Introduction
Gastric cancer is one of the major public health
problems and the main factor of cancer-related deaths in Eastern
Europe and East Asia (1).
Chemotherapy, including 5-FU, cisplatin and adriamycin, is a
commonly used treatment method in gastric cancer (2). A significant survival advantage of
5-FU-based chemotherapy has been reported in patients with
metastatic cancer as well as in those who have undergone surgery
(3,4). Although such treatments have
increased the survival rate of gastric cancer patients, many
patients treated with 5-FU-based chemotherapy have recurrence.
Although resistance to 5-FU-based treatments is a major cause for
recurrence, the mechanisms that drive the development of 5-FU
resistance in cancer patients are poorly understood.
The cyclin D1 gene encodes for the proteins in the
cyclin-dependent kinase (CDK)4/CDK6 complex. Cyclin D1 and CDK4/6
form a complex that phosphorylates and inactivates retinoblastoma
(RB) protein, a tumor suppressor (5). The phosphorylation of RB results in
the release of E2Fs, which then continues to activate genes that
are necessary for advancing into the G1/S phases of the cell cycle
(6). In accordance with its
growth-promoting role, cyclin D1 can behave as an oncogene. Indeed,
overexpression and/or rearrangement of the cyclin D1 gene is seen
in several types of human cancers, including gastric cancer
(7,8). Therefore, suppression of cyclin D1
expression with a specific targeting method may serve as a powerful
treatment for human gastric cancers and can be achieved via RNA
interference (RNAi), one of the most effective methodologies for
gene targeting.
Previous studies have demonstrated that
overexpression of cyclin D1 increases resistance to radiotherapy or
chemo-therapeutic drugs in various cancer cells (9–13).
Moreover, downregulation of cyclin D1 is related to induction of
apoptosis and chemosensitivity in TTn cells (14). However, downregulation of cyclin D1
induces chemosensitivity to cis-diamminedichloroplatinum in
human oral squamous cell carcinoma (15). The disparate effects of cyclin D1
downregulation may arise from the differential responses to the
different drugs and cell type-specific effects, among other
reasons. Therefore, cyclin D1 appears to act not only as a
pro-survival factor but also as a pro-apoptotic factor depending on
specifics of the experiment, such as the cell type and chemotherapy
drug (16). AKT is a crucial
molecule in protecting against cellular apoptosis and it plays a
pivotal function in the regulation of normal cell growth and
proliferation (17,18). Previous studies have reported that
the expression of AKT is changed in several human cancers and that
this dysregulated expression may contribute to chemoresistance
(19–21). Therefore, AKT is an attractive
target of strategies aimed at overcoming chemoresistance in
cancers.
The NFκB pathway is one of the main anti-apoptotic
signaling pathways and is aberrantly activated in many cancer
cells. Previous studies have described that some chemo-therapeutic
drugs, including 5-FU, can activate NFκB and, consequently,
markedly suppress apoptosis (22,23).
Therefore, NFκB is closely linked to 5-FU chemoresistance in many
cancer cells (24).
The aim of this study was to develop a
lentivirus-mediated shRNA expression system targeting cyclin D1 to
generate a stable silencing effect of sufficient efficiency for
delivery of cyclin D1-specific shRNA (ShCCND1). We also sought to
examine whether treatment with ShCCND1 or 5-FU, alone or in
combination, influences the activation of phosphorylated AKT (pAKT)
and pNFκB and to determine the effect of the combined treatment
with ShCCDN1 and 5-FU on cell growth and chemosensitivity to 5-FU
in AGS cells.
Materials and methods
Cell line and chemicals
AGS gastric carcinoma cells were purchased from the
Korean Cell Line Bank (KCLB, Seoul, Korea). Cells were maintained
in RPMI-1640 medium with 10% fetal bovine serum and 1%
penicillin/streptomycin at 37°C in an atmosphere of 5%
CO2 in a humidified chamber. The 293TN human embryonic
kidney cells were purchased from System Biosciences (SBI, Mountain
View, CA, USA) and maintained in high-glucose Dulbecco’s modified
Eagle’s medium supplemented with 10% fetal bovine serum, glutamax
and 1% penicillin/streptomycin at 37°C in an atmosphere of 5%
CO2 within a humidified chamber. The anticancer agent
5-FU was purchased from Sigma-Aldrich (St. Louis, MO, USA).
ShRNA-expressing plasmid DNA
The lentiviral expression vector (pGreenPuro™
vector) was purchased from SBI. Three different targeted sequences
were designed to be homologous to the cyclin D1 gene (CCND1;
GeneBank NM_053056). Target sites in human CCND1 were as
follows: site 1, 5′-GCCC TCGGTGTCCTACTTCAAAT-3′; site 2,
5′-GCACGATTTCA TTGAACACTTCC-3′; and site 3, 5′-GGAAGTGTTCAATGA
AATCGTGC-3′. These sequences were followed by a 12-bp ‘loop’
(CTTCCTGTCAGA) and the inverted repeat. These primer pairs were
annealed and inserted into the BamHI and EcoRI sites
of the pGreenPuro™ vector (25).
In addition, a negative control shRNA sequence (ShScramble), which
had no homology to human genes (GACTTCATAAGGCGCATGC), was also
designed using the same process described above (26). These plasmid DNAs were transformed
into the E. coli DH5α competent cells and purified using an
endotoxin-free plasmid purification kit (Qiagen, Valencia, CA,
USA). Successful ligation was confirmed by PCR and sequencing
analyses. The resulting plasmids containing the scrambled and
cyclin D1-specific shRNA sequences are hereafter referred to as
pScramble and pCCND1, respectively.
Lentivirus generation and infection
293TN cells were plated on 10-cm culture plates at a
density of 3×106 cells per plate. After a 24-h
incubation, the cells were co-transfected with pScramble or pCCND1
and a lentiviral expression construct using Lipofectamine
(Invitrogen, Grand Island, NY, USA). After 48 h, the supernatant
was collected and cleared through a 0.45-μm filter. PEG-it™
virus precipitation solution was added to the clarified supernatant
and the mixture was centrifuged at 1,500 × g for 30 min at 4°C. The
pellet was resuspended in 1/100th of the original volume of
RPMI-1640 medium. One day prior to transduction, AGS cells were
seeded at a density of 3×104 cells/well in 24-well
plates. After 24 h, the culture medium was replaced by RPMI-1640
containing hexadimethrine bromide (5 μg/ml; Sigma-Aldrich).
Then, AGS cells were infected with the pseudovirus stock. From the
next day onward, AGS cells were maintained in RPMI-1640 medium
containing puromycin (1 μg/ml). The stable
puromycin-resistant cancer cell lines containing pScramble or
pCCND1 obtained by subcloning on the selective medium were named as
ShScramble and ShCCND1, respectively. The transduction efficiency
of the stable AGS cells expressing shRNA was measured by flow
cytometry.
