Introduction
Colon cancer, the second most deadly malignancy in
the USA and a serious public health problem worldwide, has seen a
growing incidence in South Korea (1,2).
Since the accumulation of a variety of genetic alterations drives
colon cancer progression, much attention has been paid to the
PI3K/Akt pathway, which is responsible for carcinogenesis and
metastasis of colon cancer because it regulates the cell cycle,
growth, proliferation and survival (3–7).
Several studies have revealed that the PI3K/Akt pathway has an
important role in the early stages of sporadic colorectal cancer
and is activated and overexpressed in colon cancers (5,8,9). The
PI3K/Akt pathway has also been demonstrated to be responsible for
carcinogenesis of colon cancer (3,10).
Given its prominent role in cancer development, inhibition of this
pathway might be one of the most effective ways to conquer colon
cancer. Selective inhibitors of different molecules in this pathway
have been developed as molecular targeted anticancer therapies and
a number of studies are currently being conducted to investigate
the role of PI3K/Akt inhibitors in patients with advanced tumors
(5,11–13).
However, it has been found that a single agent is unable to disrupt
the proliferation of the cancer cells due to its modest activity
(5,13,14).
The Hippo signaling pathway was first identified in
nematodes and is evolutionarily conserved with mammals. Although
the mechanism by which Hippo signaling regulates cell growth has
not been clearly understood, it appears to play a major role in
controlling organ size and cell proliferation (15). Loss of Hippo signaling elicits
cancer development due to unlimited cell proliferation and
deregulation of the Hippo signaling pathway has been observed in
various cancers, including colon cancer. The main core mediators of
the Hippo pathway are Mst1/2, LATS1/2, Mob and Sav. When the Hippo
pathway is active, Mst1/2, Lats1/2 and Mob form a complex that
prevents the nuclear localization of YAP, a key effector protein of
the Hippo pathway, by direct phosphorylation. On the other hand,
when the Hippo pathway is inactive, YAP moves into the nucleus,
which eventually leads to YAP accumulation in the nucleus and
activation of transcription factors. Moreover, YAP expression has
been observed in colon cancer (16–18)
and it is overexpressed in human colon cancer specimens.
Overexpression of YAP stimulates cell growth and survival in colon
cancer cells (19,20).
DIM (3, 3′-diindolylmethane) is a natural compound
derived from cruciferous vegetables such as broccoli, cabbage and
cauliflower. Although several studies have shown that DIM has
anti-proliferative effects in a variety of cancer cell types
including human colon, pancreas, prostate and breast cancer
(13–23), the cellular apoptotic mechanism of
DIM on cancer cells has not been fully elucidated. We have recently
studied the antitumor effect of DIM in gastric cancer in
vivo and in vitro through activation of Hippo signaling
(unpublished data). Since PI3K/Akt signaling is known to play a
critical role in growth control, we wondered whether DIM has an
effect on Hippo signaling, mediated via the PI3K/Akt signaling
pathway in colon cancer cells. We demonstrate that DIM induces
apoptosis in human colon cancer cells by activating the Hippo
signaling pathway followed by inactivation of the PI3K/Akt
signaling pathway. The present findings have important implications
for the clinical use of DIM in colon cancer prevention.
Materials and methods
Cell culture
The human colon cancer cell line HCT116 was obtained
from the University of Texas M.D. Anderson Cancer Center (Houston,
TX, USA). HCT116 was cultured in DMEM-F12 medium (Gibco, Grand
Island, NY, USA) supplemented with 10% fetal bovine serum (Gibco),
100 mg/ml streptomycin and 100 IU/ml penicillin as a monolayer in
100-mm dishes (BD Biosciences, Sparks, MD, USA) under standard
conditions at 37°C in a 5% CO2 humidified
atmosphere.
Reagent
DIM was purchased from LKT Laboratories (St. Paul,
MN, USA). Antibodies to cleaved-caspase-9, caspase-3, cleaved
poly(ADP-ribose) polymerase (PARP), p-PTEN, Akt, p-S473-Akt,
p-T308-Akt, p-GSK-3β, p-PDK1, YAP and p-YAP were purchased from
Cell Signaling Technology (Beverly, MA, USA).
MTT assay
Cell viability of DIM, LY294002 and DIM plus
LY294002 on HCT116 cells was determined by MTT assay
(3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) as
described previously (21,22). In brief, HCT116 cells were seeded
with 1×104 cells per well into 96-well plates (SPL,
Seoul, Korea). After 24 h of cell seeding, HCT116 cells were
treated with DIM, LY294002 and DIM plus LY294002 in a
dose-dependent manner for 1, 2 and 3 days. The cells were then
incubated with 50 μl of MTT (2 mg/ml in PBS) for 3 h at 37°C
and 200 μl of DMSO (Sigma) was added to solubilize the
crystals for 30 min at room temperature. Cell viability was
determined by a scanning multiwall spectrophotometer (SpectraMAX
340, Molecular Devices Co., Sunnyvale, CA, USA).
Soft agar colony formation assay
Survival of colon carcinoma cells was tested by soft
agar colony formation assay, as described before. Briefly, cells
were exposed to DIM (50 μM), LY294002 (20 μM) and DIM
(50 μM) plus LY29402 (20 μM) in 6-well plates and
were cultured in an incubator at 37°C with 5% CO2 for 2
weeks. Colony formation was observed by microscopy and the counted
and quantitated.
Western blotting
Cells with or without DIM or LY294002 were harvested
and suspended in lysis buffer (Intron Biotechnology, Korea). Cell
extracts were incubated on ice for 20 min and centrifuged at 13,000
× g for 5 min at 4°C. The protein concentration was determined
using a BSA Protein Assay kit (Pierce, Rockford, IL, USA). Whole
lysate was resolved on an SDS-PAGE gel, transferred to a PVDF
membrane (Bio-Rad, Hercules, CA, USA) by electroblotting and then
probed with mouse anti-human cyclin D1, rabbit anti-human CDK2,
mouse anti-human CDK4, mouse anti-human CDK6, rabbit anti-human
p27, rabbit anti-human p15, rabbit anti-human cleaved-caspase-9,
rabbit anti-human cleaved PARP, rabbit anti-human Akt, rabbit
anti-human p-S473-Akt, rabbit anti-human p-T308-Akt, rabbit
anti-human p-PTEN, rabbit anti-human p-GSK3β, rabbit anti-human
p-PDK1, rabbit anti-human p-YAP and YAP antibodies (Cell Signaling
Technology). The membrane was then washed with TBS-T (10X TBS and
0.1% Tween-20) and incubated for an additional 1 h with HRP-linked
anti-rabbit and anti-mouse antibodies (Cell Signaling Technology).
