Introduction
Globins are hemo-containing proteins, have the
ability to bind gaseous ligands [oxygen (O2), nitric
oxide (NO) and carbon monoxide (CO)] reversibly. They have been
described in prokaryotes, fungi, plants and animals with an
enormous diversity of structure and function (1). To date, hemoglobin, myoglobin,
neuroglobin (Ngb) and cytoglobin (Cygb) represent the vertebrate
globin family with distinct function and tissue distributions
(2). During ontogeny, developing
erythroblasts sequentially express embryonic {[Gower 1 (ζ2ε2),
Gower 2 (α2ε2), and Portland 1 (ζ2γ2)] to fetal [Hb F(α2γ2)] and
then adult [Hb A (α2β2), 97%; Hb A2 (α2δ2), <3%]} hemoglobin
(3). Adult hemoglobin, the most
well studied globin in terms of structure, function and evolution
(5), consists of two α and β
polypeptides globin chains (heterotetrameric protein) where each
globin molecule contains a hydrophobic pocket that non-covalently
binds an iron-protoporphyrin IX molecule (4).
In contradiction to previous thought, recently
hemoglobin has been reported to be expressed not only in enucleated
red cells, but also in other cell types. Hemoglobin expression has
been reported in rodent brain (neurons of the cortex, hippocampus
and cerebellum but not astrocytes and oligodendrocytes) (6); embryonic and adult mouse endometrium
(7); alveolar epithelial type II
cells of both rat (8) and human
(9); and human breast cancer cells
(10). Generally, vertebrate
hemoglobin is known to function as a carrier protein of
O2 and CO2 (9). However, recently hemoglobin has been
reported to generate and transport NO or to scavenge NO and its
metabolic derivatives (11,12).
As well as, other potential functions, including anti-oxidant and
superoxide anion and H2O2 scavenging
properties have been reported (13,14).
Glioblastoma multiforme (GBM) is the most common and
the most aggressive of astrocytic tumors, constituting 25% of all
malignant nervous system tumors (15). Despite aggressive multimodality
treatments, GBM is characterized by a very poor prognosis with
median survival rate of about one year (16). The capability of GBM cells to
infiltrate surrounding normal tissues (17), along with unidentified glial
defence mechanisms due to expression of unknown protectant
molecules, makes curative treatment by surgery, radiation and
chemotherapies difficult, if not impossible.
We have previously reported that Ngb and Cygb are
expressed in human GBM cell lines (18–20),
as well as in human primary tumors including brain tumors (19). In this study, we examined whether
hemoglobins (α, β, γ, δ, ζ and ε) are also expressed in human GBM
cell lines.
Materials and methods
Cell lines and in vitro culture
conditions
The characterization and origin of the GBM cell
lines have been published previously: the M059J (ATCC no. CRL2366,
Manassas, VA, USA) is radio-sensitive and hypoxia-sensitive; M059K
(ATCC no. CRL-2365) is radio-resistant and hypoxia-tolerant and
M006x cell lines are hypoxia-tolerant (21–24).
The U87T (tumor) and U87R (invasive) cell lines are established GBM
cell lines (25) and were kindly
provided by Dr Donna Senger (University of Calgary, Calgary, AB,
Canada). All cells were maintained as monolayer cultures in
DMEM/F12 media supplemented with 10% fetal calf serum and 1 mM
L-glutamine in a humidified atmosphere of 5% CO2 in air
at 37°C. All tissue culture supplies were purchased from Gibco
(Carlsbad, CA, USA).
RNA extraction and reverse
transcription
RNeasy mini kit (Qiagen, Valencia, CA, USA) was used
to isolate total RNA from GBM cultured cell lines. Reverse
transcription (RT) was carried out with 0.1–1 μg total RNA
per 20 μl reaction volume using QuantiTect reverse
transcription kit (Qiagen).
Reverse transcription-PCR
Conventional RT-PCR was carried out with GeneAmp PCR
system 9700 (Applied Biosystems, Foster City, CA, USA) using
Platinum Pfx DNA polymerase (Invitrogen, Carlsbad, CA, USA) for 35
cycles. To detect α, β, γ, δ, ζ and ε globin genes, specific
oligonucleotide primer pairs (Table
I) were designed using primer 3 plus program (26). For β, γ and ε globin genes, two
primer pairs were designed to ensure their presence. For reaction
specificity, different annealing temperatures were applied
(Table I) where the annealing
temperature for each set of primers was determined empirically by
repeating the RT-PCR reaction at many different temperatures.
Amplified RT-PCR products were separated in 2% agarose gel
electrophoresis and visualized by ethidium bromide.
 | Table I.Oligonucleotide primer pairs. |
Table I.
