH 6.8, 4%
SDS, 20% glycerol, 0.1% bromophenol blue, 10% β-mercaptoethanol).
Equal amount of the total proteins were subjected to 6–15% SDS-PAGE
and transferred to PVDF. The membranes were blocked with 5% non-fat
dry milk in Tris-buffered saline with Tween-20 buffer (TBS-T) (20
mM Tris, 100 mM NaCl, pH 7.5 and 0.1% Tween-20) for 1 h at room
temperature. Then, the membranes were incubated overnight at 4°C
with the primary antibodies. The membranes were washed once for 10
min with 4X TBS-T buffer and incubated for 1 h with horseradish
peroxidase-conjugated anti-rabbit or anti-mouse immunoglobin (Santa
Cruz Biotechnology Inc., Santa Cruz, CA). The membranes were washed
again for 10 min with 1X TBS-T buffer. Antigen-antibody complexes
were detected by the enhanced chemiluminescence (ECL) detection
system (GE Healthcare Biosciences, Pittsburgh, PA).
Cell motility assay
HCT116 cells were grown to confluence on 35-mm cell
culture dishes at 90% confluence, wounded with a 200-μl
pipette tip, and marked at the injury line. After washing with
phosphate-buffered saline (PBS), serum-free media (to prevent cell
proliferation) containing either vehicle (DMSO) or various
concentrations of baicalein was added for the indicated times.
Wound closure of cells was observed and photographed under the
microscope at ×50 magnification.
In vitro migration assay
The migration capacity of HCT116 cells was
determined using a modified 24-well Boyden chamber
(8-μm-pore size) (Corning, Tewksbury, MA). The cells were
seeded at a density of 6×104 cells in 100 μl
serum-free medium to the upper compartment of the Transwell and
incubated in lower chamber containing either DMSO or baicalein for
24 h at 37°C in 5% CO2. Cells that did not penetrate the
filter were completely wiped off with cotton swabs, and cells that
had migrated to the lower surface of the filter were fixed with
methanol. The cells were then stained with methylene blue and eosin
and observed under a phase contrast microscope and photographed at
×50 magnification.
Gelatin zymographic analysis of secreted
MMPs
Following incubation with baicalein, cell culture
supernatants were collected and centrifuged at 400 × g for 5 min.
Cell-free supernatant was mixed with 2X sample buffer (Invitrogen,
Carlsbad, CA) and zymography was performed using precast gels (10%
polyacrylamide and 0.1% gelatin). Following electrophoresis, gels
were washed twice at room temperature for 30 min in 2.5% Triton
X-100, and subsequently washed in buffer containing 50 mM Tris-HCl,
150 mM NaCl, 5 mM CaCl2, 1 μM ZnCl2,
and 0.02% NaN3 at pH 7.5, and incubated in this buffer
at 37°C for 24 h. Thereafter, gels were stained with 0.5% (w/v)
Coomassie brilliant Blue G-250 (Bio-Rad, Hercules, CA) for 1 h,
then lightly destained in methanol:acetic acid:water (3:1:6). Clear
bands appeared on the Coomassie stained Blue background in the
areas of gelatinolytic activity. Gels were scanned and images were
processed for extraction of the blue channel signal, which
converted it to black and white.
Animal study
The animal protocol used in this study was reviewed
by the Pusan National University-Institutional Animal Care and Use
Committee (PNU-IACUC, Busan, Korea) on the ethics of animal
procedures and scientific care, were approved. Five-week-old male
ICR mice were purchased from Samtako Co., Ltd. (Osan, Korea). All
animals were housed in plastic cages (4 mice/cage) and had free
access to drinking water and a basal diet (Formula M07; Feed lab)
under controlled conditions of humidity (50±10%), light (12/12 h
light/dark cycle), and temperature (∼23°C). After arrival, the
animals were quarantined for the first 7 days, and then randomized
by body weights into experimental and control groups. A colonic
carcinogen AOM was purchased from Sigma-Aldrich. DSS with a
molecular weight of 36,000–50,000 (cat. no. 160110) was purchased
from MP Biomedicals, LLC (Aurora, OH). For the induction of
colitis, DSS was dissolved in water at a concentration of 1.5%
(w/v). Experimental groups included group 1 (control group, n=6);
group 2 (n=8) was treated with AOM and DSS; groups 3–5 (n=8 for
each group) were treated with AOM, DSS and baicalein (1 mg/kg for
group 3, 5 mg/kg for group 4, 10 mg/kg for group 5). Mice in groups
2–5 were given a single intraperitoneal injection of AOM (10 mg/kg
body weight). Starting 1 week after the injection, animals received
1.5% DSS in the drinking water for 7 days. Subsequently, groups 3–5
received the diets containing 1, 5 and 10 mg/kg baicalein for 16
weeks, respectively. All animals were sacrificed at week 16 after
administration of baicalein. At sacrifice, complete necropsies were
done on all mice. Histopathological examination was performed on
paraffin-embedded sections after hematoxylin and eosin (H&E)
staining.
