Introduction
Malignant mesothelioma, a highly aggressive tumor
which arises from mesothelial cells on serosal surfaces, is most
commonly seen in the pleura, followed by the peritoneum,
pericardium and male genitalia (1,2).
Mesothelioma formation is mostly attributed to asbestos exposure,
and the lag time between exposure and disease is 30–40 years
(3). Although mesothelioma has
been considered to be a rare tumor, its incidence is anticipated to
increase globally (3,4). Malignant mesothelioma has a poor
prognosis; a large population-based study reported that 6-month,
1-year, and 5-year overall survival rates were 55, 33, and 5%,
respectively (5). The median
survival of mesothelioma has been reported to be 9–10 months from
the time of diagnosis (6). In the
classification based on histological categories, three main types
of mesothelioma are reported: epithelioid, sarcomatoid and
biphasic. The epithelioid subtype is the most common and it has a
better prognosis than the sarcomatoid and biphasic subtypes.
Several markers for epithelioid subtype, such as podoplanin,
calretinin, WT-1, cytokeratin 5, thrombomodulin and ERC/mesothelin,
are widely used in the clinical diagnosis of epithelioid
mesothelioma (7,8). However, as no adequate marker is
currently available for sarcomatoid mesothelioma, accurate
diagnosis of the sarcomatoid subtype is difficult. As sarcomatoid
mesothelioma rarely responds to any of the currently available
treatments, development of a new effective treatment is awaited. To
precisely evaluate the efficacy of new types of therapy, an
accurate diagnosis is essential. Immunohistochemistry of specific
markers in cancer specimens plays an important role in the
definitive diagnosis. Therefore, there is a critical need to
determine a new reliable marker for sarcomatoid mesothelioma.
We previously reported that coatomer protein
complex, subunit α (COPA) is highly expressed not only in
epithelioid but also in sarcomatoid mesothelioma, suggesting that
it is a candidate protein as a new marker for the diagnosis of
sarcomatoid (9). Unfortunately,
there was no suitable antibody against COPA for immunohistochemical
staining. To develop a new anti-COPA antibody applicable to
immunohistochemistry, we made four antibodies by immunizing rabbits
with four types of synthetic peptides and evaluated each one as a
candidate antibody for the immunohistochemical staining of COPA,
however, none of these antibodies was suitable. Unexpectedly, one
of these antibodies detected an unknown protein of 120 kDa that was
expressed only in the membrane fraction of the mesothelioma cell
line 211H, which formed a sarcomatoid tumor; this protein was not
expressed in the mesothelial cell line MeT-5A by western blotting.
This unknown protein was considered to be expressed specifically in
mesothelioma and to have the potential to serve as a new molecular
marker for sarcomatoid mesothelioma. In this study, to characterize
this unknown protein, we conducted proteomic analysis of the
membrane fraction of 211H and MeT-5A cells, and identified that the
protein is AHNAK. Then, the expression of AHNAK was evaluated in
mesothelioma cell lines, xenografts and human specimens.
Furthermore, we investigated whether AHNAK is associated with
migration and/or invasion in mesothelioma cell lines.
Materials and methods
Cell culture
We obtained five human mesothelioma cell lines
(211H, H28, H226, H2052, and H2452) and the human mesothelial cell
line MeT-5A from American Type Culture Collections (Manassas, VA,
USA). Two human mesothelioma cell lines ACC-MESO-1 (MESO1) and
ACC-MESO-4 (MESO4) were obtained from RIKEN Cell Bank (Tsukuba,
Japan). The cells were maintained in RPMI-1640 medium supplemented
with 5% FBS (JRH Biosciences, Lenexa, KS, USA) in a humidified
incubator maintained at 37°C with 5% CO2.
Western blotting
We obtained an anti-COPA antibody by immunizing
rabbits with the synthetic peptide CAQFHPTEDL VVSASLDQTV that is a
component part of human COPA. The membrane and cytoplasmic protein
fractions were separated using a ProteoExtract Transmembrane
Protein Extraction kit (Merck Millipore, Billerica, MA, USA).
Western blotting was conducted using anti-COPA or anti-β-actin
antibody (Sigma).
Proteomic analysis
The unknown protein band was detected by western
blotting using the anti-COPA antibody, and gel pieces of the
corresponding size were excised. Proteins in the gel pieces were
analyzed by a liquid chromatography-tandem mass spectrometry
(LC-MS/MS) system and mass spectrometry data were processed to
provide protein identifications using the non-redundant NCBI
protein database with MASCOT software (Matrix Science, Boston, MA,
USA). LC-MS/MS analysis and database search were conducted by APRO
Science (Tokushima, Japan).
Quantitative real-time RT-PCR
We synthesized first-strand cDNAs from the cells
using a FastLane Cell cDNA kit (Qiagen, Hilden, Germany).
Predesigned and preoptimized TaqMan probes to detect AHNAK,
non-erythroid α-spectrin, and 18S rRNA were purchased from Applied
Biosystems (Foster City, CA, USA). Real-time RT-PCR was conducted
in triplicate using a Premix Ex Taq reagent (Takara Bio, Otsu,
Japan). Gene expression levels were normalized to 18S rRNA
expression in each sample. Three independent experiments were
conducted.
Immunofluorescence staining
Cells were grown on glass coverslips and fixed with
4% paraformaldehyde. Immunofluorescence staining was conducted
using anti-AHNAK (Abcom, Cambridge, UK) and Alexa Fluor 594 goat
anti-mouse antibodies (Molecular Probes, Eugene, OR, USA). The
coverslips were mounted in mounting medium with DAPI (Vector
Laboratories, Burlingame, CA, USA). Fluorescence images were
captured with an exposure time of 1/1.5 sec for AHNAK.
Mesothelioma xenografts
The animal experiment was approved by the
Institutional Animal Care and Use Committee of the National
Institute of Radiological Sciences. Mesothelioma xenografts were
established by injecting mesothelioma cells subcutaneously into the
flanks of BALB/c-nu/nu male mice (CLEA Japan, Tokyo, Japan).
Human specimens
Mesothelioma tissue specimens were resected from 10
patients (six sarcomatoid, two epithelioid, and two biphasic
subtypes) in Juntendo University Hospital. This study was approved
by the Institutional Review Boards of Juntendo University School of
Medicine and the National Institute of Radiological Sciences.
Immunohistological analysis
Xenografted tumors and human specimens were fixed in
10% neutral buffered formalin and embedded in paraffin for
sectioning. The tissue sections were stained with the anti-AHNAK or
anti-ERC/mesothelin (22A31) (10)
antibody and counterstained with hematoxylin. The adjacent sections
were stained with hematoxylin-eosin (H&E) staining.
