Introduction
Epstein-Barr virus (EBV) is a member of the
ubiquitous human γ herpes virus family and ∼90% of the world-wide
population is thought to be infected (1). In vitro infection of resting
human B lymphocytes with EBV results in immortalization of infected
B cells, yielding lymphoblastoid cells, which are characterized by
continuous growth and resistance to various apoptotic signals.
Moreover, lymphoblastoid cells have a phenotype related to that of
activated B-lymphoblasts. However, despite clarification of the
role of some viral proteins, such as latent membrane protein (LMP),
in EBV-related resistance, the molecular mechanisms of resistance
to apoptosis are not yet fully understood (2,3).
CD40 ligand (CD40L) signals from CD4+ T
cells induce the expression of CD95 (Fas) on activated B cells that
then become sensitive to the apoptotic effects of CD95 ligand
(CD95L, FasL) (4). Signaling from
the B cell antigen receptor (BCR) regulates whether B cells
proliferate or undergo Fas-induced apoptosis (5,6).
Fas-FasL interaction plays an important role in preventing delivery
of help signals from CD4+ T cells to self-reactive B
cells (7). The EBV-encoded protein
LMP1 is essential for EBV-mediated B cell transformation (8). LMP1 functionally mimics the tumor
necrosis factor receptor (TNFR) superfamily member CD40, although
each is controlled by a different mechanism (9). EBV infection-induced LMP1 signals are
amplified by both early kinase activation and downstream B cell
effector functions and are sustained compared to CD40 (10).
Naïve resting B cells express low levels of CD86,
whereas CD80 expression in these cells is either absent or present
at very low levels. CD86 expression by B cells can be upregulated
by triggering BCR, whereas CD80 expression can be upregulated by a
variety of stimuli such as lipopolysaccharides (LPS), CD40L and
many cytokines (11–15). Surface expression of the
co-stimulatory molecule, CD86, is one of the early responses to BCR
stimulation (16). CD86 transgenic
mice that were modified to display co-stimulatory molecules on
tolerant B cells showed a reversal in peripheral tolerance, T
cell-dependent clonal expansion and antibody secretion (17). Several studies have reported that
CD80- and CD86-mediated signaling pathways may affect B cell
responses and the production of immunoglobulins (18–21).
Moreover, triggering of CD80 specifically inhibits proliferation by
upregulating the expression of pro-apoptotic molecules and
downregulating levels of anti-apoptotic molecules. In contrast,
CD86 enhances B cell activity (22).
Previously, we reported that several B7 family
members were upregulated by EBV infection, such as B7-H1 (PD-L1)
and B7-H4. These proteins induced apoptosis both through increasing
levels of reactive oxygen species (ROS) and FasL expression
(23,24) and reducing proliferation via cell
cycle arrest at the G0-G1 phase (25). However, the role of CD80 or CD86 in
EBV-transformed B cells is unclear. Thus, we aimed to explore the
expression of CD86 and CD80 by EBV-transformed B-cells by
investigating several known apoptosis-related events after
stimulation of cells with anti-CD80 or CD86 antibodies. Our results
provide insight into the functions and characteristics of
upregulated CD80 and CD86 on EBV-transformed B cells and can
potentially be exploited for immunotherapy against malignant
diseases involving EBV.
Materials and methods
Cell culture, antibodies and
reagents
EBV-transformed B cells and IM-9 cells (EBV-positive
human B lymphoblastoid cell line), the latter which were obtained
from the American Type Culture Collection (Rockville, MD, USA),
were maintained in RPMI-1640 medium (Hyclone) containing 10% fetal
bovine serum (FBS, Hyclone) and antibiotics in a 5% CO2
atmosphere at 37°C. Anti-CD80 (B7-1; BB1), anti-CD86 (B7-2; IT2.2),
anti-Fas-PE, anti-FasL-PE, anti-CD80-FITC, anti-CD86-PE,
anti-CD22-FITC, anti-CD54-PE and anti-CD71-PE were purchased from
BD Pharmingen (San Jose, CA, USA). Goat anti-mouse IgG, MOPC21 as a
control antibody and FITC-conjugated goat anti-mouse IgG were
purchased from Sigma-Aldrich (St. Louis, MO, USA). Anti-human Fas
antibody ZB4 used for blocking experiments was purchased from Abcam
(Cambridge, MA, USA). Mouse anti-human apoptosis-inducing factor
(AIF) and mouse anti-human cytochrome c were purchased from
Santa Cruz Biotechnologies (Santa Cruz, CA, USA). N-acetylcysteine
(NAC) was purchased from Sigma-Aldrich Z-VAD-fmk
(N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone) was
purchased from Calbiochem (La Jolla, CA, USA). Propidium iodide
(PI) was purchased from Sigma-Aldrich.
Preparation of EBV-infectious culture
supernatant and generation of EBV-transformed B cells
EBV stock was prepared from an EBV-transformed B95-8
marmoset-cell line (a gift from Dr B.G. Han, National Genome
Research Institute, National Institute of Health, Seoul, Korea).
These cells were grown in RPMI-1640 media (Hyclone, Logan, UT, USA)
supplemented with 10% fetal bovine serum (Hyclone) for 7 days at
37°C in 5% CO2. The culture supernatant was centrifuged
(1,000 rpm for 10 min), filtered using a 0.2-μm pore filter
(Corning, Acton, MA, USA) to remove cell debris and stored at −80°C
for the next experiments. To establish an EBV-infected B-cell line
from normal PBMCs, 10 ml of peripheral blood was collected from
five healthy human donors (informed consent was obtained from each
participant). PBMCs were isolated from whole blood by Ficoll-Paque
gradient centrifugation (Amersham Biosciences, Uppsala, Sweden) and
B cells were purified from PBMCs using a MACS B-cell-negative
depletion kit. Purified B cells were added to B95-8 supernatant in
a culture flask and after a 2-h incubation at 37°C, an equal volume
of complete medium (RPMI-1640 medium; 10% fetal bovine serum) and 1
μg/ml cyclosporin A (Sigma-Aldrich Inc.) was added
(1×106 cells/ml). Cultures were incubated for 2–4 weeks
until clumps of EBV-infected B cells were visible and the medium
turned yellow (23,24). The study was approved by the
Institutional Bioethics Review Board of the Medical College of Inje
University and all donors provided informed consent for the
study.
Detection of B7 molecules (CD80 and
CD86), Fas and FasL
Surface or intracellular expression of CD80 and CD86
was detected by flow cytometry or confocal laser-scanning
microscopy (Carl Zeiss, 510 META, Jena, Germany). Briefly,
EBV-transformed B cells or IM-9 cells were incubated with
FITC-conjugated anti-CD80 or anti-CD86 antibodies and
EBV-transformed B cells were permeabilized with permeabilization
buffer (0.1% saponin in PBS) to detect intracellular molecules and
then stained with the same antibodies. To detect expression of Fas
and FasL molecules in EBV-transformed B cells and IM-9 cells, cells
stimulated with anti-CD80 or anti-CD86 antibodies were washed twice
with ice-cold PBS. After two washes with ice-cold PBS, cells were
incubated with mouse anti-human Fas or FasL antibodies for 30 min
on ice. After two washes, cells were further incubated with the
appropriate FITC-conjugated secondary antibodies in PBS for 30 min
on ice. All samples were subjected to flow cytometry analysis using
a FACSCalibur (BD Pharmingen) and data were processed using the
program CellQuest (BD Pharmingen).