Oligonucleotide primer pairs.
| Globin target
sequence | Forward primer
5′→3′ | Reverse primer
5′→3′ | Annealing temperature
(°C) | PCR product size
(bp) | Lane number on the
agarose gel (Fig. 1) |
|---|
| Alpha |
atgttcctgtccttccccaccaccaag |
gcttaacggtatttggaggtcagcacg | 62 | 334 | 2 |
| Beta |
gtgaacgtggatgaagttggtggtgag |
ttggacagcaagaaagcgagcttagtg | 54 | 411 | 3 |
| Beta |
tctgtccactcctgatgctg |
tgctcaaggcccttcataat | 52 | 394 | 4 |
| Gamma |
acactcgcttctggaacgtc |
gaattctttgccgaaatgga | 48 | 422 | 5 |
| Gamma |
gtggaagatgctggaggaga |
gaattctttgccgaaatgga | 48 | 309 | 6 |
| Delta |
ctgggcagattactggtggt |
ggcattgtgttcccaagttc | 44 | 440 | 8 |
| Epsilon |
gaatgtggaagaggctggag |
atgtgcagaaggagggtgtc | 55 | 443 | 9 |
| Epsilon |
gcacatatctgcttccgaca |
ccttgccaaagtgagtagcc | 55 | 421 | 10 |
| Zeta |
gaggaccatcattgtgtcca |
gaattctttgccgaaatgga | 44 | 377 | 12 |
DNA sequencing
Bands of RT-PCR amplicons were excised from the
agarose gel and purified using QIAEX II Gel Extraction Kit
(Qiagen). Cycle sequencing protocol was carried out using
BigDye® Terminator v3.1 (Applied Biosystems), BigDye
Terminator v1.1 and v3.1 5X sequencing buffer (Applied Biosystems)
and one of the primer pairs used in RT-PCR. The sequence reaction
was cycled for 25 cycles (96°C for 1 min, 96°C for 10 sec, 50°C for
5 sec, 60°C for 4 min, rapid thermal ramp to 4°C and hold) using
thermal cycler. Sequencing reaction was precipitated
(ethanol/EDTA), washed, dried out, resuspended in 10 μl
de-ionized formamide (injection buffer) and sequenced using (ABI
PRISM® 310 Genetic Analyzer, Applied Biosystems).
Sequencing data were analysed by NCBI BLAST engine.
Western blot analysis
To detect globin proteins, M006x cells were washed
four times with PBS. Whole-cell lysates were prepared using
complete Lysis-M buffer (Roche Diagnostics, Indianapolis, IN, USA)
and protein content determined using a protein assay kit (Pierce,
Rockford, IL, USA). Equal amounts of protein (50 μg) were
resolved using Tris-Tricine-PAGE or 13% SDS-PAGE under reducing
condition and electro-transferred to polyvinylidene difluoride
(PVDF) membranes (Bio-Rad Laboratories, Hercules, CA, USA).
Membranes were blocked with 5% serum derived from the same animal
species in which the secondary antibody is raised. Different
primary antibodies were used in this study: 1:100 human anti-human
hemoglobin monoclonal antibody (HCA042, AbD Serotec, Kidlington,
UK); 1:100 goat anti-human hemoglobin (α chain) polyclonal antibody
(SC-31110, Santa Cruz Biotechnology Inc, Santa Cruz, CA); 1:100
mouse anti-human hemoglobin monoclonal antibodies, γ chain
(SC-21756) and β chain (SC-21757, Santa Cruz Biotechnology); and
1:500 mouse anti-human hemoglobin (ε chain) monoclonal antibodies
(10C-CR8008M1, Fitzgerald Industries International Inc, Concord,
MA). Membranes were incubated with primary antibodies diluted in 5%
serum for 2 h. In consistent with the primary antibodies host
species, different secondary antibodies conjugated with horseradish
peroxidase were used: 1:1,000 goat anti-human IgG
F(ab′)2-polyclonal antibody (0500-0099, AbD Serotec); 1:6,000 goat
anti-mouse IgG and 1:4,000 rabbit anti-goat polyclonal antibodies
(DakoCytomation Denmark A/S, Glostrup, Denmark). Membranes were
incubated with secondary antibodies diluted in 5% serum for 1 h.
Bound proteins were detected using chemiluminescence reagents
(SuperSignal West Pico Chemiluminescent Substrate, Thermo
Scientific, Rockford, IL) and visualized by exposing to X-ray film
(Fuji Photo Film, Tokyo, Japan) that was developed using a Kodak
X-OMAT 2000A processor (Eastman Kodak Company, Tokyo, Japan).
