Contents
Introduction
Vitamin D and cancer
Vitamin D receptor
Common VDR polymorphisms and their associated
alleles
Exploration of VDR polymorphisms
Ethnic variation is crucial in predicting effects of
VDR gene polymorphisms
Vitamin D and breast cancer
Vitamin D and renal cancer
Search methods for the meta-analysis
Results of the meta-analysis
Concluding remarks
Introduction
Cancer is the leading cause of death worldwide, and
accounted for 7.6 million deaths in 2008. The incidence of cancer
continues to rise with an estimated 13.1 million deaths in 2030
(1). Breast cancer is a common
disease and the leading cause of death among women around the
world, with its incidence is increasing annually. It is estimated
that 1 in 12 British women and 1 in 8 American women will develop
breast cancer at some time in her life, the overall death ratio
among women with this disease has been established at 20%, and the
expected time of survival, at 5 years after the diagnosis (2). There are several constant risk
factors for breast cancer, namely age, gender, density of breast
tissue, benign breast conditions, early menarche, late menopause,
previous chest exposure to radiation, exposure to
diethylstilbestrol and genetic risk factors (BRCA-1 and BRCA-2)
(3,4). As for the risk factors that may
differ across patients, they include nulliparity or the first
pregnancy after the age of 30, postmenopausal hormone treatment,
combined use of estrogen and progesterone, breast feeding, alcohol
consumption, postmenopausal obesity and insufficient physical
activity (90). When renal cancer
is taken into consideration, it accounts for 3% of total human
malignancies and is the most common type of heterogeneous
urological cancer with high prevalence in elderly men (>60 years
old) (5). Because of the high
mortality level due to metastasis (Czech Republic, Poland) renal
cancer is considered to be one of the most important urological
cancers in Central and Eastern Europe (6,89).
There are several risk factors linked to the development of renal
cancer, such as obesity, smoking, high blood pressure, ethnicity,
age and family history or genetic risk factors [the most common one
being the von Hippel-Lindau (VHL) syndrome] (7). Therefore, in this review we aimed to
assess the correlation between the different alleles of VDR gene
polymorphisms and renal cell cancer and breast cancer risks
separately, through a systematic review of the present
literature.
Vitamin D and cancer
There are two major forms of vitamin D: D2
[calciferol or 1,24-dihydroxyvitamin D(2)] and D3 [calcitriol or
1,25-dihydroxyvitamin D(3)]. Anticancer properties have been
attributed primarily to vitamin D3 (8). Vitamin D3 binds to the vitamin D
receptor (VDR). This ligand-receptor complex regulates
transcription of >60 genes involved in anti-proliferative,
pro-differentiating, anti-metastatic and pro-apoptotic effects on
cells and the cell cycle (Fig. 1:
vitamin D conversion pathways) (9–12).
In the mammary gland, vitamin D3 regulates calcium transport during
lactation and acts together with mammary cell differentiation
hormones and milk protein production (13). Laboratory studies have also
demonstrated that vitamin D3 and its analogues inhibit cell
proliferation and promote apoptosis in cancer cells in culture
(14–18). These findings have led to the
development of vitamin D analogues that could serve as potential
new therapeutic agents in breast cancer treatment in humans. The
observation of a reduced risk of breast cancer among women with
high vitamin D status also supports the hypothesis that vitamin D
plays a crucial role in cancer development (19–21).
However, breast cancer is also known to be strongly influenced by
the hormonal milieu and mutations in genes involved in hormone
metabolism. The kidney has a unique function in mineral homeostasis
especially for calcium and phosphorous. The vitamin D-endocrine
system plays a key role in controlling the reabsorption of calcium
by the kidney (22,23). The kidney is the major site for
synthesis of vitamin D3 (24).
Other vitamin D3-dependent proteins and the VDR that are important
in calcium reabsorption are also expressed in the kidney (25,26).
All in all, biological and epidemiological data suggest that
vitamin D3 levels influence the development of renal cancer
(27–29).
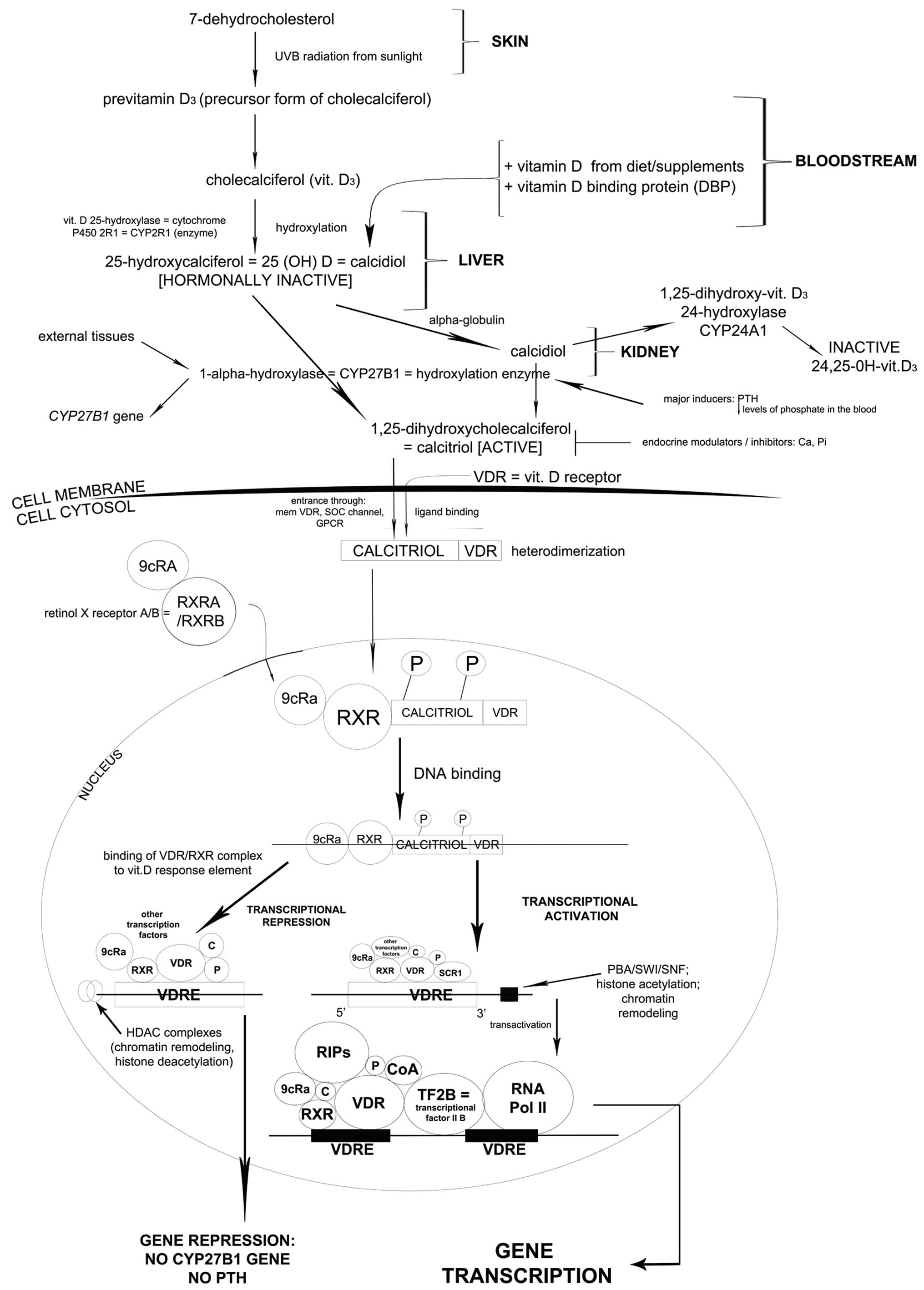 | Figure 1.7-Dehydrocholesterol undergoes
specific alterations in the skin, liver and kidney. As a result
calcitriol is formed and enters the cell cytosol through specific
channels together with vitamin D receptor (VDR). After
heterodimerization, the complex enters the nucleus and as a result,
a multi-component complex is formed (phosphorylated calcitriol-VDR
complex, retinol X receptor, 9cRa transcription factor) which
subsequently binds to DNA. In the presence of HDAC complexes and
other transcription factors, CYP27B1 gene responsible for
parathormone production is repressed. However, in the presence of
PBA/SWI/SNF complex other compounds are added (regulators of
interaction, transcriptional factor IIB and most important of all,
RNA polymerase II). As a consequence, CYP27B1 transcription occurs.
