Introduction
The human BCCIP gene has been characterized
based on its interaction with BRCA2 and p21 protein (1,2), and
its protein is an evolutionarily conserved nuclear protein with
multiple interacting domains: an N-terminus acidic domain, an
internal conserved domain, and the C-terminus variable domain
(2). The N-terminal half of BCCIP
shares moderate homology with regions of calmodulin and M-calpain,
suggesting that BCCIP may be an important cofactor for BRCA2 in
tumor suppression (2). The
BCCIP gene is alternatively spliced to produce two commonly
expressed isoforms, BCCIPα (322 aa) and BCCIPβ (314
aa) (3). Experimental evidence
indicates that both BCCIPα and BCCIPβ can interact
with p21 and BRCA2 (3–5). Although human cells express both
isoforms, BCCIPβ expression is relatively consistent in
cancer cells, whereas expression of BCCIPα varies among
cancer cell lines (2). Research
suggests that BCCIP is important in many cellular processes.
Overexpression of BCCIPβ delays G1-to-S cell cycle
progression and elevates p21 expression, suggesting that BCCIPβ can
regulate control of cell growth (6). In contrast, BCCIP protein depletion
leads reduced p21 expression and impairs G1-S checkpoint activation
in response to ionizing radiation in HT1080 cells. This regulation
of p21 expression by BCCIP depends on p53 (7). Downregulation of BCCIP in HT1080
cells results in chromosomal polyploidization, centrosome
amplification, and abnormal mitotic spindle formation, pointing to
BCCIP for the maintenance of genomic integrity (8). BCCIP may also interact in the BRCA2-
and RAD51-dependent response to DNA damage and homologous
recombinational repair (HRR) (5,9).
Recent findings suggest that BCCIP is required for mouse embryonic
development and structural stability of chromosomes (10). Collectively, this evidence
illustrates a critical role for BCCIP in fundamental cellular
processes.
Ovarian cancer, RCC and CRC are cancers with
relatively high clinical incidence rates. Ovarian cancer is the
most common cause of cancer death arising from gynecologic tumors
(11). RCC is a common
genitourinary malignancy, accounting for 3% of all cancers
worldwide (12), and CRC is a
major cause of mortality and morbidity, representing the third most
common cancer in men and the second most common cancer in women
worldwide (13). Due to early
detection difficulties, most ovarian cancers, RCC and CRC are
diagnosed at advanced stages. Various diagnostic and prognostic
biomarkers of these cancers have been identified, including
potential tumor hypoxic markers HIF1α (hypoxia inducible factor-1α)
and its regulated genes such as vascular endothelial growth factor
(VEGF) and carbonate anhydrate IX (CA9) (14–16).
However, these markers are not sufficiently specific or sensitive
to predict accurately the survival of ovarian cancer patients
(17,18). The BCCIP gene, an important
cofactor for BRCA2 in tumor suppression, is implicated in
many important cellular processes with obvious links to cancer.
Thus, we investigated whether BCCIP expression is lost in
human tumor tissues, and if so, whether the correlation between the
loss of BCCIP expression and clinicopathological features is
similar in different tumor tissues. Previously, reduction in BCCIP
protein have been reported in brain, breast and endometrial cancer
cell lines, suggesting a potential role of BCCIP in cancer etiology
(2). Although Meng et al
detected BCCIP mRNA expression in a limited number of cancer
tissues (15 kidney, 11 colon, 9 breast, 8 stomach, 7 rectal, 7
uterine, 3 prostate, 3 lung, 3 ovarian, 1 cervical and 1 small
intestine) (3), the role of BCCIP
protein and the relationship between BCCIP expression and
the clinico pathological features of cancers have not been
reported. Here, we measured BCCIP mRNA and protein expression in
multiple primary ovarian cancer, RCC and CRC cases using qPCR, WB
and IHC. In addition, we analyzed the relationship between
BCCIP gene expression and the clinicopathological features
of these three cancer types.
Materials and methods
Antibodies and tissue collection
Anti-BCCIP (16043-1-AP) polyclonal antibody was
purchased from Protech Group (Wuhan, China). Rabbit polyclonal
anti-GAPDH was raised against bacterially expressed proteins (Jilin
University, Changchun, China). Human clinical tumor tissues
(ovarian cancer, RCC and CRC) and normal tissues were collected
from patients with primary ovarian cancers between January 2010 and
July 2012, from patients with primary RCC between May 2009 and May
2012, and primary CRC cases between July 2012 and March 2013.
Cancer patients underwent radical tumor surgery at the First
Hospital of Jilin University. The study was approved by the Ethics
Committee of the First Hospital of Jilin University and all
patients gave informed consent for the use of their tissue samples.
Patient medical records including patient age and gender, tumor
staging, pathological diagnosis and surgical records were reviewed.
Tumors were staged according to the 2010 TNM classification system
using the American Joint Committee on Cancer (AJCC) stage grouping
(19). No patients received
chemotherapy or radiotherapy before surgery.
Reverse transcription PCR (RT-PCR)
Total RNA from tumor (ovarian cancer, RCC or CRC) or
normal tissues were isolated using TRIzol LS Reagent (Invitrogen).
Then, 1 μg of total RNA from each sample was used as a template to
produce cDNA with the PrimeScript 1st Strand cDNA Synthesis Kit
(Takara). BCCIP and GAPDH mRNA were measured by PCR using a C1000™
Thermal Cycler (Bio-Rad) and quantitative real-time PCR with an Eco
Real-Time PCR System (Illumina). All PCR reactions were finished as
follows: initial denaturation step at 95°C for 30 sec, followed by
40 cycles of denaturation at 95°C for 5 sec, annealing at 60°C for
30 sec and extension at 72°C for 30 sec. Primer sets used for PCR
were as follows: GAPDH, 5′-ATCACTGCCACCCAGA AGAC-3′ (forward) and
5′-ATGAGGTCCACCACCCTGTT-3′ (reverse), yielding a 460-bp product;
BCCIP, 5′-TCAAGA GTTGGTTCTACGCTTC-3′ (forward) and 5′-CATGGG
CAGAGCGATCTGT-3′ (reverse), yielding a 110-bp product.
Western blot analysis
Cancer tissue or normal tissue samples (200 mg) were
homogenized with liquid nitrogen and solubilized in 200 μl cold PBS
containing 1.0% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS,
0.05 mM PMSF and protease inhibitor cocktail. The homogenate was
swirled and kept on ice for 30 min. Whole-cell extracts were
sonicated (Scientz-IID, Zhejiang, China) for 10 sec with 50% duty
cycle and centrifugation (13,400 × g for 30 min). The protein
concentration of the resulting supernatant was measured using the
Bio-Rad Protein Assay kit (500-0201). Equal amounts of protein from
tissue whole-cell lysates were mixed with 4X SDS-containing sample
buffer and boiled for 5 min at 95°C. Denatured proteins were then
separated by 12% SDS-PAGE. Specific proteins were detected by WB
using BCCIP and GAPDH polyclonal antibodies.
