Introduction
There is currently no effective therapy against
recurrent tumors, which may occur after surgical treatment of the
primary tumor. Thus, suppression of tumor recurrence in cancer
patients is important. It was recently reported that gastric cancer
patients receiving postoperative adjuvant chemotherapy showed a
5.8% increase in their 5-year overall survival compared to those
who received surgery alone (1).
This indicates the significance of adjuvant chemotherapy and also
suggests the necessity for a more powerful adjuvant therapy to
resist tumor recurrence.
CD8+ cytotoxic T lymphocytes (CTLs) are a
major component of antitumor immune responses and CTL responses
have been shown to be effective in eliminating tumors in animal
models (2). Thus, tumor
immunotherapy that induces tumor-reactive CTLs is a good approach
to investigate because CTLs are expected to disperse throughout the
whole body in a cancer patient and destroy tumor cells if the tumor
cells exist in the patient. Short peptides and proteins recognized
by CD8+ T cells have been used as cancer vaccines
(3); however, alone, these
antigens elicit weak antitumor immune responses in vivo and
the efficacy of the therapies has shown only modest benefit so far
in clinical studies (4). Previous
studies have also shown that vaccination with peptides recognized
by CD8+ T cells caused tolerance against tumors, so that
the tumor growth was enhanced (5–7).
These studies suggest the limitation of traditional approaches for
stimulating CD8+ T cells with peptides containing only
killer epitopes.
Several studies have shown that CD4+ T
cells also play a critical role in the development of a therapeutic
antitumor immune response (8–10).
In addition, CD4+ T cells are essential for generating
CD8+ T memory cells (11,12).
Therefore, autologous tumor tissue surgically removed from a
patient should be suitable to use as a source of cancer vaccine for
suppressing tumor recurrence in the patient, because, unlike
peptide-based vaccines, tumor cells should contain all potential
MHC class I and MHC class II epitopes capable of stimulating
CD8+ and CD4+ T cells, respectively. However,
in some experiments, when tumor lysates were employed as vaccines,
dendritic cells (DCs) often failed to function efficiently in
tumor-bearing hosts due to pronounced immune suppression (13–15).
Tumor cells are also known to alter expression of DC cell-surface
molecules (e.g., MHC class II, CD116), decrease migration to
draining lymph nodes and suppress cytokine release (16–18).
Therefore, development of a new effective adjuvant that will
improve the effectiveness of a cancer vaccine based on tumor
lysates and stimulate Th1-type antitumor immunity represents a
potential new therapeutic tool to prevent tumor recurrence.
Baculovirus Autographa californica multiple
nuclear polyhedrosis virus, which has a 130-kb double-stranded
circular DNA genome, is pathogenic for insects of Lepidoptera.
Baculovirus has been widely used as a biopesticide (19,20)
and as a tool in recombinant protein-expression systems (21,22).
The virus can infect a range of mammalian cell types, but it does
not replicate in the cells; this property leads to recombinant
baculoviruses with a mammalian expression promoter that can be used
as a tool for gene therapy (23–27).
It was recently shown that baculovirus strongly stimulated the
production of cytokines such as type I interferon, tumor necrosis
factor-α and interleukin (IL)-1 in mammalian cells (28,29).
It was also demonstrated that intravenous injection of mice with
baculovirus markedly activated natural killer (NK) cells, resulting
in the induction of antitumor immunity (30). In addition, intratumoral
inoculation with DCs that were stimulated in vitro with
baculovirus suppressed tumor growth in mouse models (31,32).
These reports indicate that baculovirus efficiently activates
innate immunity in mice. The next step in the study of the
baculovirus adjuvant effect is to investigate whether or not
baculovirus-stimulated innate immunity can lead to the induction of
antigen-specific acquired immunity.
In the present study, we focus on the feasibility of
baculovirus as an adjuvant for cancer immunotherapy. We prepared a
tumor vaccine consisting of baculovirus and tumor cell lysate and
examined whether or not intradermal (i.d.) inoculation with the
combined baculovirus and tumor cell lysate vaccine effectively
induces tumor-specific acquired immunity in a mouse model.
Materials and methods
Mice and cell lines
Four-week-old female BALB/c mice (Nippon SLC,
Shizuoka, Japan) were used in a P2 level animal facility at Chiba
Institute of Technology, Chiba, Japan. The study was conducted in
the experimental animal area under the guidance of an institutional
committee for biosafety and animal experiments. Spodoptera
frugiperda (Sf-9) cells were cultured at 28°C in Sf-900 II
medium (Invitrogen, Carlsbad, CA, USA). CT26 murine colon carcinoma
cell line was purchased from American Type Culture Collection
(ATCC; Manassas, VA, USA) and maintained in RPMI-1640 (Wako Pure
Chemical Industries, Osaka, Japan) supplemented with 10% fetal calf
serum (Invitrogen), 100 U/ml penicillin and 100 μg/ml
streptomycin (Sigma-Aldrich, St. Louis, MO, USA).