The protein encoded by this gene localizes to the inner
mitochondrial membrane where it hydroxylates 25-hydroxyvitamin D3
at the 1α position. This reaction synthesizes
1α,25-dihydroxyvitamin D3, the active form of vitamin D3, which
binds to the VDR. |
Vitamin D receptor
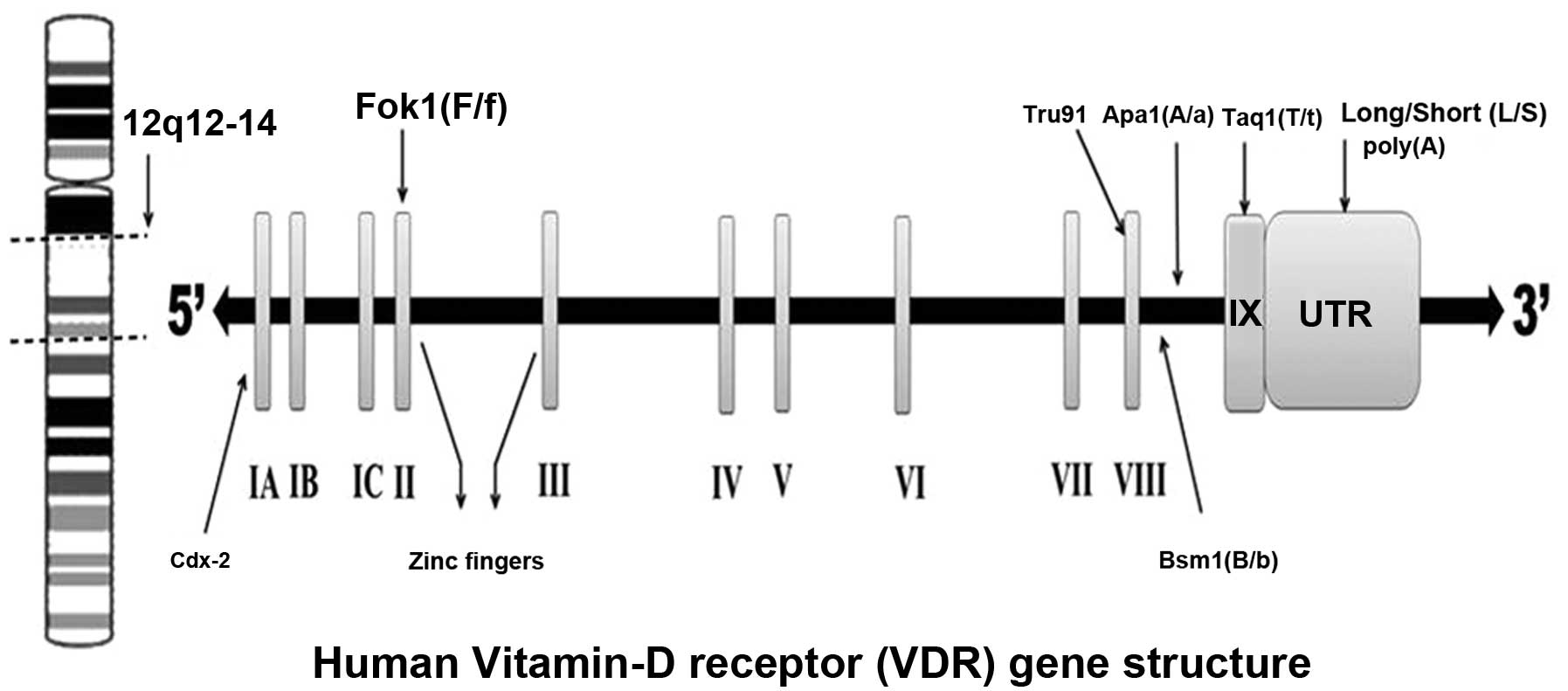
The VDR was discovered in 1969 (30). The VDR receptor gene is located on
the long arm of chromosome 12 (12q12–14) with at least five
promoter regions, and is composed of at least 11 exons that span 60
kb of DNA (31,32). The first exon is not translated
(33), while exons 2–8 encode the
VDR protein (Fig. 2).
Polymorphisms of the VDR gene have been associated with several
forms of cancer and other chronic diseases (30). The associations between the VDR
gene polymorphisms and breast and renal cancers have been
investigated by a number of studies (20,29,34–36).
It has been postulated that the VDR gene polymorphisms may
influence both the risk of cancer occurrence and its prognosis. The
purpose of this review is to analyze the association of some common
VDR gene polymorphisms, such as Taq1, Fok1, Apa1, Bsm1, Cdx2,
poly(A) and Bgl1, with breast and renal cancers.
Common VDR polymorphisms and their
associated alleles
Most studies on the VDR gene polymorphisms
association with breast and renal cancers have focused on seven
types of polymorphisms: Fok1 polymorphism in exon II (37), Bsm1 (38) and Apa1 (39) in intron VIII, Cdx2 (40) in exon I, Taq1 (41) in exon IX, Tru91 (42) in intron VIII, and the poly(A)
(43) mono-nucleotide repeat in
the 3’-untranslated region (3’-UTR) section of the gene.
Individuals are generally classified as tt, Tt or TT for Taq1
polymorphism (T allele: absence of Taq1 restriction site; t allele:
presence of Taq1 restriction site). The Fok1 polymorphism is
located near the 5’-UTR region of the gene. The f allele for the
Fok1 polymorphism shows less transcriptional activity than the F
allele (44,45). All other polymorphisms have been
found close to the 3’-UTR region of the gene (46). The polymorphisms in the 3’-UTR
region of the gene appear to be in linkage disequilibrium (LD), and
the allele frequencies for these polymorphisms appear to vary
across populations (47).
Individuals with the Bsm1 polymorphism are classified as bb, Bb, BB
genotypes. With respect to poly(A), a bi-allelic polymorphism, an
individual can be classified as: S (short, with <18 A’s) and L
(long, with >168 A’s) poly(A) stretches (45). The individuals associated with Apa1
polymorphism are classified as aa, Aa and AA genotypes. In general,
most of the polymorphisms in the VDR gene are located in regulatory
areas rather than in the coding exons (48).
Exploration of VDR polymorphisms
The VDR polymorphisms that have been studied using
the restriction fragment length polymorphism (RFLP) technique
involve Apa1 (39), Bsm1 (38) and Taq1 (41) restriction polymorphisms at the
3’-end of the VDR gene. The Fok1 polymorphism occurs due to a
thymine/cytosine (T/C) change (44). It basically alters an ACG codon to
an ATG codon located ten base pairs upstream from the translation
start codon, which results in the formation of an additional start
codon. If protein translation starts from this altered site, a
larger VDR protein with three additional amino acids will be formed
(49). Taq1 is a substitution of
nucleotide ATT for ATC in exon IX, leading to a synonymous change
at codon 252 (isoleucine) (50,51).
Although Bsm1 and Apa1 are considered as silent single nucleotide
polymorphisms (SNPs) that do not change the sequence of coding
amino acids like in Fok1 (52),
they may influence gene expression through regulation of mRNA
stability. The Cdx2 polymorphism is a guanine (G) to adenine (A)
alteration in the promoter region of the VDR gene, specifically at
the binding site for an intestinal specific transcription factor
known as Cdx2 (53). The A allele
binds to the Cdx2 transcription factor with a higher affinity, and
yields increased transcriptional activity (40). As a consequence, the A allele may
result in a higher VDR expression in the intestine, and therefore
in an increased bone mineral density (BMD) through a better
intestinal absorption of calcium (35,40,54).
The poly(A) polymorphism occurs in the 3’-UTR region of the VDR
gene, which is characterized by a variable number of tandem repeats
(VNTR) (43). Finally, two
polymorphisms, namely A-1012G and Tru91, have been poorly
investigated. The A-1012G polymorphism consists in a substitution
of A for G (55). The Tru91 is a G
(U allele) to A (u allele) polymorphism in the VDR gene (42).
Ethnic variation is crucial in predicting
effects of VDR gene polymorphisms
Genetic studies based on different ethnic
backgrounds provide excellent opportunities to link molecular
insight with epidemiological data (35). Variation in DNA sequences occurs
frequently in the population, and has a substantial biological
impact on the development of certain diseases, including cancer.
The Cdx2 polymorphism was first discovered in the Japanese
(40), and has recently been
studied in a Caucasian population by Fang et al (54). However, the study revealed that the
Cdx2 polymorphism A allele occurs more commonly in African (74%)
and Asian (43%) populations than among the Caucasians (19%). The
data obtained from their research has shown that the f allele for
the Fok1 polymorphism occurs less frequently among the Africans
(24%) than among the Caucasians (34%) and the Asians (51%), while
the frequency of the B allele for Bsm1 is much lower in the Asian
(7%) population than among the Caucasians (42%) and the Africans
(36%) (35). On the other hand,
the Apa1 A allele is exhibited at a higher frequency in the Asian
population (74%) than among the Caucasians (44%) and Africans
(31%).
The LD describes the co-occurrence of the alleles of
adjacent polymorphisms. In consequence, the presence of one type of
polymorphism may serve as indication of the presence of another
polymorphism that is linked to it. It is noticeable due to a very
slight recombination that has occurred between them during
evolution. LD is present in the case of both Taq1 and poly(A),
since they occur in similar ratios in different ethnic groups, with
a lower percentage of the Taq1 T allele among Asians (8%) compared
to Caucasians (43%) and Africans (31%); similar results have been
observed for the poly(A) S allele, which is less frequent among the
Asians (12%) than among the Caucasians (41%) and the Africans (29%)
(52). In contrast to the
Bsm-Apa-Taq haplotypes, haplotype 3 (bAT) is common among the
African population (59%), while haplotype 1 (baT) and haplotype 2
(BAt) are mostly observed in Asian (75%) and Caucasian populations
(39%) respectively (35).
Vitamin D and breast cancer
Currently, in vitro, preclinical and clinical
findings support the hypothesis that low levels of vitamin D are
linked to an increased risk of breast cancer (56). Anti-carcinogenic effects of vitamin
D are mediated via the estrogen pathway by downregulation of the
estrogen receptor (ER), which inhibits cancer cell proliferation,
induces cell apoptosis, and prevents carcinogenesis in vitro
and in animal models (57–59). On the other hand, epidemiological
data also show that vitamin D supplementation in invasive breast
cancer patients did not reduce breast cancer incidence in one trial
conducted on post-menopausal women (60). These contradictory findings could
be related to tumor heterogeneity, which suggests that effects of
vitamin D may only be exhibited in specific subtypes of breast
cancer. Therefore, additional functional experiments with vitamin D
supplementation should be conducted on specific breast cancer
subtypes and polymorphisms in the VDR gene and other gene variants
(Fig. 3).