B7 molecule-mediated apoptosis by
cross-linking or immobilization of antibodies
To immobilize anti-CD80 and CD86 (IgG1κ)
or MOPC21 (IgG1κ; Sigma-Aldrich) antibodies, antibodies
[50 μg/ml in phosphate-buffered saline (PBS)] were coated on
a 96-well culture plate (0.1 ml/well; washed with PBS before use)
after overnight incubation at 4°C. EBV-transformed B cells (4
weeks, 5.0×105 cells/well, 200 μl) or IM-9 cells
(5.0×105 cells/well, 200 μl) were incubated in
plates coated with these antibodies at 37°C for 1 h. To cross-link
antibodies, EBV-transformed B cells (1.0×106 cells) and
IM-9 cells (1.0×106 cells) were incubated in tubes with
each antibody (2 μg/ml) at 37°C for 40 min. MOPC21 (2
μg/ml) was used as an isotype control. Cells were washed in
PBS and resuspended in 100 μl of PBS and then incubated with
goat anti-mouse IgG (2 μg/ml) for 15 min at 37°C. After
cells were washed, they were further cultured in RPMI-1640 medium
for 16 h at 37°C. These cells were harvested, washed twice with PBS
and then resuspended in 100 μl of Annexin V-binding buffer
(10 mM HEPES/NaOH pH 7.4, 140 mM NaCl, 2.5 mM CaCl2).
After 2 μl of FITC-conjugated Annexin V (BD Pharmingen) was
added, cells were incubated in the dark at room temperature for 15
min with gentle vortexing. Finally, 400 μl of Annexin
V-binding buffer was added to each tube and cells were analyzed
using a FACSCalibur (BD Pharmingen).
Cell cycle analysis to detect a sub-G1
peak
Cellular DNA was stained with propidium iodide (PI)
and quantified by flow cytometry. Briefly, EBV-transformed B cells
and IM-9 cells were harvested after treatment with anti-CD80 or
anti-CD86 antibodies and then washed twice in PBS (2% FBS). After
fixing the cells in 70% cold aqueous ethanol, they were stored at
4°C for at least 24 h. Cells were washed twice in PBS after being
centrifuged and cell pellets were stained with PI staining solution
containing RNase A (10 μg/ml) and PI (10 μg/ml) in
PBS. The cell suspension was then incubated in the dark at room
temperature for 30 min and DNA content was determined using a
FACSort flow cytometer (BD Pharmingen). ModFIT LT software was used
for apoptotic sub-G1 peak detection (25).
Apoptosis blocking experiments
To investigate the effects of caspase inhibitors and
reactive oxygen species on CD80- or CD86-induced apoptosis,
EBV-transformed B cells and IM-9 cells were pre-treated with
z-VAD-fmk (20 μM in DMSO, a broad-spectrum caspase
inhibitor) or NAC for 2 h before stimulation with antibodies. Cells
were incubated with anti-CD80 or anti-CD86 antibodies (2
μg/ml) at 37°C for 40 min followed by cross-linking with
goat anti-mouse IgG (2 μg/ml) for 15 min at 37°C. The
blocking effects of caspase inhibitor or NAC on apoptosis of
EBV-transformed B cells and IM-9 cells were detected by Annexin V
staining and cell cycle analysis as described above. To block the
Fas-FasL interaction, antagonistic anti-Fas Ab ZB4 (0.5
μg/ml) was added 1 h before treatment with anti-CD80 or
anti-CD86 antibodies. ZB4 was removed from cell cultures before
stimulation with anti-CD80 or anti-CD86 antibodies. Apoptosis was
determined by flow cytometry after staining with Annexin V.
Confocal microscopy to detect
apoptosis-related intracellular molecules
EBV-transformed B cells and IM-9 cells were
cross-linked with anti-CD80 or anti-CD86 antibodies followed by
secondary antibodies to induce apoptosis. To detect intra-cellular
apoptosis-related molecules, cells were permeabilized with
permeabilization buffer (0.1% saponin in PBS). Cells were incubated
with primary antibodies against cytochrome c (mouse IgG2b)
or AIF (mouse IgG2b) and were then incubated with FITC-conjugated
goat anti-mouse IgG for 30 min. Nuclei were stained with PI for 10
min at room temperature. After three washes with PBS, cells were
mounted on microscope slides under coverslips using a fluorescent
mounting medium (Dako Cytomation, Denmark). Fluorescent cells were
examined under a confocal laser-scanning microscope (Carl Zeiss,
510 META) at ×400 original magnification and images were acquired
using Confocal Microscopy Software Release 3.0 (Carl Zeiss, 510
META).
Results
EBV transformation of primary B cells
increases the expression of CD80 and CD86
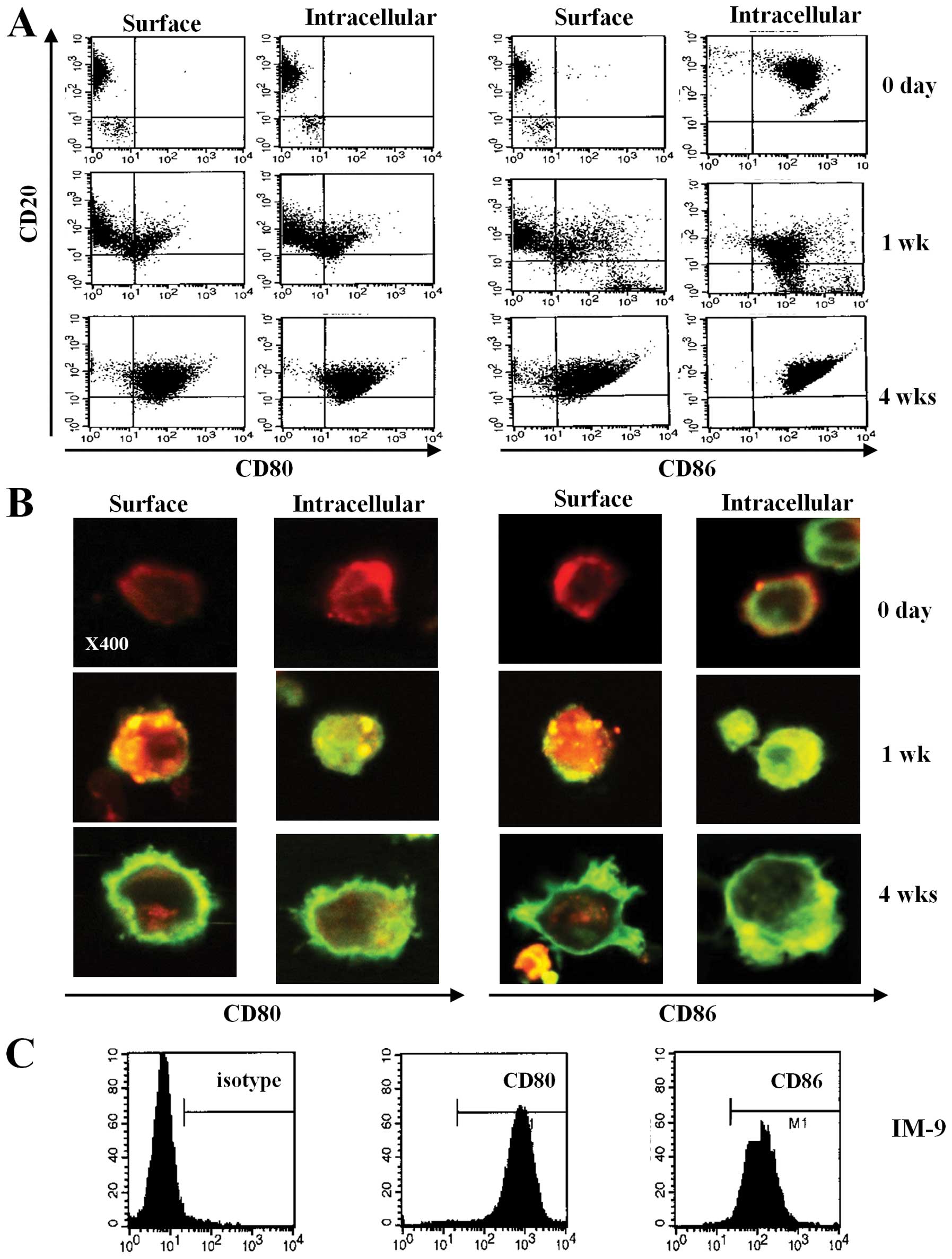
To investigate the expression of CD80 and CD86 on
human B cells through EBV transformation, we observed the surface
phenotype of primary B cells after transformation by EBV. After EBV
infection, the size of primary B cells increased and the cells
clumped. Using flow cytometric analysis, we found that CD80 and
CD86 were barely expressed on fresh primary B cells purified from
blood (Fig 1A and B, upper panel).