Purification of wild-type
baculovirus
Wild-type baculovirus was purchased from BD
Biosciences (San Jose, CA, USA) and propagated in Sf-9 cells.
Baculovirus was purified as previously described (31) and the virus titer was determined
using a plaque assay.
Preparation of vaccines
To prepare tumor cell lysate, CT26 cells were
divided into 1.5 ml tubes (1×106/50 μl PBS/tube)
and the tubes were treated with 5 freeze-thaw cycles using liquid
nitrogen and a 37°C water bath. The lysates were stored at −80°C
until use. To prepare combined baculovirus and tumor cell lysate
vaccine, baculovirus [1×108 plaque forming units (pfu)]
was added into the 1.5-ml tube described above and stored on ice
until administration. One tube was used per individual mouse
(1×106 cell lysate plus 1×108 pfu
baculovirus/mouse). As controls, CT26 cell lysate alone or
baculovirus alone was also prepared.
Immunization with vaccines
CT26 cell lysate alone, baculovirus alone, or
combined CT26 cell lysate and baculovirus were inoculated
intradermally into the upper right flank of mice. Each immunization
was performed once per week for three consecutive weeks. Seven days
after the final vaccination, the CT26 cells (5×104) were
transplanted subcutaneously in the lower right flank of the
immunized mice. Tumor volume was measured using a slide caliper
according to the following formula: tumor volume (mm3) =
length × (width)2 /2. Mice were monitored twice weekly
for tumor growth and survival.
In vitro cytotoxicity assay
As effector cells, splenocytes were isolated from
mice immunized with PBS, tumor cell lysate alone, baculovirus
alone, or the combined baculovirus and tumor cell lysate 7 days
after the final vaccination. CT26 cells (1×104) were
used as target cells. The effector cells were co-cultured with the
target cells at ratios of 50:1, 25:1 and 12.5:1 for 8 h. The
cytolytic activity was assessed using the CytoTox 96
Non-Radioactive Cytotoxicity Assay kit (Promega, Madison, WI, USA),
according to the manufacturer’s instructions.
FITC-dextran or tumor cell lysate uptake
by DCs
To prepare murine DCs, bone marrow cells were
harvested from the tibiae and femurs of BALB/c mice and depleted of
red blood cells using red blood cell lysis buffer (Sigma-Aldrich).
Bone marrow cells were cultured in RPMI-1640 medium containing 10%
FCS, 100 U/ml penicillin, 100 μg/ml streptomycin and 50
μM 2-mercaptoethanol (Invitrogen), supplemented with 20
ng/ml of murine granulocyte-macrophage colony-stimulating factor
(GM-CSF) (Miltenyi Biotech, Bergisch Gladbach, Germany). On days 3
and 6, the culture medium was replaced with fresh medium
supplemented with GM-CSF. On day 8, non-adherent cells were
collected and positively selected with anti-mouse CD11c microbeads
(Miltenyi Biotech).
To assess how baculovirus affects the ability of DCs
to capture an antigen, DCs (5×105) were suspended in
culture medium and FITC-dextran (MW=40,000) (Sigma-Aldrich) was
added at a final concentration of 1 mg/ml, with or without
baculovirus at a multiplicity of infection (MOI) of 50. The cells
were incubated at 37°C for 2 h. Stimulation with lipopolysaccharide
(LPS, 1 μg/ml) was employed as a positive control and the
incorporation of FITC-dextran at 4°C was used as a negative control
(non-specific binding). Cells were washed 3 times with cold PBS
containing 2% FCS and blocked followed by labeled with anti-mouse
CD16/32 and PE-conjugated anti-mouse CD11c monoclonal antibodies
(mAbs), respectively (eBioScience, San Diego, CA, USA).
To analyze how baculovirus affects DC activation,
DCs (5×105) were co-cultured with CT26 tumor cell lysate
(1×106) in the presence or absence of the virus (MOI =
50). Stimulation with LPS (1 μg/ml) was used as a positive
control. The cells were incubated at 37°C for 48 or 96 h and then
labeled with PE-conjugated anti-mouse CD11c and FITC-conjugated
CD86 mAbs (eBioScience). Cells were analyzed using FACSCalibur with
the CellQuest software (BD Bioscience).
ELISA
DCs (5×105) were co-cultured with CT26
cell lysate (1×106) in the presence or absence of
baculovirus (MOI = 50) or LPS (1 μg/ml) for 48 h and then
the culture supernatants were harvested. The production levels of
mouse IL-6, IL-10 and IL-12p70 were measured using ELISA kits
(eBioScience).
Statistical analysis
Statistical analyses were performed using a one-way
analysis of variance (ANOVA) followed by the Tukey test for
pair-wise comparisons; calculations were performed using the
Statistica program (StatSoft, Tulsa, OK, USA). Survival data were
plotted using the method of Kaplan-Meier and were analyzed using
the log-rank test. Data are expressed as the mean ± standard
deviation (SD) and P-values <0.05 were considered
significant.
