Introduction
Gliomas are the most common brain tumors of the
adult central nervous system. Astrocytomas, which are tumors
composed predominantly of neoplastic astrocytes, account for 80–85%
of all gliomas (1). Grade IV
astrocytomas, also referred to as glioblastoma multiforme (GBM), is
the most common primary malignant brain cancer (2). The proliferation rates of GBM are two
to five times higher than grade III tumors, and patients with GBM
have a dismal prognosis, with a median survival time of less than
15 months despite aggressive therapy (3). A characteristic of GBM is its ability
to infiltrate and invade the surrounding normal brain tissue.
Despite advances in techniques for administering radiotherapy,
local recurrence of glioblastomas typically leads to patient
mortality. Thus, therapies that effectively target invasive glioma
cells may significantly improve clinical outcomes.
The underlying molecular mechanisms of brain tumor
invasion are complex and involve integrated biochemical processes
requiring a coordinated effort of intracellular and extracellular
interactions (1). For effective
invasion, tumor cells must first detach from the nascent tumor mass
and invade the surrounding stroma, which is composed of parenchymal
cells and the extracellular matrix (ECM). Cell surface adhesion
molecules play an important role in the interaction between the
cells and ECM. Cell adhesion molecules (CAMs) are expressed on a
variety of cells, including vascular endothelial cells (ECs) and
tumor cells (4–7), that have been activated by cytokines
such as IL-1α, IL-6 or TNF-α (8,9).
Specifically, TNF-α induces the upregulation of intracellular
adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1
(VCAM-1) in ECs (10,11). ICAM-1 and VCAM-1 have been shown to
be involved in cell-cell and cell-ECM interactions and are
mechanistically important for the extravasation of cancer cells
during metastasis (12,13).
Cadherins are important molecules involved in tumor
progression. Different cadherins, including E-cadherin, N-cadherin,
T- (or H-) cadherin and VE-cadherin, are reported to have different
functions and are expressed in different tissues. E- and N-cadherin
are the most thoroughly studied cadherins in terms of the EMT
process; the loss of E-cadherin expression in epithelial tumors is
associated with a more invasive phenotype and metastasis (14). N-cadherin has been shown to promote
cell motility and migration, the opposite effect to that of
E-cadherin (14). VE-cadherin is
an endothelium-specific member of the cadherin family of adhesion
proteins and regulates transmembrane endothelial adherens
junctions. Thus, cadherins, including E-cadherin, N-cadherin and
VE-cadherin, are involved in tumor metastasis.
Honokiol is a well-known bioactive constituent of
the bark of Magnolia officinalis and has been reported to
prevent and protect the brain from damage (15) as well as to exert antitumor
efficacy in vitro and in vivo (16–19).
Notably, treatment with honokiol may be a potential strategy to
overcome immunoresistance in glioma (20), as honokiol can cross the BBB and
the BCSFB (21). In addition, our
previous study demonstrated that honokiol induces apoptotic cell
death through the upregulation of the Bax/Bcl-2 ratio and inhibits
invasion through the regulation of ICAM-1 and VCAM-1 in human
glioblastoma T98G cells (22).
Based on these results, honokiol may represent an effective drug
for the treatment of brain tumors, specifically glioblastoma. In
this study, we were interested in the effect of honokiol on
glioblastoma invasion. Thus, we investigated whether honokiol
affects glioblastoma invasion through the regulation of adhesion
molecules and VE-cadherin as well as the EMT process in U87MG, a
commonly studied grade IV glioma cell line that has been analyzed
in at least 1,700 publications over four decades (23).