Transfection of siRNA
Stealth RNAi siRNAs for AHNAK and negative control
were purchased from Applied Biosystems. The cells were transfected
with 50 nM siRNA using Lipofectamine 2000 transfection reagent
(Invitrogen, Carlsbad, CA, USA). Forty-eight hours after
transfection, the knockdown efficiency was confirmed by
quantitative real-time PCR as mentioned above.
Cell migration and invasion assays
Migration and invasion assays were conducted in
24-well plates using 8-μm pore size inserts
(Becton-Dickinson Bioscience, Bedford, MA, USA) without Matrigel
coating (for the migration assay) and with Matrigel coating (for
the invasion assay). The inserts were placed into the wells of the
24-well plates containing RPMI-1640 with 10% FBS. Cells
(5×104 cells) in RPMI-1640 with 1% FBS were seeded to
the inserts. After 22 h, non-migrating and non-invasive cells were
removed from the top of the filter, and migrating cells and
invasive cells on the bottom of the filter were fixed with 100%
methanol and stained with 0.125% crystal violet (Wako Pure Chemical
Industries). The cells were counted in five randomly selected
fields at ×200 magnification.
Statistical analysis
The data were statistically analyzed by ANOVA with
the Dunnett’s multiple comparison test, or by Student’s t-test.
Results
Identification of the unknown protein
expressed only in the membrane fraction of 211H cells
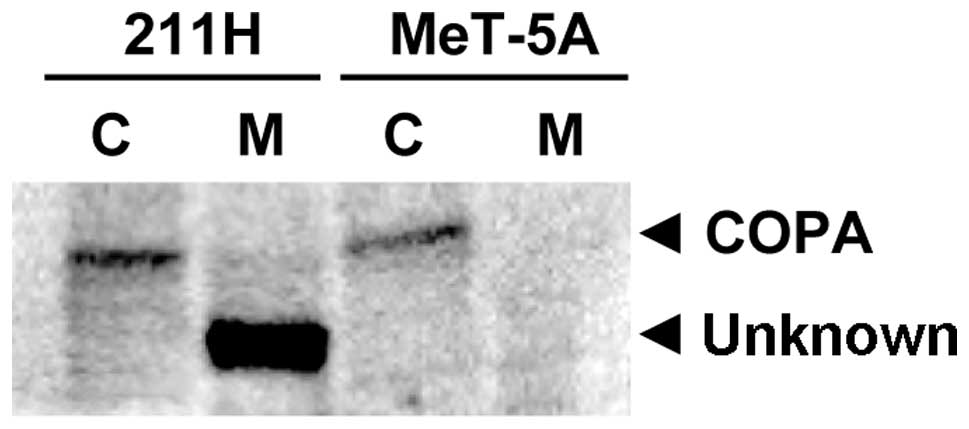
Western blotting with the anti-COPA antibody for
membrane and cytosolic protein fractions of 211H and MeT-5A cells
detected a protein band with the same molecular weight as COPA (140
kDa) in the cytosolic fraction of 211H and MeT-5A cells (Fig. 1). In addition, another protein band
with a smaller molecular weight (120 kDa) compared to COPA was
detected only in the membrane fraction of 211H cells (Fig. 1). To explore whether this protein
band is a fragment of COPA or a different protein, the piece
corresponding to the protein band of 120 kDa was excised from the
gel and subjected to LC-MS/MS analysis. According to the results of
a protein database search of mass spectrometry data, the peptides
in the gel piece of 211H and MeT-5A cells were matched to nine and
12 proteins, respectively. The identified proteins are listed in
Table I with their accession
number (GI no.), name, total score and matched peptide number. Four
proteins (talin, fatty acid synthase, filamin A and nuclear pore
complex protein Nup214) were detected in both 211H and MeT-5A
cells. Although filamin A α (actin binding protein 280), isoform
CRA_a in 211H cells, and filamin-A isoform 1 in MeT-5A cells, have
a distinct accession number (119593150 and 116063573,
respectively), these two proteins are isoforms of filamin A and
these amino acid sequences are 99.5% identical. Five other proteins
(neuroblast differentiation-associated protein AHNAK, non-erythroid
α-spectrin, Treacher Collins syndrome, pro-ubiquitin and protein
BAT2-like 2) were identified only in 211H cells. The remaining
eight proteins (β-spectrin, spectrin-α, β-filamin, non-muscle
myosin heavy chain, retinoid-acid induced protein 1, plectin,
NUP210 protein, and eukaryotic protein synthesis initiation factor)
were identified only in MeT-5A cells. There was no peptide sequence
matching COPA. Between the five proteins identified only in 211H
cells, the total score and matched peptide number of AHNAK (total
score, 1,186; matched peptide number, 24) were markedly higher than
those of the others. Although AHNAK and non-erythroid α-spectrin
were matched by multiple peptides (24 and 5 peptides,
respectively), three other proteins (Treacher Collins syndrome,
pro-ubiquitin, and protein BAT2-like 2) were matched by only one
peptide.
 | Table I.The identified proteins. |
Table I.
The identified proteins.