Statistical analysis
Results are expressed as the mean ± SD of three
separate experiments and analyzed by Student’s t-test. Means were
considered significantly different at *p<0.05 or
**p<0.01.
Results
Baicalein reduces the viability of HCT116
cells
To investigate the effects of baicalein on the
viability of HCT116 cells, the MTT assay was performed. As shown in
Fig. 2A, cell viability was
significantly decreased by treatment of baicalein in a
concentration-dependent manner. The concentrations required for
half-maximal inhibition (IC50) of the cells were about
100 μM for HCT116 cells after 24 h and about 50 μM
after 48 h. Treatment with baicalein for 24 h showed distinct
morphological changes compared with control (Fig. 2B). They were rounded and more
dispersed with aggregation in a concentration-dependent manner.
Baicalein induces apoptosis in HCT116
cells
To investigate whether the growth inhibitory effects
of baicalein were due to the induction of apoptosis in HCT116
cells, morphological changes of cellular structures were assessed
with Hoechst 33342 staining. As shown in Fig. 3A, nuclei with chromatin
condensation and formation of apoptotic bodies, which are
characteristics of apoptosis, were seen in cells cultured with
baicalein in a concentration-dependent manner, whereas the control
cells maintained their nuclear structure intact. During apoptotic
process, caspases play major roles in both the intrinsic and
extrinsic pathways. Thus, the levels of caspase-3, -8 and -9 were
investigated in order to determine whether caspases are associated
with baicalein-induced apoptosis in HCT116 cells. Treatment with
baicalein showed decreased pro-caspase-3 and -8 levels and induced
cleavage of PARP (Fig. 3B). These
results indicated that growth inhibitory effects of baicalein were
due to the induction of apoptosis in HCT116 cells.
 | Figure 3.Induction of apoptosis in HCT116 cells
by baicalein. (A) The cells were treated with baicalein for 24 h,
then stained nuclei with fluorescent DNA-binding dye, Hoechst
33342, and then photographed with a fluorescent microscope using a
blue filter at ×320 magnification. Arrows, apoptotic cells. (B)
HCT116 cells were treated with indicated concentrations of
baicalein for 24 h, collected, lysed and then cellular proteins
were separated and immunoblotted. The membranes were probed with
pro-caspase-3, pro-caspase-8, pro-caspase-9, PARP (116 kDa), and
cleaved PARP (85 kDa). Proteins were visualized using the ECL
detection system. Representative results from three independent
experiments are shown. Actin was used as a loading control. Con,
control. |
Baicalein suppresses the NF-κB activity
through the PPARγ activation
PPARγ agonists can inhibit the activities of signal
dependent transcription factors, such as NF-κB. This function could
contribute to the anti-inflammatory actions of PPARγ. To verify
whether baicalein modulates the NF-κB activity through the PPARγ
activation, western blot analysis was performed and expression
levels measured of NF-κB-related protein. Treatment of baicalein
induced PPARγ activation and inhibited pIκBα, p50, p65 and iNOS
levels (Fig. 4). These results
suggested that NF-κB activity was suppressed through PPARγ
activation by baicalein treatment in HCT116 cells.