Materials and methods
Materials
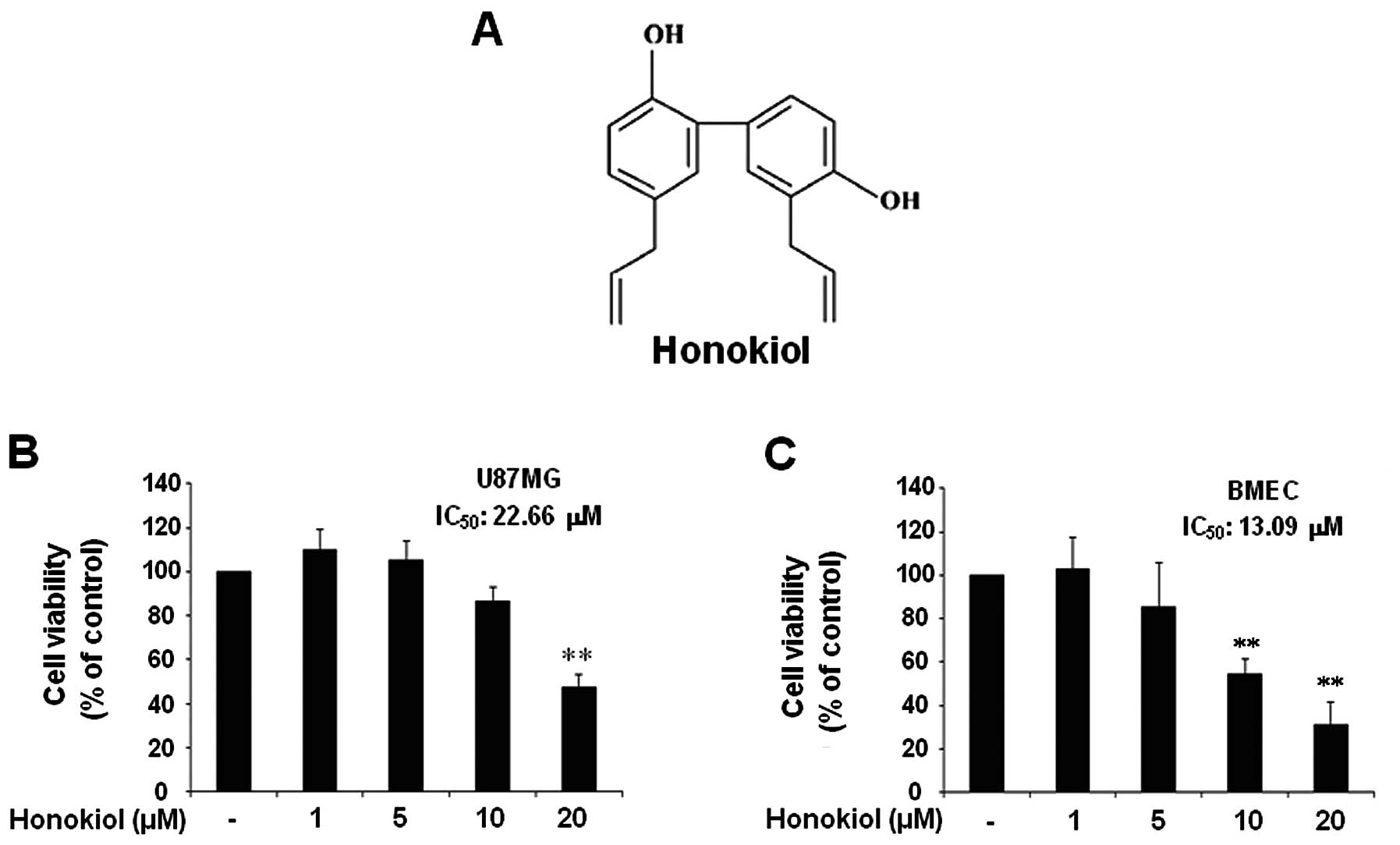
Honokiol (Fig. 1A)
was supplied by Wako Chemical (Wako, Japan). Anti-VCAM-1,
anti-VE-cadherin, anti-Snail, anti-N-cadherin, anti-β-catenin and
anti-E-cadherin antibodies were purchased from Santa Cruz
Biotechnology (Santa Cruz, CA, USA). Anti-phospho-VE-cadherin
(phospho-Y658) antibody was purchased from Abcam (Cambridge, MA,
USA). Enhanced chemiluminescence (ECL) western blotting detection
reagent was obtained from Amersham (Buckinghamshire, UK). All other
chemicals, including Evans blue dye, were purchased from
Sigma-Aldrich (St. Louis, MO, USA).