| Cell line | Accession no. (GI
no.) | Protein name | Total score | No. of peptide |
|---|
| 211H | 6739602 | Talin | 1732 | 37 |
| 61743954 | Neuroblast
differentiation-associated protein AHNAK isoform 1 | 1186 | 24 |
| 38648667 | fatty acid
synthase | 585 | 12 |
| 119593150 | Filamin A, α (actin
binding protein 280), isoform CRA_a | 258 | 7 |
| 33946327 | Neuclear pore complex
protein Nup214 | 181 | 6 |
| 179106 | Non-erythroid
α-spectrin | 150 | 5 |
| 1778432 | Treacher Collins
syndrome | 57 | 1 |
| 340062 | Pro-ubiquitin | 55 | 1 |
| 115298682 | Protein BAT2-like
2 | 45 | 1 |
| MeT-5A | 338443 | β-spectrin | 1403 | 30 |
| 62089306 | Spectrin, α,
non-erythrocytic 1 variant | 1291 | 23 |
| 6739602 | Talin | 837 | 15 |
| 116063573 | Filamin-A isoform
1 | 635 | 17 |
| 3298597 | β-filamin | 197 | 5 |
| 38648667 | Fatty acid
synthase | 161 | 3 |
| 33946327 | Neuclear pore complex
protein Nup214 | 120 | 3 |
| 189036 | Non-muscle myosin
heavy chain (NMHC) | 109 | 1 |
| 12053793 | Retinoid-acid induced
protein 1 | 96 | 1 |
| 1296662 | Plectin | 68 | 1 |
| 45595564 | NUP210 protein | 65 | 1 |
| 3941724 | Eukaryotic protein
synthesis initiation factor | 54 | 1 |
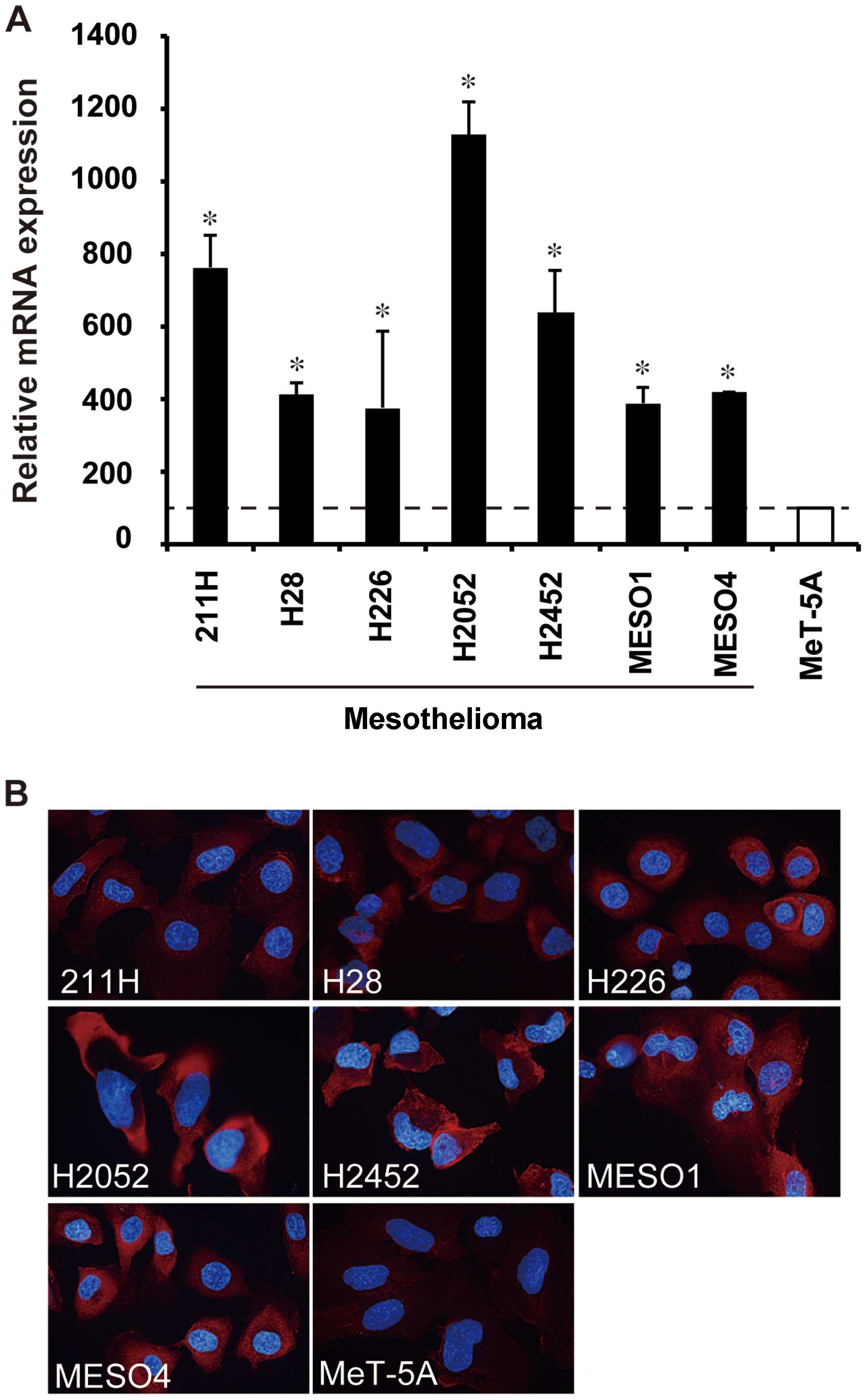
AHNAK expression analysis in cultured
cells derived from human mesothelioma
The mRNA expression of AHNAK and non-erythroid
α-spectrin in mesothelioma and mesothelial cell lines was measured
by real-time RT-PCR. AHNAK mRNA expression in all seven
mesothelioma cell lines (211H, H28, H226, H2052, H2452, MESO1 and
MESO4) was significantly higher compared with that in the
mesothelial cell line MeT-5A (P<0.05) (Fig. 2A). AHNAK mRNA levels of
mesothelioma cell lines increased by 1.5- to 5.1-fold in comparison
with that of MeT-5A (Fig. 2A),
whereas the non-erythroid α-spectrin mRNA level of 211H cells was
less than half of MeT-5A (data not shown). By immunofluorescence
staining, a high protein expression of AHNAK was observed in all
mesothelioma cell lines, and particularly in H2052 cells (Fig. 2B), whereas the AHNAK expression in
MeT-5A cells was faint (Fig. 2B).
The protein expression levels seemed to be correlated with the mRNA
levels.