Mass spectrometry and protein
sequencing
M006x cells were lysed in complete Lysis-M buffer
(Roche Diagnostics) and the protein content was determined using a
protein assay kit (Pierce). Cellular lysates were incubated with
the appropriate antibodies; goat anti-human hemoglobin (α chain,
SC-31110, and β/γ/δ/ε chain, SC-22718, Santa Cruz Biotechnology);
polyclonal antibody, mouse anti-human (γ chain, SC-21756, and β
chain, SC21757) hemoglobin monoclonal antibodies (Santa Cruz
Biotechnology); and mouse anti-human hemoglobin [ε chain]
monoclonal antibodies (10C-CR8008M1, Fitzgerald Industries
International). Immunoprecipitation was carried out using an
immunoprecipitation kit (Dynabeads Protein G, Invitrogen) according
to manufacturer’s instructions. Eluted proteins were reduced,
heated and subjected to SDS-PAGE. The gel was stained (Bio-Safe
Coomassie Stain, Bio-Rad) and the excised bands were analyzed by
mass spectrometry at Institute of Biomolecular Design (IBD),
University of Alberta (Edmonton, AB, Canada). In-gel tryptic
digestion, peptide extraction and mass spectrometric analysis were
done as described by Lopez-Campistrous et al (27) except that the ion trap mass
spectrometry (LCQ™ Deca XP ion trap mass spectrometer,
Thermo Finnigan, San Jose, CA, USA) was used.
Immunofluorescence cell staining
Cultured cells were grown overnight on sterile
coverslips. Cells were washed four times with PBS, fixed in cold
methanol for 5 min and air dried for 15 min. The cells were washed
with three changes of PBS, incubated with 10% normal serum (serum
derived from the same animal species in which the secondary
antibody is raised) for 30 min and then washed with PBS. Different
primary antibodies were used; goat anti-human hemoglobin (1:75)
polyclonal antibody (α chain, SC-31110, Santa Cruz Biotechnology);
mouse anti-human (β chain, SC21757) and (γ chain, SC-21756)
hemoglobin (1:75) monoclonal antibodies (Santa Cruz Biotechnology)
and mouse anti-human hemoglobin (1:250) monoclonal antibodies (ε
chain, 10C-CR8008M1, Fitzgerald Industries International). Cells
were incubated with primary antibody diluted in 1% serum for 1 h.
Different secondary antibodies were used [1:250 goat anti-mouse IgG
polyclonal antibody Cy3 conjugated (610-704-124, Rockland
Immunochemicals for Research, Gilbertsville, PA, USA); 1:250 donkey
anti-goat IgG polyclonal antibody Alexa Flour 488 conjugated
(A11055, Molecular Probes Inc., Eugene, OR, USA)]. Cells were
washed three times with PBS and then incubated for 45 min with
secondary antibody diluted in 1% serum. Cells were washed three
with PBS (5 min each) and then the coverslips were mounted on
slides with mounting media containing DAPI (ProLong Gold antifade
reagent with DAPI, P36935, Invitrogen).
Results
Expression of α, β, γ, δ, ζ and ε globin
m RNA in GBM cells in vitro
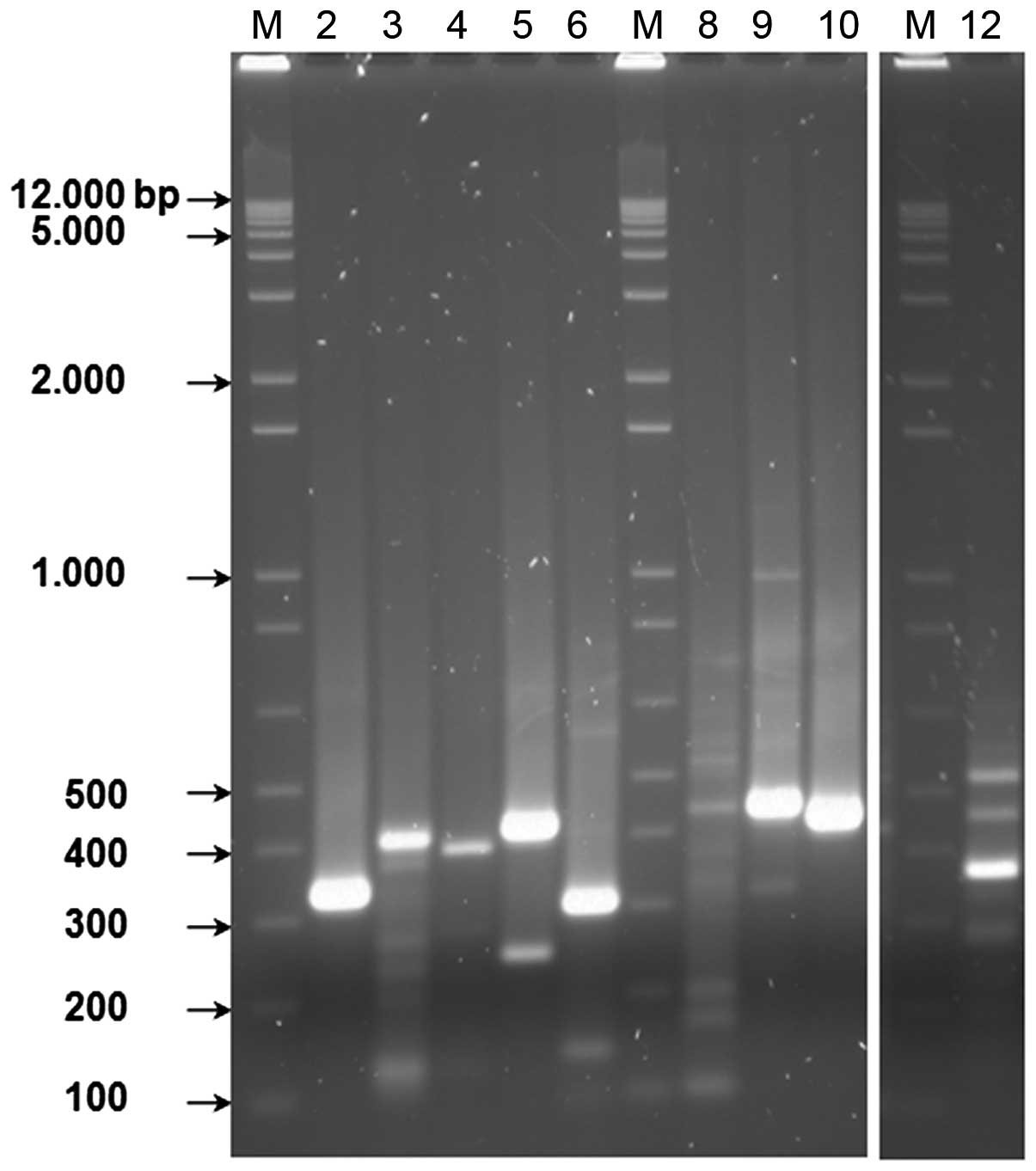
Amplified RT-PCR products separated on agarose gel
revealed clear bands that coincide with their predicted sizes using
different primer pairs (Fig. 1).