However, CD80 and CD86 surface expression increased on both the
surface and in the cytoplasm at 1 week after EBV infection
(Fig. 1A and B, middle panel). At
4 weeks after infection, most of the transformed B cells showed
significantly increased expression of CD80 and CD86 (Fig. 1A and B, bottom panel). Moreover,
flow cytometric data revealed upregulated expression of CD80 and
CD86 molecules on IM-9 cells, an EBV-transformed lymphoblastoid
cell line (Fig. 1C). These results
suggest that EBV infection induces CD80 and CD86 expression and
upregulates the expression of these molecules over time.
EBV infection-induced increase in CD80
and CD86 expression induces apoptosis of both EBV-transformed B
cells and IM-9 cells
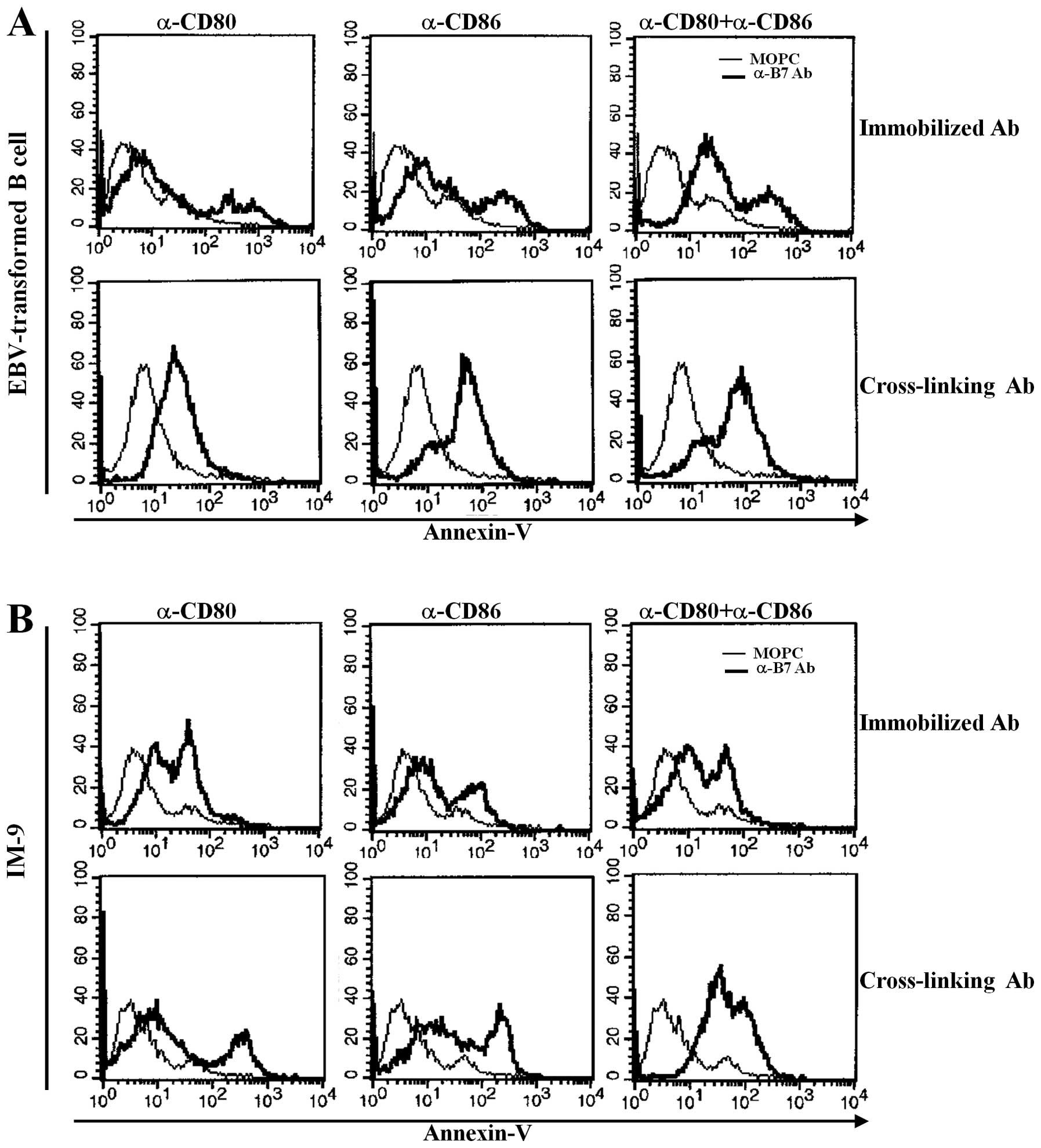
Previously, we reported that other B7 family members
upregulated in EBV-transformed B cells significantly induced
apoptosis after stimulation with antibody (23,24).
EBV-transformed primary B cells and IM-9 cells were stimulated with
anti-CD80 and anti-CD86-plated immobilizing antibodies for 1 h or
were cross-linked with secondary antibody for 15 min to determine
whether upregulation of CD80 and CD86 influenced EBV-transformed B
cell apoptosis. Twenty-four hours after stimulation, cells were
stained with FITC-labeled Annexin-V and were analyzed by flow
cytometry. CD80 or C86 stimulation with immobilized antibodies
induced apoptosis of some EBV-transformed B cells and IM-9 cells.