Cell culture
Human glioblastoma cells (U87MGs) and mouse brain
microvascular endothelial cells (BMECs) were purchased from ATCC
and grown in DMEM medium supplemented with 10% fetal bovine serum
(FBS), 2 mM L-glutamine, 100 IU/ml penicillin and 10 μg/ml
streptomycin and incubated in a humidified 5% CO2
incubator.
Cell viability assay
Cell viability was determined colorimetrically using
a 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide
(MTT) assay. Cells were plated at 1×104 cells per well
in 24-well plates. After drug treatments, MTT solution was added to
each well (0.1 mg/well) and incubated for 4 h. Supernatants were
removed, and formazan crystals in the wells were dissolved in 200
μl of dimethyl sulfoxide for 30 min at 37°C and the optical
density at 570 nm was measured using a microplate reader (Bio-Rad,
Hercules, CA, USA).
Western blot analysis
Cells were lysed using PRO-PREP protein extraction
solution. Aliquots of 40 μg of protein were subjected to 10%
SDS-polyacrylamide gel electrophoresis. Separated proteins in
SDS-polyacrylamide gel were transferred onto Hybond-P+
polyvinylidene difluoride membranes (Amersham Biosciences UK Ltd.).
The membrane was blocked with 5% non-fat milk in Tris-buffered
saline containing 0.05% Tween-20 (TBS-T) for 2 h at room
temperature and the membranes were incubated with the indicated
primary antibodies. Proteins were detected with ECL western
blotting detection reagent according to the manufacturer’s
instructions.
Fractionation of cell extracts
The cellular compartment was extracted as previously
described with a minor modification (11). Briefly, cells were washed with
ice-cold phosphate-buffered saline (PBS; pH 7.4) and lysed in
buffer A (10 mM HEPES, pH 8.0, 1.5 mM MgCl2, 0.5 mM
dithiothreitol and 1X protease inhibitors). The supernatant
(cytoplasmic extract) was obtained by centrifugation at 10,000 × g
for 15 min. The pellets were washed once with buffer A and
resuspended in buffer B (10 mM Tris-Cl, pH 7.5, 0.5% deoxycholate,
EDTA, 0.5 mM, 0.5 mM dithiothreitol, and 1% Nonidet P-40). The
suspension was agitated for 30 min at 4°C and centrifuged at 10,000
× g for 20 min. The supernatant fraction containing nuclear
proteins was collected.
Adhesion assay
BMECs were treated with honokiol for 24 h and
subsequently stimulated with TNF-α for 6 h. Thereafter, U87MG
1×106 cells were added to BMEC layers. After 30 min,
cell suspensions were removed, and BMECs were gently washed with
DMEM medium. The cells were counted under a light microscope, and
images were taken using an Olympus microscope (CKX41) equipped with
a camera (Nikon, DS-U3).
Matrigel invasion assay
The Matrigel invasion assays were performed in two
ways. First, the insert wells (8-μm pore size, BD Falcon,
Franklinlakes, NJ, USA) were coated with 100 μl of Matrigel
(1 mg/ml, BD Falcon), and BMECs were then added (2×105
cells per well). After 2 h, U87MG cells (2×105) that had
been pretreated with honokiol for 24 h were added to the BMEC
layers on Matrigel in the inserts. The inserts were incubated for
24 h in a 37°C cell culture incubator. In the second method, BMECs
were pretreated with honokiol for 24 h and then washed with PBS
three times. After BMECs were stimulated with TNF-α for 6 h, U87MG
cells were added to BMEC-Matrigel coated wells and incubated for 24
h. The non-invasive cells that remained on the upper side of the
insert were removed. The cells on the lower part of insert
membranes were stained with 4′,6-diamidino-2-phenylindole (DAPI)
and counted under a light microscope.