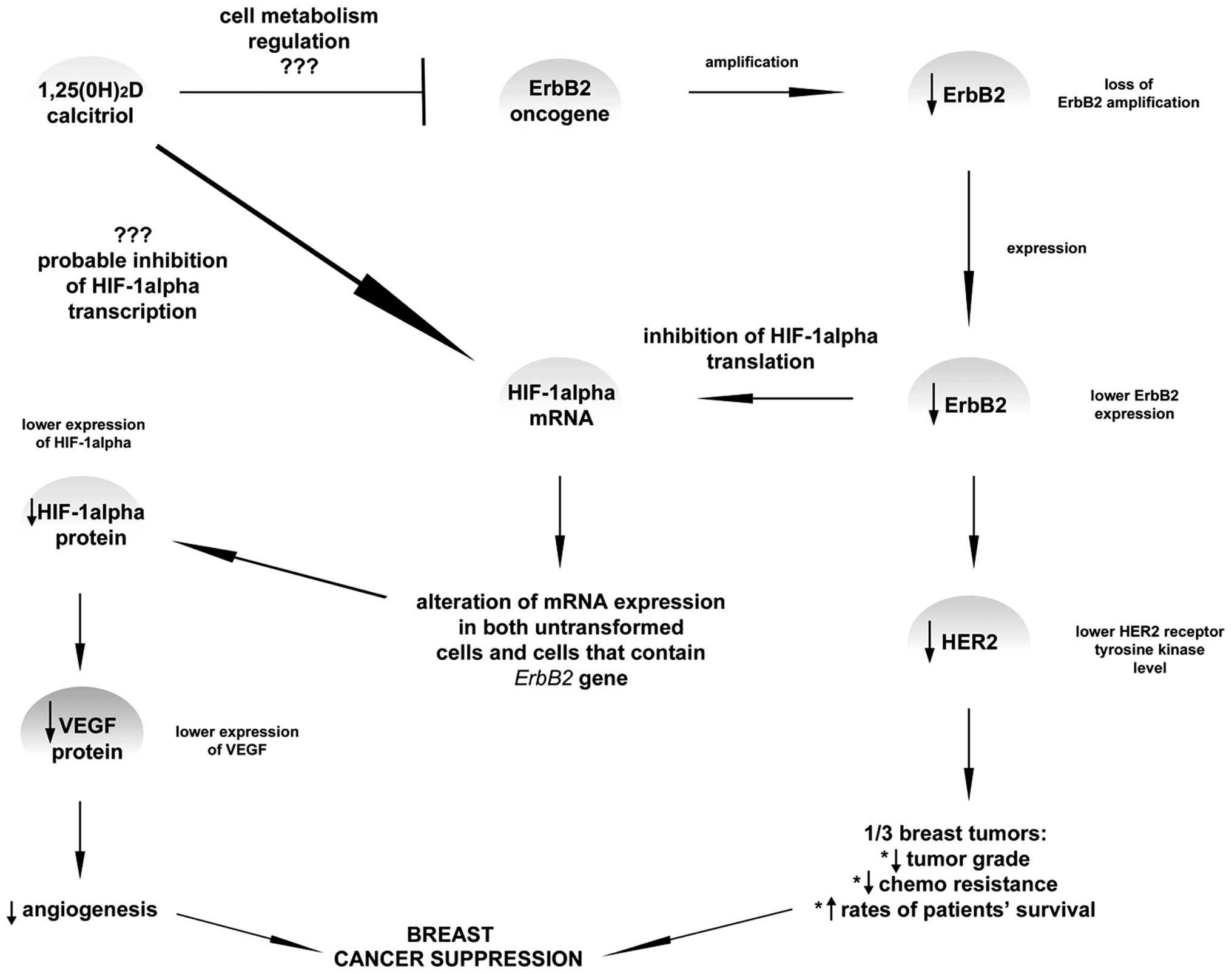 | Figure 3.It is known that vitamin D plays a
crucial role in reducing the risk of breast cancer development,
basically when the prevention of angiogenesis is taken into
account. VEGF is a potent angiogenic factor which was shown in
several publications to limit the vessels production due to the
1,25(0H)2D impact via the hypoxia-inducible factor-1α (HIF-α). It
has been proposed that dihydroxyvitamin D does not directly affect
VEGF expression. In some of the research, ErbB, a gene responsible
for breast cancer invasiveness, has been shown to increase HER2
expression. Amplification or overexpression of this gene is
perceived as one of the major factors contributing to pathogenesis
and progression of certain aggressive types of breast cancer and in
recent years it has become a significant biomarker and target of
therapy for this disease. However, it was suggested that lower
ErbB2 expression leads to the inhibition of HIF-α translation which
subsequently leads probably to lower VEGF and HER2 expression. This
means that higher rates of patient survival may be achieved.
Currently, the role of calcitriol in connection with HIF-1α
expression level is still not fully confirmed, however, inhibition
mechanism appears to be the most probable among other theory.
Moreover, ErbB2 is also probably inhibited by calcitriol; yet, the
mechanism of such action is still unknown. |
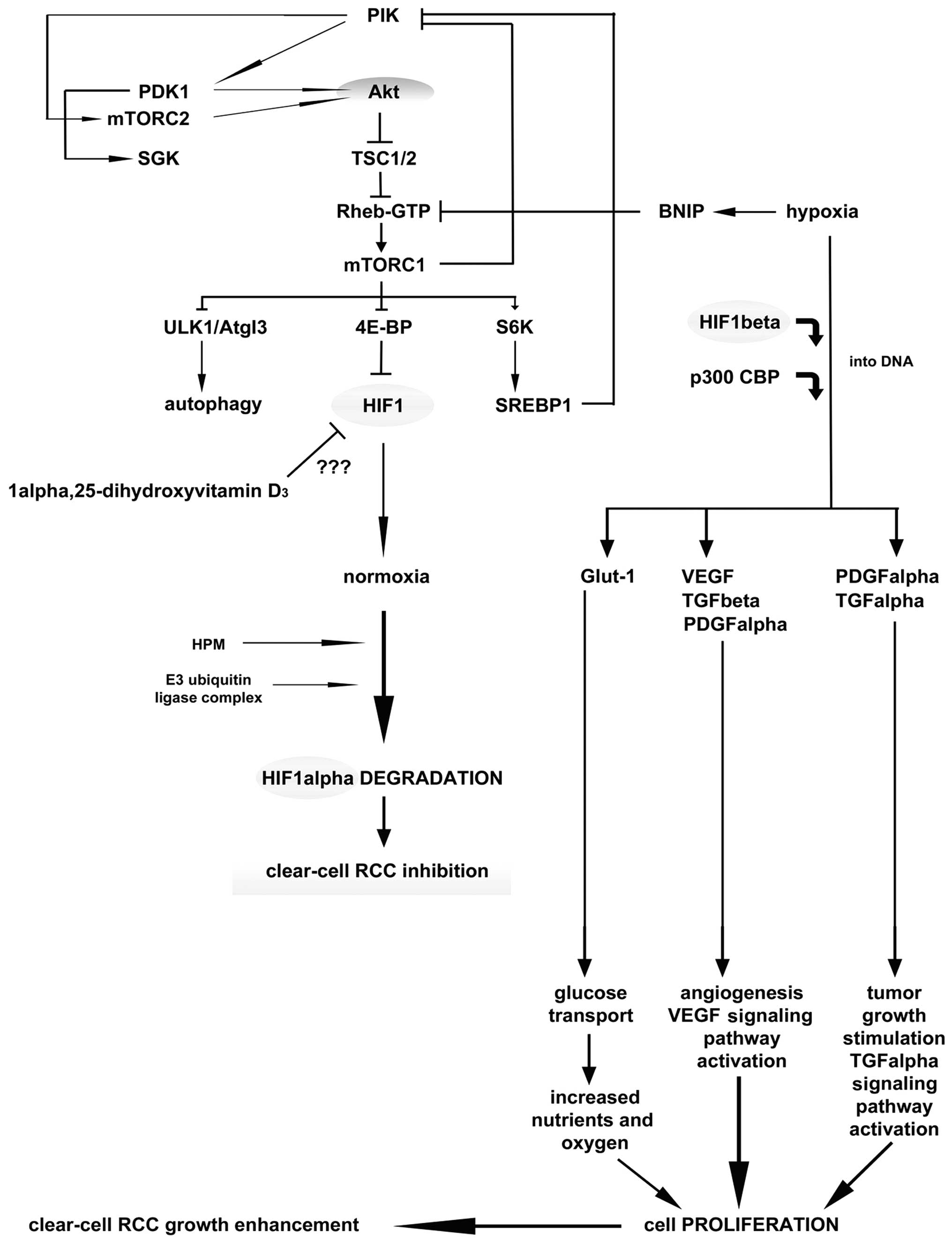
Vitamin D and renal cancer
In the kidney, the major site of vitamin D3
formation, metabolism, activity and calcium homeostasis under
physiological conditions is the renal proximal tubule (61). Epidemiological data suggest that
vitamin D3, obtained either from dietary intake or as a result of
the body’s exposure to ultraviolet light, is inversely correlated
with renal cancer risk (62,63).
Vitamin D3 serum concentrations have been found to be significantly
decreased in patients with renal cancer compared to the control
population (28,64). However, the exact role of
anti-carcinogenic mechanism of vitamin D3 has not been studied
widely nor is it completely understood. Nevertheless, it can be
postulated that vitamin D3 impedes carcinogenesis via the VDR, and
stimulates cell differentiation by inhibiting cell proliferation,
inducing apoptosis and suppressing invasiveness, angiogenesis and
metastasis (Fig. 4) (63,65,66).
The kidney is the most vital organ for vitamin D metabolism and
calcium homeostasis, though there is still scarcity of data on the
association between dietary vitamin D3, VDR gene polymorphism and
renal cancer etiology.
Search methods for the meta-analysis
Strategies to recover the published
data
Most epidemiological studies of breast and renal
cancers have been concentrated on seven polymorphisms of the VDR
gene that are potentially important for the cancer etiology. We
performed a meta-analysis of the published literature using the
PubMed Central® (PMC), a biomedical and life sciences
journal literature from the National Library of Medicine (NLM). The
search was conducted using literature data from January, 1997 until
November, 2012. The search employed combinations of keywords ‘VDR
gene polymorphisms’ with ‘breast cancer’ and ‘renal cell cancer’,
as well as of the keywords: Fok1, Bsm1, Taq1, Apa1, poly(A), Cdx2,
Bgl1, A-1012G and Tru91 with ‘breast cancer’ and ‘renal cell
cancer’. In the case of breast cancer, 52 results were generated,
while only 12 results were found in the case of VDR polymorphisms
and renal cancer.