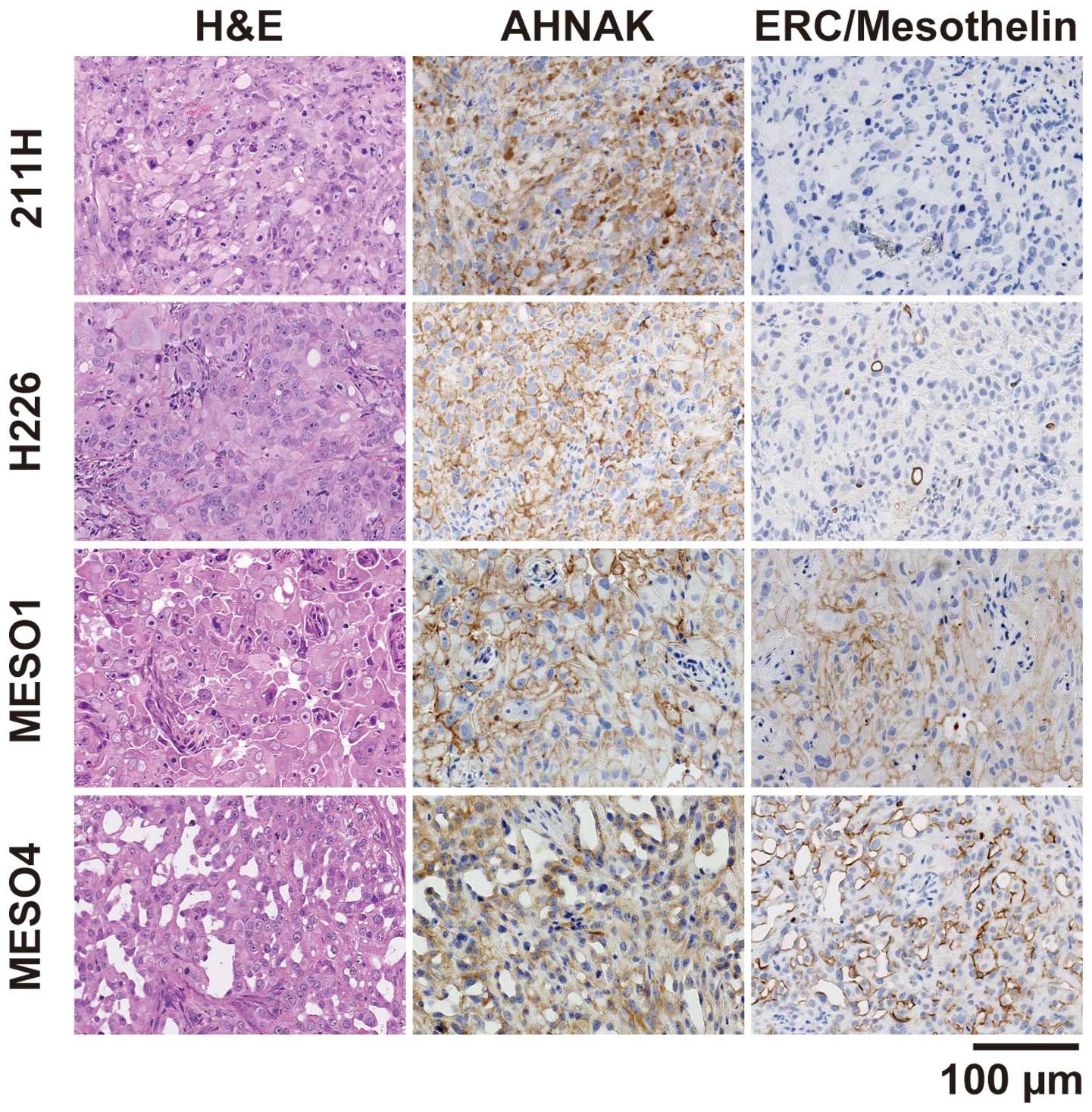
AHNAK expression analysis in mesothelioma
xenograft tumors
To examine the protein expression of AHNAK in
xenograft tumors, the mesothelioma cell lines were injected
subcutaneously into the flank of nude mice. Four cell lines (211H,
H226, MESO1 and MESO4) formed tumors at the site of inoculation,
but the remaining three cell lines (H28, H2052, and H2452) failed
to induce tumor formation. AHNAK protein was expressed in all four
xenograft tumors as determined by immunohistochemical staining
using the anti-AHNAK antibody (Fig.
3). The cytoplasm and plasma membrane of 211H tumors was
intensely stained with the anti-AHNAK antibody and the plasma
membrane of the other xenografts was intensely stained. The
epithelioid marker ERC/mesothelin was detected in H226, MESO1 and
MESO4 xenografts, but not in 211H. The 211H xenograft had spindle
elongated cell morphology and was negative for ERC/mesothelin,
indicating that it formed a tumor with sarcomatoid features. The
other xenografts showed glandular and solid epithelial patterns and
were positive for ERC/mesothelin, indicating that they formed
tumors with epithelioid features.
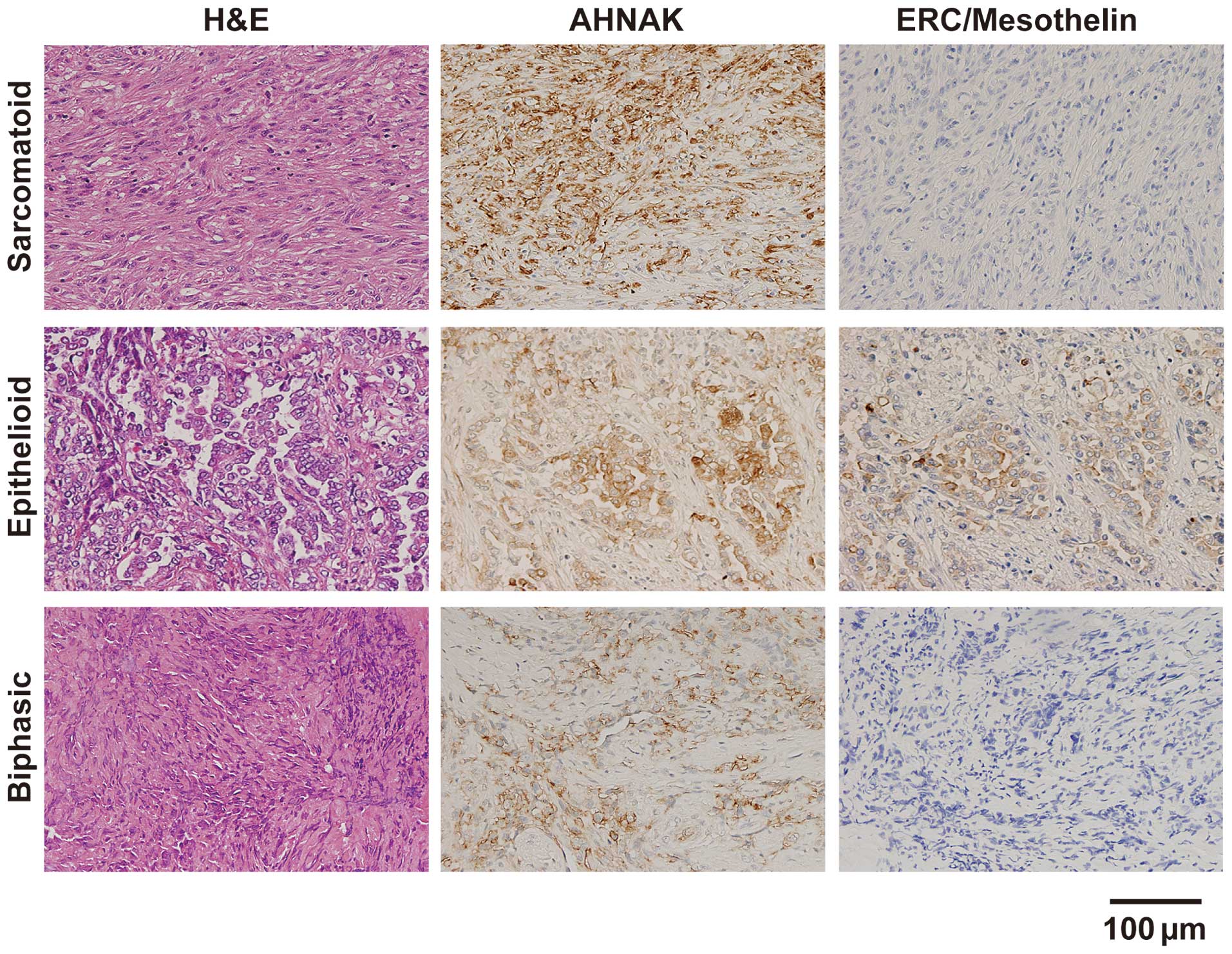
AHNAK expression analysis in surgical
specimens of mesothelioma
We assessed the AHNAK and ERC/mesothelin expression
in resected specimens from patients with sarcomatoid, epithelioid,
or biphasic mesothelioma by immunohistochemical staining (Fig. 4). In the sarcomatoid specimens, the
cytoplasm and plasma membrane of the tumor cells were intensely
stained with the anti-AHNAK antibody, but the intensity of the
plasma membrane tended to be stronger compared with that of the
cytoplasm. ERC/mesothelin expression was not observed. In the
epithelioid specimens, strong expression of both AHNAK and
ERC/mesothelin was detected on the plasma membrane, but not in the
cytoplasm. In one of two biphasic specimens, strong AHNAK
expression was observed on the plasma membrane, but not in the
other biphasic specimen. ERC/mesothelin expression was not observed
in either of the biphasic cases. The summary of immunohistochemical
staining in 10 specimens is shown in Table II. The expression of AHNAK was
detected in 90% (9/10) of all mesothelioma specimens: 100% (6/6) of
sarcomatoid, 100% (2/2) of epithelioid and 50% (1/2) of biphasic.