Western blot analysis
AGS cells were lysed in radio-immunoprecipitation
assay buffer (Sigma-Aldrich) containing a protease inhibitor
cocktail (Sigma-Aldrich). AGS cells (ShScramble and ShCCND1) were
centrifuged at 13,000 rpm for 10 min. The protein concentration of
the cell extract was determined using the BCA™ Protein Assay kit
(Thermo Scientific, Rockford, IL, USA). The protein samples were
boiled in sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) loading buffer and then electrophoresed on 10%
SDS-polyacrylamide gels. The proteins were transferred onto
nitrocellulose membranes and the membranes were blocked with 5%
non-fat dry milk and incubated overnight with antibodies against
cyclin D1 (Santa-Cruz Biotechnology, Santa Cruz, CA, USA), pRB
(Cell Signaling Technology, Beverly, MA, USA), pAKT (Cell Signaling
Technology), pNFκB (Cell Signaling Technology) and β-actin
(Santa-Cruz Biotechnology) at 4°C. Subsequently, the membranes were
incubated with either anti-rabbit or anti-mouse secondary
antibodies (Santa-Cruz Biotechnology). Specific antibody-protein
complexes were detected with the ECL Test kit (KPL, Gaithersburg,
MD, USA). Densitometric analysis was performed using ImageJ
software.
Cell proliferation and colony formation
assay
Cell proliferation was analyzed using the Cell
Counting kit-8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan).
ShScramble and ShCCND1 cells were plated on 96-well plates at a
density of 104 cells/well and cultured for 24 h. Next, 10 μl
of CCK-8 solution was added to each well. After a 2-h incubation,
an enzyme-linked immunosorbent assay (Tecan Sunrise, Sunnyvale, CA,
USA) was performed and the absorbance of the samples was measured
at 450 nm.
To assess the colony formation ability, AGS cells
containing shRNA were seeded (500 cells/well) in 6-well plates.
Cell culture medium was replaced fresh RPMI-1640 every 2 days.
After 3 weeks, cells were stained with 1% crystal violet for 30
min. To identify colonies, the medium was removed and 3.7%
formaldehyde was added. Subsequently, the number of colonies in
each well was determined by counting the stained colonies under a
light microscope. Image analysis was conducted using Metamorph
version 7.5.6.0 software (Molecular Devices, CA, USA).
Scratch wound-healing assay
AGS cells were seeded on 60-mm plates. When cells
were >90% confluent, a scratch was made using the tip of a
pipette (27). After incubation
for 48 h, cells that were protruding from the border of the wound
were observed and photographed using a Zeiss Axiovert 200 inverted
microscope (Carl Zeiss MicroImaging, Thornwood, NY, USA) equipped
with a 10x objective. Image analysis was conducted using Metamorph
version 7.5.6.0 software.
In vitro cellular chemosensitivity to
5-FU
To evaluate the cytotoxic effect of different
concentrations of 5-FU, cells were seeded in 96-well plates at a
density of 2×104 cells/well. After overnight incubation,
the culture medium was replaced with fresh medium containing
various concentrations of 5-FU (0.1, 1, 5 and 15 μg/ml); a
control set was included in which fresh medium alone was added,
without any 5-FU. Cell viability was evaluated with the CCK-8 assay
72 h after exposure to 5-FU.
To evaluate the cytotoxic effect of the combined
treatment with ShCCND1 and 5-FU, a time-course cell viability assay
was performed using the CCK-8 kit. Cells were seeded in 96-well
plates at a density of 2×104 cells/well. After overnight
incubation, the cells were treated with 5-FU (15 μg/ml).
Cell viability was evaluated with a CCK-8 assay after 24, 48 and 72
h.
Analysis of cell cycle and apoptosis
To analyze the cell cycle status, 106 AGS
cells were washed and resuspended in phosphate-buffered saline
containing 5 μl of RNase A (10 mg/ml; Sigma-Aldrich) and 10
μl of propidium iodide (PI; 1 mg/ml; Sigma-Aldrich). After
incubating the samples for 1 h in the dark, the cell cycle
distribution was assessed using a fluorescence-associated
cell-sorting (FACS) assay with FACSCalibur (Becton-Dickinson,
Rutherford, NJ, USA) and the results were analyzed with the
CellQuest software (Becton-Dickinson).
To evaluate apoptosis, AGS cells were suspended in
binding buffer at a cell density of ∼106 cells/ml.
Aliquots (100 μl) of this cell suspension were incubated
with Annexin-V-allophycocyanin and propidium iodide (BD Biosciences
Pharmingen). After incubation, the samples were mixed with binding
solution and analyzed on a flow cytometer (FACSCalibur,
Becton-Dickinson). The data were analyzed using the CellQuest
software (Becton-Dickinson).
Statistical analysis
For statistical analysis, all data obtained were
analyzed using the Prism 5 software for Windows (GraphPad Software,
San Diego, CA, USA). Statistically significant differences between
the various groups were evaluated using the unpaired Student’s
t-test and Fisher’s exact test. The level of statistical
significance was set at values of P<0.05.
Results
ShCCND1 inhibits the expression of cyclin
D1 and cell proliferation in AGS cells
AGS cells stably transduced with lentivirus were
successfully generated by selection on puromycin-containing medium.