Results of the meta-analysis
Fok1 polymorphism vs. breast cancer and
renal cancer
Fok1 polymorphism and breast
cancer
Most of the case control studies performed on
different ethnic populations did not show that the risk of breast
cancer was associated with the Fok1 polymorphism (34,67–70).
An analysis conducted recently on Chinese women also supported this
statement (70). The Fok1
polymorphism was not found to be associated with breast cancer when
analyzed separately. However, Fok1 did modulate the increased risk
of other VDR genotypes (bb/LL genotype Bsm1 b allele, poly(A) L
allele) together with the bb/LL genotype, which resulted in an
increased breast cancer risk (68). In one population-based case control
study, no effect of the concentration of 25(OH)D serum and VDR
genotype was observed, and none of the analyzed polymorphisms were
correlated with breast cancer risk (34). Another study conducted among the UK
Caucasian population showed similar results, with no association of
Fok1 with breast cancer (67). In
the investigations conducted on 500 breast cancer cases in
post-menopausal women and 500 controls matched by age, ethnicity
and blood collection date, similar results were obtained (71). Others have also obtained the same
result in their analysis (20).
Curran et al (72) have
shown in the Australian population that VDR initiation codon Fok1
polymorphism is not associated with breast cancer occurrence.
Interestingly, a significantly increased risk of breast cancer was
observed in one large study (1,234 cases and 1,676 controls) among
carriers of the ff genotype of Fok1 (multivariate OR=1.34) compared
with those with the FF genotype (73). In that study, the Fok1 association
was influenced by the menopausal status, estrogen, the progesterone
receptor status of the tumors, and plasma levels of 25(OH)D or
1,25(OH)2D3. Furthermore, a meta-analysis of 21 case-control
studies with Fok1, Bsm1, Apa1 and Taq1 polymorphisms has shown that
the Fok1 polymorphism was associated with an increased risk of
breast cancer development (ff vs. FF: OR=1.15; 95% CI, 1.03–1.28;
the recessive model ff vs. Ff + FF: OR=1.14; 95% CI, 1.03–1.26)
(74), while in a sub-analysis a
significant association was found between the Fok1 polymorphism and
breast cancer in the European population. Current meta-analysis has
shown that Fok1 may serve as a diagnostic biomarker for breast
cancer susceptibility especially in the European population. Taking
all these factors into consideration, the above studies showed an
uncertain association of Fok1 with breast cancer. Further studies
are still needed to clarify the observations based on the Fok1
polymorphism association with that cancer. Table I summarizes the reference analysis
of the Fok1 polymorphism association with breast cancer.
 | Table I.Fok1 and breast cancer. |
Table I.
Fok1 and breast cancer.
| Population
studied | Study type | Polymorphism | Cases/controls | Results | Refs. |
|---|
| Latinas | CCS | Fok1, Bsm1,
poly(A) | 143/300 | No association | (69) |
| Caucasian | CCS | Fok1, Bsm1 | 313/410 | No association | (77) |
| Caucasian | CCS | Fok1, Bsm1,
poly(A) | 398/427 | No association | (68) |
| Caucasian | CCS | Fok1, Bsm1,
poly(A) | 181/241 | No association | (67) |
| German | PCCS | Fok1, Taq1, Cdx2,
VDR-5132 | 1408/2612 | No association | (34) |
| Caucasian | NCCS | Fok1, Bsm1, Taq1,
Apa1, poly(A) | 500/500 | No association | (71) |
| Australian | CCS | Fok1, Taq1,
Apa1 | 135/410 | No association | (72) |
| USA | CCS | Fok1 | 1234/1676 | The ff genotype
associated with breast cancer risk | (73) |
| French
Canadian | CCS | Fok1, Bsm1 | Two independent
studies (225/463 and 622/974) | The ff genotype
linked to higher breast cancer risk | (78) |
| Hispanic,
non-Hispanic | CCS | Fok1, Taq1,
Bgl1 | 814/910 | No association | (20) |
| Chinese | PCCS | Fok1, Bsm1,
CYP24A1 | 2919/2313 | No association | (70) |
Fok1 polymorphism and renal
cancer
The association of Fok1 with renal cell cancer has
not been studied widely. The scarce published data comprise the
analysis of Fok1 and its role in renal cell cancer development;
however, a recent study yielded contradictory results, it was
conducted in Central and Eastern Europe, and showed that the
subjects over 60 years of age who were carriers of the f alleles in
the Fok1 SNP had reduced renal cancer risk compared to the subjects
with the FF genotype (75).
However, recent research on the North Indian population has shown
contradictory findings, in which an increased number of the Fok1
polymorphism alleles was linked to a high risk of renal cell cancer
considering other risk factors, such as: hypertension, smoking and
improper body mass index (BMI) (76). These results show the association
of Fok1 polymorphism with renal cancer. However, the result varies
depending on the ethnic background, so further research is needed
for confirmation. Table II
summarizes the reference analysis of the Fok1 polymorphism
association with renal cancer.
 | Table II.Fok1 and renal cancer. |
Table II.
Fok1 and renal cancer.
| Population
studied | Study type | Polymorphism | Cases/controls | Results | Refs. |
|---|
| Central and Eastern
European | CCS | Fok1, Bsm1,
Taq1 | 925/1192 | The f allele
associated with renal cancer risk | (75) |
| Indian | CCS | Fok1, Bsm1 | 196/250 | The ff genotype
associated with high renal cancer risk | (76) |
Bsm1 polymorphism vs. breast cancer and
renal cancer
Bsm1 polymorphism and breast
cancer
A strong association has been found between the bb
genotype of the Bsm1 VDR polymorphism and increased breast cancer
incidence. A great number of research studies have been published
to support this statement, but some conclusions differed across the
individual authors and ethnic groups. Research based on UK
Caucasian women has shown a 1.8-fold increase of cancer risk for
the bb genotype individuals (OR=1.79) (77). In addition, >70% of seven
commonly used breast cancer cell lines have been found to possess
the risky bb genotype. Guy et al (68) have further confirmed that Bsm1 is
associated with a high risk of breast cancer development, and the
likelihood of developing breast cancer is nearly twice higher for a
woman with the bb genotype than for a woman with BB or Bb genotype
(bb genotype OR=1.92; BB genotype OR=1.00; and Bb genotype
OR=1.00). Similar results have been reported for the Caucasian
women, but not for the African-American women, where this
polymorphism increased the risk of breast cancer development in
postmenopausal carriers of the bb genotype of Bsm1 (OR=1.53)
(78). It is also worth noticing
that the smoking status modified this association of Bsm1 genotype
and breast cancer risk. Premenopausal Caucasian women who had
reported smoking at least once had an increased risk of breast
cancer. However, there was no such association in case of the
smoking habit among postmenopausal Caucasian women, premenopausal
African-American women or postmenopausal African-American women.
The respective association between Bsm1 genotypes and breast cancer
risk does not vary significantly with oral contraceptive use,
hormone replacement therapy, or BMI (78).
Another study has reported that the Bsm1
polymorphism was in LD with the poly(A) sequence in the
3’-non-translated region, and the ‘L’ poly(A) variant was also
associated with breast cancer susceptibility similarly to Bsm1 (bb
vs. BB genotype OR=2.32) (67).
The study by Lowe et al (12) also reported that low levels of
circulating 25(OH)D (<50 nM) occurring in association with the
bb Bsm1 VDR genotype may increase the risk of breast cancer more
than levels of 25(OH)D >50 nM in either the BB or the Bb
genotype. Another study shows that VDR polymorphism Bsm1
distributions in the case group and in the control group of
patients did not exhibit any statistical difference. On the other
hand, the metastatic cancer group with prevalence of the bb
genotype (14/38; 37%) was twice as large as the corresponding
percentage of control subjects, whereas the percentage of BB women
with metastases was half lower than in the control group (2/38; 5%)
(79). Moreover, homozygous bb
women have been shown to develop metastases four times more often
than BB women.
Ingles et al (69) have reported that when the bb
genotype was compared with the Bb and BB genotypes separately, it
was shown to have a 1.6- and 2.2-fold increased breast cancer risk,
respectively. In Taiwanese women, the Bsm1 B allele was also
associated with an increased breast cancer risk (80). The data showed that 79% of the
breast cancer group and 76% of the benign breast tumor group had
the bb genotype compared to 91% of the control population. Only 9%
of the control population had the B allele, in contrast to the
breast cancer group (21%) and the benign breast tumor group (24%),
which supports the B allele association with an increased breast
cancer risk. However, a number of studies have also yielded
conflicting data on association of the Bsm1 VDR polymorphism with
breast cancer risk. Two independent case-control studies conducted
on the same population have shown that the Bsm1 Bb + bb genotype is
not associated with an increased risk of breast cancer in the
French Canadian population (OR=1.22) without interaction with
family history (81). Similar
results have been obtained by another group, who has shown the
absence of any significant association between Bsm1 polymorphisms
and breast cancer development on a small group (78 patients) of the
Turkish population (82). In
Caucasian women, no association was found between polymorphisms in
Bsm1 and breast cancer risk, OR=0.93 for BB vs. bb (73). However, these results suggest that
the VDR polymorphism may be a mediator of cancer risk, and could be
a target for cancer prevention efforts. Some results showed that
breast cancer incidence was not associated with any genotype
(71). However, women with the
Bsm1 bb genotype that consumed >902 mg/day of calcium had lower
susceptibility to breast cancer than those with the Bb or BB
genotype (OR=0.61). In conclusion, the Bsm1 bb genotype is
associated with breast cancer risk in Caucasian and
African-American women, despite the fact that this association is
influenced by other factors, such as smoking. Table III summarizes the reference
analysis of the Bsm1 polymorphism association with breast
cancer.
 | Table III.Bsm1 and breast cancer. |
Table III.