IHC staining
Formalin-fixed and paraffin-embedded ovarian cancer
and CRC tissue blocks were obtained from the First Clinical
Hospital of Jilin University. Tissue blocks were sectioned and
deparaffinized in xylene and rehydrated through a graded ethanol
series. Tissue slides were then subjected to antigen retrieval by
boiling in 0.01 M sodium citrate buffer (pH 6.0) in a microwave
oven for 10 min. Endogenous peroxidase was blocked by incubation
for 10 min in 3% hydrogen peroxide in methanol. Finally, the
reactions were detected using a DAB detection kit (Dako).
Anti-BCCIP (16043-1-AP) polyclonal antibody was used at a 1:500
dilution.
Statistical analysis
Differences in gene and protein expression between
tumor and normal tissues were statistically analyzed using SPSS
17.0 (SPSS Inc., Chicago, IL). Statistical comparisons were
analyzed using the Student's t-test. Values of p<0.05 were
considered to be statistically significant.
Results
BCCIP expression and clinicopathological
features of ovarian cancer
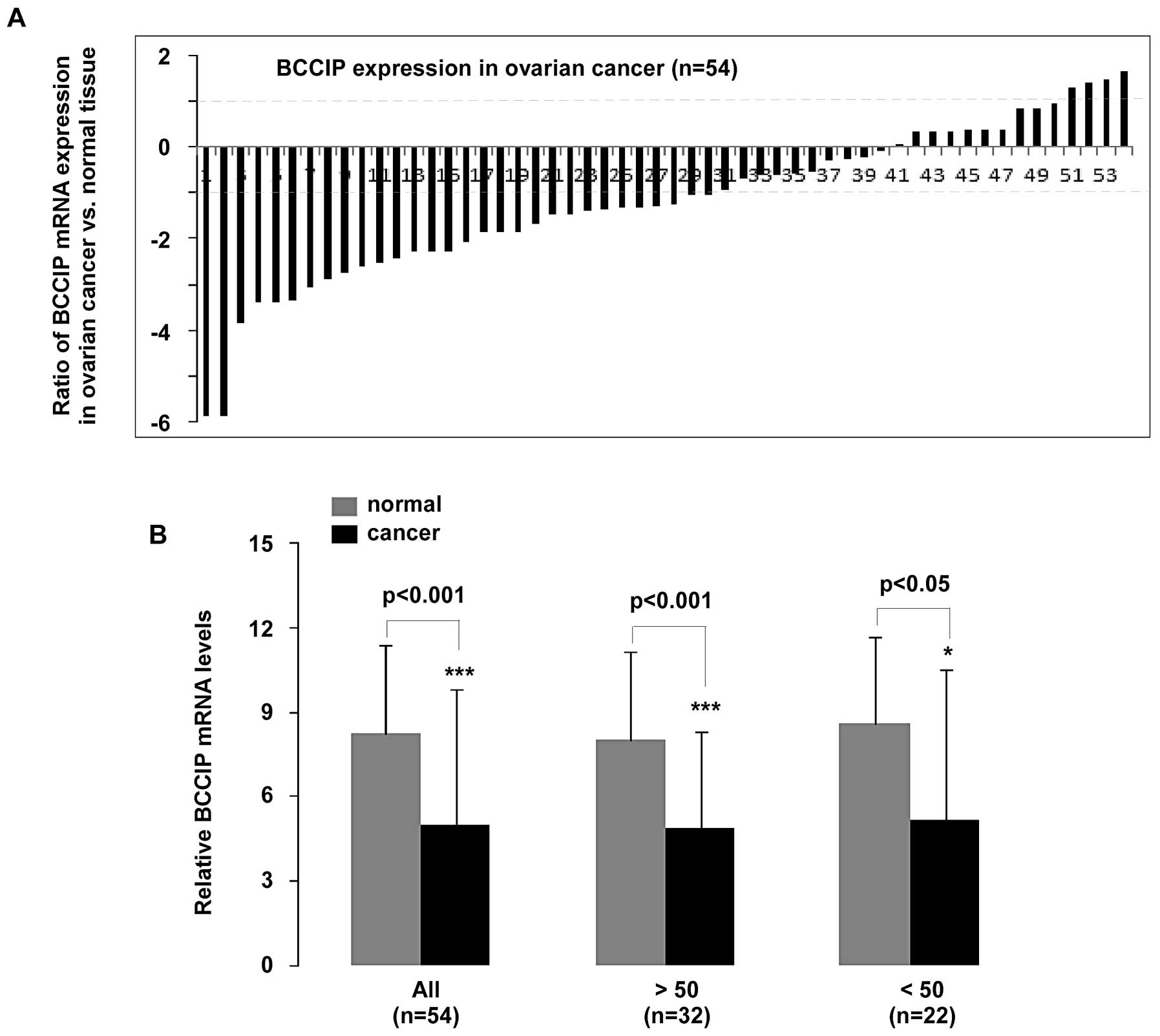
To understand BCCIP expression in the
pathogenesis of primary ovarian cancer, we measured BCCIP
mRNA using qPCR in 54 patients diagnosed with ovarian cancer.
Patients aged >50 years accounted for 32 cases, and the
remaining 22 cases were <50. Statistical analysis of qPCR data
revealed that BCCIP expression was significantly decreased
in ovarian cancer compared to normal tissues (p<0.001, n=54).
Moreover, BCCIP expression in patients >50 or <50
years was reduced (p<0.001 and p<0.05, respectively)
(Fig. 1B). As shown in Fig. 1A, mRNA expression in the 54 samples
was significantly downregulated (>2-fold decrease) in 56%
(30/54) of tissue samples, whereas 7% (4/54) of samples had
significantly upregulated (>2-fold increase) BCCIP. In
addition, a <2-fold reduction in BCCIP expression was
observed in 19% (10/54) of the ovarian cancer tissue samples.