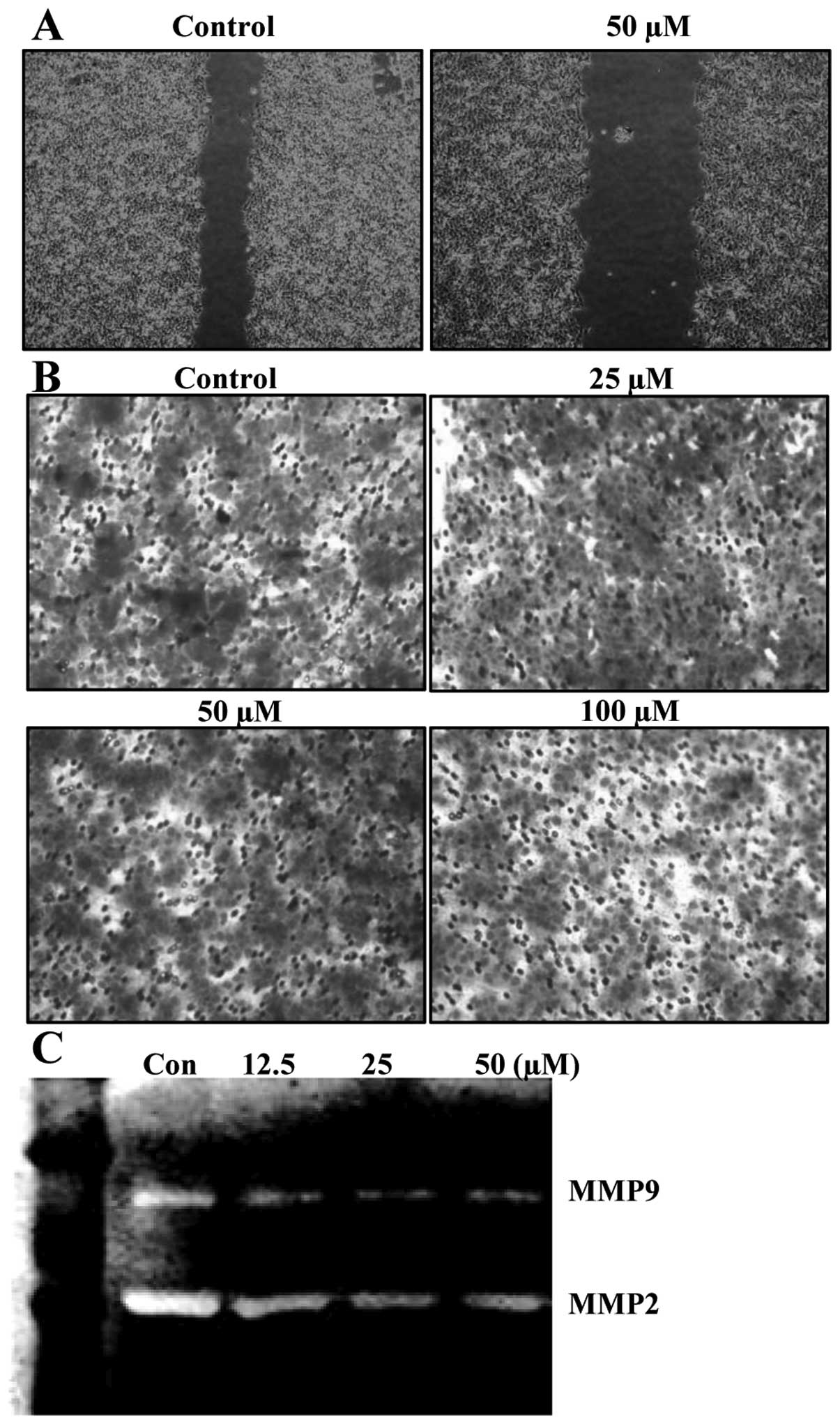
Baicalein suppresses migration
To determine whether or not biacalein inhibits
migration of HCT116 cells, wound-healing experiments were
performed. Results demonstrated that 50 μM of baicalein for
24 h, which was not cytotoxic, as shown by the MTT assay, delayed
the migration of HCT116 cells compared to that of control cells
(Fig. 5A). Using a Boyden chamber
migration assay, we next examined the question of which baicalein
decreases the activity of cell migration. As shown in Fig. 5B, treatment of cells with 50
μM of baicalein markedly reduced cell invasion through the
Matrigel chamber. Because cell migration plays an important role in
the metastasis process, and migration of the basement membrane is
primarily mediated by gelatinase matrix metalloproteinases (MMPs),
we tested the effects of baicalein on MMP gelatin zymography.
Treatment of baicalein reduced the expression of the MMP-2 and -9
activities (Fig. 5C). These
results suggested that the anti-migration effect of baicalein is
associated with inhibition of MMP-2 and -9 activities in HCT116
cells.