Bsm1 and breast cancer.
| Population
studied | Study type | Polymorphism | Cases/controls | Results | Refs. |
|---|
| Caucasian | CCS | Bsm1, Fok1 | 313/410 | The bb genotype is
associated with high breast cancer risk | (77) |
| Caucasian | CCS | Bsm1, Fok1,
poly(A) | 398/427 | Breast cancer
occurrence among women with the bb genotype is twice higher than
among women with the BB or Bb genotypes | (68) |
| Caucasian,
African-American | PCCS | Bsm1, poly(A) | 1631/1435 | Postmenopausal
Caucasian women with the bb genotype are at higher risk of
developing breast cancer | (79) |
| Caucasian | CCS | Bsm1, Fok1 | 1180/1547 | No association | (73) |
| Caucasian | CCS | Bsm1, Fok1,
poly(A) | 181/241 | Bsm1 is LD with
poly(A) and is associated with breast cancer | (67) |
| Caucasian | CCS | Bsm1 | 179/179 | Low level of
25(OH)D and the bb genotype increase breast cancer
susceptibility | (12) |
| French Canadian
population | CCS | Bsm1, Fok1 | Two independent
study (225/463 and 622/974) | Bb + bb was
associated with slightly increased risk of breast cancer in both
studies | (78) |
| Italian | CCS | Bsm1 | 88/167 | Women with
homozygous bb are at higher risk of developing metastases of breast
cancer than BB women | (80) |
| Turkish | CCS | Bsm1, Taq1 | 78/27 | No association | (82) |
| Caucasian | NCCS | Bsm1, Fok1, Taq1,
Apa1, poly(A) | 500/500 | No association | (71) |
| Taiwanese | CCS | Bsm1, Taq1,
Apa1 | 80/169 | The B allele is
associated with breast cancer | (81) |
| Latinas | CCS | Bsm1, Fok1,
poly(A) | 143/500 | The bb genotype is
associated with higher breast cancer risk than BB and Bb | (69) |
| Chinese | PCCS | Fok1, Bsm1,
CYP24A1 | 2919/2313 | No association | (70) |
Bsm1 polymorphism and renal
cancer
Although many detailed studies have been conducted
in the field of renal cell cancer, more data are undoubtedly
required to determine the importance of the Bsm1 polymorphism
association with renal cell cancer development. Only a few studies
have been published that showed its connection with renal cancer. A
previously conducted study in the Japanese population showed that
the Bsm1 variant did not have any statistically significant effect
on the risk of renal cancer (36).
However, when the BB genotype of the Bsm1 gene polymorphism was
analyzed in Central and Eastern Europe, an individual with a
positive family history of cancer had a lower renal cancer risk
compared to an individual with the bb allele (75). Similar results have been obtained
for the Indian population, showing that Bsm1 polymorphism (bb
genotype) can significantly modify the risk of renal cancer
(76). All these findings show the
potential of Bsm1 in predicting the risk of developing renal cell
cancer, though further studies taking into consideration other
ethnic groups are needed for confirmation. Table IV summarizes the reference analysis
of the Bsm1 polymorphism association with renal cancer.
 | Table IV.Bsm1 and renal cancer. |
Table IV.
Bsm1 and renal cancer.
| Population
studied | Study type | Polymorphism | Cases/controls | Results | Refs. |
|---|
| Japanese | CCS | Bsm1, Apa1,
Taq1 | 135/150 | Bsm1 variant not
found to be associated with renal cancer | (36) |
| Central and Eastern
European | CCS | Bsm1, Fok1,
Taq1 | 925/1192 | The BB genotype is
associated with lower risk of renal cancer occurrence | (75) |
| Indian | CCS | Bsm1, Fok1 | 196/250 | The bb genotype is
associated with lower risk of renal cancer | (76) |
Taq1 polymorphism vs. breast cancer and
renal cancer
Taq1 polymorphism and breast
cancer
A large number of studies have shown the absence of
any association between the Taq1 VDR polymorphism and breast cancer
susceptibility. A population-based case-control study (PCCS) has
revealed that high sun exposure reduces the risk of advanced breast
cancer development among women with light constitutive skin
pigmentation (OR=0.53), and that this association does not depend
on the Taq1 VDR genotype (20).
Similar results showing no significant association between breast
cancer risk and the Taq1 genotype have been obtained in different
ethnic backgrounds (71,82–84).
In the Taiwanese population, where low frequency of breast cancer
was found, no association of Taq1 with breast cancer has been
established (80). On the other
hand, a tendency for a decreased risk of breast cancer has been
observed for the Taq1 T allele (OR=0.68), but proper estimation of
the potential impact of Taq1 polymorphism on breast cancer was
impossible to perform (85). A
number of Taq1 analyses comprising the Taq1 polymorphism in
combination with dietary factors have also been reported. Women
with the TT genotype who consumed >902 mg total calcium per day
exhibited lower breast cancer risk compared to genotypes Tt or tt
(71). The Taq1 polymorphism
association with a significantly increased risk of estrogen
receptor positive tumors (OR=1.18) for t allele carriers compared
to non-carriers (OR=0.88) has also been reported (34,52).
In a case-control study (CCS) of Australian women, a tendency for
the Taq1 T allele association with an ∼1.5-fold increased breast
cancer risk has been found (72).
Finally, for patients with the TT genotype, a significantly
increased risk (OR=1.8) for lymph node metastasis has been
reported. Furthermore, among patients with the tt genotype, a
tendency towards prolonged survival has been noted among
ER-positive, tamoxifen-treated patients (86). In conclusion, polymorphisms in the
VDR gene may influence tumor progression and tamoxifen treatment
response in early-onset breast carcinomas (86). It appears that the T allele is a
‘risk allele’, while the t allele is a ‘protective allele’.
Table V summarizes the reference
analysis of the Taq1 polymorphism association with breast
cancer.
 | Table V.Taq1 and breast cancer. |
Table V.
Taq1 and breast cancer.
| Population
studied | Study type | Polymorphism | Cases/controls | Results | Refs. |
|---|
| Hispanic,
non-Hispanic | CCS | Taq1, Fok1,
Bgl1 | 814/910 | No association | (20) |
| Caucasian | NCCS | Taq1, Fok1, Bsm1,
Apa1, poly(A) | 500/500 | Women with the TT
genotype consuming 902 mg/day calcium have lower risk | (71) |
| Caucasian | CCS | Taq1 | 951/627 | No association | (83) |
| Turkish | CCS | Taq1, Bsm1 | 78/27 | Taq1 polymorphism
in VDR gene is not linked to breast cancer susceptibility | (84) |
| Taiwanese | CCS | Taq1, Bsm1,
Apa1 | 80/169 | No association | (81) |
| Finnish | CCS | Taq1, Apa1 | 483/482 | The T allele is
associated with lower breast cancer incidence | (86) |
| German | PCCS | Taq1, Fok1, Cdx2,
VDR-5132 | 1408/2612 | In estrogen
receptor positive tumor, the t allele is linked to increased risk
of breast cancer | (34) |
| Australian | CCS | Taq1, Fok1,
Apa1 | 135/410 | The TT genotype is
associated with breast cancer | (72) |
| Swedish | CCS | Taq1 | 111/130 | The tt genotype is
associated with prolonged survival in breast cancer patients | (87) |
| Indian | CCS | Taq1, Apa1,
Poly(A) | 160/140 | No association | (85) |
Taq1 polymorphism and renal
cancer
There is scarce data concerning the effect of the
Taq1 polymorphism on renal cancer occurrence and outcome. A lower
risk of renal cancer has been observed among individuals with the t
allele in the Taq1 gene polymorphism in the Central and Eastern
European population (75), and
this polymorphism was not differentiated by the tumor stage or
grade. Previously, a study of the Japanese population has also been
carried out, revealing that: i) the TT genotype was statistically
more frequent among renal cancer patients (80.4%); ii) TT frequency
was higher compared to rapid tumor growth type group (92.1%) and
slow tumor growth type group (73.4%) (87). All these analyses have shown that
the T allele is associated with determining the risk of developing
renal cancer. On the contrary, a recent study of the Japanese
population has shown contradictory results, and has not found any
statistical difference (36).
Therefore, further research is needed on the basis of cancer stage
to define or confirm the role of the T allele in renal cancer risk.
Table VI summarizes the reference
analysis of the Taq1 polymorphism association with renal
cancer.
 | Table VI.Taq1 and renal cancer. |
Table VI.
Taq1 and renal cancer.
| Population
studied | Study type | Polymorphism | Cases/controls | Results | Refs. |
|---|
| Central and Eastern
European | CCS | Taq1, Bsm1,
Fok1 | 925/1192 | Lower risk is
associated with the Taq1 t variant | (75) |
| Japanese | CCS | Taq1 | 102/204 | The TT genotype is
more frequent in renal cancer patients | (88) |
| Japanese | CCS | Taq1, Bsm1,
Apa1 | 135/150 | No association | (36) |
Apa1 polymorphism vs. breast cancer and
renal cancer
Apa1 polymorphism and breast
cancer
The association of Apa1 with breast cancer has not
been widely studied. Different approaches towards the Apa1
association analysis have been used, and some studies have revealed
that the Aa and aa genotypes were significantly associated with an
increased breast cancer risk (OR=1.56) (72). Contradictory results have been
obtained among the Taiwanese population (80). Those results have shown that the AA
genotype has a tendency to increase cancer risk, whereas decreasing
copies of the A allele have been found to be associated with a
reduced cancer risk (OR=0.333 for the Aa genotype and OR=0.515 for
the aa genotype). In other studies conducted on the Finnish
population, a statistically significant difference was observed in
the Apa1 genotype distribution between the cases and the controls
(85). Women with the VDR variant
‘a’ genotypes had a decreased risk of breast cancer (OR=0.73)
compared with women carrying the AA genotype. If the family history
of breast cancer was taken into consideration, this association
became strong (OR=0.14). The results also suggest that the AA
genotype is more common among women with breast cancer, whereas the
lowest risk of breast cancer is found in women with the aa genotype
(OR=0.03) (85). On the other
hand, other research groups have not found such an association
(71,84). In conclusion, the Apa1 allele
association has not been analyzed widely in different racial
groups, but in most of the studies it has been found to be linked
with breast cancer risk. Table VII
summarizes the reference analysis of the Apa1 polymorphism
association with breast cancer.
 | Table VII.Apa1 and breast cancer. |
Table VII.