Membrane permeability study
Cell culture inserts (0.4-μm pore size, BD
Falcon) were placed in a 24-well plate. BMECs were seeded on the
inserts and treated with honokiol for 1 h. The cells were washed
with PBS and treated with TNF-α. Four hours later, the media were
removed, the cells were washed with PBS and 100 μl of 200
μg/ml Evans blue suspended in 0.1% bovine serum albumin in
transport buffer (10 mM HEPES, 132 mM NaCl, 4 mM KCl, 1.4 mM
MgCl2, 1.2 mM H3PO4, 1 mM
CaCl2, 4.5% glucose, pH 7.4) was added to the upper
chamber (insert wells). After 4 h, aliquots (100 μl) were
collected from the lower chamber (24-well plate). Molecular
permeability across the membrane was determined by measuring
optical density of Evans blue at 620 nm using an EIA reading
photometer (US.HL 5500P0, Bio-Rad).
Statistical analysis
Scanning densitometry was performed using Image
Master® VDS (Pharmacia Biotech Inc., San Francisco, CA,
USA). All results are representative of three independent
experiments performed in triplicate (mean ± SEM). Significant
differences within data were evaluated by one-way analysis of
variance (ANOVA) and the post hoc test by Scheffe. P-values
<0.05 were treated as statistically significant.
Results
The effect of honokiol on the cell
viability of U87MG human glioblastoma cells and BMECs
In this study, we aimed to investigate the effect of
honokiol on the cell invasion process of U87MG human glioblastoma
cells through brain microvascular endothelial cells (BMECs) and its
possible mechanisms. Thus, first, we examined the cell viability of
U87MG cells and BMECs in response to honokiol in a lower range
compared to the previous study (22). When U87MG cells and BMECs were
treated with indicated honokiol (1–20 μM) for 24 h, the
results revealed that honokiol significantly decreased the cell
viability of U87MG only at doses of 20 μM (Fig. 1B). BMECs exhibited significant
reduction in viability at concentrations of 10 μM and 20
μM (Fig. 1C).
Honokiol-mediated cytotoxicity was not significant at doses <20
μM in U87MGs or 10 μM in BMECs. The IC50
of honokiol in U87MG and BMECs was 22.66 and 13.09 μM,
respectively.
Honokiol inhibited VCAM-1 expression by
TNF-α in BMECs and suppressed the adhesion of U87MG cells to
TNF-α-stimulated BMECs
Cell-cell and cell-ECM interactions are partially
regulated by adhesion molecules. Specifically, VCAM-1 has been
shown to be important in cancer cell metastasis (24,25).
Accordingly, we examined whether honokiol inhibits VCAM-1
expression by TNF-α in BMECs. BMECs exhibited a significant
induction of VCAM-1 protein levels in response to TNF-α (10 ng/ml,
6 h), which was efficiently inhibited by pretreatment with
honokiol; significant inhibition occurred at 5–20 μM
honokiol (Fig. 2A). Next, we
investigated the effect of honokiol on U87MG adhesion to BMECs.
Adhesion of U87MG cells to BMECs stimulated with TNF-α at 10 ng/ml
for 6 h was dramatically increased compared to unactivated BMECs.
In contrast, treatment of the BMECs with 5-20 μM honokiol
for 24 h before TNF-α stimulation resulted in a significant
reduction of adhesion of U87MG cells to ECs (Fig. 2B and C).
Honokiol reduces TNF-α-mediated
phosphorylation of VE-cadherin and increases membrane permeability
in BMECs
Tyrosine phosphorylation of VE-cadherin is known to
be associated with weak junctions and impaired barrier function.
Therefore, we investigated the effect of honokiol on the
phosphorylation of VE-cadherin at tyrosine residue 658 (Y658) by
western blotting. When BMECs were treated with TNF-α in a
time-dependent manner, TNF-α was able to prominently increase
phospho-VE-cadherin at 4 h (preliminary data, not shown). Thus, we
measured the phospho-VE-cadherin levels at 4 h after TNF-α
treatment. Honokiol treatment 1 h prior to TNF-α significantly
decreased TNF-α-induced phospho-VE-cadherin at very low dose (1
μM) (Fig. 3A). This result
coincided with the membrane permeability results. The concentration
of Evans blue dye across BMECs increased with TNF-α treatment and
significantly decreased with honokiol treatment (1–20 μM).