Interestingly, co-stimulation with anti-CD80 and CD86 antibodies
anchored on the plate significantly enhanced apoptosis of
EBV-transformed B cells (Fig. 2A,
upper panel). Cross-linking of CD80 and CD86 effectively induced
apoptosis of EBV-transformed B cells or IM-9 cells after treatment
of cells with a single or both antibody types (Fig. 2A and B, lower panel). These results
suggest that both CD80 and CD86 molecules are upregulated by EBV
transformation and are involved in the apoptosis of EBV-transformed
B cells.
CD80- and CD86-mediated apoptosis
involves caspases and ROS
We examined whether CD80- and CD86-mediated
apoptosis was related to caspase activity and ROS production
following cross-linking with antibodies, because caspases and ROS
are important mediators of apoptosis (26,27).
EBV-transformed B cells were pre-incubated with Z-VAD-fmk, a
pan-caspase inhibitor, or NAC, a ROS inhibitor, for 2 h before
stimulation with anti-CD80 or CD86 antibodies. Z-VAD-fmk
pre-treatment blocked anti-CD80 and anti-CD86 antibody-induced
apoptosis (Fig. 3A and B, upper
panel). Antioxidant treatment (NAC, 10 mM) also blocked the
anti-CD80 and anti-CD86 antibody-stimulated apoptosis of
EBV-transformed B cells and IM-9 cells (Fig. 3A, bottom panel).
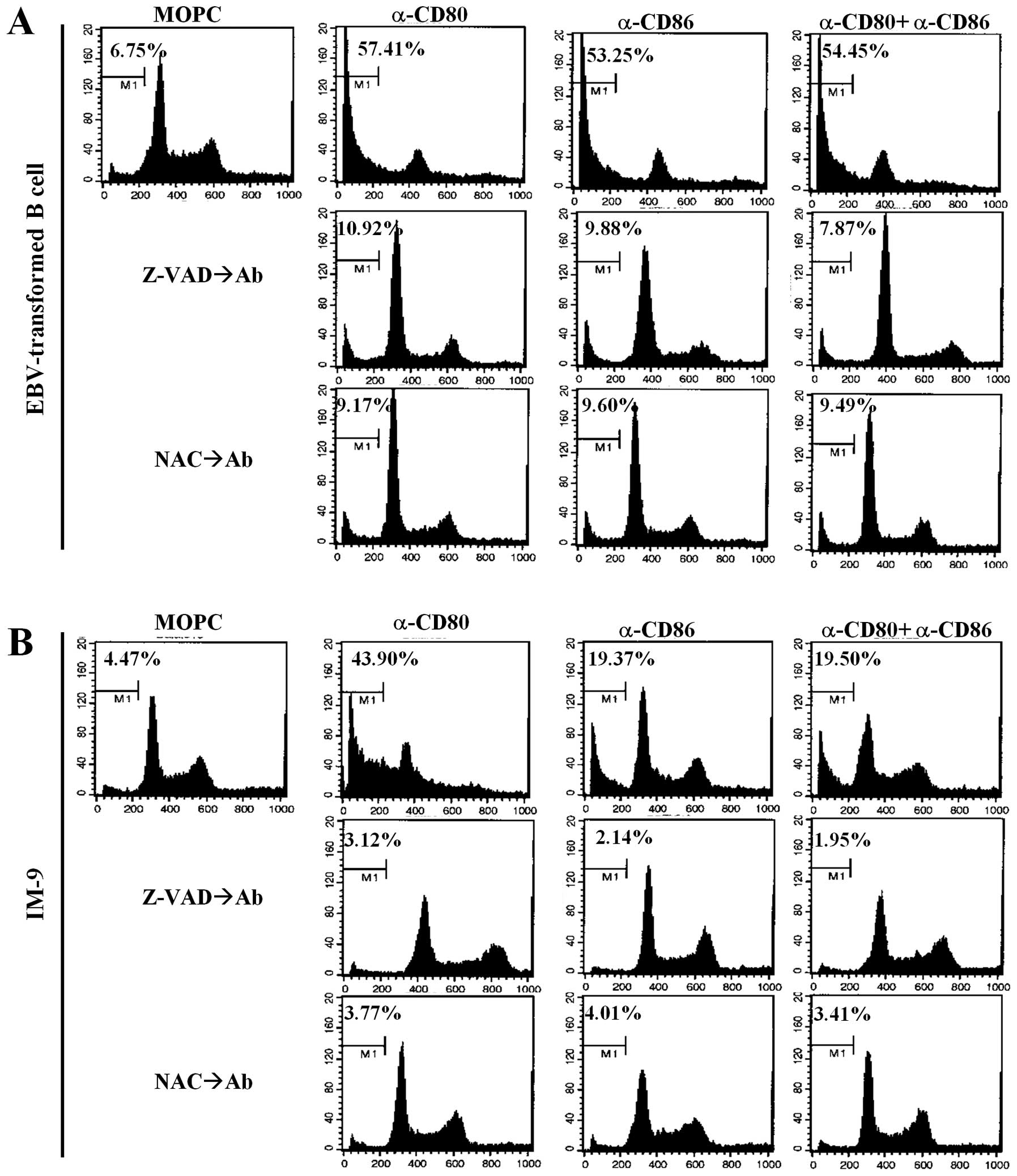
To further elucidate the mechanisms of
CD80/CD86-mediated apoptosis, cell cycle analysis was performed
using propidium iodide. Cross-linking of CD80 and CD86
significantly induced sub-G1 arrest in EBV-transformed B cells and
the IM-9 cell line (Fig. 4A and B,
upper panel). Interestingly, there was a significant increase in
G0/G1 phase IM-9 cells after treatment with the anti-CD80 antibody
compared to untreated cells, anti-CD86 stimulated cells and
combination groups (Fig. 4B, upper
panel). Pre-treatment with Z-VAD-fmk and NAC effectively restored
the sub-G1 peak to the control level (Fig. 4A and B, middle and bottom panels).
These results suggest that CD80- and CD86-mediated apoptosis of
EBV-transformed B cells is related to ROS generation and is
dependent on caspases released from mitochondria.