Apa1 and breast cancer.
| Population
studied | Study type | Polymorphism | Cases/controls | Results | Refs. |
|---|
| Australian | CCS | Apa1, Fok1,
Taq1 | 135/410 | The Aa and aa
genotypes are associated with breast cancer risk | (72) |
| Taiwanese | CCS | Apa1, Bsm1,
Taq1 | 80/169 | The AA genotype is
associated with breast cancer risk | (81) |
| Finnish | CCS | Apa1, Taq1,
Apa1 | 483/482 | Variant ‘a’
genotype is linked to lower risk | (86) |
| Caucasian | NCCS | Apa1, Bsm1, Fok1,
Taq1, poly(A) | 500/500 | No association | (71) |
| Indian | CCS | Apa1, Taq1,
poly(A) | 160/140 | No association | (85) |
Apa1 polymorphism and renal
cancer
Only one study on the Japanese population has
analysed the association between renal cancer and the AA genotype
of the Apa1 polymorphism in the VDR gene (36). The frequency of the AA genotype has
been found to be significantly higher in renal cancer patients
(17.0%) than in the control group (7.3%). More than half (52.2%) of
the patients with the AA genotype had distant or lymph node
metastases compared to the Aa + aa genotypes (6.3%). Moreover,
patients with the AA genotype were found to have poor survival
rates compared to patients with other genotypes (Aa + aa). In
conclusion, these findings suggest that the AA genotype may
influence disease progression and prognosis of renal cancer;
however, these results are limited to a certain ethnic background.
Thus, further research concerning the Apa1 polymorphism for other
populations are necessary. Table
VIII summarizes the reference analysis of the Apa1 polymorphism
association with renal cancer.
 | Table VIII.Apa1 and renal cancer. |
Table VIII.
Apa1 and renal cancer.
| Population
studied | Study type | Polymorphism | Cases/controls | Results | Refs. |
|---|
| Japanese | CCS | Taq1, Bsm1,
Apa1 | 135/150 | AA genotype is
frequent in renal cancer cases | (36) |
Poly(A) polymorphism vs. breast cancer
and renal cancer
Poly(A) polymorphism and breast
cancer
The claim concerning the presence of an association
between the poly(A) polymorphism and breast cancer occurrence is
controversial. In a recent study carried out on the Swedish
population, women carrying two short poly(A) alleles (SS genotype)
were found to have an increased risk of breast cancer incidence
(OR=1.26) (88). There was a
statistically significant interaction between the VDR genotype and
parity; women with two short alleles (SS) had a halved risk of
breast cancer, irrespective of the parity, compared with
nulliparous women with two long alleles (LL). On the other hand,
homozygosity for the long VDR allele (LL) was associated with a
more advanced clinical stage at diagnosis (88). With respect to a previous study,
when the LL genotype was compared to the S genotype with an
increased number of short poly(A) alleles, a higher risk of
developing cancer was associated with the number of S variants
(OR=1.5 for the SL genotype and OR=3.2 for the SS genotype)
(69). Some other studies carried
out on Caucasian women also suggested the association of poly(A)
with breast cancer (67). The
poly(A) variant L was associated with an increase of breast cancer
risk because, poly(A) was in LD with the Bsm1 polymorphism
(67). Similar results have also
been obtained by another group (OR=1.94 for LL vs. SS genotype)
(68). Some studies have also
yielded contradictory results for the poly(A) genotype (71). However, women with the poly(A) (LL)
repeat who consumed more than 902 mg/day of calcium exhibited
decreased risk of breast cancer incidence (71). On the other hand, breast cancer and
the poly(A) genotype were not found to be associated in a recent
study on a group of Caucasian and African-American women (78). It has been found that the
association of poly(A) with breast cancer can be modified by the
smoking status, but it did not significantly vary with oral
contraceptive use, hormone replacement therapy, or BMI (78). In conclusion, the role of poly(A)
is still controversial, but the variant LL allele is associated
with breast cancer risk, though further confirmation is undoubtedly
needed. Table IX summarizes the
reference analysis of the poly(A) polymorphism association with
breast cancer.
 | Table IX.Poly(A), Cdx2, Bgl1 and breast
cancer. |
Table IX.
Poly(A), Cdx2, Bgl1 and breast
cancer.
| Population
studied | Study type | Polymorphism | Cases/controls | Results | Refs. |
|---|
| Swedish | PCCS | Poly(A) | 1502/1510 | The SS genotype is
associated with breast cancer | (89) |
| Latinas | CCS | Poly(A), Bsm1,
Fok1 | 143/500 | Two SS allele of
the poly(A) polymorphism are associated with breast cancer
risk | (69) |
| Caucasian | CCS | Poly(A), Bsm1,
Fok1 | 181/241 | The poly(A) L
variant is in LD with Bsm1 and is associated with breast
cancer | (67) |
| Caucasian | CCS | Poly(A), Bsm1,
Fok1 | 398/427 | The poly(A) LL
genotype is associated with breast cancer | (68) |
| Caucasian | NCCS | Poly(A), Bsm1,
Fok1, Apa1, Taq1 | 500/500 | Poly(A) LL positive
women can decrease breast cancer risk by consuming 902 mg
calcium/day | (71) |
| Caucasian,
African-American | PCCS | Poly(A), Bsm1 | 1631/1435 | No association with
poly(A) | (79) |
| Indian | CCS | Poly(A), Apa1,
Taq1 | 160/140 | The long poly(A) L
allele is associated with breast cancer | (85) |
| German | PCCS | Cdx2, Fok1, Taq1,
VDR-5132 | 1408/2612 | Cdx2 is not
associated with breast cancer | (34) |
| Hispanic,
non-Hispanic | CCS | Bgl1, Fok1,
Taq1 | 814/910 | The Bgl1 BB
genotype is associated with the decrease of breast cancer
development in a group of women with medium pigmentation | (20) |
Poly(A) polymorphism and renal
cancer
No specific data basis has been found so far to
demonstrate a direct association of poly(A) with the risk of renal
cancer. However, some studies have found a correlation between Taq1
and the poly(A) polymorphism, which are in LD with each other in
the Asian, Caucasian and African populations (35). A lower risk of renal cancer has
been observed for the t allele of the Taq1 gene polymorphism
(75,87). All these studies examined solely
the role of the Taq1 polymorphism on its own. However, it is
feasible that poly(A) may show an important role in renal cancer
development when studied together with the Taq1 polymorphism.
Cdx2 and Bgl1 polymorphism vs. breast
cancer and renal cancer
Cdx2 and Bgl1 polymorphism and breast
cancer
Undoubtedly, not enough work has been done on the
potential functional association between Cdx, Bgl1 and breast
cancer risk. No LD has been found between the Cdx2 and other VDR
genes (Fok1, Taq1 and VDR-5132) with respect to the breast cancer
risk (34). In a PCCS carried out
on various racial groups, such as Hispanic, African-American and
non-Hispanic Caucasians, similar results were obtained for the
linkage of Bgl1 to breast cancer risk (20). High exposure to sunlight was linked
with reduced risk of breast cancer among women with light
constitutive skin pigmentation (OR=0.53), but not among women with
medium or dark pigmentation (20).
In conclusion, the data on VDR Cdx and Bgl1 polymorphism are still
limited.
Cdx2 and Bgl1 polymorphism and renal
cancer
There are no published data so far that would
describe the role of Cdx2 and Bgl1 with regard to the risk of
developing renal cell cancer. Table
IX summarizes the reference analysis of the Cdx2 and Bgl1
polymorphism association with breast cancer.
Concluding remarks
In this study we have analyzed the polymorphisms in
the VDR gene occurring most commonly in breast cancer and renal
cancer. Based on the set of data collected from different studies
performed on different ethnic populations, it is not possible to
make an authoritative declaration on the role of VDR polymorphisms
in breast and renal cancer development and prognosis. However, some
polymorphisms have been found to be strongly associated with breast
cancer and some with renal cancer. Understanding the role of VDR
polymorphisms provides mechanistic insight and may facilitate the
development of new preventive strategies for breast and renal
cancer.
Abbreviations:
|
CCS
|
case-control study;
|
|
PCCS
|
population-based case-control
study;
|
|
NCCS
|
nested case-control study;
|
|
OR
|
odds ratio;
|
|
UV-B
|
ultraviolet radiation;
|
|
VDR
|
vitamin D receptor;
|
|
LD
|
linkage disequilibrium;
|
|
Bsm1 alleles
|
(B, b);
|
|
poly(A) alleles
|
[L (long), S (short)];
|
|
Apa1 alleles
|
(A, a);
|
|
Taq1 alleles
|
(T, t);
|
|
Fok1 alleles
|
(F, f);
|
|
RFLP
|
restriction fragment length
polymorphism
|
Acknowledgements
This study was supported by grant
TEAM/2010-6/8 of the Foundation for Polish Science Team Programme
co-financed by the European Regional Development Fund, Operational
Program Innovative Economy 2007–2013.
References
|
1.