Analysis of the DNA sequences extracted from the agarose bands
using the NBCI BLAST engine revealed perfect alignments to their
correspondents α, β, γ, δ, ζ and ε globins mRNA in GenBank
(Table II).
 | Table II.DNA sequencing of RT-PCR
amplicons. |
Table II.
DNA sequencing of RT-PCR
amplicons.
| Globin | Primer used | mRNA
transcripts | GenBank match | Identities |
|---|
| α globin | F | Hs
hemoglobin alpha 1 (HBA1) | NM_000558.3 | 99% (NB
216–468) |
| Hs
hemoglobin alpha 2 (HBA2) | NM_000517.4 | 98% (NB
245–497) |
| R | Hs
hemoglobin alpha 1 (HBA1) | NM_000558.3 | 98% (NB
143–439) |
| Hs
hemoglobin alpha 2 (HBA2) | NM_000517.4 | 98% (NB
172–468) |
| β globin | F1 | Hs
hemoglobin beta (HBB) | NM_000518.4 | 92% (NB
145–503) |
| R1 | Hs
hemoglobin beta (HBB) | NM_000518.4 | 91% (NB
119–459) |
| F8 | Hs
hemoglobin beta (HBB) | NM_000518.4 | 94% (NB
272–586) |
| R8 | Hs
hemoglobin beta (HBB) | NM_000518.4 | 99% (NB
194–529) |
| γ globin 1 | F | Hs
hemoglobin gamma 2 (HBG2) | NM_000184.2 | 100% (NB
55–405) |
| Hs
hemoglobin gamma 1 (HBG1) | NM_000559.2 | 99% (NB
55–405) |
| R | Hs
hemoglobin gamma 2 (HBG2) | NM_000184.2 | 99% (NB
21–379) |
| Hs
hemoglobin gamma 1 (HBG1) | NM_000559.2 | 98% (NB
21–379) |
| F | Hs
hemoglobin gamma 2 (HBG2) | NM_000184.2 | 98% (NB
139–412) |
| Hs
hemoglobin gamma 1 (HBG1) | NM_000559.2 | 97% (NB
139–412) |
| R | Hs
hemoglobin gamma 2 (HBG2) | NM_000184.2 | 99% (NB
114–379) |
| Hs
hemoglobin gamma 1 (HBG1) | NM_000559.2 | 99% (NB
114–379) |
| δ globin | F | Hs
hemoglobin delta (HBD) | NM_000519.3 | 99% (NB
320–710) |
| R | Hs
hemoglobin delta (HBD) | NM_000519.3 | 100% (NB
319–679) |
| ε globin | F | Hs
hemoglobin epsilon 1 (HBE1) | NM_005330.3 | 99% (NB
348–738) |
| R | Hs
hemoglobin epsilon 1 (HBE1) | NM_005330.3 | 99% (NB
326–704) |
| F | Hs
hemoglobin epsilon 1 (HBE1) | NM_005330.3 | 100% (NB
255–601) |
| R | Hs
hemoglobin epsilon 1 (HBE1) | NM_005330.3 | 100% (NB
210–576) |
| ζ globin | F | Hs