The ERC/mesothelin expression was detected in 0% (0/6) of
sarcomatoid, 100% (2/2) of epithelioid and 0% (0/2) of biphasic
mesothelioma specimens.
 | Table II.Summary of immunohistochemical
analysis in human specimens. |
Table II.
Summary of immunohistochemical
analysis in human specimens.
| No. | Positive (%) |
|---|
| AHNAK | | |
| Sarcomatoid | 6 | 6 (100) |
| Epithelioid | 2 | 2 (100) |
| Biphasic | 2 | 1 (50) |
| Total | 10 | 9 (90) |
| ERC/mesothelin | | |
| Sarcomatoid | 6 | 0 (0) |
| Epithelioid | 2 | 2 (100) |
| Biphasic | 2 | 0 (0) |
| Total | 10 | 2 (20) |
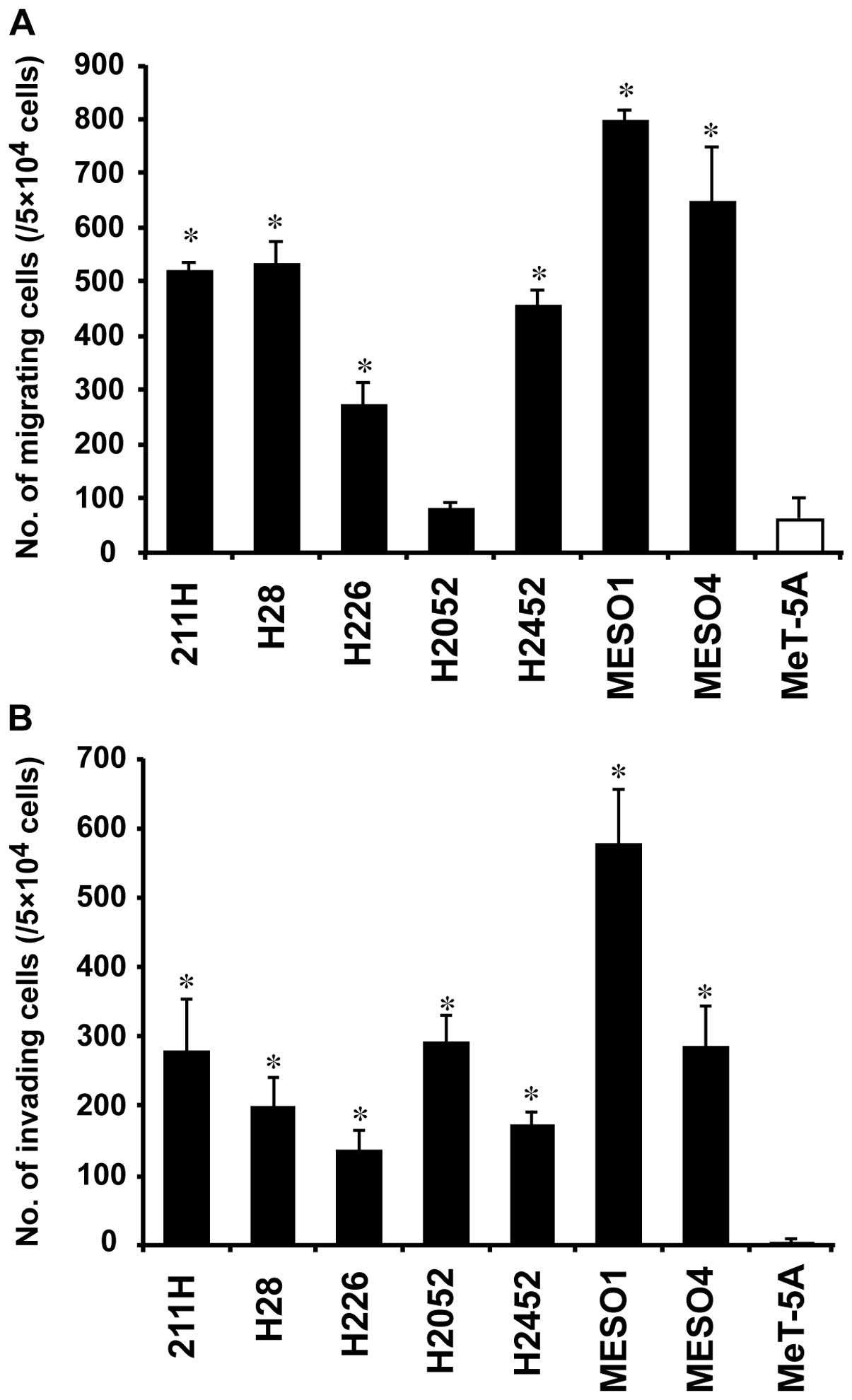
Cell migration and invasion assays
We assessed the cell migration and invasion ability
of the mesothelioma cell lines. The number of migrating cells in
six of the mesothelioma cell lines (211H, H28, H226, H2452, MESO1
and MESO4) was 4.3- to 12.5-fold higher than that in MeT-5A
(P<0.01) (Fig. 5A). No
significant difference was observed between H2052 and MeT-5A
(Fig. 5A). On the other hand, the
number of invading cells in all seven mesothelioma cell lines was
33.5-to 143.9-fold higher than that in MeT-5A (P<0.01) (Fig. 5B).