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Pharoah PDP and Mackay J: Absolute risk of
breast cancer in women at increased risk: a more useful clinical
measure than relative risk? Breast. 7:255–259. 1998. View Article : Google Scholar
|
|
3.
|
Czarnecka AM, Klemba A, Krawczyk T, et al:
Mitochondrial NADH-dehydrogenase polymorphisms as sporadic breast
cancer risk factor. Oncol Rep. 23:531–535. 2010.PubMed/NCBI
|
|
4.
|
Najm MZ, Zaidi S, Siddiqui WA and Husain
SA: Immunohistochemical expression and mutation study of Prohibitin
gene in Indian female breast cancer cases. Med Oncol. 30:6142013.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Porta C, Bellmunt J, Eisen T, Szczylik C
and Mulders P: Treating the individual: The need for a
patient-focused approach to the management of renal cell carcinoma.
Cancer Treat Rev. 36:16–23. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Hung RJ, Moore L, Boffetta P, et al:
Family history and the risk of kidney cancer: a multicenter
case-control study in Central Europe. Cancer Epidemiol Biomarkers
Prev. 16:1287–1290. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Chow WH, Dong LM and Devesa SS:
Epidemiology and risk factors for kidney cancer. Nat Rev Urol.
7:245–257. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Krishnan AV, Trump DL, Johnson CS and
Feldman D: The role of vitamin D in cancer prevention and
treatment. Endocrinol Metab Clin North Am. 39:401–418. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Ali MM and Vaidya V: Vitamin D and cancer.
J Cancer Res Ther. 3:225–230. 2007. View Article : Google Scholar
|
|
10.
|
Simboli-Campbell M, Narvaez CJ, Tenniswood
M and Welsh J: 1,25-dihydroxyvitamin D-3 induces morphological and
biochemical markers of apoptosis in MCF-7 breast cancer cells. J
Steroid Biochem Mol Biol. 58:367–376. 1996. View Article : Google Scholar
|
|
11.
|
Thibault F, Cancel-Tassin G and Cussenot
O: Low penetrance genetic susceptibility to kidney cancer. BJU Int.
98:735–738. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Lowe LC, Guy M, Mansi JL, et al: Plasma
25-hydroxy vitamin D concentrations, vitamin D receptor genotype
and breast cancer risk in a UK Caucasian population. Eur J Cancer.
41:1164–1169. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Bhattacharjee M, Wientroub S and
Vonderhaar BK: Milk protein synthesis by mammary glands of vitamin
D-deficient mice. Endocrinology. 121:865–874. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Chouvet C, Vicard E, Devonec M and Saez S:
1,25-Dihydroxyvitamin D3 inhibitory effect on the growth of two
human breast cancer cell lines (MCF-7, BT-20). J Steroid Biochem.
24:373–376. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Eisman JA, Sutherland RL, McMenemy ML,
Fragonas JC, Musgrove EA and Pang GY: Effects of
1,25-dihydroxyvitamin D3 on cell-cycle kinetics of T 47D human
breast cancer cells. J Cell Physiol. 138:611–616. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
James SY, Mackay AG and Colston KW:
Vitamin D derivatives in combination with 9-cis retinoic acid
promote active cell death in breast cancer cells. J Mol Endocrinol.
14:391–394. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Welsh J, Wietzke JA, Zinser GM, et al:
Impact of the Vitamin D3 receptor on growth-regulatory pathways in
mammary gland and breast cancer. J Steroid Biochem Mol Biol.
83:85–92. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Sergeev IN: Vitamin D and cellular
Ca2+ signaling in breast cancer. Anticancer Res.
32:299–302. 2012.PubMed/NCBI
|
|
19.
|
Freedman DM, Looker AC, Chang SC and
Graubard BI: Prospective study of serum vitamin D and cancer
mortality in the United States. J Natl Cancer Inst. 99:1594–1602.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
John EM, Schwartz GG, Koo J, Wang W and
Ingles SA: Sun exposure, vitamin D receptor gene polymorphisms, and
breast cancer risk in a multiethnic population. Am J Epidemiol.
166:1409–1419. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Shao T, Klein P and Grossbard ML: Vitamin
D and breast cancer. Oncologist. 17:36–45. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Friedman PA and Gesek FA: Cellular calcium
transport in renal epithelia: measurement, mechanisms, and
regulation. Physiol Rev. 75:429–471. 1995.PubMed/NCBI
|
|
23.
|
Kumar R: Calcium transport in epithelial
cells of the intestine and kidney. J Cell Biochem. 57:392–398.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Gray R, Boyle I and DeLuca HF: Vitamin D
metabolism: the role of kidney tissue. Science. 172:1232–1234.
1971. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Tanaka Y and DeLuca HF: Rat renal
25-hydroxyvitamin D3 1- and 24-hydroxylases: their in vivo
regulation. Am J Physiol. 246:E168–E173. 1984.PubMed/NCBI
|
|
26.
|
Johnson JA, Grande JP, Roche PC, Sweeney
WE Jr, Avner ED and Kumar R: 1 alpha, 25-dihydroxyvitamin D3
receptor onto-genesis in fetal renal development. Am J Physiol.
269:F419–F428. 1995.PubMed/NCBI
|
|
27.
|
Wilson RT, Wang J, Chinchilli V, et al:
Fish, vitamin D, and flavonoids in relation to renal cell cancer
among smokers. Am J Epidemiol. 170:717–729. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Okamoto T, Fujioka T and Horiuchi S: A
study of the metabolism of vitamin D in patients with renal cell
carcinoma - with special reference to serum concentration of 1
alpha, 25-(OH)2D and its clinical significance. Nihon Hinyokika
Gakkai Zasshi. 82:890–899. 1991.(In Japanese).
|
|
29.
|
Fujioka T, Suzuki Y, Okamoto T, Mastushita
N, Hasegawa M and Omori S: Prevention of renal cell carcinoma by
active vitamin D3. World J Surg. 24:1205–1210. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Norman AW: Minireview: vitamin D receptor:
new assignments for an already busy receptor. Endocrinology.
147:5542–5548. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Zhou H, Xu C and Gu M: Vitamin D receptor
(VDR) gene polymorphisms and Graves’ disease: a meta-analysis. Clin
Endocrinol (Oxf). 70:938–945. 2009.
|
|
32.
|
Zmuda JM, Cauley JA and Ferrell RE:
Molecular epidemiology of vitamin D receptor gene variants.
Epidemiol Rev. 22:203–217. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Tokita A, Matsumoto H, Morrison NA, et al:
Vitamin D receptor alleles, bone mineral density and turnover in
premenopausal Japanese women. J Bone Miner Res. 11:1003–1009. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Abbas S, Nieters A, Linseisen J, et al:
Vitamin D receptor gene polymorphisms and haplotypes and
postmenopausal breast cancer risk. Breast Cancer Res. 10:P312008.
View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Uitterlinden AG, Fang Y, Van Meurs JB,
Pols HA and Van Leeuwen JP: Genetics and biology of vitamin D
receptor polymorphisms. Gene. 338:143–156. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Obara W, Suzuki Y, Kato K, Tanji S, Konda
R and Fujioka T: Vitamin D receptor gene polymorphisms are
associated with increased risk and progression of renal cell
carcinoma in a Japanese population. Int J Urol. 14:483–487. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Gross C, Eccleshall TR, Malloy PJ, Villa
ML, Marcus R and Feldman D: The presence of a polymorphism at the
translation initiation site of the vitamin D receptor gene is
associated with low bone mineral density in postmenopausal
Mexican-American women. J Bone Miner Res. 11:1850–1855. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Morrison NA, Yeoman R, Kelly PJ and Eisman
JA: Contribution of trans-acting factor alleles to normal
physiological variability: vitamin D receptor gene polymorphism and
circulating osteocalcin. Proc Natl Acad Sci USA. 89:6665–6669.
1992. View Article : Google Scholar
|
|
39.
|
Faraco JH, Morrison NA, Baker A, Shine J
and Frossard PM: ApaI dimorphism at the human vitamin D receptor
gene locus. Nucleic Acids Res. 17:21501989. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Arai H, Miyamoto KI, Yoshida M, et al: The
polymorphism in the caudal-related homeodomain protein Cdx-2
binding element in the human vitamin D receptor gene. J Bone Miner
Res. 16:1256–1264. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Morrison NA, Qi JC, Tokita A, et al:
Prediction of bone density from vitamin D receptor alleles. Nature.
367:284–287. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Gong YL, Xie DW, Deng ZL, et al: Vitamin D
receptor gene Tru9I polymorphism and risk for incidental sporadic
colorectal adenomas. World J Gastroenterol. 11:4794–4799.
2005.PubMed/NCBI
|
|
43.
|
Ingles SA, Ross RK, Yu MC, et al:
Association of prostate cancer risk with genetic polymorphisms in
vitamin D receptor and androgen receptor. J Natl Cancer Inst.
89:166–170. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Arai H, Miyamoto K, Taketani Y, et al: A
vitamin D receptor gene polymorphism in the translation initiation
codon: effect on protein activity and relation to bone mineral
density in Japanese women. J Bone Miner Res. 12:915–921. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Jurutka PW, Remus LS, Whitfield GK, et al:
The polymorphic N terminus in human vitamin D receptor isoforms
influences transcriptional activity by modulating interaction with
transcription factor IIB. Mol Endocrinol. 14:401–420. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Slattery ML: Vitamin D receptor gene (VDR)
associations with cancer. Nutr Rev. 65:S102–S104. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Sweeney C, Curtin K, Murtaugh MA, Caan BJ,
Potter JD and Slattery ML: Haplotype analysis of common vitamin D
receptor variants and colon and rectal cancers. Cancer Epidemiol
Biomarkers Prev. 15:744–749. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Fang Y, van Meurs JB, d’Alesio A, et al:
Promoter and 3’-untranslated-region haplotypes in the vitamin D
receptor gene predispose to osteoporotic fracture: the rotterdam
study. Am J Hum Genet. 77:807–823. 2005.
|
|
49.
|
Saijo T, Ito M, Takeda E, et al: A unique
mutation in the vitamin D receptor gene in three Japanese patients
with vitamin D-dependent rickets type II: utility of single-strand
conformation polymorphism analysis for heterozygous carrier
detection. Am J Hum Genet. 49:668–673. 1991.
|
|
50.
|
Hustmyer FG, DeLuca HF and Peacock M:
ApaI, BsmI, EcoRV and TaqI polymorphisms at the human vitamin D
receptor gene locus in Caucasians, blacks and Asians. Hum Mol
Genet. 2:4871993. View Article : Google Scholar : PubMed/NCBI
|
|
51.
|
Bhanushali AA, Lajpal N, Kulkarni SS,
Chavan SS, Bagadi SS and Das BR: Frequency of fokI and taqI
polymorphism of vitamin D receptor gene in Indian population and
its association with 25-hydroxyvitamin D levels. Indian J Hum
Genet. 15:108–113. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52.
|
Kostner K, Denzer N, Müller CS, Klein R,
Tilgen W and Reichrath J: The relevance of vitamin D receptor (VDR)
gene polymorphisms for cancer: a review of the literature.