hemoglobin zeta 1 (HBZ1) | NM_005332.2 | 99% (NB
207–581) |
| R | Hs
hemoglobin zeta 1 (HBZ1) | NM_005332.2 | 98% (NB
167–550) |
Expression of α, β, γ, δ, ζ and ε globin
proteins in GBM cells in vitro
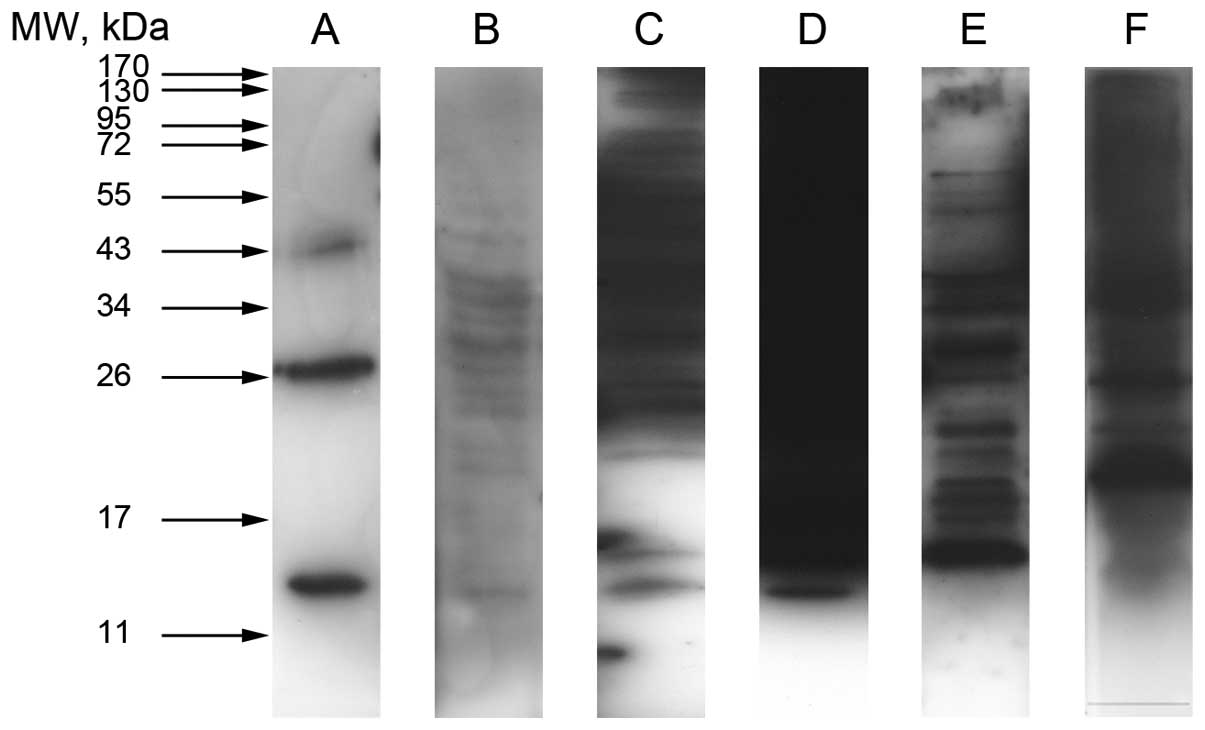
Western blot analyses of cell-lysate of M006x cells
showed the presence of α, β, γ, δ, ζ and ε globin protein (Fig. 2). As shown by others (9,28)
and as indicated in the manufacturer’s data sheet (AbD Serotec),
adult hemoglobin and globins specific antibodies detected protein
bands of 14, 26, 43 kDa and higher in cell lysate and hemoglobin
standard protein despite reduction and heat denaturing of the
samples. The heterotetramer and globin dimer were more easily
detected than monomer globins that require long exposure time
accompanied by high background. Mass spectrometry analysis of
different globin immunoprecipitates showed peptide match to α, β,
δ, and ε globin protein (Table
III). The unsuccessful detection of γ and ζ globin protein by
mass spectrometry could be due to multiple factors including
unsuitability of their relevant antibodies for the application of
immunoprecipitation.
 | Table III.Peptides of globin proteins
identified by mass spectrometry. |
Table III.
Peptides of globin proteins
identified by mass spectrometry.
| Accession no. | Protein hit | Matched
peptides | Ion score | Protein
scorea |
|---|
| gi|229751 | Chain α, structure
of haemoglobin in the deoxy quaternary state with ligand bound at
the alpha haems (Homo sapiens) | VGAHAGEYGAEALER
(pos. 17–31) | 61 | 136 |
| TYFPHFDLSHGSAQVK
(pos. 41–56) | 45 |
|
VADALTNAVAHVDDMPNALSALSDL HAHK (pos.
62–90) | 29 |
| gi|26892090 | Beta globin chain
variant (Homo sapiens) | SAVTALWGK (pos.
10–18) | 39 | 292 |
| VNVDEVGGEALGR (pos.
19–31) | 52 |
| GTFATLSELHCDK (pos.
84–96) | 50 |
| LLGNVLVCVLAHHFGK
(pos. 106–121) | 30 |
| KFTPPVQAAYQK
(pos.122–133) | 61 |
| VVAGVANALAHK (pos.
134–145) | 59 |
| gi|73762521 | Delta globin
Troodos variant (Homo sapiens) | VHLTPEEK (pos.