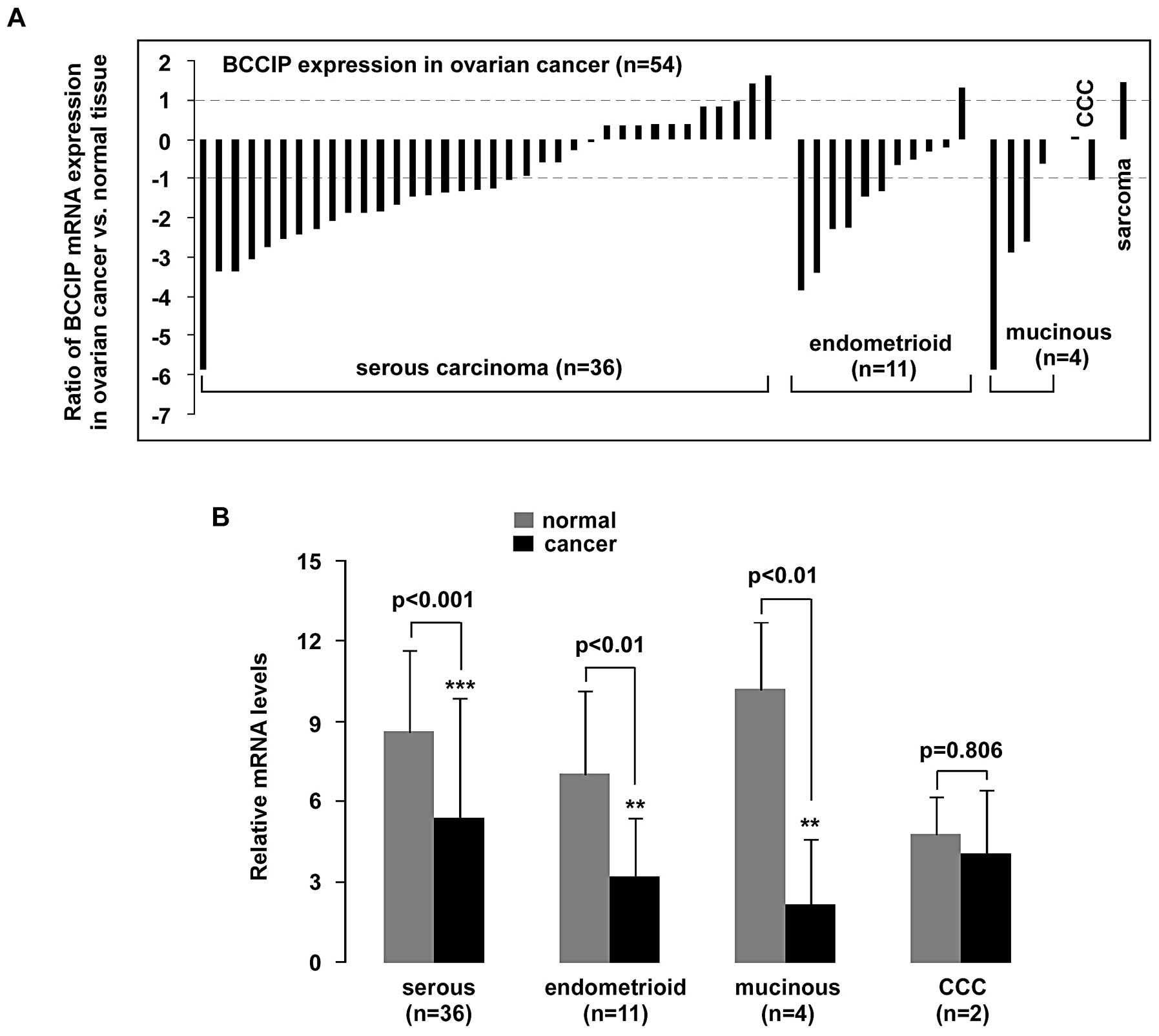
Cell subtypes were assigned according to refined
World Health Organization (WHO) criteria (20) as recently described (21). A significant (>2-fold decrease)
downregulation of BCCIP mRNA in 56% (20/36) of serous
carcinoma samples, in 55% (6/11) of endometrioid carcinoma samples
(n=11) and in 75% (3/4) of mucinous carcinoma samples (n=4) was
observed (p<0.001, p<0.01 and p<0.01, respectively). Over
2-fold elevation of BCCIP was detected in only one sarcoma
case (Fig. 2).
Next, BCCIP expression and tumor stage were
correlated (Table I).
Significantly low expression of BCCIP was observed in pT1-
and pT3-stage serous tumors (p<0.05 and p<0.001,
respectively), and in pT1- and pT2-stage endometrioid carcinomas
(p<0.05 and p<0.05, respectively). However, BCCIP
downregulation was not observed in the T2 stage of serous carcinoma
(p=0.127).
 | Table I.Relationship between BCCIP expression
and pathological diagnosis of ovarian cancer. |
Table I.
Relationship between BCCIP expression
and pathological diagnosis of ovarian cancer.
| Pathological
diagnosis | Average age | No. of cases
(n) | BCCIP mRNA (qPCR)
(average ± SD)
| P-value (normal vs.
cancer) |
|---|
| Normal | Cancer |
|---|
| Serous
carcinoma | 50 | 36 | 8.59±3.03 | 5.39±4.48 | 0.00083a |
| pT1 stage | 49.3 | 6 | 10.2±3.43 | 5.31±3.42 | 0.0482b |
| pT2 stage | 50.5 | 22 | 7.57±2.91 | 5.55±5.17 | 0.127 |
| pT3 stage | 49.5 | 8 | 10.2±1.35 | 5.05±2.81 | 0.000611a |
| Endometrioid
carcinoma | 42.5 | 11 | 7.05±3.09 | 3.19±2.18 | 0.00425c |
| pT1 stage | 48.8 | 5 | 8.98±2.33 | 4.69±2.15 | 0.0268b |
| pT2 stage | 37.3 | 6 | 5.45±2.72 | 1.94±1.22 | 0.0249b |
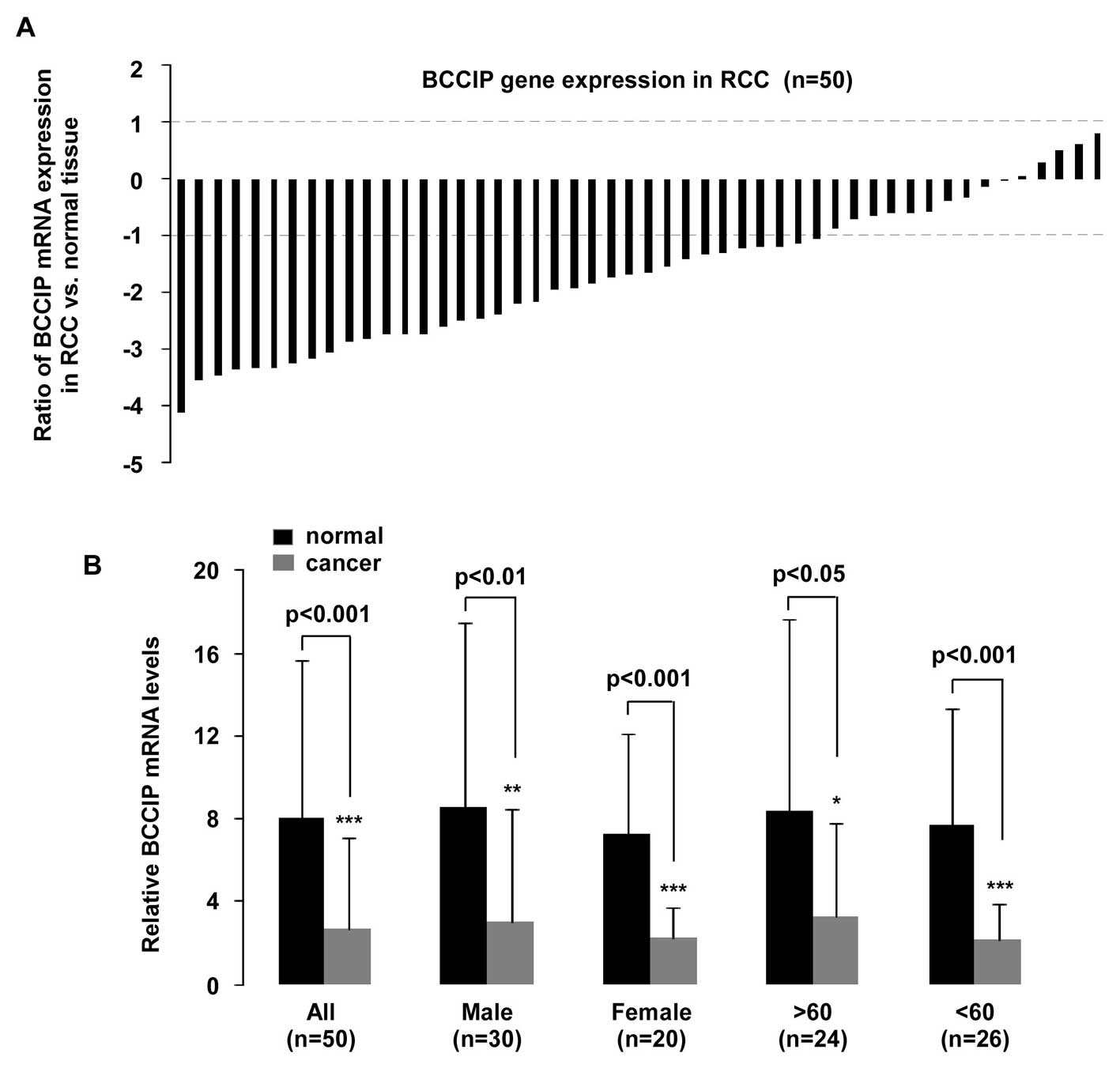
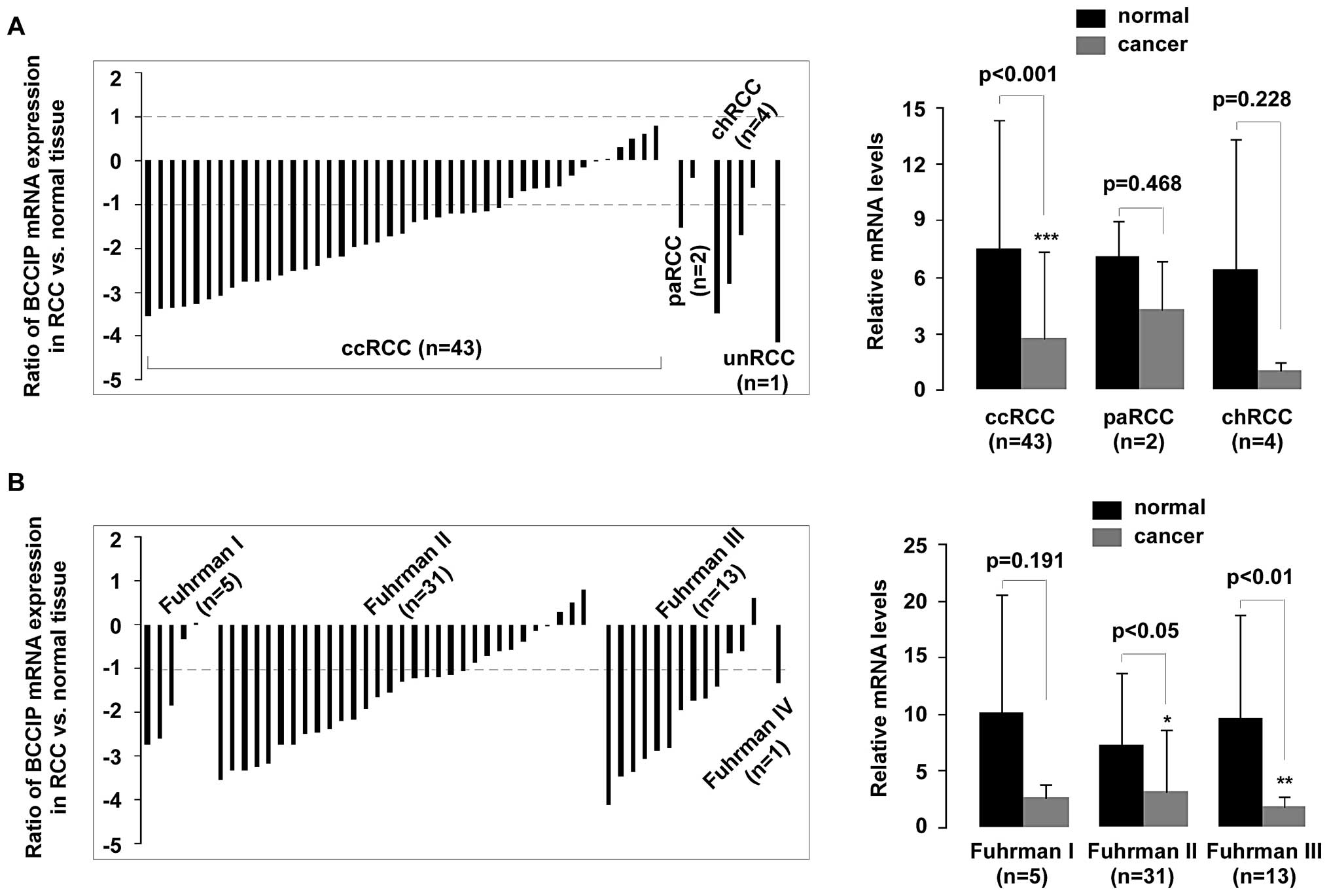
BCCIP expression in ccRCC and Fuhrman
grading.
BCCIP expression was measured in 50 samples.