TNF-α-induced membrane permeability was significantly reduced with
20 μM of honokiol, however, the permeability remained
slightly higher than with 10 μM of honokiol, possibly due to
cell toxicity caused by 20 μM honokiol (Fig. 3B).
Honokiol inhibits EMT in U87MG cells via
downregulation of the mesenchymal markers Snail, β-catenin and
N-cadherin and the upregulation of the epithelial marker
E-cadherin
Then, we assessed whether honokiol regulates EMT
proteins, including Snail, β-catenin, N-cadherin and E-cadherin.
Honokiol effectively reduced the mesenchymal markers Snail,
β-catenin and N-cadherin but increased the levels of the epithelial
marker E-cadherin. These results suggest that honokiol suppresses
EMT by downregulating the mesenchymal markers Snail, β-catenin and
N-cadherin and upregulating the epithelial marker E-cadherin
(Fig. 4).
Honokiol effectively inhibits U87MG
invasion through BMECs
We evaluated the effect of honokiol on human
glioblastoma invasion through BMECs using two different methods.
First, to test the effect of honokiol on U87MG invasion through the
regulation of EMT, U87MG cells were treated with honokiol in a
dose-dependent manner and added to BMECs that were not treated with
honokiol or TNF-α (Fig. 5A). In
our second method, BMECs were treated with honokiol and stimulated
with TNF-α and incubated with U87MG cells that were not treated
with honokiol to examine the effect of honokiol on U87MG invasion
through the regulation of VCAM-1 and membrane permeability in BMECs
(Fig. 5D). As expected, U87MG
cells treated with honokiol exhibited reduced invasion through
BMECs (Fig. 5B and C). In
addition, treatment of BMECs with honokiol suppressed U87MG cell
invasion through TNF-α-stimulated BMECs (Fig. 5E and F).
Discussion
Glioblastoma is almost uniformly fatal with only a
few patients surviving longer than 2 years (26). Two major problems impede the
success of chemotherapy. First, the delivery of sufficient amounts
of most antineoplastic drugs into brain tissue is prevented by the
BBB. Second, high-grade gliomas are often characterized by high
intrinsic chemoresistance. Honokiol is reported to cross the BBB
and the BCSFB and to overcome immunoresistance in glioma, strongly
suggesting that it could be an effective drug for the treatment of
brain tumors, including glioblastoma. Our previous study
demonstrated that honokiol induced apoptotic cell death and
inhibited cell invasion through regulation of ICAM-1 and VCAM-1 in
human glioblastoma T98G cells (22). Recently, it was reported that
tumors, including gliomas, contain a small subpopulation of cancer
stem cells (CSCs). These cells are characterized by their ability
to form neural spheres, antibiotic resistance and high cell
migration ability (27). According
to Moon and Park (28), U87MG
cells formed neural spheres and expressed CD133 and Bmil, which are
the canonical cell surface markers of brain CSCs, at much higher
levels than any other glioblastomas (A172, T98G, U138, U251, U373).
Given these characteristics of U87MG, finding new treatments that
can target this cell type may be beneficial in developing an
effective cure for glioblastoma. Therefore, we further investigated
the effect of honokiol on U87MG human glioblastoma cell invasion
and the possible mechanisms underlying this regulation by honokiol.
Our results revealed that honokiol effectively inhibited the
adhesion of U87MG cells to BMECs by inhibiting normal VCAM-1
expression. Honokiol also perturbed U87MG invasion through BMECs by
inhibiting VCAM-1 expression and suppressing
phospho-VE-cadherin-mediated BMEC permeability. TNF-α-induced
permeability of BMECs was significantly reduced by 1 μM
honokiol; however, permeability appeared to increase slightly at 20
μM, possibly due to the cytotoxicity of honokiol at 20
μM.