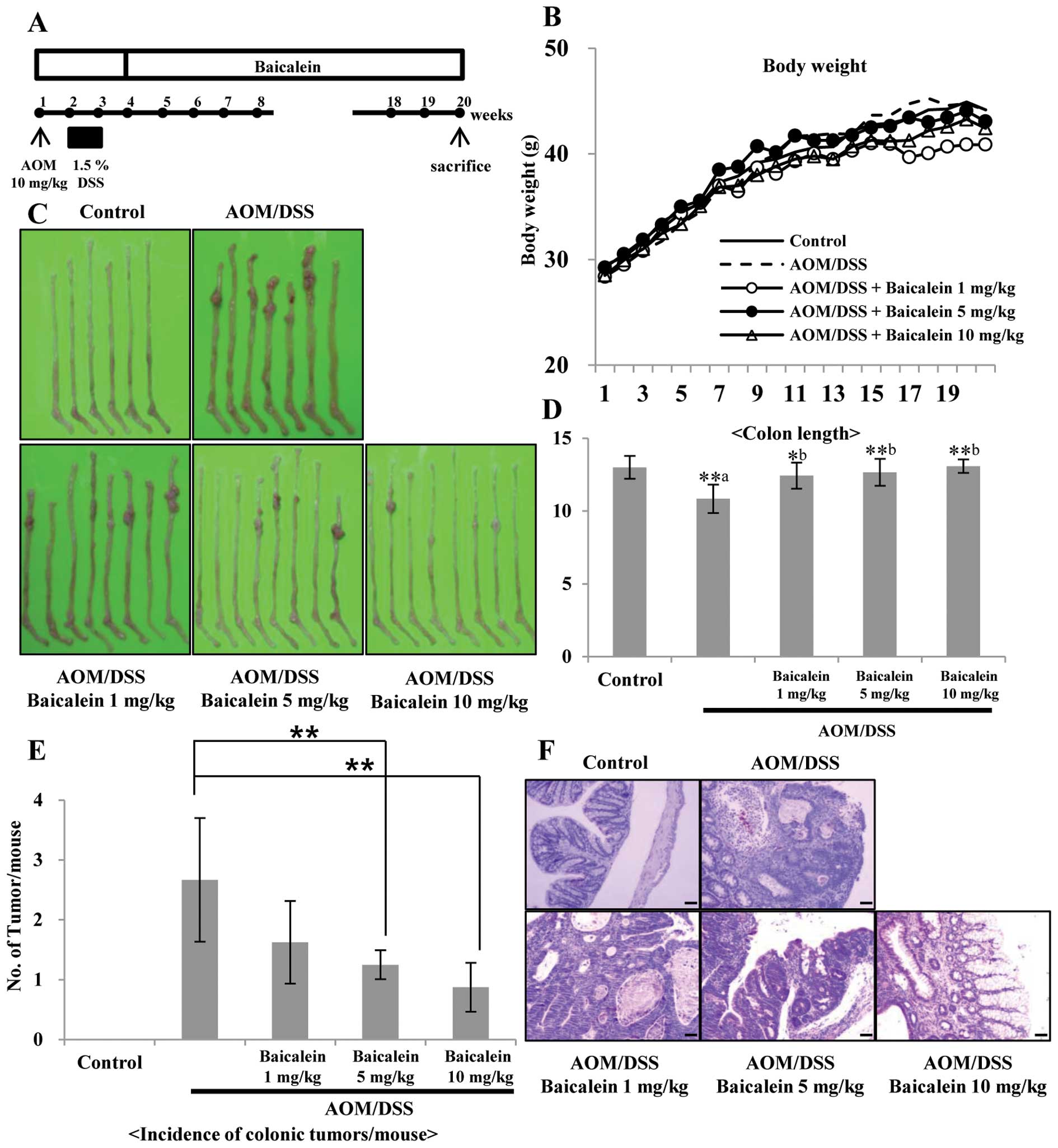
Baicalein inhibits AOM/DSS-induced
colitis and tumorigenesis
The AOM/DSS model is a widely used
inflammation-associated colon cancer model in rodents. The
antitumor activity of dietary administration of baicalein on
AOM/DSS-induced tumorigenesis was evaluated. The study protocol is
summarized in Fig. 6A. During the
experiments, feeding the mice with the three different doses of
baicalein-containing diets did not produce any observable clinical
toxicity or significant changes in body weight compared to control
(Fig. 6B). But colon length was
recovered by administration of baicalein in a dose-dependent manner
compared to the control (Fig. 6D).