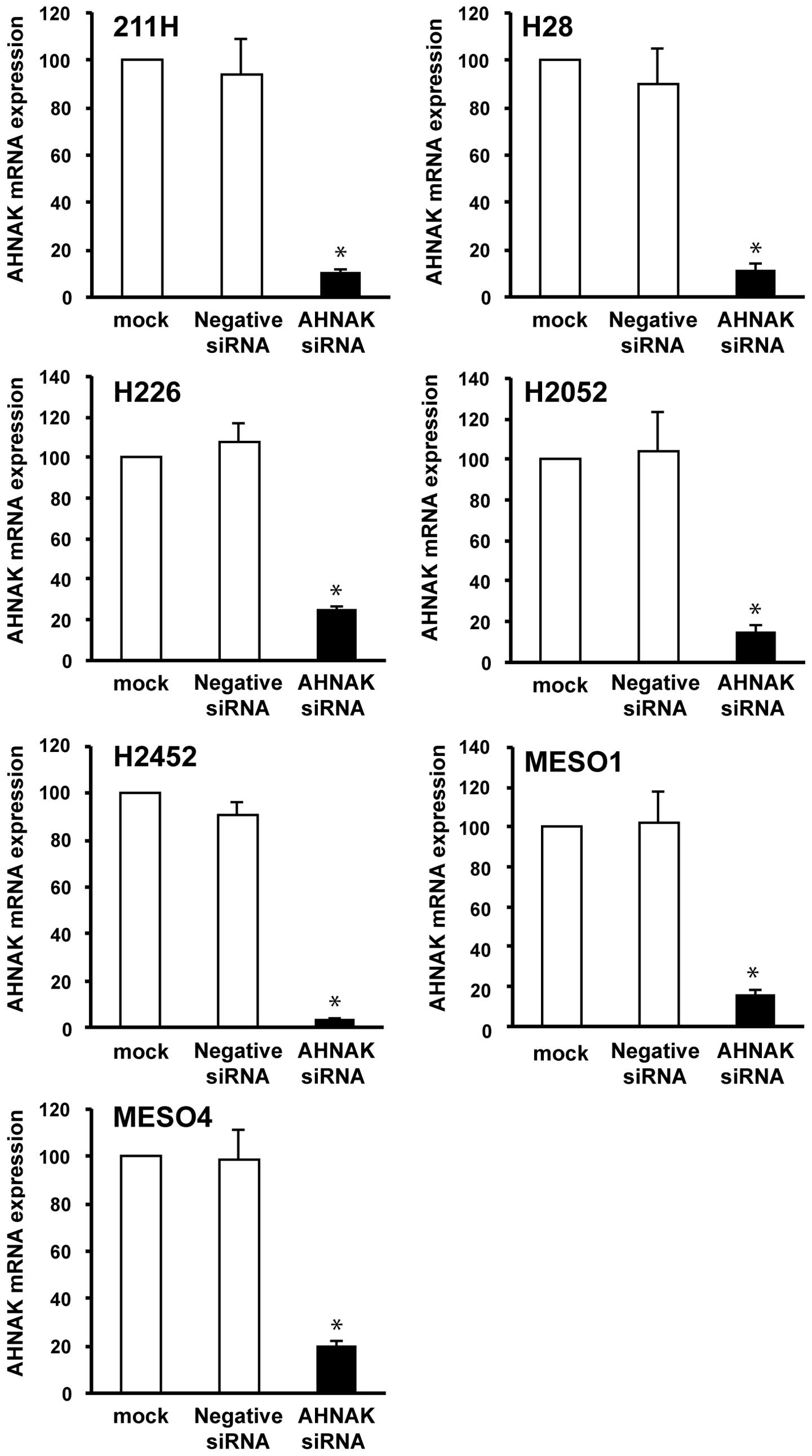
To examine the association of AHNAK and the cell
migration or invasion in mesothelioma cells, we conducted migration
and invasion assays combined with AHNAK knockdown using the siRNA
technique. The knockdown efficiency of the siRNA on AHNAK mRNA
expression was measured by real-time RT-PCR at 48 h after
transfection. The AHNAK mRNA expression in AHNAK siRNA-transfected
mesothelioma cells was reduced to <25 or 23% compared with that
in the mock-transfected cells (without siRNA) or negative control
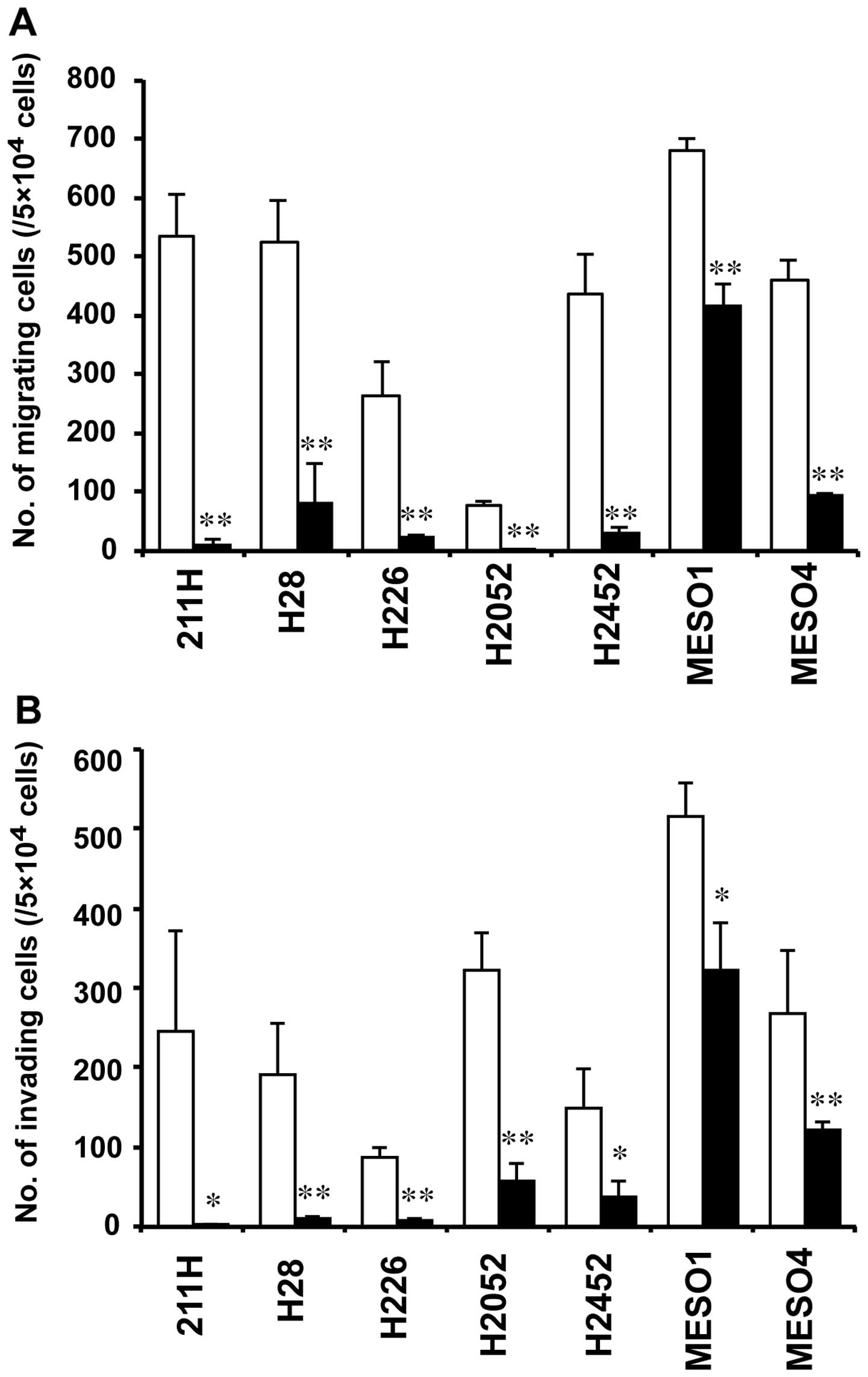
siRNA-transfected cells, respectively (Fig. 6). In six mesothelioma cells (211H,
H28, H226, H2052, H2452 and MESO4), the number of migrating cells
in AHNAK siRNA-transfected cells was decreased to <21% compared
with that in cells transfected with the negative control siRNA at
48 h after transfection (Fig. 7A).
Although the number of migrating cells in MESO1 transfected with
the AHNAK siRNA was decreased to 62% compared with that in cells
transfected with the negative control siRNA, there was a
significant difference (P<0.01) (Fig. 7A). In five mesothelioma cells
(211H, H28, H226, H2052 and H2452), the number of invading cells in
AHNAK siRNA-transfected cells was reduced to <26% compared with
that in cells transfected with the negative control siRNA (Fig. 7B). The number of invading cells in
MESO1 and MESO4 cells transfected with the AHNAK siRNA was reduced
to <63 and 46% compared with that in each negative control
siRNA-transfected cell, respectively (Fig. 7B). However, in all cell lines,
significant differences were observed between the AHNAK
siRNA-transfected and negative control siRNA-transfected cells
(P<0.05 in 211H, H2452 and MESO1; P<0.01 in H28, H226, H2052
and MESO4) (Fig. 7B).
Discussion
Malignant mesothelioma is still a very challenging
problem because its incidence has been increasing worldwide and its
prognosis remains poor (3).
Mesothelioma is classified into three histological subtypes
(epithelioid, sarcomatoid, and biphasic) by pathological
examination including immunohistochemistry. For the epithelioid
subtype, the immunohistochemical staining of diagnosis based on
pathological markers is well established and achieves an accurate
diagnosis, however, to date no adequate maker for the sarcomatoid
subtype has been found. There is a critical need to determine a
reliable diagnostic marker for sarcomatoid mesothelioma (7,8).
We previously reported that COPA is a candidate
protein for use as a pathological maker in the diagnosis of the
sarcomatoid subtype of mesothelioma because it is highly expressed
in both epithelioid and sarcomatoid mesothelioma models (9). Since there was no anti-COPA antibody
suitable for immunohistochemistry, we newly produced and evaluated
four antibodies. Unexpectedly, we found that one of these
antibodies detected an unknown protein of 120 kDa expressed only in