CD80 and CD86 stimulation of
EBV-transformed B cells and IM-9 cells results in release and
translocation of cytochrome c and AIF from the mitochondria
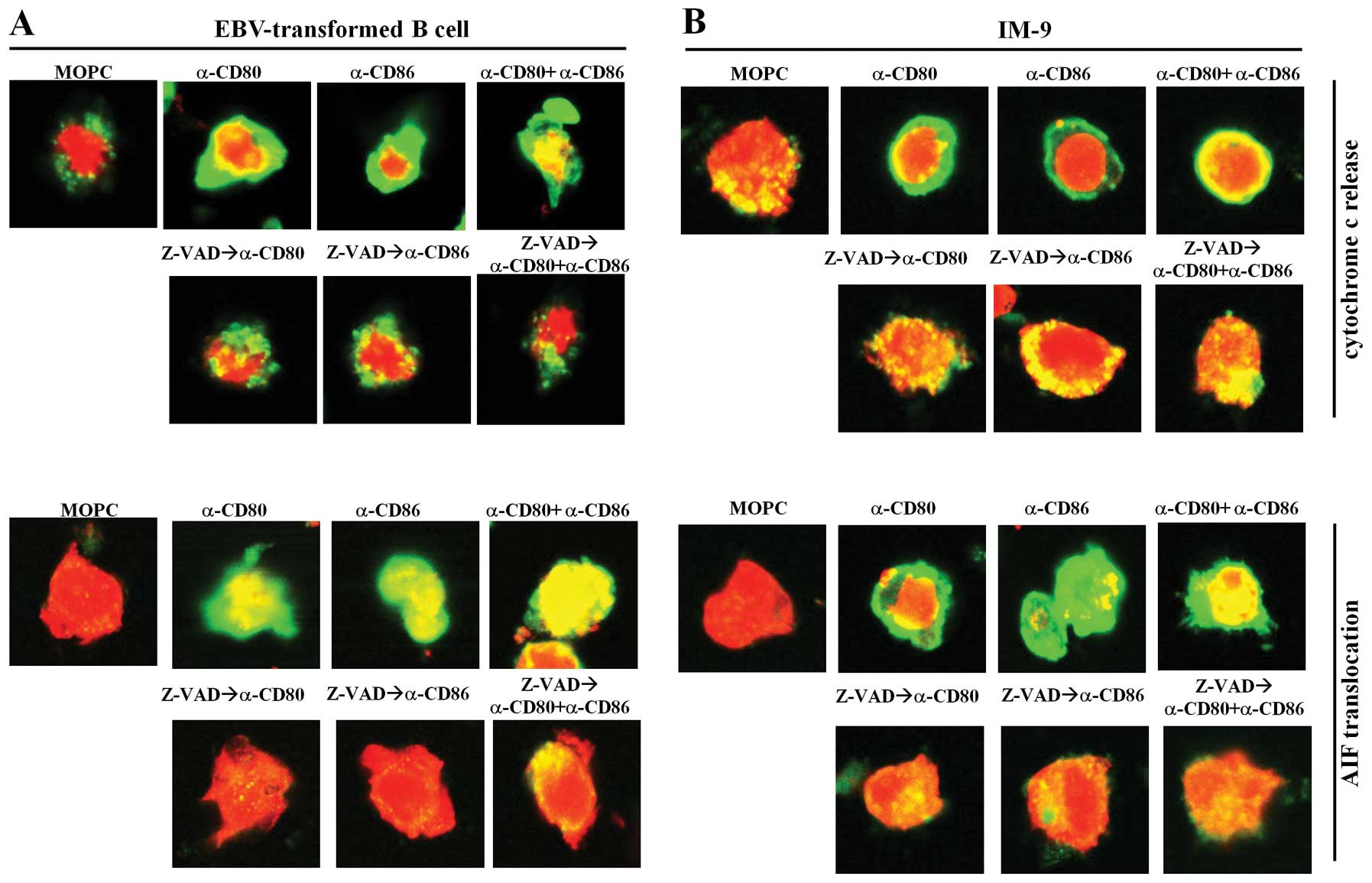
Because caspases are considered to be primary
apoptotic mediators (27,28), we next investigated if there were
changes in expression of other possible apoptotic proteins, such as
cytochrome c and apoptosis-inducing factor (AIF), which are
stored in mitochondria. We performed confocal microscopic analysis
using fluorescence-conjugated secondary anti-cytochrome c
and anti-AIF antibodies. When stimulated with MOPC isotype control
antibodies, cytochrome c and AIF were localized within small
mitochondria in EBV-transformed B cells and IM-9 cells (Fig. 5). In contrast, stimulation with
anti-CD80 and anti-CD86 antibodies caused the release of cytochrome
c from mitochondria to the cytoplasm and translocation of
AIF into the nucleus (Fig. 5A and
B, upper panel). Moreover, we found that pre-treatment with
Z-VAD-fmk and NAC largely blocked cytochrome c and AIF
release from the mitochondria in EBV-transformed B cells and IM-9
cells (Figs. 5A and B, lower
panel). These results suggest that CD80 and CD86-mediated apoptosis
of EBV-transformed B cells involves mitochondria.
CD80 and CD86 stimulation induces
apoptosis in EBV-transformed B cells and IM-9 cells through the
Fas-FasL interaction
Because Fas/FasL can initiate apoptosis, we
investigated whether stimulation of EBV-infected B cells with CD80
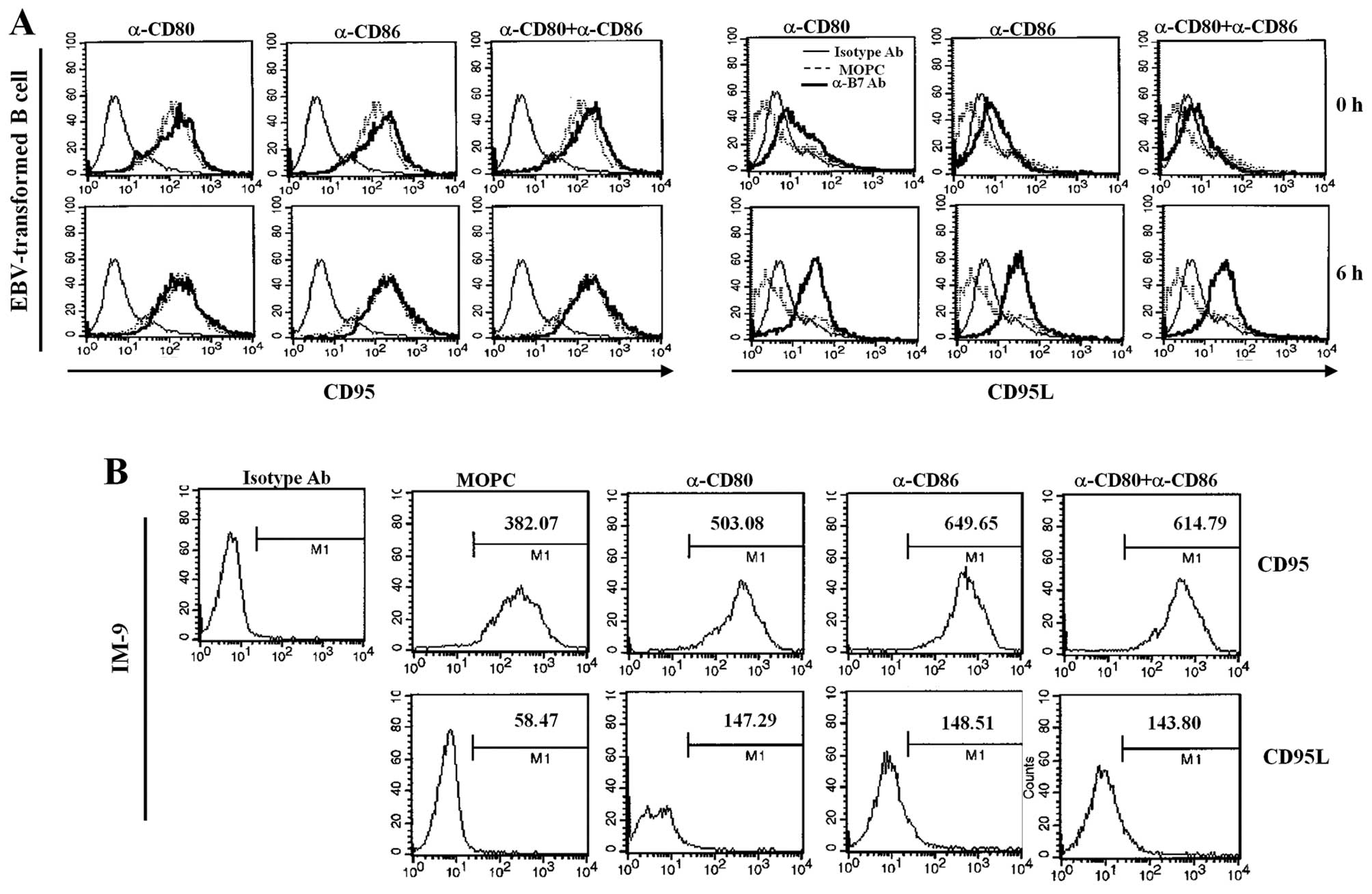
and CD86 changed the expression of Fas and FasL. Interestingly,
CD80 and CD86 ligation of EBV-transformed B cells did not change
Fas expression; Fas is constitutively expressed in EBV-transformed
B cells. In contrast, CD80 and CD86 ligation significantly
upregulated FasL expression (Fig.