The transduction efficiency of the lentivirus-transduced AGS cells,
as measured by flow cytometry, was 95.1 and 95.8% for the
ShScramble and ShCCND1 cells, respectively (data not shown). The
protein levels of cyclin D1 in AGS cells were examined by western
blotting. Compared to the AGS cells, ShScramble expressed similar
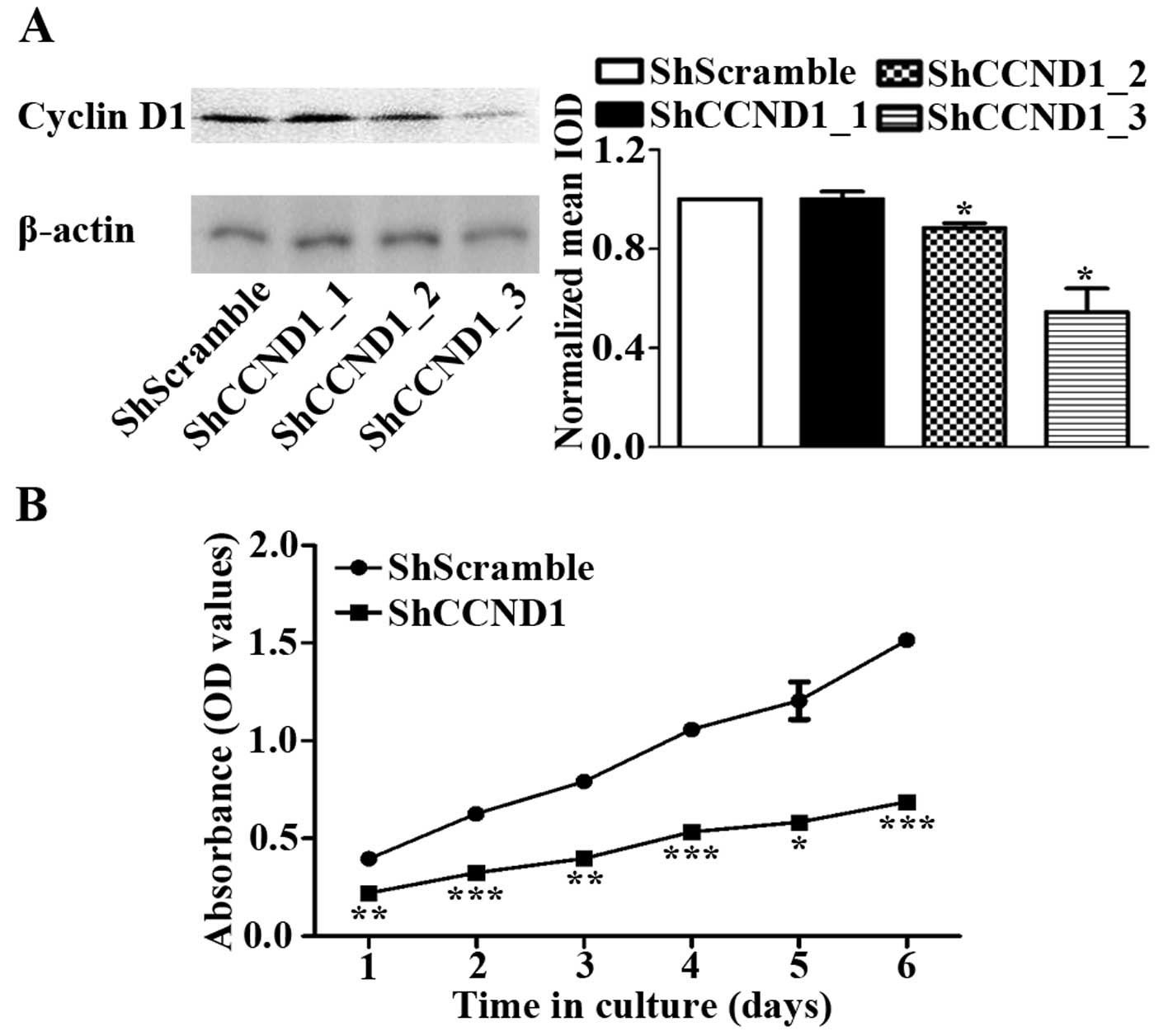
levels of cyclin D1 (data not shown). As shown in Fig. 1A, cyclin D1 expression levels were
similar in ShCCND1_1- and ShScramble-transduced cells, whereas
ShCCND1_2- and ShCCND1_3-transduced cells had decreased cyclin D1
levels compared to AGS cells (Fig.
1A). Densitometric analysis of the western blotting images
indicated a dramatic and significant decrease in cyclin D1
expression in ShCCND1_3-transduced cells, compared to the parental
AGS cells (P<0.05). The ShCCND1_3-transduced clone was then used
for subsequent studies and named as ShCCND1. To evaluate the
inhibitory effect of cyclin D1 knockdown on cancer cell
proliferation, the cell viability of ShScramble- and
ShCCND1-transduced cells was measured using the CCK-8 assay. The
viability of the ShCCND1-transduced cells was significantly
diminished compared to that of ShScramble cells (Fig. 1B). Proliferation of these cells was
considerably suppressed by ShCCND1-transduced cells after day 1 of
culture. These data suggested that ShCCND1 could effectively
knockdown the endogenous cyclin D1 expression and inhibit cell
proliferation in AGS cells.
ShCCND1 inhibits focus formation and cell
motility in AGS cells
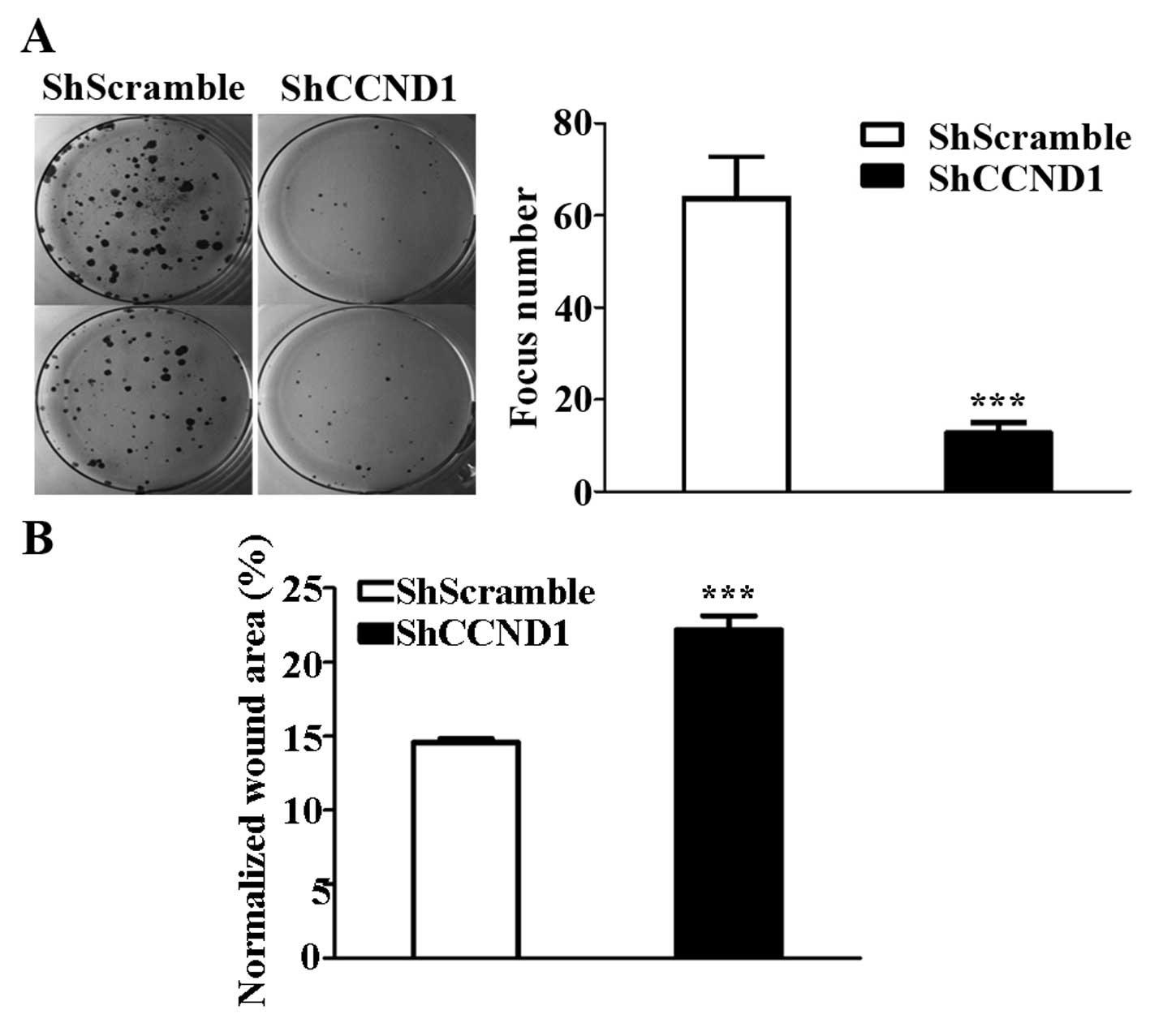
To test whether the knockdown of cyclin D1 affects
the clonogenic potential, which is one of the important
characteristics required for tumor formation in vivo, a
colony formation assay was performed, as described previously
(28). The number of foci in
ShCCND1-transduced cells (N=12.7) was lower than that in
ShScramble-transduced cells (N=63.7, P<0.001; Fig. 2A). Moreover, because decreased
clonogenic potential is usually associated with invasive ability in
cancer cells, the cell motility of AGS cells was analyzed using a
classic wound-healing assay (28).