As mentioned above, glioblastoma exhibits aggressive
proliferation and highly invasive properties and can diffusely
infiltrate various regions of the normal brain, accounting for a
poor prognosis. Tumor invasion results from vasculature leakiness
and the directional migration of tumor cells across a disrupted
endothelium; thus, cancer metastasis requires communication between
tumor cells and ECs that culminates in the disruption of EC-EC
contacts and the degradation of the vascular basement membrane.
Cell surface adhesion molecules play an important role in the
interaction between the cells and ECM, and some highly metastatic
human melanoma cells adhere and migrate to VCAM-1 rather than
ICAM-1 (24). In addition,
endothelial permeability is a major factor influencing
intravasation, extravasation and invasion in cancer metastasis.
Endothelial cells possess several molecular mechanisms by which
vascular permeability can be modulated. Such mechanisms focus on
adherens junction organization, and in several cases, target
VE-cadherin specifically. Furthermore, the phosphorylation,
cleavage and internalization of VE-cadherin are thought to affect
endothelial permeability (29). In
this study, honokiol inhibited U87MG cell invasion across ECs
through the downregulation of VCAM-1 expression and the inhibition
of phospho-VE-cadherin-mediated EC permeability.
In addition, loss of E-cadherin expression in
epithelial tumors is associated with a more invasive phenotype and
metastasis (14). N-cadherin has
been shown to promote cell motility and migration, an effect
opposite to that of E-cadherin (14). The cadherin switch may occur during
the transition from a benign to an invasive, malignant tumor
phenotype. All gliomas, regardless of grade, lack E-cadherin
expression (1). Concerning the
upstream signals that affect N- and E-cadherin, Snail suppresses
transcription of E-cadherin, and β-catenin induces N-cadherin
expression. Snail and β-catenin are negatively regulated by GSK-3β,
which is regulated by intracellular signaling pathways including
PI3K/Akt. In other words, activation of PI3K/Akt results in the
phosphorylation of GSK-3β (inactivation of GSK-3β), which in turn
increases Snail and β-catenin protein levels. In this study,
honokiol significantly reduced N-cadherin levels but increased
E-cadherin levels in cytosolic fractions. In addition, honokiol
decreased both Snail and β-catenin levels in nuclear fractions.
These results suggest that honokiol suppresses EMT through the
reduction of both Snail and β-catenin levels, which results in the
suppression of N-cadherin and the induction of E-cadherin. Further
study is needed to examine whether honokiol may decrease the
phosphorylation of Akt while increasing the phosphorylation of
GSK-3β.
Taken together, our findings suggest that honokiol
exhibits an inhibitory effect on the process of metastasis by
targeting the interaction between U87MG and BMECs; honokiol
regulates the adhesion of U87MG cells to BMECs by inhibiting VCAM-1
expression in BMECs, and reduces the invasion of U87MG cells
through BMECs by reducing BMEC permeability and inhibiting EMT in
U87MG. These findings suggest that honokiol may serve as a
therapeutic strategy against brain tumors such as glioblastoma.
Abbreviations:
|
BBB
|
blood-brain barrier;
|
|
BCSFB
|
blood cerebrospinal fluid barrier;
|
|
BMECs
|
brain microvascular endothelial
cells;
|
|
CAM
|
cell adhesion molecule;
|
|
DAPI
|
4′,6-diamidino-2-phenylindole;
|
|
ECM
|
extracellular matrix;
|
|
ECL
|
enhanced chemiluminescence;
|
|
EMT
|
epithelial-mesenchymal transition;
|
|
FBS
|
fetal bovine serum;
|
|
GBM
|
glioblastoma multiforme;
|
|
ICAM
|
intracellular adhesion molecule;
|
|
MTT
|
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide;
|
|
PBS
|
phosphate-buffered saline;
|
|
TBS
|
Tris-buffered saline;
|
|
TNF
|
tumor necrosis factor;
|
|
VCAM
|
vascular cell adhesion molecule
|
Acknowledgements
This study was supported by Basic
Science Research Program through the National Research Foundation
of Korea (NRF) funded by the Ministry of Education, Science and
Technology (2012R1A1A3003268) and by the MRC program of MOST/KOSEF
(NRF-2005-0049415).