Protein bands were visualized with the Enhanced Chemiluminescence
kit (Amersham, Arlington Heights, IL, USA).
Microarray
Total RNA was isolated from the cells by using a
mirVana™ miRNA isolation labeling kit (Ambion Inc., TX, USA)
according to the manufacturer’s protocol. Biotin-labeled cRNA was
prepared using an Illumina Total Prep RNA amplification kit (Ambion
Inc.) for hybridization. Samples were hybridized in Illumina
Human-12 BeadChip V.4 microarray (Illumina, CA, USA). Gene
expression data were extracted from the Genome Studio (Illumina).
Data were normalized using the quantile normalization method in the
Linear Models for Microarray data package in the program R. A heat
map of gene expression was generated using the Cluster and Treeview
programs (23). A microarray study
was performed by the Shared Research Equipment Assistance Program
by Korea Basic Science Institute, MEST.
Statistical analysis
The experimental results are shown as mean ± SE.
Student’s t-test and one-way ANOVA were used to test for
significant differences among the experimental groups. P-values
<0.05 were considered significant.
Results
Cell growth inhibition by LY294002 and
DIM treatment
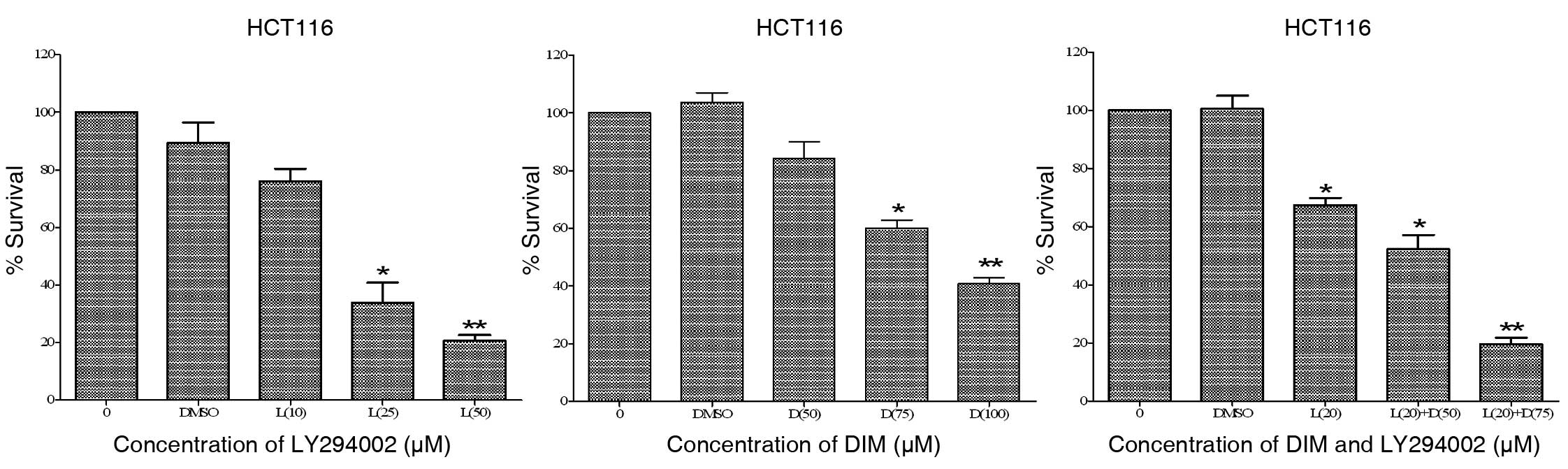
We tested several doses of DIM and LY294002 at 72 h
in HCT116 cells. As shown in Fig.
1, LY294002 inhibited cell viability in a dose-dependent
manner. The IC50 of LY294002 was ∼20–25 μM. DIM
also inhibited HCT116 cells in a dose-dependent manner. The
IC50 concentration of LY294002 (20 μM) with a
modestly toxic concentration of DIM (50 and 75 μM, 20–40%
apoptosis at 72 h) resulted in a significant growth inhibition
(60–80% at 72 h) of HCT116 cells compared with either agent alone,
suggesting a significant inhibitory effect of combination treatment
in colon carcinoma cells. These results indicate that the
combination of LY294002 with a lower dose of DIM evoked
significantly greater inhibition of colon cancer cell growth
compared with either agent alone.