Histological subtypes were classified as clear cell RCC (ccRCC),
papillary RCC (paRCC), chromophobe RCC (chRCC) and unclassified RCC
(unRCC) according to previous WHO classifications (22). qPCR revealed that BCCIP gene
expression was downregulated in RCC (p<0.001, n=50; Fig. 3B). A significant (>2-fold
decreased) reduction of BCCIP expression was detectable in 70%
(35/50) of the RCC samples, and a <2-fold reduction of BCCIP
mRNA was observed in 18% (9/50) of the RCC samples. Although BCCIP
expression was upregulated in 10% (5/50) of samples, the
fold-change of all RCC samples was less than 2-fold. In addition,
one sample was not different with respect to BCCIP expression,
compared to normal tissues (Fig.
3A).
BCCIP expression and tissue age/gender was
investigated (Fig. 3B). A
statistically significant reduction of BCCIP expression was
observed in tissues from males (n=30, p<0.01), females (n=20,
p<0.001), in tissue from persons >60 years of age (n=24,
p<0.05) and <60 years (n=26, p<0.001), compared with
normal tissues. Furthermore, mRNA expression in the 50 RCC samples
was compared to the histological tumor type and Fuhrman grading.
Downregulation of BCCIP expression in RCC was strongly associated
with ccRCC (p<0.001), but no significant difference was observed
in paRCC (p=0.468) and chRCC (p=0.228), compared with normal
tissues (Fig. 4A). Fuhrman nuclear
grading is the most widely used and most predictive grading system
for RCC, and this scale was used to assess the nuclear grade in
each RCC sample (23). The
correlation between BCCIP expression and tumor Fuhrman grading is
shown in Fig. 4B. Low BCCIP
expression in RCC was strongly associated with Fuhrman grading. A
significant reduction (>2-fold decrease) of BCCIP mRNA occurred
in 68% (21/31) of Fuhrman grade II samples and in 77% (10/13) of
Fuhrman grade III samples (p<0.05 and p<0.01,
respectively).