References
|
1.
|
Nakada M, Nakada S, Demuth T, Tran NL,
Hoelzinger DB and Berens ME: Molecular targets of glioma invasion.
Cell Mol Life Sci. 64:458–478. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Clark MJ, Homer N, O’Connor BD, Chen Z,
Eskin A, Lee H, Merriman B and Nelson SF: U87MG decoded: the
genomic sequence of a cytogenetically aberrant human cancer cell
line. PLoS Genet. 6:e10008322010. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A,
Lacombe D, Cairncross JG, Eisenhauer E and Mirimanoff RO; European
Organisation for Research and Treatment of Cancer Brain Tumor and
Radiotherapy Groups; National Cancer Institute of Canada Clinical
Trials Group: Radiotherapy plus concomitant and adjuvant
temozolomide for glioblastoma. N Engl J Med. 352:987–996. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Fox SB, Turner GD, Gatter KC and Harris
AL: The increased expression of adhesion molecules ICAM-3,
E-selectin and P-selectins on breast cancer endothelium. J Pathol.
177:369–376. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Nizamutdinova IT, Lee GW, Lee JS, Cho MK,
Son KH, Jeon SJ, Kang SS, Kim YS, Lee JH, Seo HG, Chang KC and Kim
HJ: Tanshinone I suppresses growth and invasion of human breast
cancer cells, MDA-MB-231, through regulation of adhesion molecules.
Carcinogenesis. 29:1885–1892. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Christiansen I, Sundstrom C and Totterman
TH: Elevated serum levels of soluble vascular cell adhesion
molecule-1 (sVCAM-1) closely reflect tumour burden in chronic
B-lymphocytic leukaemia. Br J Haematol. 103:1129–1137. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Maeda K, Kang SM, Sawada T, Nishiguchi Y,
Yashiro M, Ogawa Y, Ohira M, Ishikawa T, Hirakawa YS and Chung K:
Expression of intercellular adhesion molecule-1 and prognosis in
colorectal cancer. Oncol Rep. 9:511–514. 2002.PubMed/NCBI
|
|
8.
|
Becker JC, Dummer R, Hartmann AA, Burg G
and Schmidt RE: Shedding of ICAM-1 from human melanoma cell lines
induced by IFN-gamma and tumor necrosis factor-alpha. Functional
consequences on cell-mediated cytotoxicity. J Immunol.
147:4398–4401. 1991.
|
|
9.
|
Osborn L, Hession C, Tizard R, Vassallo C,
Luhowskyj S, Chi-Rosso G and Lobb R: Direct expression cloning of
vascular cell adhesion molecule-1, a cytokine-induced endothelial
protein that binds to lymphocytes. Cell. 59:1203–1211. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Kim HJ, Tsoy I, Park JM, Chung JI, Shin SC
and Chang KC: Anthocyanins from soybean seed coat inhibit the
expression of TNF-α-induced genes associated with
ischemia/reperfusion in endothelial cell by NF-κB-dependent pathway
and reduce rat myocardial damages incurred by ischemia and
reperfusion in vivo. FEBS Lett. 580:1391–1397. 2006.PubMed/NCBI
|
|
11.
|
Nizamutdinova IT, Oh HM, Min YN, Park SH,
Lee MJ, Kim JS, Yean MH, Kang SS, Kim YS, Chang KC and Kim HJ:
Paeonol suppresses intercellular adhesion molecule-1 expression in
tumor necrosis factor-α-stimulated human umbilical vein ECs by
blocking p38, ERK and nuclear factor-κB signaling pathways. Int
Immunopharmacol. 7:343–350. 2007.PubMed/NCBI
|
|
12.
|
Thompson EW and Price JT: Mechanisms of
tumour invasion and metastasis: emerging targets for therapy.