The wound area in the ShCCND1-transduced cells was significantly
decreased compared to that in ShScramble-transduced cells (Fig. 2B); the wound area was 31.3 and
14.6% in ShCCND1- and ShScramble-transduced cells, respectively
(P<0.001). These results demonstrated that ShCCND1 significantly
decreased the focus-formation potential of the cells, which
correlates with the formation of cancer in nude mice and their
migratory capacity (29).
Combined treatment with ShCCND1 and 5-FU
inhibits cell growth to a greater extent than treatment with either
agent alone
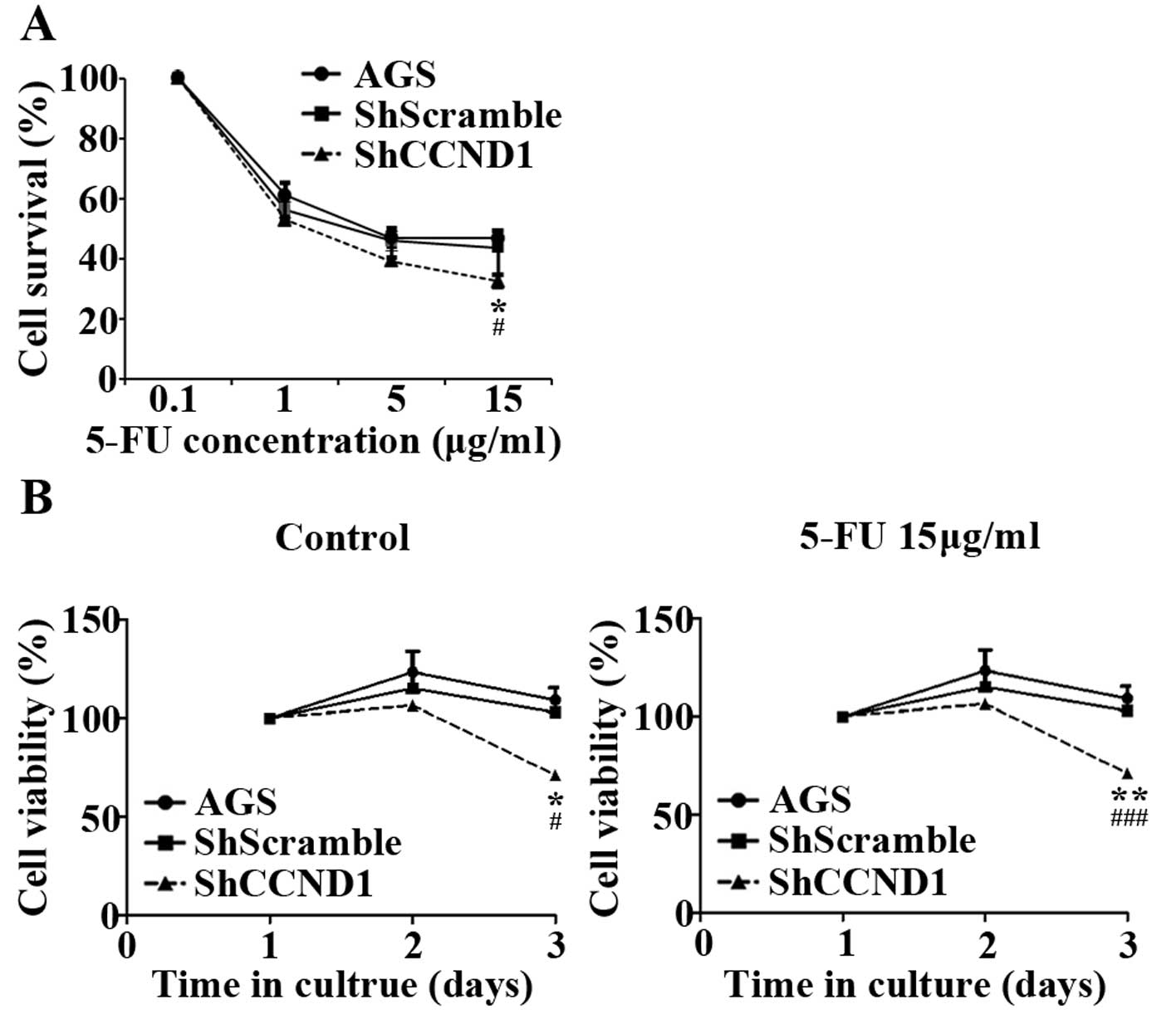
High expression of cyclin D1 protein has been
associated with increased resistance of cancer cells to
chemotherapeutic agents, such as 5-FU (12,13).
Therefore, we investigated whether ShCCND1 could enhance the
sensitivity of AGS cells to 5-FU. As shown in Fig. 3A, 5-FU treatment (0.1, 1 and 5 μg/ml)
decreased cell survival in a dose-dependent manner in AGS and in
ShScramble- and ShCCND1-transduced cells. However, treatment with a
high dose of 5-FU (15 μg/ml) decreased cell survival in
ShCCND1-transduced cells (P<0.05), but not in AGS and
ShScramble-transduced cells. As shown in Fig. 3B (left panel), cell viability
gradually increased in AGS and ShScramble- and ShCCND1-transduced
cells during culture without 5-FU. Compared to AGS and
ShScramble-transduced cells, ShCCND1 cells exhibited significantly
decreased cell viability on day 3 (P<0.05). These results
reconfirmed that ShCCND1 inhibited the proliferation of AGS cells.