Results
Induction of antitumor immunity by
intradermal immunization with combined baculovirus and tumor cell
lysate vaccine
To assess whether i.d. inoculation with baculovirus
affects the growth of mice, we monitored the body weight of mice
that received the combined baculovirus and tumor cell lysate
vaccine once per week for three consecutive weeks (Fig. 1A). The body weight was also
measured in control mice that received PBS, tumor cell lysate
alone, or baculovirus alone (Fig.
1A). There was no difference in the increase of body weight
between the four groups of mice (Fig.
1B), which suggests that the growth was not affected by i.d.
baculovirus injection. However, induration was observed at the
vaccination sites in mice receiving the combined baculovirus and
tumor cell lysate, but not in the animals receiving baculovirus
alone or tumor cell lysate alone.
To verify whether the combined baculovirus and tumor
cell lysate vaccine can induce effective antitumor immunity, the
combined vaccine, tumor cell lysate alone, or baculovirus alone was
administered intradermally into the upper right flank of mice once
per week for three consecutive weeks. At 1 week after the final
vaccination, CT26 cells (5×104) were inoculated
subcutaneously into the lower right flank of the mice (Fig. 1A) and 40% of the combined
vaccine-immunized mice did not demonstrate tumorigenesis (Fig. 1C). However, tumorigenesis was
detected in all of the mice inoculated with PBS, tumor cell lysate
alone, or baculovirus alone during the observation period (Fig. 1C). Tumors were observable at 7 days
after the tumor inoculation in all of the animals that received
PBS, tumor cell lysate alone and baculovirus alone and in 60% of
mice that received the combined vaccine. Although variance in the
tumor growth was observed among animals in each group, the tumor
growth was slower in mice immunized with the combined vaccine
compared to mice that received PBS, tumor cell lysate alone, or
baculovirus alone (Fig. 1C). The
median values of tumor size at day 38 after the tumor inoculation
were 128.4, 1322.8, 2514.3 and 463.9 mm3 for the groups
receiving the combined vaccine, PBS, tumor cell lysate alone and
baculovirus alone, respectively. Forty percent of the mice
immunized with the combined vaccine did not demonstrate
tumorigenesis during a 1-year follow-up period and the survival
rate was significantly higher in mice immunized with the combined
vaccine compared to that of mice injected with PBS, tumor cell
lysate alone or baculovirus alone (P= 0.02, Fig. 1D).
Therapeutic effect of combined
baculovirus and tumor cell lysate vaccine on established
tumors
We next investigated whether the combined vaccine
can effectively eradicate established tumors. Mice were vaccinated
intradermally with PBS, tumor cell lysate alone, baculovirus alone,
or the combined vaccines at days 0, 7 and 14, and then CT26 cells
(5×104) were inoculated subcutaneously at day 21
(Fig. 1A). There was a marked
difference in stimulation of the antitumor immunity among the four
vaccination groups (Fig. 2A).
Three out of five mice that received baculovirus plus tumor cell
lysate became resistant to a challenge with the tumor (Fig. 2A). Because the remaining mice
showed tumor growth, a therapeutic booster inoculation with
baculovirus plus tumor cell lysate was administered at 21 and 31
days after the tumor inoculation. The booster vaccine was
intradermally inoculated at a 1–2 mm distance around the tumor.
Upon the first booster vaccination, one mouse showed a rapid
regression of the tumor and the tumor volume of another mouse was
also reduced gradually following the second booster vaccination
(Fig. 2A). Eventually, the tumors
were completely eradicated in each of two mice that received a
booster dose of the combined baculovirus and tumor cell lysate
(Fig. 2A). In addition, 100% of
the mice survived for more than one year without tumor recurrence
in the group that received the combined vaccine and the survival
rate was significantly higher in this group compared to that in
groups receiving PBS, baculovirus alone, or tumor cell lysate alone
(P=0.003, Fig. 2B). However,
tumorigenesis was observed in all of the mice immunized either with
baculovirus alone or tumor cell lysate alone and mouse survival was
not significantly different compared to that seen in PBS-injected
control mice (Fig. 2). The mice
inoculated with baculovirus alone or tumor cell lysate alone also
received the i.d. therapeutic booster immunization with baculovirus
or tumor cell lysate at 21 and 31 days after the tumor inoculation;
however, the tumor in these animals did not regress (Fig. 2A).
Induction of antitumor T cell immunity by
intradermal immunization with combined baculovirus and tumor cell
lysate
To investigate whether or not CD8+ T
cells induced by the i.d. vaccination with the combined baculovirus
and tumor cell lysate function by lysing the tumor cells, we
conducted an in vitro cytotoxicity assay. Cytolytic activity
against CT26 cells was detected when spleen cells obtained from the
mice receiving the combined vaccine were co-cultured with target
CT26 cells at ratios of 50:1, 25:1 and 12.5:1 (Fig. 3). The magnitude of the cytolytic
activity in the combined vaccine group was considerably higher than
that in the other groups that received PBS, tumor cell lysate
alone, or baculovirus alone. This result demonstrates that i.d.
immunization with the combined baculovirus and tumor cell lysate
effectively induced tumor-reactive CTLs.