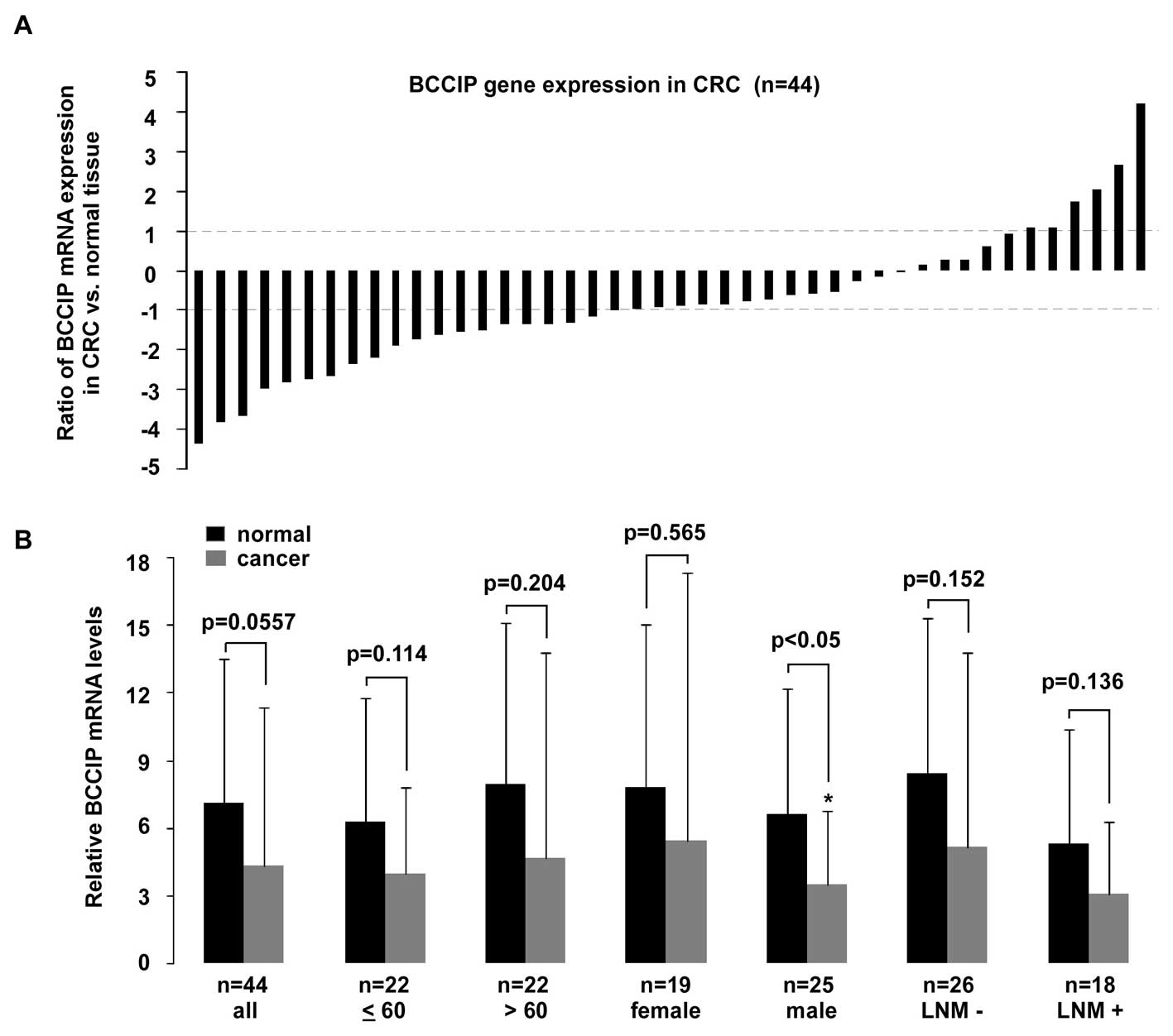
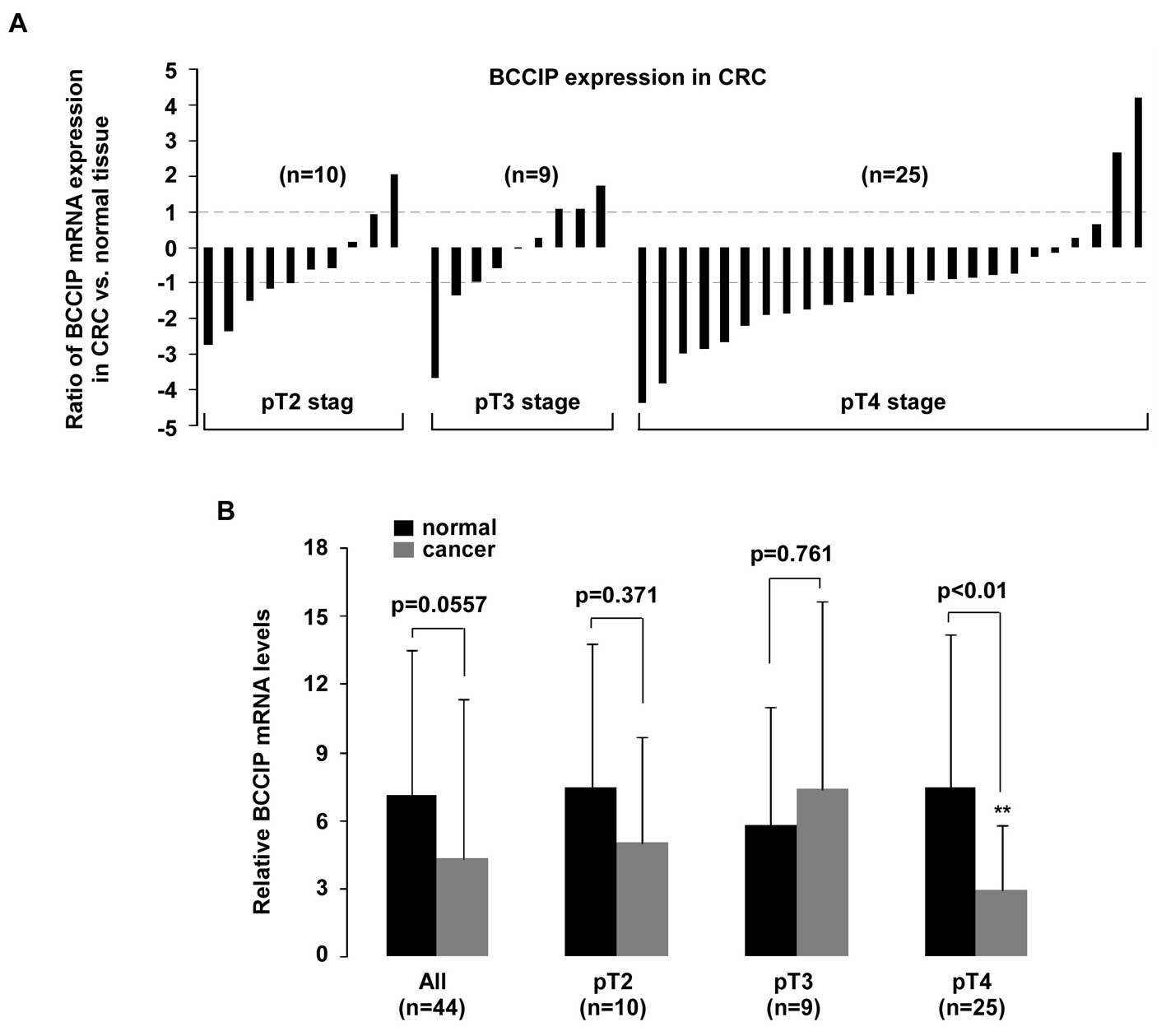
BCCIP expression in male and pT4 CRC
tumors
Compared with the previous tumor tissues studied
BCCIP expression in CRC is not as remarkable. When compared with
age- and gender-matched normal tissues, BCCIP expression in CRC
tissues decreased, but this was not statistically significant
(p=0.0577) between cancer and normal tissues (Fig. 5B). A >2-fold reduction of BCCIP
expression was observed in 45% (20/44) of CRC tissues, and a
<2-fold reduction of BCCIP mRNA was observed in 29% (13/44) of
tissues. In contrast, a >2-fold elevation of BCCIP expression
was detectable in 14% (6/44) of tissues, whereas a <2-fold
overexpression of BCCIP mRNA was present in 11% (5/44) of tissues
(Fig. 5A). As shown in Fig. 5B, BCCIP expression in tissues from
male patients with CRC was significantly decreased (p<0.05), but
no statistically significant difference existed between tumor and
normal tissues in other CRC tissue groups compared. After analysis
for TNM tumor stage (Fig. 6), a
significant reduction of BCCIP mRNA was only observed in pT4 CRC
tumors (p<0.01).