Under the 5-FU (15 μg/ml) treatment, the viability of
ShCCND1-transduced cells was significantly decreased compared to
that of AGS cells (P<0.01) on day 2 and compared to AGS
(P<0.01) and ShScramble-transduced (P<0.001) cells on day 3
(Fig. 3B right panel). These
findings indicate that combined treatments with 5-FU and ShCCND1
more effectively inhibited cell growth than treatment with 5-FU or
ShCCND1 alone. These results indicated that ShCCND1 increases the
sensitivity of AGS cells to 5-FU.
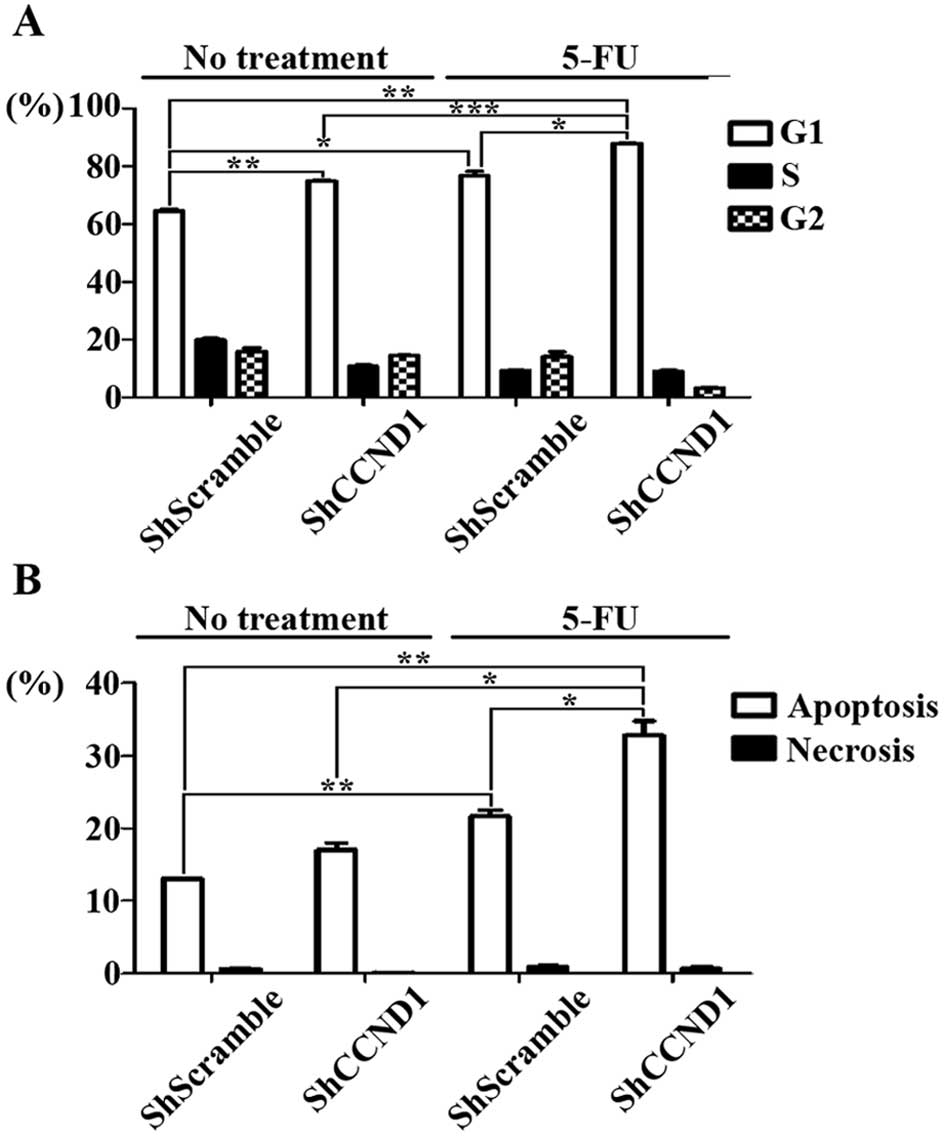
Combined treatment with ShCCND1 and 5-FU
affects apoptosis and cell cycle regulation
To determine the potential effects of ShCCND1 on the
cell cycle, ShCCND1-transduced cells were analyzed by flow
cytometry. As shown in Fig. 4A,
the G1-phase distribution was 76.7, 74.9 and 87.9% in cells treated
with 5-FU, ShCCND1 and a combination of both agents, respectively.
The percentage of cells arrested in the G1 phase in the
combination-treated cells was much higher than that in
ShScramble-transduced (P<0.001), ShCCND1-treated (P<0.001)
and 5-FU-treated cells (P<0.05). This increase was accompanied
with a concomitant reduction in the percentage of cells in the S
and G2 phases of the cell cycle. These data indicated that G1 phase
arrest is induced in ShCCND1-transduced cells and that the combined
treatment with ShCCND1 and 5-FU elicits a synergistic increase in
G1 phase arrest, compared to treatment with ShCCND1 or 5-FU as
single agents. In addition to cell cycle arrest, apoptotic cell
death was measured by flow cytometry to assess the effect of the
combined treatment on cell death. As shown in Fig. 4B, cellular apoptotic indices were
13.02% in cells without treatment, 17.03% in cells treated with
ShCCND1 alone, 21.62% in cells treated with 5-FU alone and 32.85%
in cells treated with the combination. These results show that
ShCCND1 in combination with 5-FU leads to significantly increased
apoptosis, compared to that in untreated (P<0.05), ShCCND1-
(P<0.05) and 5-FU-treated cells (P<0.05). Thus, these data
indicate that ShCCND1 increases the apoptotic cell population and
combined treatment with ShCCND1 and 5-FU synergistically increases
apoptosis. Collectively, these results clearly demonstrate that the
anti-proliferative effect of ShCCND1 is mediated via induction of
cell cycle arrest and apoptosis.