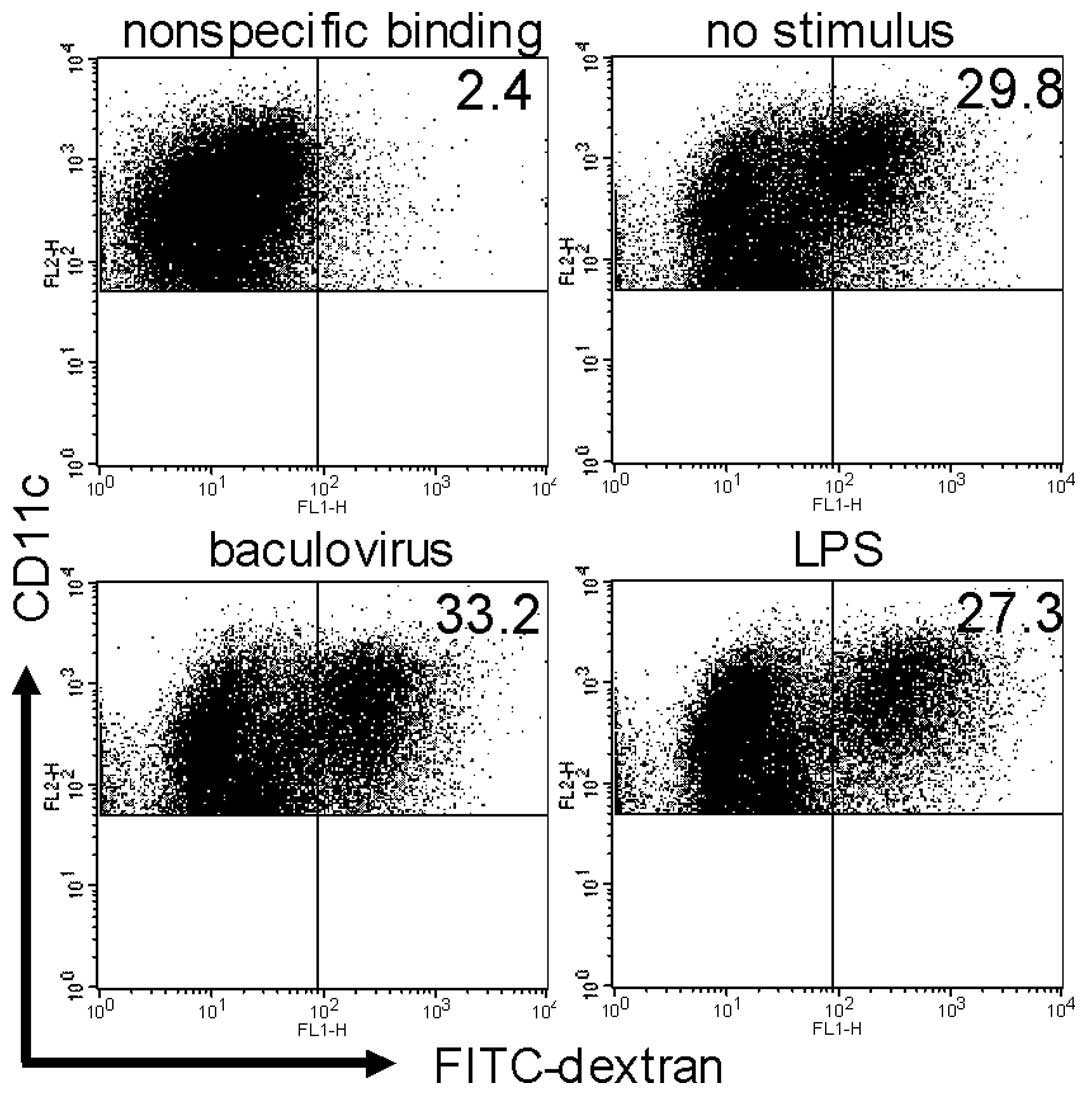
Effects of baculovirus on the ability of
DCs to capture an antigen and their activation
To assess whether baculovirus affects the ability of
DCs to take up an antigen, DCs were co-cultured with FITC-dextran
as an antigen in the presence or absence of the virus. FITC-dextran
uptake by DCs co-cultured with baculovirus was almost equivalent to
that without the virus or with LPS (32.9±5.6, 30.0±5.8 and
28.6±4.3%, respectively; Fig.
4).