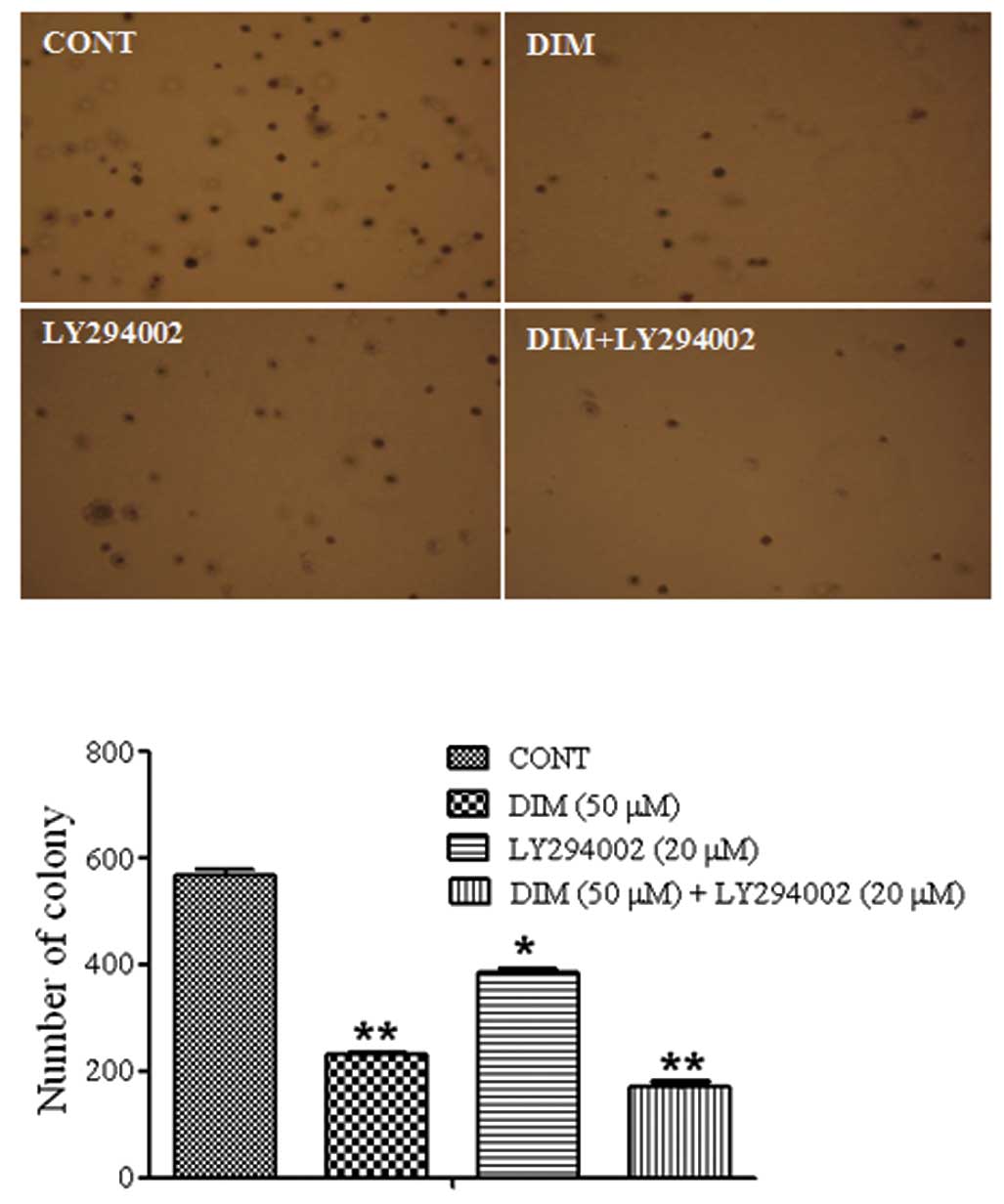
Inhibitory effect of LY294002 and DIM on
clonogenicity
The effect of LY294002 and DIM treatment in
vitro on HCT116 cell colony formation was evaluated by the soft
agar cloning assay. Treatment with LY294002 (20 μM) and DIM
(50 μM) resulted in a significant inhibition of colony
formation in HCT116 cells when compared with single agent treatment
(Fig. 2).
Induction of apoptosis by LY294002 and
DIM treatment
The cell viability was further evaluated by
determining the apoptotic effects. Sub-G1 population was
significantly increased by the combination treatment of LY294002
(20 μM) and DIM (50 μM) compared with either agent
alone (Fig. 3A). We found that the
combination of DIM and LY294002 resulted in a significant induction
of apoptosis in colon cancer cells. We further examined
cleaved-caspase-9, cleaved-PARP and pro-caspase-3 protein levels in
the HCT116 colon cancer cell line. As shown in Fig. 3B, the combination with LY294002 (20
μM) and DIM (50 μM) significantly increased the
cleaved-caspase-9 and cleaved-PARP protein levels compared with the
cells treated with LY294002 or DIM alone. Pro-caspase-3 protein
levels were also significantly decreased by the combination
treatment. These data indicate that the combination of DIM and
LY294002 induces more dramatic apoptotic cell death in the human
colon cancer cell line HCT116 than single agent treatment.
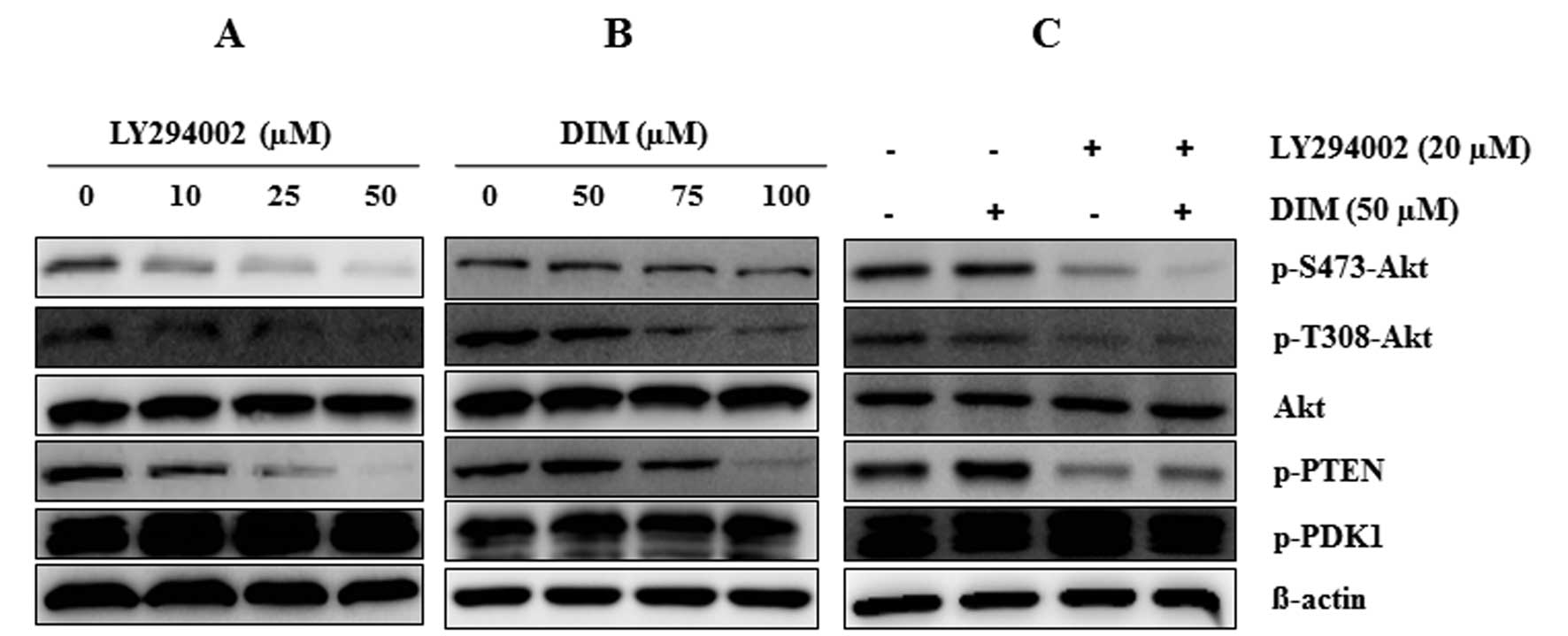
Downregulation of the Akt pathway by
DIM
To investigate the effect of DIM on the PI3K/Akt
pathway, we detected the expression of PI3K/Akt pathway proteins.