6A). CD80/CD86-stimulation of IM-9 cells resulted in more
significant induction of Fas than observed for EBV-transformed B
cells and slightly increased FasL expression (Fig. 6B). To further confirm the
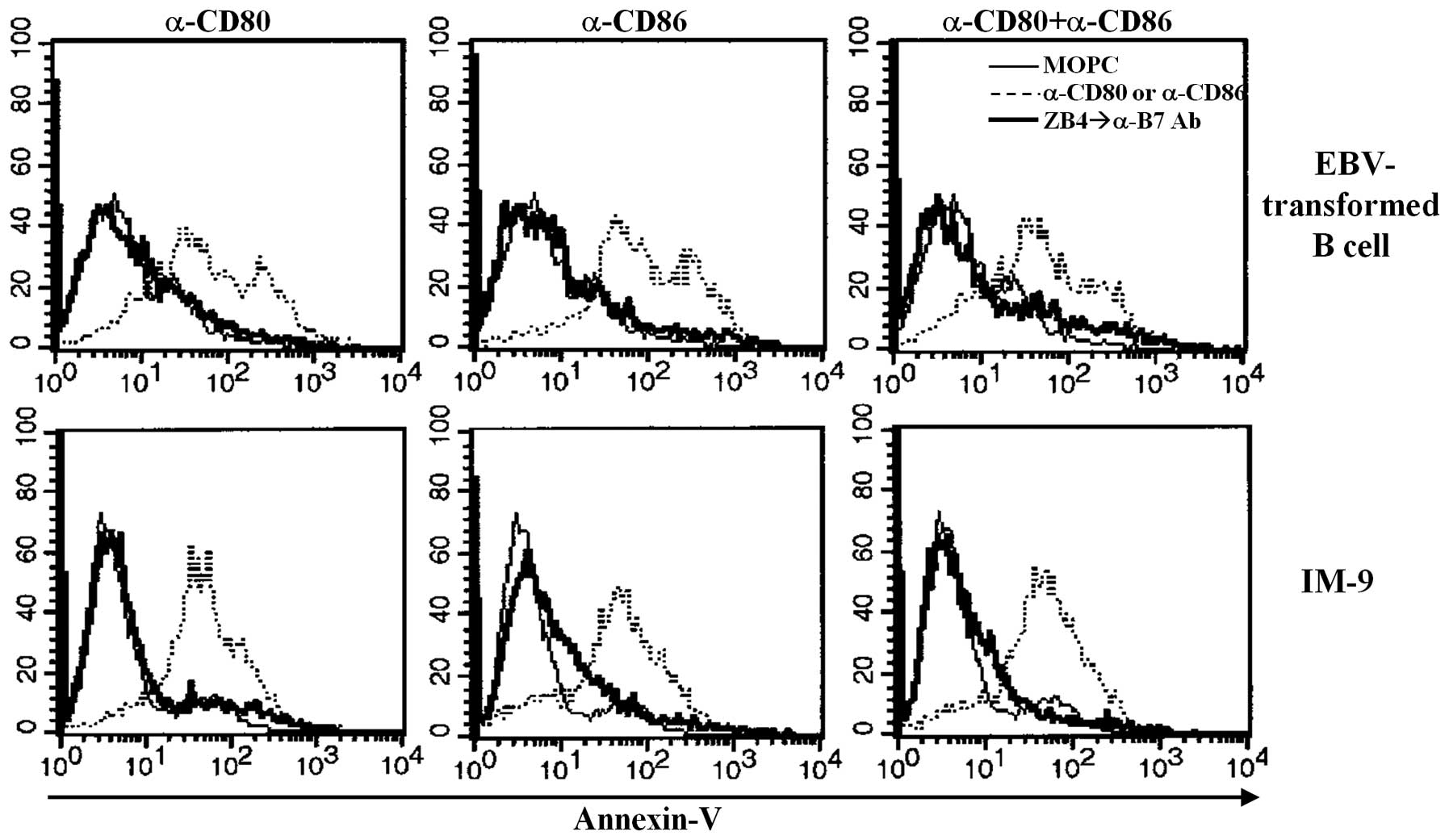
involvement of Fas/FasL in EBV-transformed B cell apoptosis after
CD80 and CD86 ligation, we examined the effect of ZB4 antibodies,
which block Fas-mediated apoptosis. Pre-treatment with ZB4
antibodies for 30 min before cross-linking using anti-CD80 and CD86
prevented apoptosis of both EBV-transformed B cells and
EBV-positive IM-9 cells (Fig. 7).
These results suggest that ligation of CD80/CD86 on EBV-transformed
B cells affects Fas or FasL expression and that Fas/FasL expression
is closely connected with apoptosis.
Discussion
Several studies have demonstrated that
lymphoblastoid cell lines (LCLs) express high levels of B-cell
activation markers (CD23, CD30, CD39 and CD70) and cell adhesion
molecules (29–31). These molecules are transiently
induced at high levels when these cells are transformed into LCLs
and the majority of them play a role in LCL proliferation. Both
CD80 and CD86 are members of the immunoglobulin (Ig) super-gene
family and are expressed on many cell types, including T cells,
macrophages and dendritic cells (32–35).
These molecules play a major role in the co-stimulation of T cells,
subsequently leading to T cell-mediated immune responses, such as
the killing of virus-infected cells. However, the role of CD80 and
CD86 molecules induced by EBV infection of B cells has not been
explored in previous studies. CD80 and CD86 expression by B cells
is strongly regulated. CD80 is normally expressed at basal levels
and is induced in B cells by various stimuli (e.g., cytokines,
ligation of MHC class II and CD40). CD86 is constitutively
expressed in B cells and is upregulated by ligation of the Ig
receptor or treatment with various cytokines (11–15).