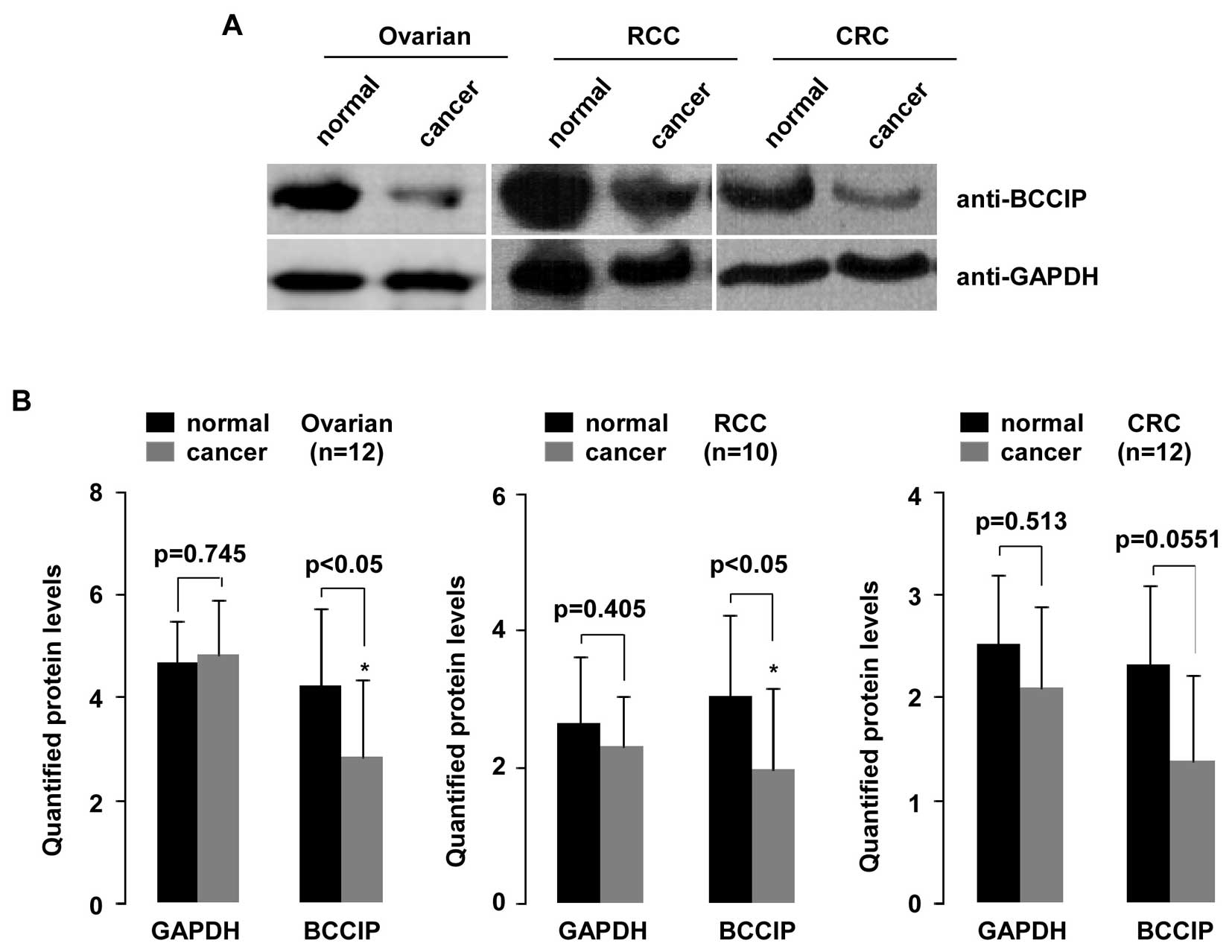
BCCIP protein in ovarian cancer, RCC and
CRC
To investigate reduction of BCCIP expression
and potentially decreased BCCIP protein, we randomly selected
samples from ovarian cancer tissue (n=12), RCC tissue (n=10) and
CRC tissue (n=12) and compared these to normal tissues (Fig. 7). As shown in the upper panel,
aliquots of whole-cell extract from all tissues were analyzed by WB
using indicated antibodies. Quantified protein was analyzed with
the Student's t-test (Quantity One software). As shown in Fig. 7B, BCCIP protein expression was
significantly decreased in ovarian cancer and RCC tissues compared
with their matched normal tissues (p=0.0404 and p=0.0421,
respectively). Although BCCIP protein in CRC tissues decreased,
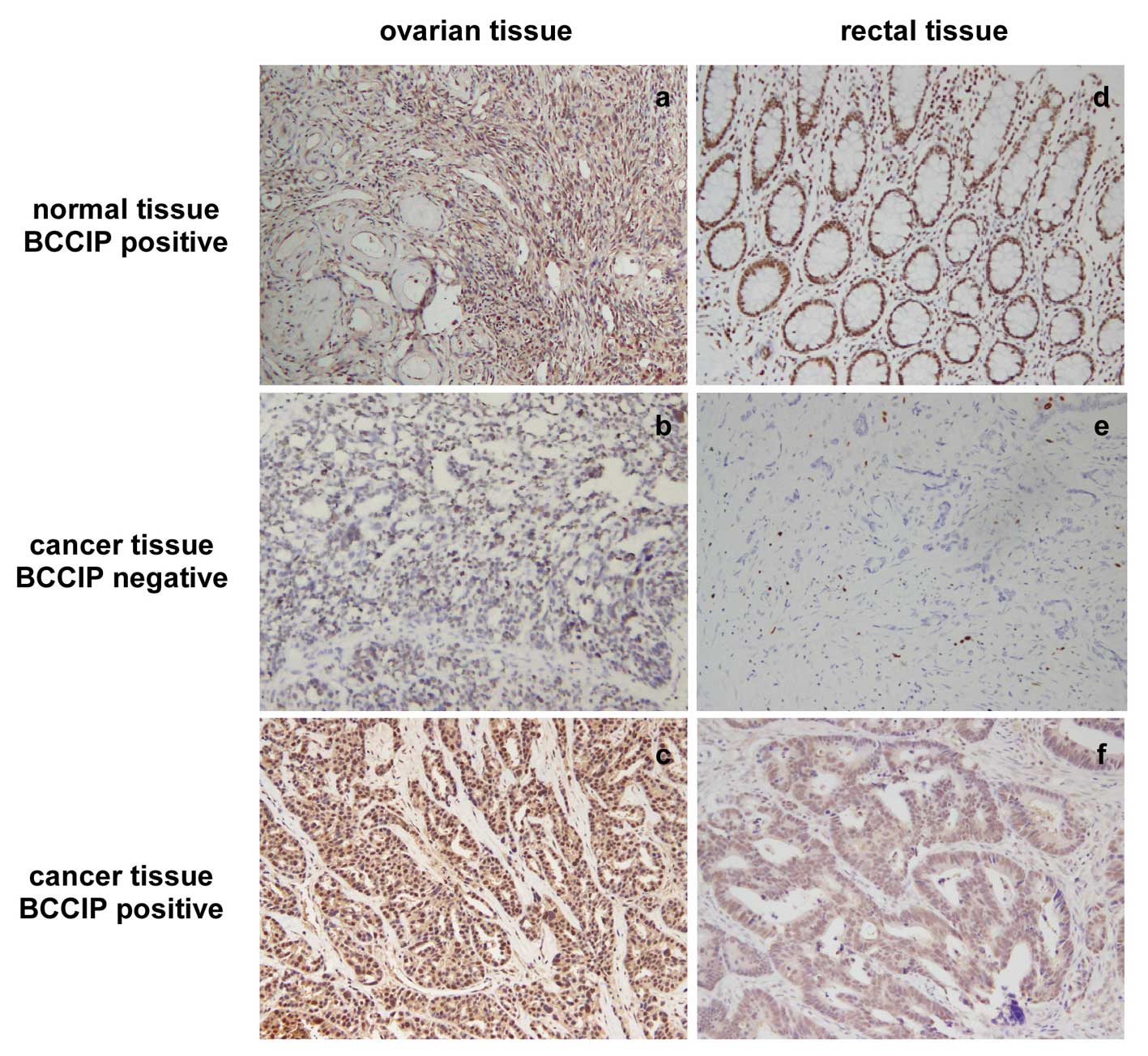
this was not statistically significant. To confirm this
observation, we performed IHC for BCCIP in formalin-fixed
paraffin-embedded tissue sections from 20 ovarian cancer tissues
and 34 CRC tissues. Data show that BCCIP protein was reduced (score
0–1 for BCCIP) in 70% (14/20) of ovarian cancer samples and in 35%
(12/34) of CRC samples. IHC staining for BCCIP in ovarian cancer
and CRC is shown in Fig. 8.
Negative (middle) or positive (bottom) staining of BCCIP in ovarian
carcinoma and CRC paraffin-embedded tissue sections are
depicted.
Discussion
The human BCCIP gene, located at 10q26 (1) has been implicated in many cellular
processes such as cell cycle regulation, DNA recombination, DNA
repair, telomere maintenance, embryonic development and genomic
stability (6–10). These data strongly suggest a role
for BCCIP in tumor development and progression. BCCIP has been
implicated in several forms of human tumors such as astrocytic
brain tumors (24,25). Here, we report data regarding
differential BCCIP gene expression in tumor tissues
including ovarian cancer (n=54), RCC (n=50) and CRC (n=44) by qPCR.
BCCIP protein was measured using WB and IHC staining. Statistical
analysis of experimental results revealed a significant reduction
of BCCIP expression in ovarian cancer (p<0.001) and RCC tissues
(p<0.001) compared with normal tissues. BCCIP expression in CRC
tissues was decreased, but this was not statistically significant
between cancer and normal tissues (p=0.0577).
Although the functions of BCCIP in tumor formation
and progression are unclear, that its interaction with BRCA2 and
p21 suggests a potential critical role in cancer pathogenesis.
Genomic instability is a major driving force in tumor progression