As shown in Fig. 4, LY294002
significantly suppressed the expression of Akt pathway proteins
(p-S473-Akt, p-T308-Akt, p-PTEN and p-GSK) in a dose-dependent
manner (0, 10, 25 and 50 μM). DIM also significantly
inhibited the expression of Akt pathway proteins as effectively as
the PI3K inhibitor. The combination treatment of LY294002 with DIM
notably suppressed the expression of p-S473-Akt, p-T308-Akt and
p-PTEN, although the expressions of Akt and p-PDK1 protein levels
were not altered by the combination treatment of DIM and LY294002
or alone. Thus, our findings suggest that the combination of
LY294002 and DIM significantly induced the inactivation of the
PI3K/Akt pathway more than the single treatment alone.
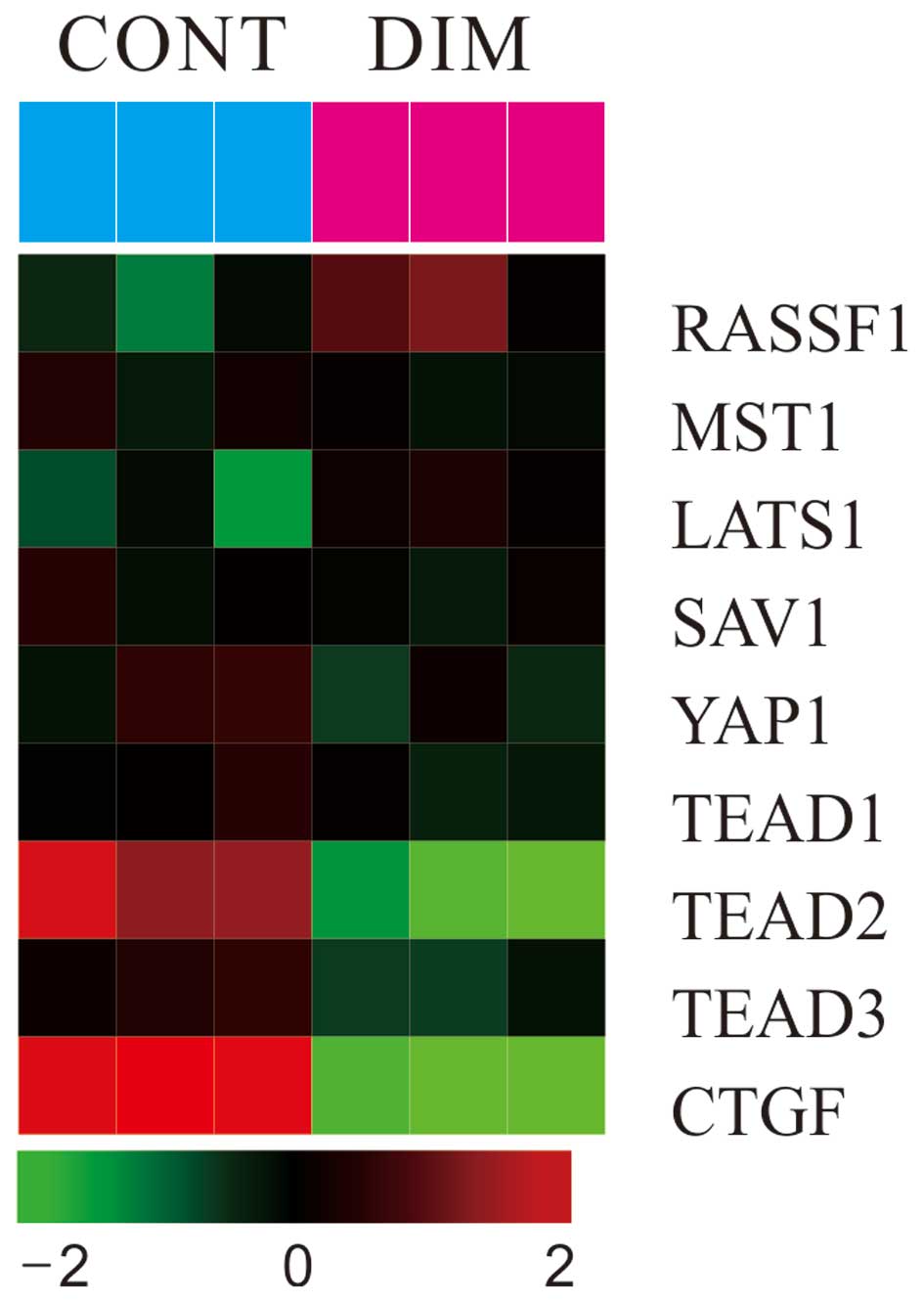
Gene expression levels of the Hippo
signaling pathway by DIM treatment
To investigate the effect of DIM on gene expression
levels in colon carcinoma cells, microarray was performed. As shown
in Fig. 5, the administration of
DIM (100 μM) significantly induced the expression of RASSF1
gene and mildly increased the Mst1, LATS1 gene expression levels.