In addition, we investigated DC activation when the
cells were co-cultured with CT26 cell lysate in the presence or
absence of the baculovirus. Following incubation with the lysate
and virus for 48 h, 56.7±5.7% of DCs became CD86-positive,
representing the activation of DCs, while the frequencies of
CD86-positive DCs were 4.4±0.6% and 21.3±2.5% after incubation
without the virus and with LPS, respectively (P=0.0002, Fig. 5A). Following incubation with the
lysate and baculovirus for 96 h, 18.1±1.8% of DCs were
CD86-positive, while the frequencies of CD86-positive DCs were
2.3±0.5% and 1.4±0.3% after incubation without the virus and with
LPS, respectively (P=0.0002, Fig.
5B).
The production level of cytokines was also measured
in the culture medium obtained from the above experiment (Fig. 5C). A high level of IL-6 production
was observed in DCs co-cultured with baculovirus plus tumor lysate
for 48 h compared to the level seen in DCs co-cultured with tumor
lysate alone or with the lysate plus LPS. No IL-12p70 secretion was
observed in DCs pulsed with tumor lysate alone; however, the
cytokine production was elevated by adding baculovirus into DCs
pulsed with tumor lysate. The level of IL-10 secretion from DCs
co-cultured with tumor lysate plus baculovirus tended to be
slightly higher, but not significantly different, compared to that
from DCs co-cultured with tumor lysate alone; however, the
production level from DCs co-cultured with baculovirus plus tumor
lysate was lower than that from DCs co-cultured with tumor lysate
plus LPS.
Discussion
Although baculovirus is widely used as both a tool
to investigate gene transfer and a vaccine vector using a
recombinant baculovirus (23–27),
it remains unclear whether wild-type baculovirus possesses an
adjuvant effect for a cancer vaccine when the virus is combined
with a tumor antigen. We report here that i.d. inoculation with
combined freeze-thaw tumor cell lysate and wild-type baculovirus
effectively induces anti-tumor immune responses, mainly mediated by
tumor-reactive CD8+ T cells, in a mouse model. Survival
was significantly prolonged in mice vaccinated with the combined
baculovirus and tumor cell lysate compared to that in animals
inoculated either with baculovirus alone or tumor cell lysate
alone. In addition, established tumors in mice that had previously
been vaccinated with the combined baculovirus and tumor cell lysate
were completely eradicated when the combined vaccine was
administered around the tumors. These findings suggest that this
vaccine strategy using wild-type baculovirus as an adjuvant is
effective in preventing postoperative tumor recurrence and for
treatment of recurrent tumors.
Many peptides containing killer epitopes were
identified and are being used as cancer vaccines to induce
tumor-reactive CTLs; however, clinical benefits of the vaccines are
modest (3,4). One reason for the modest effect of
the vaccines may be because this vaccine strategy targets CTL
stimulation only. Several studies have reported the critical role
of CD4+ T cells in inducing antitumor responses
(8–10) and in generating CD8+ T
memory cells (11,12). These reports prompted us to
consider tumor lysates as a useful cancer vaccine since tumor
lysates should contain helper epitopes and killer epitopes.
However, tumor lysate was reported to give rise to DC dysfunction
(13–15) and moreover, it was also shown that
DC was not able to enhance a cytotoxic activity even by stimulating
with cell lysate (33).
In the present study, when tumor cells were
transplanted subcutaneously into mice that had been intradermally
vaccinated with the combined baculovirus and tumor cell lysate,
baculovirus alone, or tumor cell lysate alone, the combination
vaccine was found to provide strong antitumor immunity that
suppressed tumorigenesis compared to baculovirus alone or tumor
cell lysate alone. This result indicates that baculovirus would not
share the same peptide recognized by T cells as does the CT26 cell;
baculovirus itself cannot induce antitumor immunity, but exerts a
strong adjuvant effect to evoke antitumor immunity when the virus
plus freeze-thawed tumor cell lysate is inoculated intradermally.
We further demonstrated that such antitumor immunity induced by the
i.d. immunization with the combined baculovirus and tumor cell
lysate vaccine should be associated with CD8+ T cells
using a cytotoxicity assay. A high level of cytolytic activity was
detected in mice vaccinated with the combined baculovirus and tumor
cell lysate vaccine compared to that in mice inoculated with
baculovirus alone or tumor lysate alone. These results suggest the
feasibility of using baculovirus as a vaccine adjuvant, which can
help to strongly induce tumor-reactive CTLs.
When DCs were co-cultured with FITC-dextran in the
presence or absence of baculovirus, the antigen uptake by DCs
co-cultured with the virus was almost equivalent to that without
the virus. However, when DCs were pulsed with tumor cell lysate for
48 h, the frequency of CD86-positive mature DCs was substantially
higher in the presence of baculovirus compared to that observed in
the absence of the stimulus. The mature DCs remained viable 96 h
following incubation with tumor cell lysate and the baculovirus.