and tumor suppressor gene BRCA2 participates in the regulation of
chromosomal stability, with respect to chromo-some structure and
number (26–28). BCCIP, as a BRCA2 and p21
interacting protein, co-localizes with BRCA2 on the chromatin
fraction and contributes to BRCA2 and RAD51 nuclear focus formation
(5). Furthermore, BCCIP is
implicated in homologous recombination and cell cycle regulation
(9,29). These roles support the idea of
BCCIP in the maintenance of genomic stability that may be involved
in tumorigenesis. BCCIPα protein was reduced in the 27 cancer cell
lines in 3/9 brain tumor cell lines, 3/7 breast cancer cell lines
and 2/4 endometrial tumor cell lines (3). In contrast, overexpression of BCCIPα
moderately inhibits cell growth in certain brain and breast cancers
(3), so BCCIP may be important for
various types of tumor development. Liu et al (30) reported that BCCIP expression was
not detectable in ∼45% of all astrocytic tumors and in >60% of
grade IV glioblastomas. Here, we found a significant (>2-fold
decreased) downregulation of BCCIP in 56% (30/54) of ovarian cancer
tissue samples, 70% (35/50) RCC tissues and 45% (20/44) CRC
tissues. A statistically significant reduction of BCCIP expression
was detected in all tissue samples of serous, endometrioid, and
mucinous carcinomas, and the greatest reduction of BCCIP was
detected in pT3 serous carcinomas tumors (p<0.001). In addition,
BCCIP was observed in RCC samples, data which support previous
findings in 15 kidney tumor cases (3). The relationship between BCCIP
expression and RCC clinicopathological features suggested that
downregulation of BCCIP is strongly correlated with ccRCC. Also,
low expression of BCCIP is closely related to Fuhrman tumor grading
(Fig. 4B). BCCIP expression
decreased in CRC tissues but this was not statistically significant
(p=0.0577; n=44). Only male CRC tissues (p<0.05) and CRC tissues
(p<0.01) from pT4 tumors had a statistically significant
difference in BBCIP. Western blot analysis and IHC revealed that
BCCIP protein is also significantly reduced in ovarian cancer and
RCC. These data, along with published literature confirm that BCCIP
may be common in cancer tumors and may represent a novel biomarker
for tumor diagnosis.
In conclusion, a significant reduction of BCCIP
expression in ovarian cancer and RCC tissues compared with normal
tissues was found. BCCIP expression in CRC tissues was
decreased, but this was not statistically significant between
cancer and normal tissues. Our data suggest a role for the
BCCIP in the pathogenesis of these cancers.