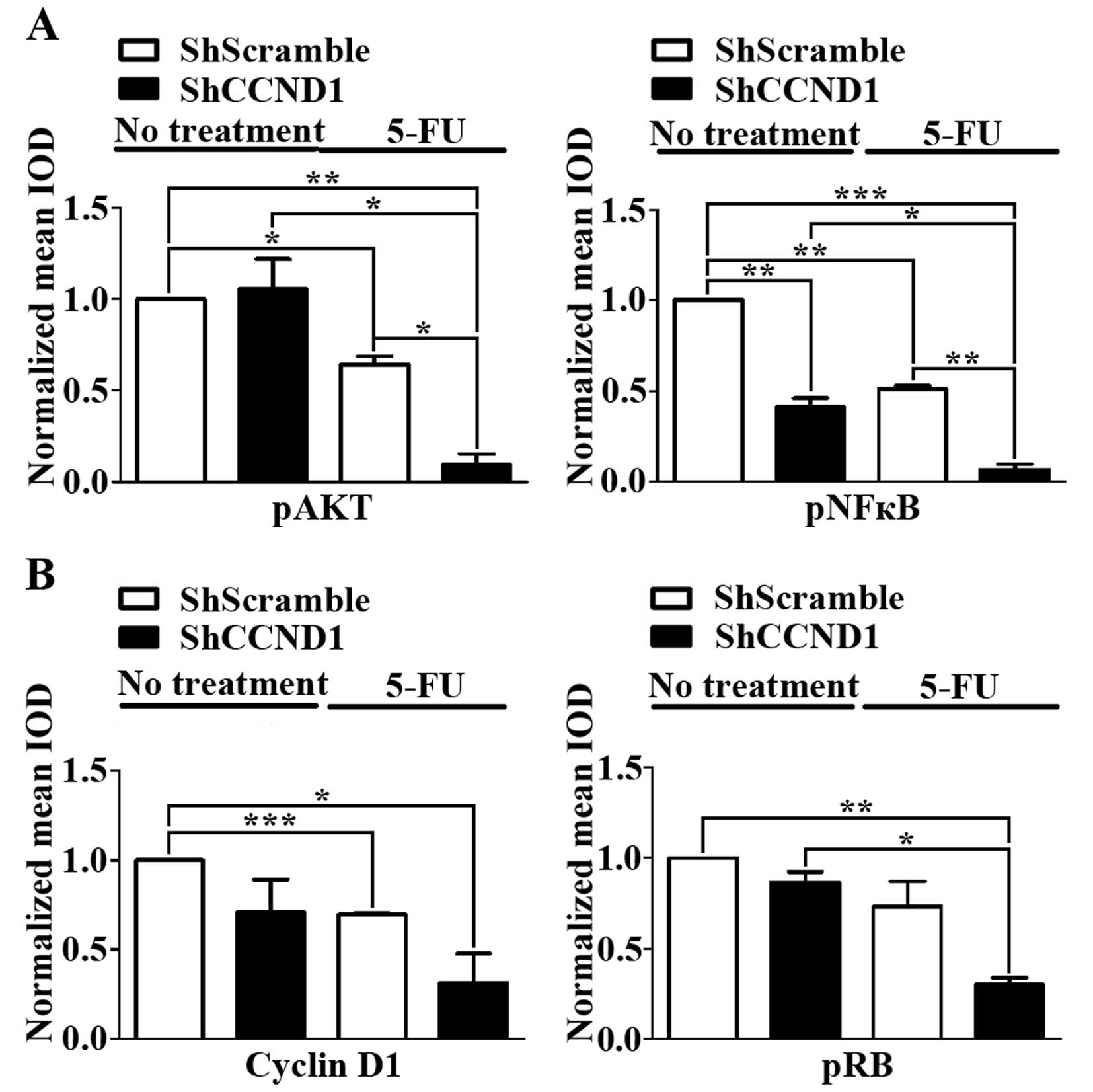
ShCCND1 or 5-FU singly or in combination
influences the expression of pAKT, pNFκB and downstream signal
molecules
The AKT/NFκB survival signaling pathway plays a
significant role in the progression of various cancers (30,31).
Chemoresistance to 5-FU has been reported to be caused by the
induction of pAKT and pNFκB (32).
Moreover, high expression of cyclin D1 protein is also associated
with increased resistance of cancer cells to chemotherapeutic
agents such as 5-FU (12,13). Therefore, to test whether the
anti-proliferative effect of the combination of 5-FU and ShCCND1 is
attributable to the inhibition of AKT/NFκB expression, we
investigated the activation status of downstream components of
AKT/NFκB by western blotting. As shown in Fig. 5A, the expression of pAKT and pNFκB
after 5-FU treatment was reduced, compared to that in untreated
cells. Combined treatment reduced pAKT expression to a greater
degree than did treatment with ShScramble (P<0.01), ShCCND1
(P<0.05), or 5-FU (P<0.05) alone. In addition, combined
treatment with 5-FU and ShCCND1 significantly reduced pNFκB
expression compared to that in cells treated with ShScramble
(P<0.001), ShCCND1 (P<0.05), or 5-FU (P<0.01) alone. These
data suggest that the reduced expression of pAKT and pNFκB by
combined treatment with ShCCND1 and 5-FU increases chemosensitivity
and overcomes 5-FU resistance in AGS cells.
The expression of cyclin D1 and pRB, as measured by
western blotting, was consistent with the cell cycle arrest at G1
phase, as determined by flow cytometry (Fig. 5B). The expression of cyclin D1 in
cells treated with 5-FU (P<0.001) or ShCCND1 was reduced
compared to that in untreated cells. Combined treatment with 5-FU
and ShCCND1 reduced cyclin D1 expression to a greater extent
compared to that in untreated cells (P<0.05) and in cells
treated with 5-FU or ShCCND1 alone. Analysis of pRB, a downstream
molecule of cyclin D1, revealed a similar pattern. The expression
level of pRB in cells treated with 5-FU or ShCCND1 was decreased
compared to that in untreated cells. Combined treatment with 5-FU
and ShCCND1 significantly reduced pRB levels compared to that in
untreated cells (P<0.01) and in cells treated with ShCCND1
(P<0.05) or 5-FU alone.
Discussion
In spite of the availability of new chemotherapeutic
treatments, the prognosis of gastric cancer is still poor (33,34).
The survival time of patients with recurrence or metastasis is
<2 years, despite application of traditional chemotherapy
(35). Moreover, the side effects
of cytotoxic chemotherapeutic drugs often result in deterioration
of the quality of life in patients. Accordingly, to improve the
clinical outcome, more effective and innovative treatments are
needed.
5-FU is the standard therapy against gastric cancer
and is a well-known apoptosis-inducing drug that has been in use
for several decades. However, resistance to 5-FU, a main cause for
failure of chemotherapy, frequently develops in human gastric
cancer. In previous studies, chemoresistance to 5-FU has been
associated with AKT and NFκB activation and was shown to increase
with increased expression of pAKT and pNFκB (32,36).
Therefore, among the numerous reported mechanisms of 5-FU
resistance, in this study, we focused on the cell signaling
proteins AKT and NFκB.