Expert Opin Ther Targets. 6:217–233. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Balkwill F and Mantovani A: Inflammation
and cancer: back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Cavallaro U and Christofori G: Cell
adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev
Cancer. 4:118–132. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Liou KT, Shen YC, Chen CF, Tsao CM and
Tsai SK: Honokiol protects rat brain from focal cerebral
ischemia-reperfusion injury by inhibiting neutrophil infiltration
and reactive oxygen species production. Brain Res. 992:159–166.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Hibasami H, Achiwa Y, Katsuzaki H, Imai K,
Yoshioka K, Nakanishi K, Ishii Y, Hasegawa M and Komiya T: Honokiol
induces apoptosis in human lymphoid leukemia molt 4B cells. Int J
Mol Med. 2:671–673. 1998.PubMed/NCBI
|
|
17.
|
Yang SE, Hsieh MT, Tsai TH and Hsu SL:
Downmodulation of Bcl-XL, release of cytochrome c and sequential
activation of caspases during honokiol induced apoptosis in human
squamous lung cancer CH27 cells. Biochem Pharmacol. 63:1641–1651.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Wang T, Chen F, Chen Z, Wu YF, Xu XL,
Zheng S and Hu X: Honokiol induces apoptosis through
p53-independent pathway in human colorectal cell line RKO. World J
Gastroenterol. 10:2205–2208. 2004.PubMed/NCBI
|
|
19.
|
Hirano T, Gotoh M and Oka K: Natural
flavonoids and lignans are potent cytostatic agents against human
leukemic HL-60 cells. Life Sci. 55:1061–1069. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Crane C, Panner A, Pieper RO, Arbiser J
and Parsa AT: Honokiol-mediated inhibition of PI3K/mTOR pathway: a
potential strategy to overcome immunoresistance in glioma, breast,
and prostate carcinoma without impacting T cell function. J
Immunother. 32:585–592. 2009. View Article : Google Scholar
|
|
21.
|
Wang X, Duan X, Yang G, Zhang X, Deng L,
Zheng H, Deng C, Wen J, Wang N, Peng C, Zhao X, Wei Y and Chen L:
Honokiol crosses BBB and BCSFB, and inhibits brain tumor growth in
rat 9L intracerebral gliosarcoma model and human U251 xenograft
glioma model. PLoS One. 6:e184902011. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Jeong JJ, Lee JH, Chang KC and Kim HJ:
Honokiol exerts an anticancer effect in T98G human glioblastoma
cells through the induction of apoptosis and the regulation of
adhesion molecules. Int J Oncol. 41:1358–1364. 2012.PubMed/NCBI
|
|
23.
|
Ponten J and Macintyre EH: Long term
culture of normal and neoplastic human glia. Acta Pathol Microbiol
Scand. 74:465–486. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Klemke M, Weschenfelder T, Konstandin MH
and Samstag Y: High affinity interaction of integrin alpha4beta1
(VLA-4) and vascular cell adhesion molecule 1 (VCAM-1) enhances
migration of human melanoma cells across activated endothelial cell
layers. J Cell Physiol. 212:368–374. 2007. View Article : Google Scholar
|
|
25.
|
Wu TC: The role of vascular cell adhesion
molecule-1 in tumor immune evasion. Cancer Res. 67:6003–6006. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Davis FG, Freels S, Grutsch J, Barlas S
and Brem S: Survival rates in patients with primary malignant brain
tumors stratified by patient age and tumor histological type:
analysis based on surveillance, epidemiology and end results (SEER)
data, 1973–1991. J Neurosurg. 88:11998.PubMed/NCBI
|
|
27.
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks PB: Identification of a cancer stem
cell in human brain tumors. Cancer Res. 63:5821–5828.
2003.PubMed/NCBI
|
|
28.
|
Moon SH and Park KS: Enrichment of cancer
stem cell of glioblastoma by neurosphere formation. Tissue Eng
Regenerative Med. 9:40–47. 2012.
|
|
29.
|
Dejana E, Orsenigo F and Lampugnani MG:
The role of adherens junctions and VE-cadherin in the control of
vascular permeability. J Cell Sci. 121:2115–2122. 2008. View Article : Google Scholar : PubMed/NCBI
|