These results indicate that although baculovirus does not enhance
the ability of DCs to take up an antigen, it strongly stimulates
DCs loaded with tumor cell lysate. DCs are needed to prime naïve T
cell responses so that they undergo the maturation process
(34). We demonstrated here that
baculovirus helps DCs stimulated with tumor lysate to become mature
and also helps to maintain them.
We also found that cytokine production was enhanced
by baculovirus-induced stimulation of tumor cell lysate-loaded DCs.
It should be noted that a higher level of IL-6 was produced by DCs
co-cultured with tumor cell lysate plus the virus compared to DCs
co-cultured with the tumor cell lysate alone. IL-6 has been shown
to play an important role in T cell activation because of its
ability to overcome the suppressive effect of regulatory T cells
(Tregs) (35). Tregs have been
reported to downregulate T cell responses (36–39)
and Treg depletion has also been shown to contribute to enhanced
antitumor immunity (40,41). Accordingly, it is possible that
high levels of IL-6 secreted by baculovirus-activated DCs might
block suppression mediated by Tregs. In addition, IL-12, which is
important to induce Th1-type immune responses, was secreted by DCs
stimulated with tumor cell lysate plus baculovirus, whereas no
IL-12 was produced by DCs co-cultured with tumor cell lysate alone.
These results also indicate that combined baculovirus and tumor
lysate vaccine can elicit effective DC activation and secretion of
IL-6 and IL-12, leading to the induction of antitumor immunity.
Why can the combined baculovirus and tumor cell
lysate vaccine elicit tumor-reactive CTLs? In general, for the
induction of antigen-specific CTLs, a killer epitope is required to
be presented on MHC class I molecules of antigen-presenting cells.
It has been reported that baculovirus infects mammalian cells
through the cell-surface phospholipid (42). Thus, one possible explanation is
that baculovirus might bind to freeze-thawed cells via a
cell-surface phospholipid and the resulting complex consisting of
the virus and lysate might be captured and endocytosed by DCs. The
complex may then be released from the endosomal compartment to the
cytosol by fusing with the viral envelope containing the tumor
lysate into endosomal membrane and possibly lead to the
cross-presentation of the tumor antigen to MHC class I molecules.
In addition, these DCs effectively mature and become active by
stimulation with baculovirus and IL-6 and IL-12 are also
simultaneously secreted by the DCs, perhaps resulting in the
induction of tumor-reactive CTLs.
As described in the present study, we observed that
i.d. immunization with tumor cell lysate alone did not elicit
effective antitumor immunity. DCs took up FITC-dextran in the
absence of baculovirus because immature DCs possess the ability to
take up an antigen. Therefore, DCs must have taken up tumor cell
lysate in the absence of baculovirus; however, only a few DCs
matured and these secreted little IL-6 and IL-12. These results may
lead to the induction of tolerance against tumors, so that such an
immune condition might increase the tumor growth acceleration in
mice immunized with tumor cell lysate alone compared to that in
mice injected with PBS (2514.3 and 1322.8 mm3,
respectively). Because it is necessary to trigger innate immunity
for subsequent effective acquired immunity (43), i.d. inoculation with tumor lysate
alone may be insufficient to stimulate innate immunity, so that
acquired antitumor immunity may not be fully stimulated.
In the present study, prophylactic vaccination with
the combined baculovirus and tumor cell lysate suppressed tumor
growth in 40–60% of mice (Figs. 1
and 2). Although the remaining
mice showed tumor growth, therapeutic booster vaccination with the
combined vaccine completely eradicated the established tumors. The
booster vaccination with the combined baculovirus and tumor cell
lysate may effectively activate the antigen-captured DCs and these
DCs may then stimulate tumor-specific memory CD8+ T
cells through the cross-presentation of the tumor antigen. These
DCs concomitantly secrete high level of IL-6 and IL-12, possibly
resulting in the induction of effective antitumor responses.
In conclusion, we have demonstrated that baculovirus
can effectively activate DCs pulsed with tumor lysate and that a
strong antitumor immunity can be induced by i.d. immunization with
combined baculovirus and tumor lysate. Baculovirus is not able to
replicate in mammalian cells and the virus is also widely used as
an agricultural insecticide, suggesting that baculovirus is safe
for humans. Our findings reported here suggest the possibilities
that baculovirus can make tumor lysate useful as a cancer vaccine
and that the virus can be used as an effective adjuvant for cancer
immunotherapy.
Acknowledgements
We would like to thank Drs Tomoyuki
Suzuki (Chiba Institute of Technology), Noboru Hagiwara, Akira
Hashimoto and Kazuhiro Matsuo (Japan BCG Laboratory) for their
insightful comments during our discussion of this study.