DIM also significantly decreased the expression of the YAP1 gene
together with downstream target genes (TEAD1/2/3 and CTGF) of YAP
in colon cancer cells.
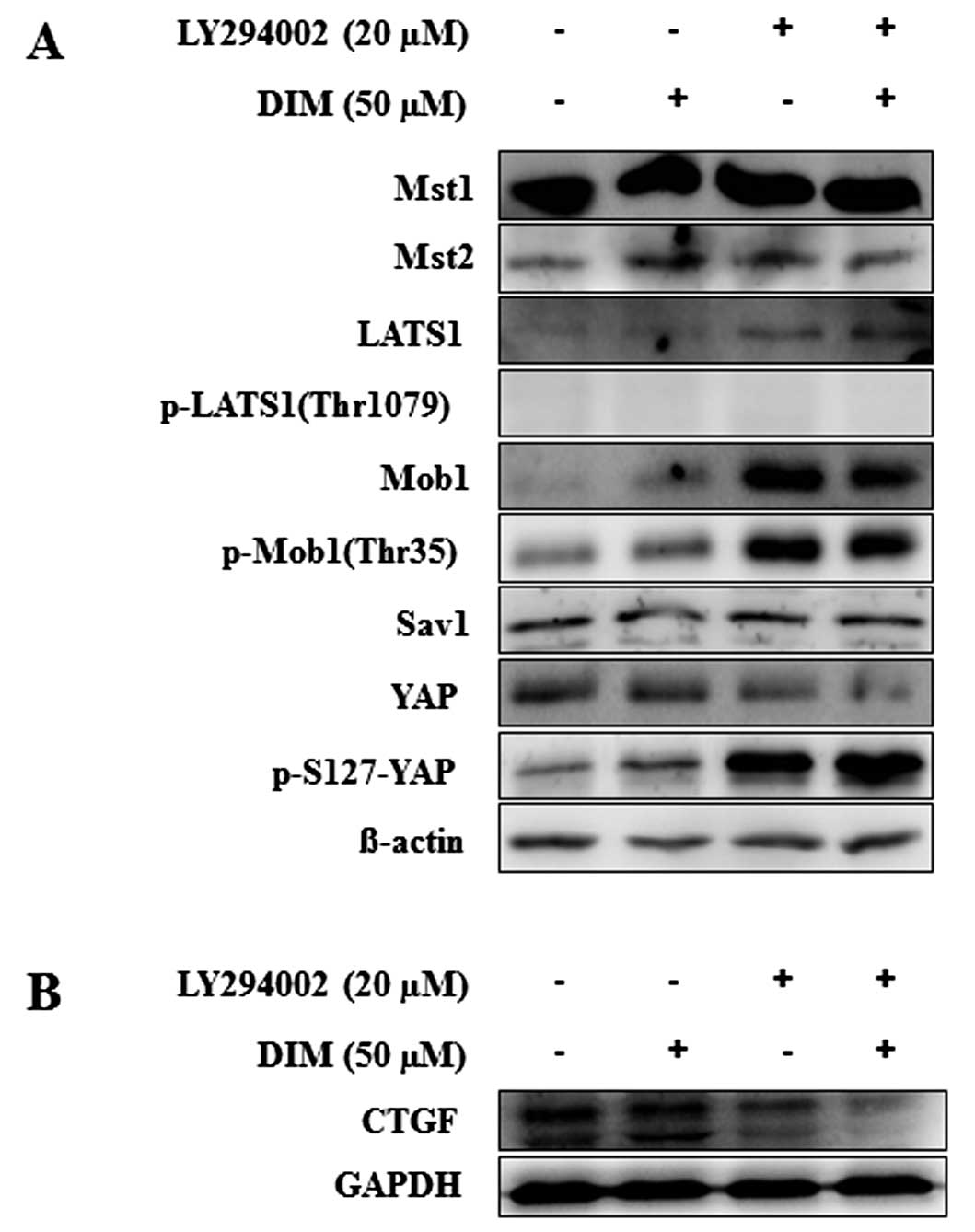
DIM activates Hippo signaling and
suppresses the YAP activity
To further investigate the effect of LY294002 and
DIM on the Hippo signaling pathway, we examined the protein levels
of Mst1/2, LATS1, p-LATS1, Mob1, p-Mob1, Sav1, YAP and p-YAP after
LY294002 (20 μM) and DIM (50 μM) treatment at 72 h.
As shown in Fig. 6A, the
expression of LATS1, Mob1 and p-Mob1 proteins was significantly
increased in HCT116 cells by the treatment of LY294002 and DIM. We
found that the production of p-YAP protein expression was increased
and YAP protein levels were decreased by the treatment of LY294002
and DIM in HCT116 cells. The combination of LY294002 and DIM
significantly increased the expression of p-YAP protein, an
inactive form of YAP, compared to single treatment alone. The
downstream target gene of YAP, the CTGF protein, was also
significantly decreased by the combination treatment of LY294002
and DIM (Fig. 6B). These results
suggest that the combination treatment of LY294002 with DIM
significantly enhanced the activation of the Hippo signaling
pathway, which inhibits colon cancer cell growth and
proliferation.