2–9) | 30 | 124 |
| LLGNVLVCVLACNFGK
(pos. 106–121) | 38 |
| VVAGVANALAHK (pos.
134–145) | 55 |
| gi|78099200 | Hemoglobin subunit
epsilon Bradypus tridactylus | LLVVYPWTQR (pos.
32–41) | 35 | 80 |
| LVGGVANALAHK
(pos.134–145) | 45 |
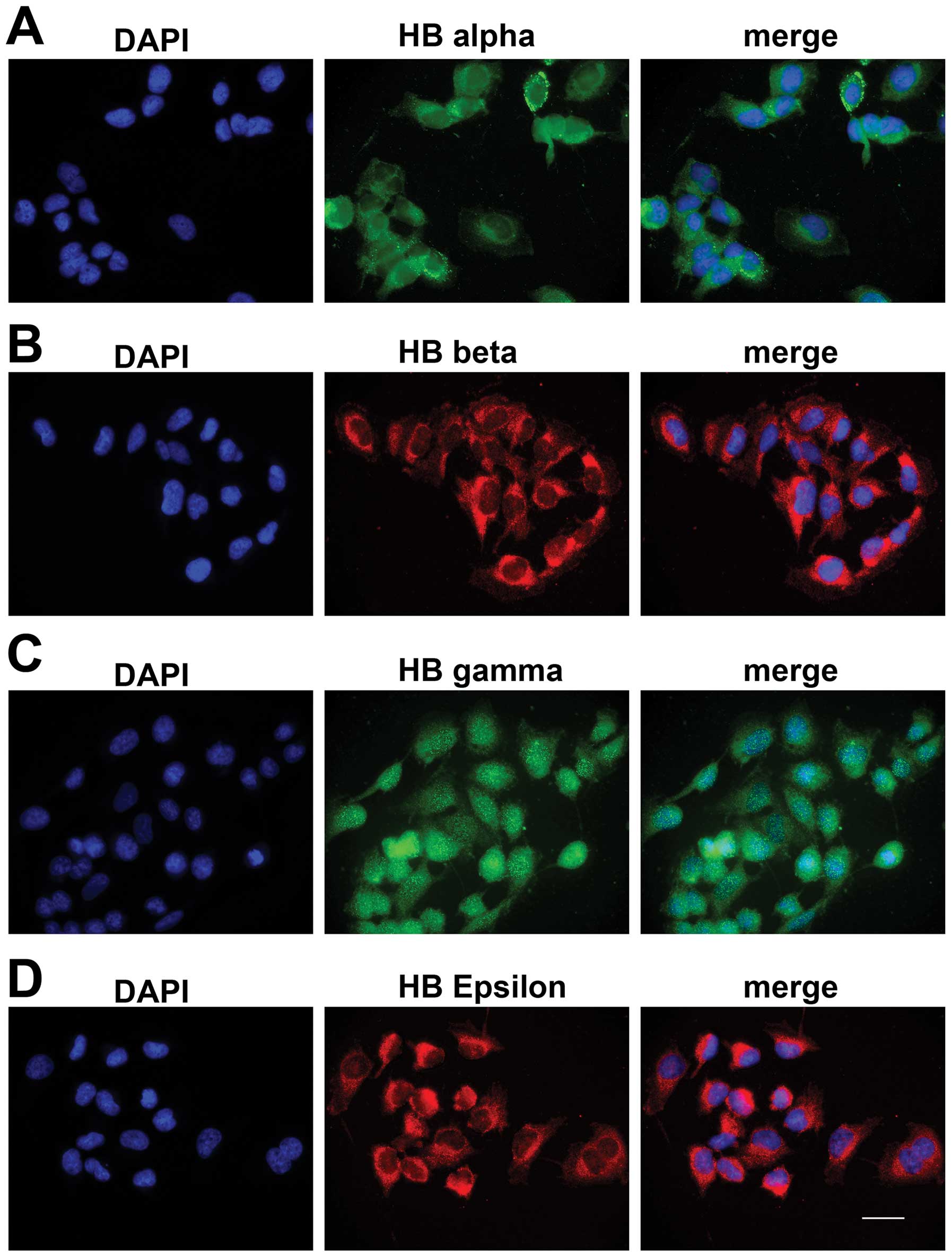
Immunofluorescence analysis
To confirm the expression of globin proteins,
immunofluorescence experiments were carried out. Anti-human α, β, γ
and ε antibody incubated with fixed M006x cells that grown under
aerobic conditions (Fig. 3)
displayed both cytoplasmic and nuclear staining. This is consistent
with the staining pattern of α, β globin previously observed in rat
neurons (6,28), murine and human A9 dopaminergic
neurons, a subpopulation of cortical and hippocampal astrocytes,
and in mature oligodentrocytes (29). Expression of α, β and ε globin was
higher in the cytoplasm whereas γ globin was predominant in the
nucleus.
Discussion
We have previously reported that two newly
discovered globins, neuroglobin and cytoglobin, are expressed in
human primary GBM tumors and cell lines (18,19).