References
|
1.
|
Paoletti X, Burzykowski T, Michiels S,
Ohashi Y, Pignon JP, Rougier P, Sakamoto J, Sargent D, Sasako M,
Van Cutsem E and Buyse M: Benefit of adjuvant chemotherapy for
resectable gastric cancer: a meta-analysis. JAMA. 303:1729–1737.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Ochsenbein AF: Principles of tumor
immunosurveillance and implications for immunotherapy. Cancer Gene
Ther. 9:1043–1055. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Kirkwood JM, Butterfield LH, Tarhini AA,
Zarour H, Kalinski P and Ferrone S: Immunotherapy of cancer in
2012. CA Cancer J Clin. 62:309–335. 2012. View Article : Google Scholar
|
|
4.
|
Rosenberg SA, Yang JC and Restifo NP:
Cancer immunotherapy: moving beyond current vaccines. Nat Med.
10:909–915. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Toes RE, Blom RJ, Offringa R, Kast WM and
Melief CJ: Enhanced tumor outgrowth after peptide vaccination.
Functional deletion of tumor-specific CTL induced by peptide
vaccination can lead to the inability to reject tumors. J Immunol.
156:3911–3918. 1996.PubMed/NCBI
|
|
6.
|
Toes RE, Offringa R, Blom RJ, Melief CJ
and Kast WM: Peptide vaccination can lead to enhanced tumor growth
through specific T-cell tolerance induction. Proc Natl Acad Sci
USA. 93:7855–7860. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Muraoka D, Kato T, Wang L, Maeda Y,
Noguchi T, Harada N, Takeda K, Yagita H, Guillaume P, Luescher I,
Old LJ, Shiku H and Nishikawa H: Peptide vaccine induces enhanced
tumor growth associated with apoptosis induction in CD8+
T cells. J Immunol. 185:3768–3776. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Bour H, Horvath C, Lurquin C, Cerottini JC
and MacDonald HR: Differential requirement for CD4 help in the
development of an antigen-specific CD8+ T cell response
depending on the route of immunization. J Immunol. 160:5522–5529.
1998.PubMed/NCBI
|
|
9.
|
Pardoll DM and Topalian SL: The role of
CD4+ T cell responses in antitumor immunity. Curr Opin
Immunol. 10:588–594. 1998.
|
|
10.
|
Toes RE, Ossendorp F, Offringa R and
Melief CJ: CD4 T cells and their role in antitumor immune
responses. J Exp Med. 189:753–756. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Janssen EM, Lemmens EE, Wolfe T, Christen
U, von Herrath MG and Schoenberger SP: CD4+ T cells are
required for secondary expansion and memory in CD8+ T
lymphocytes. Nature. 421:852–856. 2003.PubMed/NCBI
|
|
12.
|
Shedlock DJ and Shen H: Requirement for
CD4 T cell help in generating functional CD8 T cell memory.
Science. 300:337–339. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Lizée G, Rasvanyi LG, Overwijik WW and Hwu
P: Immunosuppression in melanoma immunotherapy: potential
opportunities for intervention. Clin Cancer Res. 12:S2359–S2365.
2006.PubMed/NCBI
|
|
14.
|
Jackson AM, Mulcathy LA, Zhu XW, O’Donnell
D and Patel PM: Tumor-mediated disruption of dendritic cell
function: inhibiting the MEK1/2-p44/42 axis restores IL-12
production and Th1-generation. Int J Cancer. 123:623–632. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Yang DH, Park JS, Jin CJ, Kang HK, Nam JH,
Rhee JH, Kim YK, Chung SY, Choi SJN, Kim HJ, Chung IJ and Lee JJ:
The dysfunction and abnormal signaling pathway of dendritic cells
loaded by tumor antigen can be overcome by neutralizing VEGF in
multiple myeloma. Leuk Res. 33:665–670. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Schnurr M, Toy T, Stoitzner P, Cameron P,
Shin A, Beecroft T, Davis ID, Cebon J and Maraskovsky E: ATP
gradients inhibit the migratory capacity of specific human
dendritic cell types: implications for P2Y11 receptor signaling.
Blood. 102:613–620. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Pinzon-Charry A, Maxwell T and Lopez JA:
Dendritic cell dysfunction in cancer: a mechanism for
immunosuppression. Immunol Cell Biol. 83:451–461. 2005. View Article : Google Scholar
|
|
18.
|
Bennaceur K, Popa I, Portoukalian J,
Bethier-Vergnes O and Peguet-Navarro J: Melanoma-derived
gangliosides impair migratory and antigen-presenting function of
human epidermal Langerhans cells and induce their apoptosis. Int
Immunol. 18:879–886. 2006. View Article : Google Scholar
|
|
19.
|
Stewart LM, Hirst M, López-Ferber M,
Merryweather AT, Cayley PJ and Possee RD: Construction of an
improved baculovirus insecticide containing an insect-specific
toxin gene. Nature. 352:85–88. 1991. View
Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Jennifer SC, Mark LH, Trevor W, Rosemary
SH, David G, Bernadette MG, Timothy MC, Robert DP, Cayley PJ and
Bishop DHL: Field trial of a genetically improved baculovirus
insecticide. Nature. 370:138–140. 1994. View Article : Google Scholar
|
|
21.
|
Matsuura Y, Possee RD, Overton HA and
Bishop DHL: Baculovirus expression vectors: the requirements for
high level expression of proteins, including glycoproteins. J Gen
Virol. 68:1233–1250. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Luckow VA and Summers MD: Trends in the
development of baculovirus expression vectors. Biotechnology.