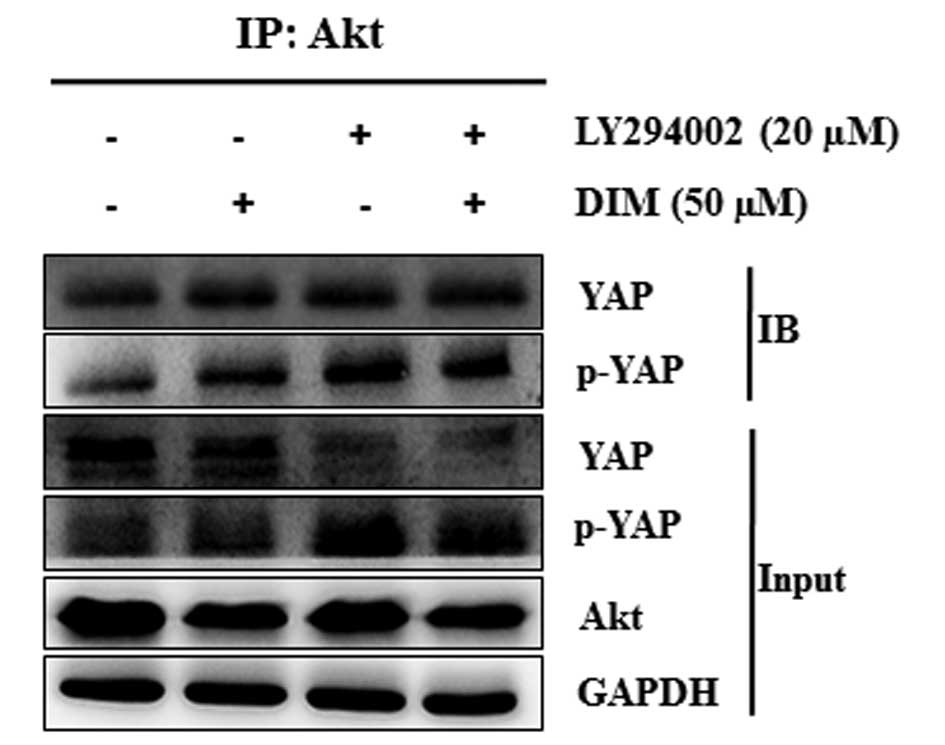
DIM enhances the binding capacity between
Akt and YAP in colon cancer cells
IP was performed to detect the interaction between
Akt and YAP. As shown in Fig. 7,
we found that the Akt-YAP binding complex significantly increased
after treatment with LY294002 and DIM. The combination treatment of
LY294002 with DIM significantly increased the binding ability of
Akt and YAP proteins. The ability of p-YAP to bind with Akt was
also increased by the combination treatment of LY294002 with DIM.
The increased binding ability between Akt and YAP due to the
combination treatment of LY294002 and DIM may prevent the nuclear
localization of YAP and induce the proapoptotic gene expression.
Our results showed that the inactivation of Akt together with the
induced phosphorylation of YAP, due to the treatment of LY294002
with DIM, subjected the cells to apoptosis.
Discussion
Cancer development is a complex process governed by
the interaction of several signaling pathways that regulate normal
cell growth and proliferation. The Hippo and PI3K/Akt pathways have
been shown to play a critical role in monitoring tissue growth
involved in the regulation of organ size all the way through their
individual functions and in the regulation of cell proliferation
(15). Our study provides new
insight into the mechanisms of a crosstalk between Hippo signaling
and the Akt pathway controlling cell proliferation by DIM treatment
in colon cancer cells. We report for the first time that the
co-administration of LY294002 with DIM enhanced upregulation of
Hippo signaling through downregulation of Akt activity in HCT116
cells and this combination therapy is significantly more effective
at killing cancer cells than either agent alone. These findings
highlight the potential usefulness of DIM and can help develop
therapeutic strategies for the prevention and treatment of colon
cancer.
DIM is a natural compound that selectively kills
cancer cells without toxicity to normal cells (24–27).
A number of studies have reported that DIM has antitumor effects
and induces apoptosis in various cancer cells including colon
cancer, suggesting that DIM is a new promising candidate as a
chemotherapeutic agent (27–29).
In the present study, we found that LY294002 and DIM induced
dose-dependent inhibition of growth in HCT116 colon cancer cells,
respectively. The combination of DIM and LY294002 had even more
significant anti-proliferative effects against HCT116 colon cancer
cells. In agreement with MTT assay, significant inhibition of
colony formation of HCT116 cells was observed with the combination
treatment of LY294002 with DIM compared to the single agent alone.
Significantly increased sub-G1 population and apoptotic proteins
(cleaved-caspase-9 and cleaved-PARP) were shown by the combination
treatment of LY294002 with DIM compared with the cells treated with
either agent alone. These findings are in agreement with another
previously published study described by Gao et al, where
co-administration of LY294002 (20 μM) and DIM (40 μM)
resulted in a pronounced increase in apoptosis (∼60%) and inhibited
cell proliferation in human leukemia cells (30). Our findings are consistent with the
study of Gao et al in leukemia cells in demonstrating that
the PI3K inhibitor with a low dose of DIM has a more effective
synergistic antitumor and cancer prevention effect in colon cancer
cells.
Akt has been shown to be activated in various
cancers including colon cancer (31) and plays a critical role in
controlling cell survival. Because activated Akt was found in a
variety of cancers, it is believed to be an attractive target for
cancer treatment. Akt is activated by phosphorylation at Thr308 by
PDK1 or at Ser473 by mTORC2 (30,32).
PTEN, which is also well known as a tumor suppressor,
dephosphorylates PIP3 and inactivates the Akt pathway (5). Akt activation generally involves PTEN
inactivation and phosphorylation of PTEN stabilizes the PTEN
protein, making it less active towards its substrate, PIP3
(5). The present findings showed
that the administration of LY294002 resulted in suppression of Akt
activation and the co-administration of LY294002 and DIM
significantly attenuated the phosphorylation of Akt. In addition,
phosphorylated PTEN expression levels were significantly reduced by
the co-administration of LY294002 and DIM and by an individual
treatment in a dose-dependent manner. These findings are in
agreement with previous reports of the anti-cancer activity of DIM,
which has been linked with inhibition of the Akt signaling pathway
in leukemia cells (25,30). DIM inhibited cell proliferation
through the downregulation of Akt signaling and the PI3K inhibitor
can enhance DIM-mediated inhibition of Akt in colon cancer
cells.