the membrane fraction of the sarcomatoid mesothelioma cell line
211H, and not in the mesothelial cell line MeT-5A. By proteomic
analysis of the gel pieces corresponding to the protein band of 120
kDa, five proteins (neuroblast differentiation-associated protein
AHNAK, nonerythroid α-spectrin, Treacher Collins syndrome,
pro-ubiquitin and protein BAT2-like 2) were found only in 211H
cells as candidates of the unknown protein. Although AHNAK and
non-erythroid α-spectrin were matched by multiple peptides, the
three other proteins were matched by only one peptide, suggesting
that these three proteins were unlikely to be the unknown protein.
The non-erythroid α-spectrin mRNA level of 211H cells was half of
MeT-5A, in contrast to AHNAK mRNA which was highly expressed in all
seven mesothelioma cell lines. These results suggest that AHNAK is
the most likely to be the unknown protein. Although the original
molecular weight of AHNAK was 680 kDa (11,12),
Huang et al reported that AHNAK is cleaved by calpain 3,
resulting in the production of an AHNAK fragment of 120 kDa
(13). This fragment size is
consistent with the molecular weight of the unknown protein
detected by the anti-COPA antibody. Unfortunately, we could not
verify whether the AHNAK fragment is the same molecular weight of
the unknown protein in the membrane fraction of 211H cells by
western blotting, because we could not find any antibody to work
well in western blotting for AHNAK. However, these findings can
support the fact that our anti-COPA antibody detected the AHNAK
fragment in the membrane fraction of 211H cells.
We conducted further experiments to evaluate the
expression of AHNAK in mesothelioma tissues. Immunohistochemical
staining in xenograft tumors showed that AHNAK was highly expressed
in all of four xenografts 211H, H226, MESO1 and MESO4 that could
form tumors in nude mice. The 211H xenograft had sarcomatoid
features and the others had epithelioid features. These results
suggest that AHNAK is expressed in both sarcomatoid and epithelioid
mesothelioma. To evaluate the AHNAK protein expression in clinical
specimens of mesothelioma, we conducted immunohistochemical
analysis of 10 mesothelioma specimens including sarcomatoid,
epithelioid and biphasic subtypes. Tumor cells in 90% of
mesothelioma specimens (except for one case with the biphasic
subtype) were intensely stained with the anti-AHNAK antibody.
ERC/mesothelin was expressed in 100% of the epithelioid cases, but
0% of the sarcomatoid cases. Although further studies will be
necessary to confirm our findings in a larger number of human
specimens, the present study suggests that AHNAK has the potential
to be a new molecular marker for detecting mesothelioma and that
the combined immunohistochemical staining of AHNAK with
conventional marker(s) for epithelioid, such as ERC/mesothelin,
could provide helpful information for diagnosing sarcomatoid
mesothelioma.