Cyclin D1, a cell cycle regulator, is involved in
the regulation of the G1-S phase transition of the cell cycle
(37). Overexpression of cyclin D1
is associated with rapid cell growth, poor prognosis and increased
chemoresistance in various cancers (25,38).
Previous studies have distinctly shown that treatment of cancer
cells with cyclin D1 antisense results in considerable inhibition
of cell growth (38). Recently,
silencing cyclin D1 has also been shown to induce apoptosis in some
cancer cell lines (25,39). To analyze the effects of silencing
cyclin D1 expression on gastric cancer cell function, we first
established a stable cell line expressing ShCCND1 that effectively
inhibits expression of the cyclin D1 protein. As expected, the
results of this study showed that cells stably transduced with
ShCCND1 exhibited decreases in cyclin D1 protein levels, cell
proliferation rate, cell motility and the ability to form foci.
These results are in agreement with those reported in previous
studies (9,10,25).
If ShCCND1 can decrease the resistance of gastric
cancer to 5-FU, it might be an encouraging potential anticancer
therapy that can be administered along with 5-FU for treatment of
gastric cancer. To review the role of ShCCND1 in regulating 5-FU
sensitivity in gastric cancer, a stable cell line expressing
ShScramble or ShCCND1 was used. In this study, AGS gastric cells
were insensitive to 5-FU concentrations >5 μg/ml, whereas
AGS cells treated with ShCCND1 were sensitive to lower
concentrations of 5-FU. Combined treatment with ShCCND1 and 5-FU
was more effective than treatment with either agent alone. As
expected, cell proliferation was inhibited, G1 arrest was enhanced
and apoptosis was induced in AGS cells exposed to ShCCND1 or 5-FU
alone or in combination. These results demonstrate that the
expression level of cyclin D1 is related to the chemosensitivity to
5-FU and that chemosensitivity might be generated or restored by
decreasing the expression of cyclin D1.
The AKT and NFκB proteins play important roles in
cancer progression, including cell proliferation, cell invasion,
apoptosis and metastasis (30).
Furthermore, their activation is also associated with
chemosensitivity to 5-FU (40). In
a previous report, chemoresistance to 5-FU was shown to be caused
by an increase in pAKT and pNFκB expression (32). Moreover, overexpression of cyclin
D1 protein also increases resistance of cancer cells to
chemotherapeutic agents such as 5-FU (12,13).
In this study, combined treatment with ShCCND1 and 5-FU suppressed
the expression of pAKT, pNFκB and cyclin D1 proteins to a greater
extent than that in cells treated with ShCCND1 or 5-FU alone. In
addition, the expression of pRB, which is directly regulated by
cyclin D1, was significantly downregulated in cells that received
the combined treatment than in those that received the single
treatment. These results support the notion that the 5-FU and
ShCCND1 combination exerts a synergistic killing effect in human
gastric cancer cells and that this effect is attributable to the
suppression of pAKT and pNFκB expression. Taken together, the
results of this study provide further evidence that therapeutic
strategies targeting cyclin D1 may have the dual advantage of
suppressing the growth of cancer cells, while concomitantly
enhancing their chemosensitivity.
Acknowledgements
This study was supported by Konkuk
University in 2011.
References
|
1.
|
Leung WK, Wu MS, Kakugawa Y, Kim JJ, Yeoh
KG, Goh KL, Wu KC, Wu DC, Sollano J, Kachintorn U, Gotoda T, Lin
JT, You WC, Ng EK and Sung JJ; Asia Pacific Working Group on
Gastric C: Screening for gastric cancer in Asia: current evidence
and practice. Lancet Oncol. 9:279–287. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Ohtsu A: Chemotherapy for metastatic
gastric cancer: past, present and future. J Gastroenterol.
43:256–264. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Sakuramoto S, Sasako M, Yamaguchi T,
Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi
Y, Imamura H, Higashino M, Yamamura Y, Kurita A and Arai K:
Adjuvant chemotherapy for gastric cancer with S-1, an oral
fluoropyrimidine. N Engl J Med. 357:1810–1820. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
GASTRIC (Global Advanced/Adjuvant Stomach
Tumor Research International Collaboration) Group; Paoletti X, Oba
K, Burzykowski T, Michiels S, Ohashi Y, Pignon JP, Rougier P,
Sakamoto J, Sargent D, Sasako M, Van Cutsem E and Buyse M: Benefit
of adjuvant chemotherapy for resectable gastric cancer: a
meta-analysis. JAMA. 303:1729–1737. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Sherr CJ: G1 phase progression: cycling on
cue. Cell. 79:551–555. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Weinberg RA: The retinoblastoma protein
and cell cycle control. Cell. 81:323–330. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Gao P, Zhou GY, Liu Y, Li JS, Zhen JH and
Yuan YP: Alteration of cyclin D1 in gastric carcinoma and its
clinicopathologic significance. World J Gastroenterol.
10:2936–2939. 2004.PubMed/NCBI
|
|
8.
|
Motokura T and Arnold A: Cyclin D and
oncogenesis. Curr Opin Genet Dev. 3:5–10. 1993. View Article : Google Scholar
|
|
9.
|
Coco Martin JM, Balkenende A, Verschoor T,
Lallemand F and Michalides R: Cyclin D1 overexpression enhances
radiation-induced apoptosis and radiosensitivity in a breast tumor
cell line. Cancer Res. 59:1134–1140. 1999.PubMed/NCBI
|
|
10.
|
Han EK, Begemann M, Sgambato A, Soh JW,
Doki Y, Xing WQ, Liu W and Weinstein IB: Increased expression of
cyclin D1 in a murine mammary epithelial cell line induces p27kip1,
inhibits growth and enhances apoptosis. Cell Growth Differ.
7:699–710. 1996.PubMed/NCBI
|
|
11.
|
Kuroda Y, Sakai A, Tsuyama N, Katayama Y,
Munemasa S, Asaoku H, Okikawa Y, Nakaju N, Mizuno M, Ogawa K,
Nishisaka T, Matsui H, Tanaka H and Kimura A: Ectopic cyclin D1
overexpression increases chemosensitivity but not cell
proliferation in multiple myeloma. Int J Oncol. 33:1201–1213.
2008.PubMed/NCBI
|
|
12.
|
Kornmann M, Arber N and Korc M: Inhibition
of basal and mitogen-stimulated pancreatic cancer cell growth by
cyclin D1 antisense is associated with loss of tumorigenicity and
potentiation of cytotoxicity to cisplatinum. J Clin Invest.