Increasing numbers of studies have validated the
importance of the Hippo pathway in human cancers (33). Perturbation of this pathway has
been shown to lead to an acceleration of tumorigenesis in mice
(34) and loss of expression of
the Hippo signaling pathway genes have been reported in a variety
of cancers (33,35). We have previously reported that the
Hippo signaling is a potent in vivo growth pathway and a
potent suppressor of liver tumor formation (36). Besides liver cancer, Hippo
signaling also plays an important role in many cancers including
colon cancer. In fact, deregulation of the Hippo pathway has been
elicited in intestinal tumorigenesis (17,19,37,38)
and the overexpression of YAP has been shown in human primary
colonic tumors (16,17). YAP has been revealed to play a
crucial role in tumorigenesis of esophageal squamous cell carcinoma
and is associated with clear cell ovarian tumors with poor
prognosis. Recent findings show that TAZ and YAP are associated
with colorectal cancer and are predictors of patient survival
(20). In our study, DIM slightly
increased the expression of Hippo signaling genes (RASSF1 and
LATS1) but great suppression was observed in the expression of
downstream genes of Hippo such as YAP1, TEAD1/2/3 and CTGF by DIM
treatment in the microarray experiment. In agreement with
microarray data, western blot analysis also revealed that the
administration of LY294002 and DIM induced the expression of Mst,
LATS1, Mob and p-YAP protein levels and suppressed the YAP protein
levels in HCT116 colon cancer cells. The co-administration of
LY294002 and DIM significantly decreased the expression of YAP.
Phosphorylated YAP protein expression was significantly increased
by administration of both LY294002 and DIM. CTGF, the downstream
target gene of YAP, was also suppressed by administration of
LY294002 and DIM. Based on our findings, DIM could enhance the
activation of the Hippo signaling pathway and induces the
phosphorylation of YAP, which causes the down regulation of the YAP
target genes.
Since inactivation of Akt and YAP were observed by
the administration of LY294002 and DIM, we further investigated the
relationship between the Hippo and Akt pathways. A recent study
showed that the loss of Hippo signaling increased Akt expression as
well as Akt activity, whereas the activation of Hippo signaling
reduced Akt expression in Drosophila developing tissues
(39). Cinar et al also
reported that Mst1 induced functionally antagonized activated Akt1
in vivo in the zebrafish (40), suggesting that the Hippo pathway
may negatively regulate cell growth by reducing Akt pathway
activity. In agreement with previous reports in Drosophila
and zebrafish, our present study showed that suppression of Akt
activity significantly induced phosphorylation of YAP. In addition,
the binding interaction of Akt and YAP was enhanced after LY29400
treatment and was strongly increased by the combination treatment
of LY294002 with DIM. Inactivation of Akt may interact with Hippo
signaling because some of the Hippo pathway signaling protein
activity was increased by LY294002 treatment alone and was even
more strongly increased by the combination of LY294002 with DIM.
The binding of Akt with YAP may prevent the localization of YAP in
the nucleus and induce the phosphorylation of YAP in the cytoplasm.
However, further research is needed to discern how inactivated Akt
bound to YAP controls the Hippo signaling pathway. Thus, induction
of Hippo signaling activity in conjunction with the inactivation of
Akt in colon cancer cells by LY294002 and DIM treatment indicated
that DIM potentiates the inhibition of colon cancer cell
proliferation mediated through Akt via the Hippo signaling pathway.
Moreover, inactivation of Akt induced by the gain of Hippo
signaling by LY294002 and DIM treatment is dependent on YAP, which
supports the notion that Hippo signaling may negatively regulate
cell growth by Akt activity.
In conclusion, our present study shows that DIM
potentiates the inhibition of the proliferation of colon cancer by
inhibiting the PI3K/Akt pathway mediated through the activation of
the Hippo signaling pathway. DIM has dramatic therapeutic effects
when it is combined with the PI3K inhibitor in the treatment of
colon cancer cells. These findings highlight the potential
usefulness of DIM, a natural chemopreventive agent for colon cancer
therapy. However, further molecular investigations are needed to
fully address the mechanisms by which DIM potentiates PI3K/Akt
effects through the Hippo signaling pathway.
Acknowledgements
This study was supported by a grant
(17) from Kye-Nam, Kim Jae Jung
Memorial Fund and by the Basic Science Research Program through the
National Research Foundation of Korea (NRF) funded by the Ministry
of Education, Science and Technology 2011-0014864 and 2008-0062611
(MEST).
References
|
1.
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar
|
|
2.
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: the impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Leystra AA, Deming DA, Zahm CD, et al:
Mice expressing activated PI3K rapidly develop advanced colon
cancer. Cancer Res. 72:2931–2936. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Pal I and Mandal M: PI3K and Akt as
molecular targets for cancer therapy: current clinical outcomes.
Acta Pharmacol Sin. 33:1441–1458. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Oldham S and Hafen E: Insulin/IGF and
target of rapamycin signaling: a TOR de force in growth control.
Trends Cell Biol. 13:79–85. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Hietakangas V and Cohen SM: Regulation of
tissue growth through nutrient sensing. Annu Rev Genet. 43:389–410.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Michlig S, Harris M, Loffing J, Rossier BC
and Firsov D: Progesterone down-regulates the open probability of
the amiloride-sensitive epithelial sodium channel via a
Nedd4-2-dependent mechanism. J Biol Chem. 280:38264–38270. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Saif MW and Chu E: Biology of colorectal
cancer. Cancer J. 16:196–201. 2010. View Article : Google Scholar
|
|
10.
|
Walther A, Johnstone E, Swanton C, Midgley
R, Tomlinson I and Kerr D: Genetic prognostic and predictive
markers in colorectal cancer. Nat Rev Cancer. 9:489–499. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Tenbaum SP, Ordonez-Moran P, Puig I, et
al: beta-catenin confers resistance to PI3K and AKT inhibitors and
subverts FOXO3a to promote metastasis in colon cancer. Nat Med.