AHNAK is reported to be a membrane scaffold protein
(14) and the expression is
downregulated in several cell lines derived from neuroblastoma,
small cell lung carcinomas and Burkitt lymphomas (15,16);
and upregulated in several metastatic tumor cell lines derived from
prostate cancer, breast cancer, fibrosarcoma and glioma (17). To our knowledge, the present study
is the first showing that AHNAK is highly expressed in
mesothelioma. According to a previous study of metastatic tumor
cell lines, AHNAK is considered to be one of the essential proteins
for pseudopod protrusion and it is involved in tumor cell migration
and invasion (17). AHNAK
knockdown via RNA interference induced pseudopod retraction,
inhibited cell migration and invasion, reduced actin cytoskeleton
dynamics, and induced mesenchymal to epithelial transition
(17). Many mesothelioma patients
receive a diagnosis of local invasion into the endothoracic fascia,
mediastinal fat, chest wall or pericardium (18). The local invasion is a major factor
preventing surgical resection; therefore, 40% of mesothelioma
patients had unresectable tumors at the time of the initial
diagnosis (19). We hypothesized
that the high expression of AHNAK is involved in the local invasion
of mesothelioma. According to cell migration and invasion assays,
six of the seven mesothelioma cell lines showed a higher cell
migration rate and all seven mesothelioma cell lines showed
increased invasion ability compared with the mesothelial cell line
MeT-5A. The AHNAK expression level was not correlated with
migration or invasion potency in the seven mesothelioma cell lines.
Taken together, AHNAK above a certain expression level might
promote cell migration and invasion. The inhibition effect on
migration and invasion by AHNAK knockdown in MESO1 cell lines was
somewhat lower compared with that in the other lines, suggesting
that there could be additional molecule(s) associated with
migration and invasion in mesothelioma. Our findings suggest that
AHNAK is one of the key molecules of cell migration and invasion in
mesothelioma.
In conclusion, we report that the unknown protein of
120 kDa that is expressed only in the membrane fraction of 211H
mesothelioma cells was identified as AHNAK by proteomic and
expression analyses. AHNAK was highly expressed not only in
xenograft tumors but also in mesothelioma specimens including
sarcomatoid, epithelioid, and biphasic subtypes. These results
suggest that AHNAK has the potential to be a new marker for
detecting mesothelioma, although further clinical investigation is
needed. Furthermore, we demonstrated that AHNAK could be involved
in the cell migration and invasion of mesothelioma cell lines. Our
findings support further clinical investigation to evaluate whether
AHNAK expression is associated with metastasis in mesothelioma.
Acknowledgements
The authors thank Yuriko Ogawa for
technical assistance.
References
|
1.
|
Robinson BW and Lake RA: Advances in
malignant mesothelioma. N Engl J Med. 353:1591–1603. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Tsiouris A and Walesby RK: Malignant
pleural mesothelioma: current concepts in treatment. Nat Clin Pract
Oncol. 4:344–352. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Kanazawa N, Ioka A, Tsukuma H, Ajiki W and
Oshima A: Incidence and survival of mesothelioma in Osaka, Japan.
Jpn J Clin Oncol. 36:254–257. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Peto J, Decarli A, La Vecchia C, Levi F
and Negri E: The European mesothelioma epidemic. Br J Cancer.
79:666–672. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Milano MT and Zhang H: Malignant pleural
mesothelioma: a population-based study of survival. J Thorac Oncol.
5:1841–1848. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Nojiri S, Gemba K, Aoe K, et al: Survival
and prognostic factors in malignant pleural mesothelioma: a
retrospective study of 314 patients in the west part of Japan. Jpn
J Clin Oncol. 41:32–39. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Ordonez NG: Immunohistochemical diagnosis
of epithelioid mesothelioma: an update. Arch Pathol Lab Med.
129:1407–1414. 2005.PubMed/NCBI
|
|
8.
|
Sterman DH and Albelda SM: Advances in the
diagnosis, evaluation, and management of malignant pleural
mesothelioma. Respirology. 10:266–283. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Sudo H, Tsuji AB, Sugyo A, et al:
Knockdown of COPA, identified by loss-of-function screen, induces
apoptosis and suppresses tumor growth in mesothelioma mouse model.
Genomics. 95:210–216. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Ishikawa K, Segawa T, Hagiwara Y, Maeda M,
Abe M and Hino O: Establishment of novel mAb to human
ERC/mesothelin useful for study and diagnosis of
ERC/mesothelin-expressing cancers. Pathol Int. 59:161–166. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Hieda Y and Tsukita S and Tsukita S: A new
high molecular mass protein showing unique localization in
desmosomal plaque. J Cell Biol. 109:1511–1518. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Hashimoto T, Amagai M, Parry DA, et al:
Desmoyokin, a 680 kDa keratinocyte plasma membrane-associated
protein, is homologous to the protein encoded by human gene AHNAK.
J Cell Sci. 105:275–286. 1993.PubMed/NCBI
|
|
13.
|
Huang Y, de Morree A, van Remoortere A, et
al: Calpain 3 is a modulator of the dysferlin protein complex in
skeletal muscle. Hum Mol Genet. 17:1855–1866. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Pankonien I, Otto A, Dascal N, Morano I
and Haase H: Ahnak1 interaction is affected by phosphorylation of
Ser-296 on Cavbeta(2). Biochem Biophys Res Commun. 421:184–189.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Shtivelman E, Cohen FE and Bishop JM: A
human gene (AHNAK) encoding an unusually large protein with a
1.2-microns polyionic rod structure. Proc Natl Acad Sci USA.
89:5472–5476. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Shtivelman E and Bishop JM: The human gene
AHNAK encodes a large phosphoprotein located primarily in the
nucleus. J Cell Biol. 120:625–630. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Shankar J, Messenberg A, Chan J, Underhill
TM, Foster LJ and Nabi IR: Pseudopodial actin dynamics control
epithelialmesenchymal transition in metastatic cancer cells. Cancer
Res. 70:3780–3790. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Servais EL, Colovos C, Rodriguez L, et al:
Mesothelin over-expression promotes mesothelioma cell invasion and
MMP-9 secretion in an orthotopic mouse model and in epithelioid
pleural mesothelioma patients. Clin Cancer Res. 18:2478–2489. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Flores RM, Zakowski M, Venkatraman E, et
al: Prognostic factors in the treatment of malignant pleural
mesothelioma at a large tertiary referral center. J Thorac Oncol.
2:957–965. 2007. View Article : Google Scholar : PubMed/NCBI
|