Several previous studies have reported that
engagement of CD80 and CD86 by their counterpart molecules affects
B cell responses and the regulation of humoral immunity (18,20).
LPS-activated B cells derived from mice expressed CD80 and the
growth of activated B cells was regulated by upregulating the
expression of caspase-3, caspase-8, Fas and FasL in the presence of
anti-CD80 (16-10A1). In contrast, activation of cells via CD86
(GL1) augmented the levels of the anti-apoptotic molecules Bcl-w
and Bcl-x(L) and decreased the expression of caspase-8 (22). We found that both CD80 and CD86
expressed by EBV-transformed B cells derived from humans
contributed to apoptosis after stimulation with antibodies.
Anti-CD80 or CD86 antibodies also induced apoptosis in IM-9 cells,
which are EBV-positive human B lymphoblastoid cells.
EBV-transformed B cells are known to resist
Fas-mediated apoptosis due to defects in the proximal Fas signaling
pathway (36) or expression of the
FLICE-inhibitory protein (FLIP) (37). In contrast, another study reported
that EBV-positive LCL cells established from allograft recipients
were sensitive to apoptosis triggered by high-dose agonistic
anti-Fas antibody and that CD95 surface expression sensitized
EBV-infected B cells to the induction of CD95-mediated apoptosis
(38,39). ROS also regulate apoptosis
signaling through the Fas and redox-sensitive transcription
factors, NF-κB, AP-1 and p53 and proinflammatory lymphokines
(40). CD80 and CD86-ligation of
EBV-transformed B cells resulted in the immediate production of ROS
(data not shown). Stimulation of EBV-transformed B cells with CD80
or CD86 significantly increased the expression of FasL, but not
Fas. In contrast, Fas and FasL expression was upregulated in IM-9
cells after stimulation with anti-CD80 or CD86 antibodies. Fas
blocking antibody treatment (ZB4) successfully blocked Fas-mediated
apoptosis of EBV-transformed B cells and EBV-positive IM-9 cells.
This result is supported by the observation that the ROS inhibitor,
NAC, not only blocked ROS generation but also prevented CD80- and
CD86-ligation-induced apoptosis. However, apoptosis induced by
co-ligation of anti-CD80 and CD86 antibodies in EBV-transformed B
cells was not significantly enhanced compared to control cells. Our
findings indicate that CD80 and CD86 ligation of EBV-transformed B
cells may contribute to the induction of apoptosis and that
stimulation with CD80 and CD86 induces apoptosis of EBV-transformed
lymphoblastoid B cells via the Fas/FasL pathway. However, the
differences in expression of Fas and FasL in EBV-transformed B
cells and IM-9 cells after CD80 or CD86 stimulation need to be
investigated.
CD80 and CD86 ligation of EBV-transformed B cells
resulted in the immediate release of apoptosis-related molecules,
such as cytochrome c and AIF, from mitochondria. These
results suggest that CD80- and CD86-mediated apoptosis may be
induced through the formation of apoptosomes containing Apaf-1 and
caspase-9, which ultimately activate caspase-3, an effector of
apoptosis. AIF translocates directly to the nucleus and is involved
in caspase-independent apoptosis (27,28).
Furthermore, we found that Z-VAD-fmk, a pancaspase inhibitor,
interfered with CD80- and CD86-mediated apoptosis. Using confocal
microscopy, we observed that cytochrome c was translocated
to the cytosol and AIF was translocated to the nucleus after CD80
and CD86 ligation. These results demonstrated that ligation of CD80
and CD86 induced apoptosis through caspase- and
mitochondria-dependent pathways.
Taken together, our results suggest that CD80 and
CD86 ligation provoke caspase-dependent apoptosis in association
with cytochrome c and AIF released from mitochondria in
EBV-transformed B cells through the Fas-FasL interaction. This
study furthers our understanding of the functions of CD80 and CD86
in activated B cells and provides basic data that can potentially
be exploited to develop therapeutic options for EBV-associated
cancers.
Abbreviations:
|
EBV
|
Epstein-Barr virus
|
|
ROS
|
reactive oxygen species
|
|
NAC
|
N-acetyl-l-cysteine
|
Acknowledgements
This study was supported by the 2013
Inje University Research Grant.
References
|
1.
|
Epstein MA, Barr YM and Achong BG: Virus
particles in cultured lymphoblasts from Burkitt’s lymphoma. Lancet.
15:702–703. 1964.PubMed/NCBI
|
|
2.
|
Caldwell RG, Wilson JB, Anderson SJ and
Longnecker R: Epstein-Barr virus LMP2A drives B cell development
and survival in the absence of normal B cell receptor signals.
Immunity. 9:405–411. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Henderson S, Huen D, Rowe M, Dawson C,
Johnson G and Rickinson A: Epstein-Barr virus-coded BHRF1 protein,
a viral homologue of Bcl-2, protects human B cells from programmed
cell death. Proc Natl Acad Sci USA. 90:8479–8483. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Rathmell JC, Townsend SE, Xu JC, Flavell
RA and Goodnow CC: Expansion or elimination of B cells in vivo:
dual roles for CD40-and Fas (CD95)-ligands modulated by the B cell
antigen receptor. Cell. 87:319–329. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Rathmell JC, Cooke MP, Ho WY, Grein J,
Townsend SE, Davis mM and Goodnow CC: CD95 (Fas)-dependent
elimination of self-reactive B cells upon interaction with
CD4+ T cells. Nature. 376:181–184. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Lagresle C, Mondière P, Bella C, Krammer
PH and Defrance T: Concurrent engagement of CD40 and the antigen
receptor protects naive and memory human B cells from
APO-1/Fas-mediated apoptosis. J Exp Med. 183:1377–1388. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Jacobson BA, Panka DJ, Nguyen KA, Erikson
J, Abbas AK and Marshak-Rothstein A: Anatomy of autoantibody
production: dominant localization of antibody-producing cells to T
cell zones in Fas-deficient mice. Immunity. 3:509–519. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Arcipowski KM, Stunz LL, Graham JP, Kraus
ZJ, Vanden Bush TJ and Bishop GA: Molecular mechanisms of
TNFR-associated factor 6 (TRAF6) utilization by the oncogenic viral
mimic of CD40, latent membrane protein 1 (LMP1). J Biol Chem.
286:9948–9955. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Arcipowski KM and Bishop GA: Roles of the
kinase TAK1 in TRAF6-dependent signaling by CD40 and its oncogenic
viral mimic, LMP1. PLoS One. 7:e424782012. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Graham JP, Arcipowski KM and Bishop GA:
Differential B lymphocyte regulation by CD40 and its viral mimic,
latent membrane protein 1. Immunol Rev. 237:226–248. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Mongini PK, Tolani S, Fattah RJ and Inman
JK: Antigen receptor triggered upregulation of CD86 and CD80 in
human B cells: augmenting role of the CD21/CD19 co-stimulatory
complex and IL-4. Cell Immunol. 216:50–64. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Evans DE, Munks MW, Purkerson JM and
Parker DC: Resting B lymphocytes as APC for naive T lymphocytes:
dependence on CD40 ligand/CD40. J Immunol. 164:688–697. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Clatza A, Bonifaz LC, Vignali DA and
Moreno J: CD40-induced aggregation of MHC class II and CD80 on the
cell surface leads to an early enhancement in antigen presentation.