Macroscopically, colonic tumors developed in the mice of groups 2
through 5 with different incidence rate and multiplicity (Fig. 6C and E). Group 2 (AOM/DSS group)
had mainly adenocarcinoma (ADC, Fig.
6F) with a multiplicity of 2.67±1.03. The incidence of ADC in
groups 3–5 was less than that of group 2 and the multiplicity of
ADC in groups 3–5 is 1.63±0.69, 1.25±0.24 and 0.88±0.41,
respectively. The multiplicity of colonic ADC in groups 4 and 5 was
significantly smaller than group 2 (p<0.01) (Fig. 6E). In H&E staining, group 2
(AOM/DSS group) animals showed increased tissue inflammation, but
administration of baicalein reduced tissue inflammation compared to
control dose-dependently (Fig.
6F).
Discussion
In this study, we found concentration-dependent cell
growth inhibition in response to baicalein in HCT116 cells, in
accordance with previous research in bladder cancer (16). Cell growth was maximally inhibited
by the treatment of 100 μM baicalein. The concentrations of
50% inhibition of tumor proliferation range between 20 and 200
μM, depending on the type of tumor cells tested.
Baicalein is a natural plant flavone originally
isolated from the roots of Scutellaria baicalensis. This
compound exhibits various biological effects, including
anti-inflammatory (17) and
antitumor activity (18). Although
Scutellaria has been shown to have almost no or very low
toxicity to animals and humans (The grand dictionary of Chinese
herbs, 1977). So far, baicalein, at doses that are toxic to
malignant cells have been shown to have no or very little toxicity
to normal myeloid cells (19) and
also no effect on the viability of normal human prostate epithelial
cells (20). In contrast,
baicalein has been shown to inhibit growth of various human cancer
cell lines (21,22). Baicalein also possesses a direct
cytotoxicity to a large panel of human malignant cell lines by
inducing apoptotic cell death. Oral administration of 20 mg/kg
baicalein was also shown to inhibit growth of established prostate
tumors by approximately 55% (23).
These data demonstrate that baicalein has therapeutic potential
against cancers.
Apoptosis is an important process required for
homeostasis. Apoptosis occurs through two broad pathways: the
intrinsic pathway and extrinsic pathway (24). To clarify whether the effect on
cell growth inhibition was due to apoptosis in colon cancer cells,
we performed morphological experiments and western blot analysis
after treatment of baicalein for 24 h. Treatment with baicalein
decreased the expression levels of pro-caspase-3 and -8, and
induced cleavage of PARP. Thus, it involved the extrinsic pathway
to induce apoptosis.
The PPARγ agonists affect cell proliferation,
differentiation, and apoptosis in a PPARγ-dependent and/or
-independent manner, and thereby represent a potentially important
family of therapeutic compounds for cancer treatment. Many studies
show that PPARγ agonists such as thiazolidinedione have
anti-tumorigenic properties in colorectal cancer by increasing the
expression of tumor suppressor genes (25). Activation of PPARγ results in
anti-inflammatory effects in several cell types, including smooth
muscle cells, endothelial cells. PPARs act as anti-inflammatory
agents by interfering with the transcriptional pathways involved in
inflammatory responses, such as the modulation of NF-κB signaling.
NF-κB is the key transcriptional factor for synthesis of
pro-inflammatory mediators, including iNOS, COX-2 and TNF-α. NF-κB
also plays central roles in carcinogenesis and inflammation, and
thus it is one of the molecular targets of cancer chemoprevention
and therapy (26). NF-κB
activation is reported to be involved in colon carcinogenesis and
certain NF-κB inhibitors are able to suppress cancer development in
these tissues (27). Treatment of
HCT116 cells with baicalein resulted in increase in the expression
of PPARγ in a concentration-dependent manner and baicalein
inhibited p50, p65 and iNOS levels concentration-dependently.
Baicalein not only directly affects NF-κB transcription factors but
also affects invasion and migration signaling molecules such as
MMP-2 and MMP-9 (28). Treatment
with baicalein inhibited cell migration through inhibiting,
respectively, MMP-2, MMP-9 expression concentration-dependently as
detected by gelatin zymography assay. Therefore, baicalein have
effective anti-metastatic activity for the treatment of colon
cancer by inhibiting the expression of MMP-9 and MMP-2, thus
blocking cell migration and invasion pathways. Baicalein is a
potent PPARγ activator that inhibits NF-κB and mediates
inflammatory responses in colon cancer cells.