Here, using conventional RT-PCR and DNA sequencing, western blot
analysis, immunofluorescence staining and mass spectrometry, we
confirmed the presence of different hemoglobins (α, β, γ, δ, ζ and
ε) in GBM cells under normal physiological conditions. Others have
reported α and β globin expression in rodent neurons, but not in
astrocytes and oligodendrocytes (6,28),
whereas Biagioli et al (29) reported their presence in A9
dopaminergic neurons, a subpopulation of cortical and hippocampal
astrocytes and in mature oligodentrocytes. However, the
heterogeneity of gliomas (30) and
ability of gliobalstoma cell lines to co-express glial and neuronal
markers (31) may partially
explain the presence of neuroprotective globin molecules such as
neuroglobin in GBM cells (18), as
well as hemoglobin.
The most important functions of erythroid hemoglobin
are the transport of O2 and CO2 (9), to bind CO, and to scavenge and
release NO (32). Anti-oxidative
function of hemoglobin has been manifested by reducing production
of hydrogen peroxide-induced intracellular radical oxygen species
and enhancing cell viability after overexpression of the detected
hemoglobin in rat mesangial cells (14). Moreover, human hemoglobin can
detoxify highly oxidizing radicals yielding the respective
non-toxic ferric states (33).
Interestingly, hemoglobin was detected in breast cancer tissues and
MCF7 cell line but not in normal breast tissue (10) suggesting a functional role for
hemoglobin in carcinogenesis.
The expression of different types of hemoglobins (α,
β, γ, δ, ζ and ε) representing fetal, embryonic and adult
hemoglobin in GBM cells may imply an undefined potential role for
these hemoglobins at different stages of cellular growth and
adaptation, assisting those cells to resist aggressive
multimodality treatments and consequently enabling cell survival.
In addition, expression of the hemoglobins in all tested cell
lines; M006x (hypoxia-tolerant) M059J (radio-sensitive and
hypoxia-sensitive), M059K (radio-resistant and hypoxia-tolerant)
(21–24) U87T (tumor) and U87R (invasive) cell
lines (25) may indicate that
hemoglobin expression is not limited to GBM cells with certain cell
characteristics but it is a common property of all tested cell
lines.
Although α and β globin expression has been reported
in many non-erythroid cells, to our knowledge, we are the first to
report the expression of α, β, γ, δ, ζ and ε globins in human GBM
cell lines under normal physiological conditions. Our results,
together with the known non-oxygen transport related functions of
hemoglobin suggest that hemoglobin expression in GBM cells may be a
part of series of active defence and adaptation mechanisms by which
these cells resist even aggressive multimodality treatments.
Further experiments are required to investigate whether hemoglobin
is upregulated under hypoxic conditions simulating hypoxic tumor
microenvironment, and whether those hemoglobins are differentially
regulated.
Acknowledgements
We thank Dr Richard Fahlman and Jack
Moore for help with mass spectrometry, Dr Xuejun Sun for help with
immunofluorescence imaging, and Bonnie Andrais for assistance with
tissue culture. This study was supported by an award from the
Canadian Cancer Society Research Institute with funds provided by
the Canadian Cancer Society.
References
|
1.
|
Hardison RC: A brief history of
hemoglobins: plant, animal, protist, and bacteria. Proc Natl Acad
Sci USA. 93:5675–5679. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Roesner A, Fuchs C, Hankeln T and
Burmester T: A globin gene of ancient evolutionary origin in lower
vertebrates: evidence for two distinct globin families in animals.
Mol Biol Evol. 22:12–20. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Bunn HF and Forget BG: Hemoglobin:
Molecular, Genetic, and Clinical Aspects. WB Saunders Co;
Philadelphia, PA: 1986
|
|
4.
|
Hardison RC: Globin genes on the move. J
Biol. 7:352008. View
Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Pesce A, Bolognesi M, Bocedi A, et al:
Neuroglobin and cytoglobin. Fresh blood for the vertebrate globin
family EMBO Rep. 3:1146–1151. 2002.
|
|
6.
|
Schelshorn DW, Schneider A, Kuschinsky W,
et al: Expression of hemoglobin in rodent neurons. J Cereb Blood
Flow Metab. 29:585–595. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Dassen H, Kamps R, Punyadeera C, et al:
Haemoglobin expression in human endometrium. Hum Reprod.
23:635–641. 2008. View Article : Google Scholar
|
|
8.
|
Bhaskaran M, Chen H, Chen Z and Liu L:
Hemoglobin is expressed in alveolar epithelial type II cells.
Biochem Biophys Res Commun. 333:1348–1352. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Newton DA, Rao KM, Dluhy RA and Baatz JE:
Hemoglobin is expressed by alveolar epithelial cells. J Biol Chem.