J Immunol. 171:6478–6487. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Bluestone JA: New perspectives of
CD28-B7-mediated T cell costimulation. Immunity. 2:555–559. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Jirapongsananuruk O, Hofer MF, Trumble AE,
Norris DA and Leung DY: Enhanced expression of B7.2 (CD86) in
patients with atopic dermatitis: a potential role in the modulation
of IgE synthesis. J Immunol. 160:4622–4627. 1998.PubMed/NCBI
|
|
16.
|
Lenschow DJ, Walunas TL and Bluestone JA:
CD28/B7 system of T cell costimulation. Annu Rev Immunol.
14:233–258. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Rathmell JC, Fournier S, Weintraub BC,
Allison JP and Goodnow CC: Repression of B7.2 on self-reactive B
cells is essential to prevent proliferation and allow Fas-mediated
deletion by CD4(+) T cells. J Exp Med. 17:651–659. 1998.PubMed/NCBI
|
|
18.
|
Ikemizu S, Gilbert RJ, Fennelly JA,
Collins AV, Harlos K, Jones EY, Stuart DI and Davis SJ: Structure
and dimerization of a soluble form of B7-1. Immunity. 12:51–60.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Bajorath J, Peach RJ and Linsley PS:
Immunoglobulin fold characteristics of B7-1 (CD80) and B7-2 (CD86).
Protein Sci. 3:2148–2150. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Heath AW, Chang R, Harada N, Argumedo LS,
Gordon J, Hannum C, Campell D, Shanafelt AB, Clark EA, Torres R and
Howard M: Antibodies to murine CD40 stimulate normal B lymphocytes
but inhibit proliferation of B lymphoma cells. Cell Immunol.
152:468–480. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Borriello F, Sethna MP, Boyd SD,
Schweitzer AN, Tivol EA, Jacoby D, Strom TB, Simpson EM, Freeman GJ
and Sharpe AH: B7-1 and B7-2 have overlapping, critical roles in
immunoglobulin class switching and germinal center formation.
Immunity. 6:303–331. 1997. View Article : Google Scholar
|
|
22.
|
Suvas S, Singh V, Sahdev S, Vohra H and
Agrewala JN: Distinct role of CD80 and CD86 in the regulation of
the activation of B cell and B cell lymphoma. J Biol Chem.
277:7766–7775. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Kim YS, Park GB, Lee HK, Song H, Choi IH,
Lee WJ and Hur DY: Cross-linking of B7-H1 on EBV-transformed B
cells induces apoptosis through reactive oxygen species production,
JNK signaling activation, and fasL expression. J Immunol.
181:6158–6169. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Song H, Park G, Kim YS, Hur I, Kim H, Ryu
JW, Lee HK, Cho DH, Choi IH, Lee WJ and Hur DY: B7-H4 reverse
signaling induces the apoptosis of EBV-transformed B cells through
Fas ligand up-regulation. Cancer Lett. 266:227–237. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Park GB, Song H, Kim YS, Sung M, Ryu JW,
Lee HK, Cho DH, Kim D, Lee WJ and Hur DY: Cell cycle arrest induced
by engagement of B7-H4 on Epstein-Barr virus-positive B-cell
lymphoma cell lines. Immunology. 128:360–368. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Andreyev AY, Kushnareva YE and Starkov AA:
Mitochondrial metabolism of reactive oxygen species. Biochemistry.
70:200–214. 2005.PubMed/NCBI
|
|
27.
|
Saelens X, Festjens N, Vande Walle L, van
Gurp M, van Loo G and Vandenabeele P: Toxic proteins released from
mitochondria in cell death. Oncogene. 23:2861–2874. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Cregan SP, Dawson VL and Slack RS: Role of
AIF in caspase-dependent and caspase-independent cell death.
Oncogene. 23:2785–2796. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Bornkamm GW and Hammerschmidt W: Molecular
virology of Epstein-Barr virus. Phil Trans R Soc Lond B Biol Sci.
356:437–459. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Kempkes B, Spitkovsky D, Jansen-Durr P,
Ellwart GW, Kremmer E, Delecluse HJ, Rottenberger C, Bornkamm GW
and Hammerschmidt W: B cell proliferation and induction of early
G1-regulating proteins by Epstein-Barr virus mutants conditional
for EBNA2. EMBO J. 14:88–96. 1995.PubMed/NCBI
|
|
31.
|
Yamada S, Shinozaki K and Agematsu K:
Involvement of CD27/CD70 interactions in antigen-specific cytotoxic
T-lymphocyte (CTL) activity by perforin-mediated cytotoxicity. Clin
Exp Immunol. 130:424–430. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Gordon J, Millsum MJ, Guy GR and Ledbetter
JA: Resting B lymphocytes can be triggered directly through the
CDw40 (Bp50) antigen. A comparison with IL-4-mediated signaling. J
Immunol. 140:1425–1430. 1988.PubMed/NCBI
|
|
33.
|
Goldstein MD and Watts TH: Identification
of distinct domains in CD40 involved in B7-1 induction or growth
inhibition. J Immunol. 157:2837–2843. 1996.PubMed/NCBI
|
|
34.
|
Nakajima A, Kodama T, Morimoto S, Azuma M,
Takeda K, Oshima H, Yoshino S, Yagita H and Okumura K: Antitumor
effect of CD40 ligand: elicitation of local and systemic antitumor
responses by IL-12 and B7. J Immunol. 161:1901–1907.
1998.PubMed/NCBI
|
|
35.
|
Bergamo A, Bataille R and
Pellat-Deceunynck C: CD40 and CD95 induce programmed cell death in
the human myeloma cell line XG2. Br J Haematol. 97:652–655. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Snow AL, Chen LJ, Nepomuceno RR, Krams SM,
Esquivel CO and Martinez OM: Resistance to Fas-mediated apoptosis
in EBV-infected B cell lymphomas is due to defects in the proximal
Fas signaling pathway. J Immunol. 167:5404–5411. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Tepper CG and Seldin MF: Modulation of
caspase-8 and FLICE-inhibitory protein expression as a potential
mechanism of Epstein-Barr virus tumorigenesis in Burkitt’s
lymphoma. Blood. 94:1727–1737. 1999.PubMed/NCBI
|
|
38.
|
Le Clorennec C, Youlyouz-Marfak I,
Adriaenssens E, Coll J, Bornkamm GW and Feuillard J: EBV latency
III immortalization program sensitizes B cells to induction of
CD95-mediated apoptosis via LMP1: role of NF-kappaB, STAT1, and
p53. Blood. 107:2070–2078. 2006.PubMed/NCBI
|
|
39.
|
Durandy A, Le Deist F, Emile JF, Debatin K
and Fischer A: Sensitivity of Epstein-Barr virus-induced B cell
tumor to apoptosis mediated by anti- CD95/Apo-1/fas antibody. Eur J
Immunol. 27:538–543. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Li-Weber M and Krammer PH: Function and
regulation of the CD95 (APO-1/Fas) ligand in the immune system.
Semin Immunol. 15:145–157. 2003. View Article : Google Scholar : PubMed/NCBI
|