Accumulative evidence has shown a significant
association between deficiency of PPARγ and IBD (29). In addition, activation of PPARγ
attenuated the inflammation in the gut (30). These results suggested that colonic
PPARγ may be a promising therapeutic target in patients suffering
from IBD. In this study chemopreventive ability of baicalein at 3
different dose levels (1, 5, 10 mg/kg in diet) using
inflammation-induced colon carcinogenesis model in mice were
assessed. All doses of baicalein suppressed colonic inflammation
and reduced the tumor incidence induced by AOM/DSS in a
dose-dependent manner.
In conclusion, our findings indicate that the
flavone, baicalein, being a major constituent of the Scutellaria
baicalensis Georgi is one of the good candidates with multiple
targets for cancer chemoprevention in colon related to inflammation
associated-carcinogenesis.
Acknowledgements
This study was supported by National
Research Foundation of Korea (NRF) grant funded by the Korea
government (MOST) (no. 20120009374). We thank Aging Tissue Bank for
providing research information.
References
|
1.
|
Tenesa A and Dunlop MG: New insights into
the aetiology of colorectal cancer from genome-wide association
studies. Nat Rev Genet. 10:353–358. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Rubin DC, Shaker A and Levin MS: Chronic
intestinal inflammation: inflammatory bowel disease and
colitis-associated colon cancer. Front Immunol. 3:1072012.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Loftus EV Jr: Clinical epidemiology of
inflammatory bowel disease: Incidence, prevalence, and
environmental influences. Gastroenterology. 126:1504–1517. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Kim ES and Kim WH: Inflammatory bowel
disease in Korea: epidemiological, genomic, clinical, and
therapeutic characteristics. Gut Liver. 4:1–14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Asakura K, Nishiwaki Y, Inoue N, Hibi T,
Watanabe M and Takebayashi T: Prevalence of ulcerative colitis and
Crohn’s disease in Japan. J Gastroenterol. 44:659–665. 2009.
|
|
6.
|
Dubuquoy L, Rousseaux C, Thuru X, et al:
PPARgamma as a new therapeutic target in inflammatory bowel
diseases. Gut. 55:1341–1349. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Bassaganya-Riera J, Reynolds K,
Martino-Catt S, et al: Activation of PPAR gamma and delta by
conjugated linoleic acid mediates protection from experimental
inflammatory bowel disease. Gastroenterology. 127:777–791. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Liang HL and Ouyang Q: A clinical trial of
rosiglitazone and 5-aminosalicylate combination for ulcerative
colitis. Zhonghua Nei Ke Za Zhi. 45:548–551. 2006.(In Chinese).
|
|
9.
|
Bassaganya-Riera J, DiGuardo M, Climent M,
et al: Activation of PPARgamma and delta by dietary punicic acid
ameliorates intestinal inflammation in mice. Br J Nutr.
106:878–886. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Ricote M and Glass CK: PPARs and molecular
mechanisms of transrepression. Biochim Biophys Acta. 1771:926–935.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Li-Weber M: New therapeutic aspects of
flavones: the anti-cancer properties of Scutellaria and its
main active constituents Wogonin, Baicalein and Baicalin. Cancer
Treat Rev. 35:57–68. 2009. View Article : Google Scholar
|
|
12.
|
Peng CY, Pan SL, Huang YW, Guh JH, Chang
YL and Teng CM: Baicalein attenuates intimal hyperplasia after rat
carotid balloon injury through arresting cell-cycle progression and
inhibiting ERK, Akt, and NF-kappaB activity in vascular
smooth-muscle cells. Naunyn Schmiedebergs Arch Pharmacol.
378:579–588. 2008. View Article : Google Scholar
|
|
13.
|
Lee EK, Kim JM, Choi J, et al: Modulation
of NF-kappaB and FOXOs by baicalein attenuates the
radiation-induced inflammatory process in mouse kidney. Free Radic
Res. 45:507–517. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Lea MA, Ibeh C, Deutsch JK, Hamid I and
desBordes C: Inhibition of growth and induction of alkaline
phosphatase in colon cancer cells by flavonols and flavonol
glycosides. Anticancer Res. 30:3629–3635. 2010.PubMed/NCBI
|
|
15.