18:892–901. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Abdul-Ghani R, Serra V, Gyorffy B, et al:
The PI3K inhibitor LY294002 blocks drug export from resistant colon
carcinoma cells overexpressing MRP1. Oncogene. 25:1743–1752. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Martini M, Ciraolo E, Gulluni F and Hirsch
E: Targeting PI3K in cancer: any good news? Front Oncol. 3:1082013.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Vlahos CJ, Matter WF, Hui KY and Brown RF:
A specific inhibitor of phosphatidylinositol 3-kinase,
2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J Biol
Chem. 269:5241–5248. 1994.PubMed/NCBI
|
|
15.
|
Tumaneng K, Russell RC and Guan KL: Organ
size control by Hippo and TOR pathways. Curr Biol. 22:R368–R379.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Steinhardt AA, Gayyed MF, Klein AP, et al:
Expression of Yes-associated protein in common solid tumors. Hum
Pathol. 39:1582–1589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Konsavage WM Jr, Kyler SL, Rennoll SA, Jin
G and Yochum GS: Wnt/beta-catenin signaling regulates
Yes-associated protein (YAP) gene expression in colorectal
carcinoma cells. J Biol Chem. 287:11730–11739. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Avruch J, Zhou D and Bardeesy N: YAP
oncogene overexpression supercharges colon cancer proliferation.
Cell Cycle. 11:1090–1096. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Zhou D, Zhang Y, Wu H, et al: Mst1 and
Mst2 protein kinases restrain intestinal stem cell proliferation
and colonic tumorigenesis by inhibition of Yes-associated protein
(Yap) overabundance. Proc Natl Acad Sci USA. 108:E1312–1320. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Yuen HF, McCrudden CM, Huang YH, et al:
TAZ expression as a prognostic indicator in colorectal cancer. PloS
One. 8:e542112013. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Kim SJ, Chung MJ, Kim JS, et al:
Deciphering the role of paclitaxel in the SKGT4 human esophageal
adenocarcinoma cell line. Int J Oncol. 39:1587–1591.
2011.PubMed/NCBI
|
|
22.
|
Kim SJ, Lee JS and Kim SM:
3,3′-Diindolylmethane suppresses growth of human esophageal
squamous cancer cells by G1 cell cycle arrest. Oncol Rep.
27:1669–1673. 2012.
|
|
23.
|
Eisen MB, Spellman PT, Brown PO and
Botstein D: Cluster analysis and display of genome-wide expression
patterns. Proc Natl Acad Sci USA. 95:14863–14868. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Ahmad A, Ali S, Ahmed A, et al: 3,
3′-Diindolylmethane enhances the effectiveness of herceptin against
HER-2/neu-expressing breast cancer cells. PloS One.
8:e546572013.
|
|
25.
|
Bhatnagar N, Li X, Chen Y, Zhou X, Garrett
SH and Guo B: 3,3′-diindolylmethane enhances the efficacy of
butyrate in colon cancer prevention through down-regulation of
survivin. Cancer Prev Res. 2:581–589. 2009.
|
|
26.
|
Kim YH, Kwon HS, Kim DH, et al:
3,3′-diindolylmethane attenuates colonic inflammation and
tumorigenesis in mice. Inflamm Bowel Dis. 15:1164–1173. 2009.
|
|
27.
|
Pappa G, Strathmann J, Lowinger M, Bartsch
H and Gerhauser C: Quantitative combination effects between
sulforaphane and 3,3′-diindolylmethane on proliferation of human
colon cancer cells in vitro. Carcinogenesis. 28:1471–1477.
2007.
|
|
28.
|
Li Y, Li X and Guo B: Chemopreventive
agent 3,3′-diindolylmethane selectively induces proteasomal
degradation of class I histone deacetylases. Cancer Res.
70:646–654. 2010.
|
|
29.
|
Choi HJ, Lim do Y and Park JH: Induction
of G1 and G2/M cell cycle arrests by the dietary compound
3,3′-diindolylmethane in HT-29 human colon cancer cells. BMC
Gastroenterol. 9:392009.PubMed/NCBI
|
|
30.
|
Gao N, Cheng S, Budhraja A, et al:
3,3′-Diindolylmethane exhibits antileukemic activity in vitro and
in vivo through a Akt-dependent process. PloS One.
7:e317832012.
|
|
31.
|
Kim AH, Khursigara G, Sun X, Franke TF and
Chao MV: Akt phosphorylates and negatively regulates apoptosis
signal-regulating kinase 1. Mol Cellular Biol. 21:893–901. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Alessi DR, Andjelkovic M, Caudwell B, et
al: Mechanism of activation of protein kinase B by insulin and
IGF-1. EMBO J. 15:6541–6551. 1996.PubMed/NCBI
|
|
33.
|
Harvey KF, Zhang X and Thomas DM: The
Hippo pathway and human cancer. Nat Rev Cancer. 13:246–257. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Halder G and Johnson RL: Hippo signaling:
growth control and beyond. Development. 138:9–22. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Harvey K and Tapon N: The
Salvador-Warts-Hippo pathway - an emerging tumour-suppressor
network. Nat Rev Cancer. 7:182–191. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Lu L, Li Y, Kim SM, et al: Hippo signaling
is a potent in vivo growth and tumor suppressor pathway in the
mammalian liver. Proc Natl Acad Sci USA. 107:1437–1442. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Camargo FD, Gokhale S, Johnnidis JB, et
al: YAP1 increases organ size and expands undifferentiated
progenitor cells. Curr Biol. 17:2054–2060. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Cai J, Zhang N, Zheng Y, de Wilde RF,
Maitra A and Pan D: The Hippo signaling pathway restricts the
oncogenic potential of an intestinal regeneration program. Genes
Dev. 24:2383–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Ye X, Deng Y and Lai ZC: Akt is negatively
regulated by Hippo signaling for growth inhibition in Drosophila.
Dev Biol. 369:115–123. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Cinar B, Fang PK, Lutchman M, et al: The
pro-apoptotic kinase Mst1 and its caspase cleavage products are
direct inhibitors of Akt1. EMBO J. 26:4523–4534. 2007. View Article : Google Scholar : PubMed/NCBI
|