101:344–352. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Warenius HM, Seabra LA and Maw P:
Sensitivity to cis-diamminedichloroplatinum in human cancer cells
is related to expression of cyclin D1 but not C-RAF-1 protein. Int
J Cancer. 67:224–231. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Simizu S, Shibasaki F and Osada H: Bcl-2
inhibits calcineurin-mediated Fas ligand expression in antitumor
drug-treated baby hamster kidney cells. Jpn J Cancer Res.
91:706–714. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Zhou X, Zhang Z, Yang X, Chen W and Zhang
P: Inhibition of cyclin D1 expression by cyclin D1 shRNAs in human
oral squamous cell carcinoma cells is associated with increased
cisplatin chemosensitivity. Int J Cancer. 124:483–489. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Sun Y, Luo D and Liao DJ: Cyclin D1
protein plays different roles in modulating chemoresponses in MCF7
and MDA-MB231 cells. J Carcinog. 11:122012. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Cardone MH, Roy N, Stennicke HR, Salvesen
GS, Franke TF, Stanbridge E, Frisch S and Reed JC: Regulation of
cell death protease caspase-9 by phosphorylation. Science.
282:1318–1321. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo
P, Hu LS, Anderson MJ, Arden KC, Blenis J and Greenberg ME: Akt
promotes cell survival by phosphorylating and inhibiting a Forkhead
transcription factor. Cell. 96:857–868. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Curnock AP, Logan MK and Ward SG:
Chemokine signalling: pivoting around multiple phosphoinositide
3-kinases. Immunology. 105:125–136. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Baldwin AS: Control of oncogenesis and
cancer therapy resistance by the transcription factor NF-kappaB. J
Clin Invest. 107:241–246. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Ozes ON, Mayo LD, Gustin JA, Pfeffer SR,
Pfeffer LM and Donner DB: NF-kappaB activation by tumour necrosis
factor requires the Akt serine-threonine kinase. Nature. 401:82–85.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Wang C-Y, Mayo MW and Baldwin AS: TNF- and
cancer therapy-induced apoptosis: potentiation by inhibition of
NF-kappaB. Science. 274:784–787. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Camp ER, Li J, Minnich DJ, Brank A,
Moldawer LL, MacKay SL and Hochwald SN: Inducible nuclear
factor-kappaB activation contributes to chemotherapy resistance in
gastric cancer. J Am Coll Surg. 199:249–258. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Lu YS, Yeh PY, Chuang SE, Gao M, Kuo ML
and Cheng AL: Glucocorticoids enhance cytotoxicity of cisplatin via
suppression of NF-κB activation in the glucocorticoid receptor-rich
human cervical carcinoma cell line SiHa. J Endocrinol. 188:311–319.
2006.PubMed/NCBI
|
|
25.
|
Sauter ER, Nesbit M, Litwin S,
Klein-Szanto AJ, Cheffetz S and Herlyn M: Antisense cyclin D1
induces apoptosis and tumor shrinkage in human squamous carcinomas.
Cancer Res. 59:4876–4881. 1999.PubMed/NCBI
|
|
26.
|
Huang WS, Wang JP, Wang T, Fang JY, Lan P
and Ma JP: ShRNA-mediated gene silencing of beta-catenin inhibits
growth of human colon cancer cells. World J Gastroenterol.
13:6581–6587. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Liang CC, Park AY and Guan JL: In vitro
scratch assay: a convenient and inexpensive method for analysis of
cell migration in vitro. Nat Protoc. 2:329–333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Tiang JM, Butcher NJ and Minchin RF: Small
molecule inhibition of arylamine N-acetyltransferase Type I
inhibits proliferation and invasiveness of MDA-MB-231 breast cancer
cells. Biochem Biophys Res Commun. 393:95–100. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Pearson G, English JM, White MA and Cobb
MH: ERK5 and ERK2 cooperate to regulate NF-kappaB and cell
transformation. J Biol Chem. 276:7927–7931. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Martin V, Herrera F, Carrera-Gonzalez P,
Garcia-Santos G, Antolin I, Rodriguez-Blanco J and Rodriguez C:
Intracellular signaling pathways involved in the cell growth
inhibition of glioma cells by melatonin. Cancer Res. 66:1081–1088.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Cheng JC, Chou CH, Kuo ML and Hsieh CY:
Radiation-enhanced hepatocellular carcinoma cell invasion with
MMP-9 expression through PI3K/Akt/NF-kappaB signal transduction
pathway. Oncogene. 25:7009–7018. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Shin JY, Kim JO, Lee SK, Chae HS and Kang
JH: LY294002 may overcome 5-FU resistance via down-regulation of
activated p-AKT in Epstein-Barr virus-positive gastric cancer
cells. BMC Cancer. 10:4252010. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Kim TW, Kang YK, Ahn JH, Chang HM, Yook
JH, Oh ST, Kim BS and Lee JS: Phase II study of capecitabine plus
cisplatin as first-line chemotherapy in advanced gastric cancer.
Ann Oncol. 13:1893–1898. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Song M, Zhang R, Dai Y, Gao F, Chi H, Lv
G, Chen B and Wang X: The in vitro inhibition of multidrug
resistance by combined nanoparticulate titanium dioxide and UV
irradition. Biomaterials. 27:4230–4238. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Yoshida K and Toge T: Combination
chemotherapy of TS-1 and docetaxel on advanced and recurrent
gastric cancer. Gan To Kagaku Ryoho. 31:1982–1986. 2004.PubMed/NCBI
|
|
36.
|
Arlt A, Gehrz A, Muerkoster S, Vorndamm J,
Kruse ML, Folsch UR and Schafer H: Role of NF-kappaB and Akt/PI3K
in the resistance of pancreatic carcinoma cell lines against
gemcitabine-induced cell death. Oncogene. 22:3243–3251. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Fu M, Wang C, Li Z, Sakamaki T and Pestell
RG: Minireview: Cyclin D1: normal and abnormal functions.
Endocrinology. 145:5439–5447. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Kornmann M, Danenberg KD, Arber N, Beger
HG, Danenberg PV and Korc M: Inhibition of cyclin D1 expression in
human pancreatic cancer cells is associated with increased
chemosensitivity and decreased expression of multiple
chemoresistance genes. Cancer Res. 59:3505–3511. 1999.
|
|
39.
|
Zhou H, Fujigaki Y, Kato A, Miyaji T,
Yasuda H, Tsuji T, Yamamoto T, Yonemura K and Hishida A: Inhibition
of p21 modifies the response of cortical proximal tubules to
cisplatin in rats. Am J Physiol Renal Physiol. 291:F225–F235. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Liu N, Zhang J, Zhang J, Liu S, Liu Y and
Zheng D: Erbin-regulated sensitivity of MCF-7 breast cancer cells
to TRAIL via ErbB2/AKT/NF-kappaB pathway. J Biochem. 143:793–801.
2008. View Article : Google Scholar : PubMed/NCBI
|