|
Pidgeon GP, Kandouz M, Meram A and Honn
KV: Mechanisms controlling cell cycle arrest and induction of
apoptosis after 12-lipoxygenase inhibition in prostate cancer
cells. Cancer Res. 62:2721–2727. 2002.PubMed/NCBI
|
|
16.
|
Chao JI, Su WC and Liu HF: Baicalein
induces cancer cell death and proliferation retardation by the
inhibition of CDC2 kinase and survivin associated with opposite
role of p38 mitogen-activated protein kinase and AKT. Mol Cancer
Ther. 6:3039–3048. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Hsieh CJ, Hall K, Ha T, Li C, Krishnaswamy
G and Chi DS: Baicalein inhibits IL-1beta- and TNF-alpha-induced
inflammatory cytokine production from human mast cells via
regulation of the NF-kappaB pathway. Clin Mol Allergy. 5:52007.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Takahashi H, Chen MC, Pham H, et al:
Baicalein, a component of Scutellaria baicalensis, induces
apoptosis by Mcl-1 down-regulation in human pancreatic cancer
cells. Biochim Biophys Acta. 1813:1465–1474. 2011.
|
|
19.
|
Ma Z, Otsuyama K, Liu S, et al: Baicalein,
a component of Scutellaria radix from Huang-Lian-Jie-Du-Tang
(HLJDT), leads to suppression of proliferation and induction of
apoptosis in human myeloma cells. Blood. 105:3312–3318.
2005.PubMed/NCBI
|
|
20.
|
Lee DH, Kim C, Zhang L and Lee YJ: Role of
p53, PUMA, and Bax in wogonin-induced apoptosis in human cancer
cells. Biochem Pharmacol. 75:2020–2033. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Zhang HB, Lu P, Guo QY, Zhang ZH and Meng
XY: Baicalein induces apoptosis in esophageal squamous cell
carcinoma cells through modulation of the PI3K/Akt pathway. Oncol
Lett. 5:722–728. 2013.PubMed/NCBI
|
|
22.
|
Zhang Y, Song L, Cai L, Wei R, Hu H and
Jin W: Effects of baicalein on apoptosis, cell cycle arrest,
migration and invasion of osteosarcoma cells. Food Chem Toxicol.
53:325–333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Bonham M, Posakony J, Coleman I,
Montgomery B, Simon J and Nelson PS: Characterization of chemical
constituents in Scutellaria baicalensis with antiandrogenic
and growth-inhibitory activities toward prostate carcinoma. Clin
Cancer Res. 11:3905–3914. 2005.PubMed/NCBI
|
|
24.
|
Lorenzo HK and Susin SA: Therapeutic
potential of AIF-mediated caspase-independent programmed cell
death. Drug Resist Updat. 10:235–255. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Yamaguchi K, Cekanova M, McEntee MF, et
al: Peroxisome proliferator-activated receptor ligand MCC-555
suppresses intestinal polyps in ApcMin/+ mice via extracellular
signal-regulated kinase and peroxisome proliferator-activated
receptor-dependent pathways. Mol Cancer Ther. 7:2779–2787.
2008.PubMed/NCBI
|
|
26.
|
Surh YJ: NF-kappa B and Nrf2 as potential
chemopreventive targets of some anti-inflammatory and antioxidative
phytonutrients with anti-inflammatory and antioxidative activities.
Asia Pac J Clin Nutr. 17(Suppl 1): 269–272. 2008.PubMed/NCBI
|
|
27.
|
Rajakangas J, Misikangas M, Paivarinta E
and Mutanen M: Chemoprevention by white currant is mediated by the
reduction of nuclear beta-catenin and NF-kappaB levels in Min mice
adenomas. Eur J Nutr. 47:115–122. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Chiu YW, Lin TH, Huang WS, et al:
Baicalein inhibits the migration and invasive properties of human
hepatoma cells. Toxicol Appl Pharmacol. 255:316–326. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Aoyagi Y, Nagata S, Kudo T, et al:
Peroxisome proliferator-activated receptor gamma 2 mutation may
cause a subset of ulcerative colitis. Pediatr Int. 52:729–734.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Yamamoto-Furusho JK, Penaloza-Coronel A,
Sanchez-Munoz F, Barreto-Zuniga R and Dominguez-Lopez A: Peroxisome
proliferator-activated receptor-gamma (PPAR-gamma) expression is
downregulated in patients with active ulcerative colitis. Inflamm
Bowel Dis. 17:680–681. 2011. View Article : Google Scholar : PubMed/NCBI
|