Abbreviations:
|
BCCIP
|
BRCA2 and CDKN1A interacting
protein
|
|
RCC
|
renal cell carcinoma
|
|
CRC
|
colorectal cancer
|
|
qPCR
|
quantitative real-time PCR
|
|
RT-PCR
|
reverse transcription polymerase chain
reaction
|
Acknowledgements
This study was supported by National
Natural Science Foundation of China (31071131, Y.C.), and in part
by the National Laboratory of Biomacromolecules, Institute of
Biophysics, Chinese Academy of Sciences (2012kf04, J.J. and
O5SY02110A, Y.C.).
References
|
1.
|
Ono T, Kitaura H, Ugai H, Murata T,
Yokoyama KK, Iguchi-Ariga SM and Ariga H: Tok-1, a novel
p21Cip1-binding protein that cooperatively enhances
p21-dependent inhibitory activity toward CDK2 kinase. J Biol Chem.
275:31145–31154. 2000.PubMed/NCBI
|
|
2.
|
Liu J, Yuan Y, Huan J and Shen Z:
Inhibition of breast and brain cancer cell growth by BCCIPα, an
evolutionarily conserved nuclear protein that interacts with BRCA2.
Oncogene. 20:336–345. 2001.PubMed/NCBI
|
|
3.
|
Meng X, Liu J and Shen Z: Genomic
structure of the human BCCIP gene and its expression in cancer.
Gene. 302:139–146. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Fan J, Wray J, Meng X and Shen Z: BCCIP is
required for the nuclear localization of the p21 protein. Cell
Cycle. 8:3019–3024. 2009.PubMed/NCBI
|
|
5.
|
Lu H, Guo X, Meng X, Liu J, Allen C, Wray
J, Nickoloff JA and Shen Z: The BRCA2-interacting protein BCCIP
functions in RAD51 and BRCA2 focus formation and homologous
recombinational repair. Mol Cell Biol. 25:1949–1957. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Meng X, Liu J and Shen Z: Inhibition of G1
to S cell cycle progression by BCCIPβ. Cell Cycle. 3:343–348.
2004.PubMed/NCBI
|
|
7.
|
Meng X, Lu H and Shen Z: BCCIP functions
through p53 to regulate the expression of p21Waf1/Cip1.
Cell Cycle. 3:1457–1462. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Meng X, Fan J and Shen Z: Roles of BCCIP
in chromosome stability and cytokinesis. Oncogene. 26:6253–6260.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Wray J, Liu J, Nickoloff JA and Shen Z:
Distinct RAD51 associations with RAD52 and BCCIP in response to DNA
damage and replication stress. Cancer Res. 68:2699–2707. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Lu H, Huang YY, Mehrotra S, Droz-Rosario
R, Liu J, Bhaumik M, White E and Shen Z: Essential roles of BCCIP
in mouse embryonic development and structural stability of
chromosomes. PLoS Genet. 7:e10022912011. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics. CA Cancer J Clin. 62:10–29. 2012.
|
|
12.
|
Jemal A, Siegel E, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics. CA Cancer J Clin. 57:43–66.
2007.
|
|
13.
|
Sameer AS: Colorectal cancer: molecular
mutations and polymorphisms. Front Oncol. 3:1142013. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Schwab LP, Peacock DL, Majumdar D, Ingels
JF, Jensen LC, Smish KD, Cushing RC and Seagroves TN:
Hypoxia-inducible factor 1α promotes primary tumor growth and
tumor-initiating cell activity in breast cancer. Breast Cancer Res.
14:R62012.
|
|
15.
|
McDonald PC, Winum JY, Supuran CT and
Dedhar S: Recent developments in targeting carbonate anhydrase IX
for cancer therapeutics. Oncotarget. 3:84–97. 2012.PubMed/NCBI
|
|
16.
|
Law AY and Wong CK: Stanniocalcin-2 is a
HIF-1 target gene that promotes cell proliferation in hypoxia. Exp
Cell Res. 316:466–476. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Choschzick M, Oosterwijk E, Müller V,
Woelber L, Simon R, Moch H and Tennstedt P: Overexpression of
carbonic anhydrase IX (CAIX) is an independent unfavorable
prognostic marker in endometrioid ovarian cancer. Virchows Arch.
459:193–200. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Seeber LM, Horrée N, Vooijs MA, Heintz AP,
van der Wall E, Verheijen RH and van Diest PJ: The role of hypoxia
inducible factor-1 alpha in gynecological cancer. Crit Rev Oncol
Hematol. 78:173–184. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. 7th edition.
Springer; Chicago, IL: 2010
|
|
20.
|
Tavassoli FA and Devilee P: Patholology
and genetics Tumors of the breast and female genital organs. World
Health Organization Classification of Tumors. IARC Press; Lyon:
2003
|
|
21.
|
Gilks CB, Ionescu DN, Kalloger SE, Köbel
M, Irving J, Clarke B, Santos J, Le N, Morravan V and Swenerton K:
Tumor cell type can be reproducibly diagnosed and is of independent
prognostic significance in patients with maximally debulked ovarian
carcinoma. Hum Pathol. 39:1239–1251. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Mostofi FK: Histological typing of kidney
tumors. World Health Organization International Classification of
Tumors. Springer-Verlag; New York, NY: 1998
|
|
23.
|
Fuhrman SA, Lasky LC and Limas C:
Prognostic significance of morphologic parameters in renal cell
carcinoma. Am J Surg Pathol. 6:655–663. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Merlo A: Genes and pathways driving
glioblastomas in humans and murine disease models. Neurosurg Rev.
26:145–158. 2003.PubMed/NCBI
|
|
25.
|
Ohgaki H, Dessen P, Jourde B, Horstmann S,
Nishikawa T, Di Patre PL, Burkhard C, Schüler D, Probst-Hensch NM,
Maiorka PC, Baeza N, Pisani P, Yonekawa Y, Yasargil MG, Lütolf UM
and Kleihues P: Genetic pathways to glioblastoma: a
population-based study. Cancer Res. 64:6892–6899. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Powell SN and Kachnic LA: Roles of BRCA1
and BRCA2 in homologous recombination, DNA replication fidelity and
the cellular response to ionizing radiation. Oncogene.
22:5784–5791. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Daniels MJ, Wang Y, Lee M and Venkitaraman
AR: Abnormal cytokinesis in cells deficient in the breast cancer
susceptibility BRCA2. Science. 306:876–879. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Rudkin TM and Foulkes WD: BRCA2: breaks,
mistakes and failed separations. Trends Mol Med. 11:145–148. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Lu H, Yue J, Meng X, Nickoloff JA and Shen
Z: BCCIP regulates homologous recombination by distinct domains and
suppresses spontaneous DNA damage. Necleic Acids Res. 35:7160–7170.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Liu J, Lu H, Ohgaki H, Merlo A and Shen Z:
Alterations of BCCIP, a BRCA2 interacting protein, in astrocytomas.
BMC Cancer. 9:2682009. View Article : Google Scholar : PubMed/NCBI
|