281:5668–5676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Gorr TA, Wichmann D, Pilarsky C, et al:
Old proteins - new locations: myoglobin, haemoglobin, neuroglobin
and cytoglobin in solid tumours and cancer cells. Acta Physiol
(Oxf). 202:563–581. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Cosby K, Partovi KS, Crawford JH, et al:
Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates
the human circulation. Nat Med. 9:1498–1505. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Huang Z, Shiva S, Kim-Shapiro DB, et al:
Enzymatic function of hemoglobin as a nitrite reductase that
produces NO under allosteric control. J Clin Invest. 115:2099–2107.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Masuoka N, Kodama H, Abe T, Wang DH and
Nakano T: Characterization of hydrogen peroxide removal reaction by
hemoglobin in the presence of reduced pyridine nucleotides. Biochim
Biophys Acta. 1637:46–54. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Nishi H, Inagi R, Kato H, et al:
Hemoglobin is expressed by mesangial cells and reduces oxidant
stress. J Am Soc Nephrol. 19:1500–1508. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Brandes AA, Tosoni A, Franceschi E, Reni
M, Gatta G and Vecht C: Glioblastoma in adults. Crit Rev Oncol
Hematol. 67:139–152. 2008. View Article : Google Scholar
|
|
16.
|
Lim SK, Llaguno SR, McKay RM and Parada
LF: Glioblastoma multiforme: a perspective on recent findings in
human cancer and mouse models. BMB Rep. 44:158–164. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Alves TR, Lima FR, Kahn SA, et al:
Glioblastoma cells: A heterogeneous and fatal tumor interacting
with the parenchyma. Life Sci. 89:532–539. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Emara M, Salloum N and Allalunis-Turner J:
Expression and hypoxic up-regulation of neuroglobin in human
glioblastoma cells. Mol Oncol. 3:45–53. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Emara M, Turner AR and Allalunis-Turner J:
Hypoxic regulation of cytoglobin and neuroglobin expression in
human normal and tumor tissues. Cancer Cell Int. 10:332010.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Fang J, Ma I and Allalunis-Turner J:
Knockdown of cytoglobin expression sensitizes human glioma cells to
radiation and oxidative stress. Radiat Res. 176:198–207. 2011.
View Article : Google Scholar
|
|
21.
|
Allalunis-Turner MJ, Barron GM, Day RS
III, Dobler KD and Mirzayans R: Isolation of two cell lines from a
human malignant glioma specimen differing in sensitivity to
radiation and chemotherapeutic drugs. Radiat Res. 134:349–354.
1993. View
Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Allalunis-Turner MJ, Barron GM, Day RS
III, Fulton DS and Urtasun RC: Radiosensitivity testing of human
primary brain tumor specimens. Int J Radiat Oncol Biol Phys.
23:339–343. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Allalunis-Turner MJ, Franko AJ and
Parliament MB: Modulation of oxygen consumption rate and vascular
endothelial growth factor mRNA expression in human malignant glioma
cells by hypoxia. Br J Cancer. 80:104–109. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Parliament MB, Allalunis-Turner MJ, Franko
AJ, et al: Vascular endothelial growth factor expression is
independent of hypoxia in human malignant glioma spheroids and
tumours. Br J Cancer. 82:635–641. 2000.PubMed/NCBI
|
|
25.
|
Johnston AL, Lun X, Rahn JJ, et al: The
p75 neurotrophin receptor is a central regulator of glioma
invasion. PLoS Biol. 5:e2122007. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Untergasser A, Nijveen H, Rao X, Bisseling
T, Geurts R and Leunissen JA: Primer3Plus, an enhanced web
interface to Primer3. Nucleic Acids Res. 35:W71–W74. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Lopez-Campistrous A, Semchuk P, Burke L,
et al: Localization, annotation, and comparison of the
Escherichia coli K-12 proteome under two states of growth.
Mol Cell Proteomics. 4:1205–1209. 2005.
|
|
28.
|
He Y, Hua Y, Liu W, Hu H, Keep RF and Xi
G: Effects of cerebral ischemia on neuronal hemoglobin. J Cereb
Blood Flow Metab. 29:596–605. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Biagioli M, Pinto M, Cesselli D, et al:
Unexpected expression of alpha- and beta-globin in mesencephalic
dopaminergic neurons and glial cells. Proc Natl Acad Sci USA.
106:15454–15459. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Valtz NL, Hayes TE, Norregaard T, Liu SM
and McKay RD: An embryonic origin for medulloblastoma. New Biol.
3:364–371. 1991.PubMed/NCBI
|
|
31.
|
Rebetz J, Tian D, Persson A, et al: Glial
progenitor-like phenotype in low-grade glioma and enhanced
CD133-expression and neuronal lineage differentiation potential in
high-grade glioma. PLoS One. 3:e19362008. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Schechter AN: Hemoglobin research and the
origins of molecular medicine. Blood. 112:3927–3938. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Goldstein S and Samuni A: Intra- and
intermolecular oxidation of oxymyoglobin and oxyhemoglobin induced
by hydroxyl and carbonate radicals. Free Radic Biol Med.
39:511–519. 2005. View Article : Google Scholar : PubMed/NCBI
|