6:47–55. 1988. View Article : Google Scholar
|
|
23.
|
Hofmann C, Sandig V, Gennings G, Rudolph
M, Schlag P and Strauss M: Efficient gene transfer into human
hepatocytes by baculovirus vectors. Proc Natl Acad Sci USA.
92:10099–10103. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Boyce FM and Bucher NLR:
Baculovirus-mediated gene transfer into mammalian cells. Proc Natl
Acad Sci USA. 93:2348–2352. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Sandig V and Strauss M: Liver-directed
gene transfer and application to therapy. J Mol Med. 74:205–212.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Pieroni L and La Monica N: Towards the use
of baculovirus as a gene therapy vector. Curr Opin Mol Ther.
3:464–467. 2001.PubMed/NCBI
|
|
27.
|
Kost TA and Condreay JP: Recombinant
baculoviruses as mammalian gene-delivery vectors. Trends
Biotechnol. 20:173–180. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Gronowski AM, Hilbert DM, Sheehan KCF,
Garotta G and Schreiber RD: Baculovirus stimulates antiviral
effects in mammalian cells. J Virol. 73:9944–9951. 1999.PubMed/NCBI
|
|
29.
|
Beck NB, Sidhu JS and Omiecinski CJ:
Baculovirus vectors repress phenobarbital-mediated gene induction
and stimulate cytokine expression in primary cultures of rat
hepatocytes. Gene Ther. 7:1274–1283. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Kitajima M, Abe T, Miyano-Kurosaki N,
Taniguchi M, Nakayama T and Takaku H: Induction of natural killer
cell-dependent antitumor immunity by the Autographa
californica multiple nuclear polyhedrosis virus. Mol Ther.
16:261–268. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Suzuki T, Chang MO, Kitajima M and Takaku
H: Baculovirus activates murine dendritic cells and induces
non-specific NK cell and T cell immune responses. Cell Immunol.
262:35–43. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Suzuki T, Chang MO, Kitajima M and Takaku
H: Induction of antitumor immunity against mouse carcinoma by
baculovirus-infected dendritic cells. Cell Mol Immunol. 7:440–446.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Schöttker B and Schmidt-Wolf IGH: Pulsing
with blast cell lysate or blast-derived total RNA reverses the
dendritic cell-mediated cytotoxic activity of cytokine-induced
killer cells against allogeneic acute myelogenous leukemia cells.
Ger Med Sci. doi: 10.3205/000141. URN: urn:nbn:de:0183-0001410.
2011.
|
|
34.
|
Reis e Sousa C: Dendritic cells in a
mature age. Nat Rev Immunol. 6:476–483. 2006.PubMed/NCBI
|
|
35.
|
Pasare C and Medzhitov R: Toll
pathway-dependent blockade of CD4+CD25+ T
cell-mediated suppression by dendritic cells. Science.
299:1033–1036. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Shevach EM: Regulatory T cells in
autoimmmunity*. Annu Rev Immunol. 18:423–449. 2000.
View Article : Google Scholar
|
|
37.
|
Maloy KJ and Powrie F: Regulatory T cells
in the control of immune pathology. Nat Immunol. 2:816–822. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Piccirillo CA and Shevach EM: Cutting
edge: control of CD8+ T cell activation by
CD4+CD25+ immunoregulatory cells. J Immunol.
167:1137–1140. 2001.PubMed/NCBI
|
|
39.
|
Sakaguchi S: Naturally arising
Foxp3-expressing CD25+CD4+ regulatory T cells
in immunological tolerance to self and non-self. Nat Immunol.
6:345–352. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Turk MJ, Guevara-Patiño JA, Rizzuto GA,
Engelhorn ME, Sakaguchi S and Houghton AN: Concomitant tumor
immunity to a poorly immunogenic melanoma is prevented by
regulatory T cells. J Exp Med. 200:771–782. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Yu P, Lee Y, Liu W, Krausz T, Chong A,
Schreiber H and Fu YX: Intratumor depletion of CD4+
cells unmasks tumor immunogenicity leading to the rejection of
late-stage tumors. J Exp Med. 201:779–791. 2005.PubMed/NCBI
|
|
42.
|
Tani H, Nishijima M, Ushijima H, Miyamura
T and Matsuura Y: Characterization of cell-surface determinants
important for baculovirus infection. Virology. 279:343–353. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Akira S, Takeda K and Kaisho T: Toll-like
receptors: critical proteins linking innate and acquired immunity.
Nat Immunol. 2:675–680. 2001. View
Article : Google Scholar : PubMed/NCBI
|