Anticancer Res. 29:3511–3536. 2009.PubMed/NCBI
|
|
53.
|
Yamamoto H, Miyamoto K, Li B, et al: The
caudal-related homeodomain protein Cdx-2 regulates vitamin D
receptor gene expression in the small intestine. J Bone Miner Res.
14:240–247. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
54.
|
Fang Y, van Meurs JB, Bergink AP, et al:
Cdx-2 polymorphism in the promoter region of the human vitamin D
receptor gene determines susceptibility to fracture in the elderly.
J Bone Miner Res. 18:1632–1641. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
55.
|
Halsall JA, Osborne JE, Potter L, Pringle
JH and Hutchinson PE: A novel polymorphism in the 1A promoter
region of the vitamin D receptor is associated with altered
susceptibilty and prognosis in malignant melanoma. Br J Cancer.
91:765–770. 2004.PubMed/NCBI
|
|
56.
|
Welsh J: Vitamin D and prevention of
breast cancer. Acta Pharmacol Sin. 28:1373–1382. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57.
|
James SY, Mackay AG, Binderup L and
Colston KW: Effects of a new synthetic vitamin D analogue, EB1089,
on the oestrogen-responsive growth of human breast cancer cells. J
Endocrinol. 141:555–563. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
58.
|
Swami S, Krishnan AV and Feldman D:
1alpha,25-Dihydroxyvitamin D3 down-regulates estrogen receptor
abundance and suppresses estrogen actions in MCF-7 human breast
cancer cells. Clin Cancer Res. 6:3371–3379. 2000.PubMed/NCBI
|
|
59.
|
Welsh J: Vitamin D and breast cancer:
insights from animal models. Am J Clin Nutr. 80(Suppl 6):
1721S–1724S. 2004.PubMed/NCBI
|
|
60.
|
Chlebowski RT, Johnson KC, Kooperberg C,
et al: Calcium plus vitamin D supplementation and the risk of
breast cancer. J Natl Cancer Inst. 100:1581–1591. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
61.
|
Fraser DR and Kodicek E: Unique
biosynthesis by kidney of a biological active vitamin D metabolite.
Nature. 228:764–766. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
62.
|
Bosetti C, Scotti L, Maso LD, et al:
Micronutrients and the risk of renal cell cancer: a case-control
study from Italy. Int J Cancer. 120:892–896. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
63.
|
Karami S, Brennan P, Navratilova M, et al:
Vitamin D pathway genes, diet, and risk of renal cell carcinoma.
Int J Endocrinol. 2010:8793622010. View Article : Google Scholar : PubMed/NCBI
|
|
64.
|
Raimondi S, Johansson H, Maisonneuve P and
Gandini S: Review and meta-analysis on vitamin D receptor
polymorphisms and cancer risk. Carcinogenesis. 30:1170–1180. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
65.
|
Trump DL, Hershberger PA, Bernardi RJ, et
al: Anti-tumor activity of calcitriol: pre-clinical and clinical
studies. J Steroid Biochem Mol Biol. 89–90:519–526. 2004.PubMed/NCBI
|
|
66.
|
Ordonez-Moran P, Larriba MJ, Pendas-Franco
N, Aguilera O, Gonzalez-Sancho JM and Munoz A: Vitamin D and
cancer: an update of in vitro and in vivo data. Front Biosci.
10:2723–2749. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
67.
|
Bretherton-Watt D, Given-Wilson R, Mansi
JL, Thomas V, Carter N and Colston KW: Vitamin D receptor gene
polymorphisms are associated with breast cancer risk in a UK
Caucasian population. Br J Cancer. 85:171–175. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
68.
|
Guy M, Lowe LC, Bretherton-Watt D, et al:
Vitamin D receptor gene polymorphisms and breast cancer risk. Clin
Cancer Res. 10:5472–5481. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
69.
|
Ingles SA, Garcia DG, Wang W, et al:
Vitamin D receptor genotype and breast cancer in Latinas (United
States). Cancer Causes Control. 11:25–30. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
70.
|
Dorjgochoo T, Delahanty R, Lu W, et al:
Common genetic variants in the vitamin D pathway including
genome-wide associated variants are not associated with breast
cancer risk among Chinese women. Cancer Epidemiol Biomarkers Prev.
20:2313–2316. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
71.
|
McCullough ML, Stevens VL, Diver WR, et
al: Vitamin D pathway gene polymorphisms, diet, and risk of
postmenopausal breast cancer: a nested case-control study. Breast
Cancer Res. 9:R92007. View Article : Google Scholar : PubMed/NCBI
|
|
72.
|
Curran JE, Vaughan T, Lea RA, Weinstein
SR, Morrison NA and Griffiths LR: Association of A vitamin D
receptor polymorphism with sporadic breast cancer development. Int
J Cancer. 83:723–726. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
73.
|
Chen WY, Bertone-Johnson ER, Hunter DJ,
Willett WC and Hankinson SE: Associations between polymorphisms in
the vitamin D receptor and breast cancer risk. Cancer Epidemiol
Biomarkers Prev. 14:2335–2339. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
74.
|
Tang C, Chen N, Wu M, Yuan H and Du Y:
Fok1 polymorphism of vitamin D receptor gene contributes to breast
cancer susceptibility: a meta-analysis. Breast Cancer Res Treat.
117:391–399. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
75.
|
Karami S, Brennan P, Hung RJ, et al:
Vitamin D receptor polymorphisms and renal cancer risk in Central
and Eastern Europe. J Toxicol Environ Health A. 71:367–372. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
76.
|
Arjumand W, Ahmad ST, Seth A, Saini AK and
Sultana S: Vitamin D receptor FokI and BsmI gene polymorphism and
its association with grade and stage of renal cell carcinoma in
North Indian population. Tumour Biol. 33:23–31. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
77.
|
Guy M, Lowe LC, Bretherton-Watt D, Mansi
JL and Colston KW: Approaches to evaluating the association of
vitamin D receptor gene polymorphisms with breast cancer risk.
Recent Results Cancer Res. 164:43–54. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
78.
|
Trabert B, Malone KE, Daling JR, et al:
Vitamin D receptor polymorphisms and breast cancer risk in a large
population-based case-control study of Caucasian and
African-American women. Breast Cancer Res. 9:R842007. View Article : Google Scholar : PubMed/NCBI
|
|
79.
|
Ruggiero M, Pacini S, Aterini S, Fallai C,
Ruggiero C and Pacini P: Vitamin D receptor gene polymorphism is
associated with metastatic breast cancer. Oncol Res. 10:43–46.
1998.PubMed/NCBI
|
|
80.
|
Hou MF, Tien YC, Lin GT, et al:
Association of vitamin D receptor gene polymorphism with sporadic
breast cancer in Taiwanese patients. Breast Cancer Res Treat.
74:1–7. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
81.
|
Sinotte M, Rousseau F, Ayotte P, et al:
Vitamin D receptor polymorphisms (FokI, BsmI) and breast cancer
risk: association replication in two case-control studies within
French Canadian population. Endocr Relat Cancer. 15:975–983. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
82.
|
Buyru N, Tezol A, Yosunkaya-Fenerci E and
Dalay N: Vitamin D receptor gene polymorphisms in breast cancer.
Exp Mol Med. 35:550–555. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
83.
|
Dunning AM, McBride S, Gregory J, et al:
No association between androgen or vitamin D receptor gene
polymorphisms and risk of breast cancer. Carcinogenesis.
20:2131–2135. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
84.
|
Chakraborty A, Mishra AK, Soni A, et al:
Vitamin D receptor gene polymorphism(s) and breast cancer risk in
north Indians. Cancer Detect Prev. 32:386–394. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
85.
|
Sillanpää P, Hirvonen A, Kataja V, et al:
Vitamin D receptor gene polymorphism as an important modifier of
positive family history related breast cancer risk.
Pharmacogenetics. 14:239–245. 2004.PubMed/NCBI
|
|
86.
|
Lundin AC, Söderkvist P, Eriksson B,
Bergman-Jungeström M and Wingren S: Association of breast cancer
progression with a vitamin D receptor gene polymorphism. South-East
Sweden Breast Cancer Group. Cancer Res. 59:2332–2334.
1999.PubMed/NCBI
|
|
87.
|
Ikuyama T, Hamasaki T, Inatomi H, Katoh T,
Muratani T and Matsumoto T: Association of vitamin D receptor gene
polymorphism with renal cell carcinoma in Japanese. Endocr J.
49:433–438. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
88.
|
Wedrén S, Magnusson C, Humphreys K, et al:
Associations between androgen and Vitamin D receptor
microsatellites and postmenopausal breast cancer. Cancer Epidemiol
Biomarkers Prev. 16:1775–1783. 2007.PubMed/NCBI
|
|
89.
|
Protzel C, Maruschke M and Hakenberg OW:
Epidemiology, aetiology, and pathogenesis of renal cell carcinoma.
Eur Urol (Suppl). 11:52–59. 2012. View Article : Google Scholar
|
|
90.
|
American Cancer Society. Breast Cancer
Facts and Figures 2009–2010. American Cancer Society; Atlanta, GA:
2010
|