Introduction
It has been estimated that nearly 22,280 new cases
of ovarian cancer were diagnosed in the US in 2012, with
approximately 15,500 people dying of the disease (1). Ovary cancer incidence in Korea was
similar to that in women worldwide and ovarian cancer-related
mortality rates has been increasing (2). High-grade serous ovarian carcinoma
(HGSOC) is the most lethal form of gynecologic cancer and mostly
advanced clinical stages (FIGO stages III and IV) of the disease at
the time of diagnosis, and they exhibit more metastatic lesions.
HGSOCs are heterogenous group of diseases involving many different
tumorigenic pathways and harboring a variety of genetic mutations.
It seems that the understanding of the possible mechanisms
underlying the aggressive progression of HGSOC is required to
elaborate their fatal clinical outcome. It has been suggested that
cancer cells become aggressive and metastatic by acquiring an
invasive or meta-static phenotype. We suggests this step requires
remodeling of actin cytoskeleton and fascin1 fundamentally
contributes to disease progression and patients prognosis.
Fascin1 (gene name FSCN1 in human) is a 55
kDa actin-bundling protein and is an important regulatory element
in the maintenance and stability of parallel bundles of filamentous
actin in a variety of cellular contexts (3). This can help form the cellular
structures critical to cell movement, such as filopodia and spikes,
and its depletion by small interfering RNA (siRNA) leads to a
substantially reduced number of filopodia (4). It is not clear how fascin1 promotes
invasive motility in cancer cells, but Li et al recently
provided new evidence that fascin1 stabilizes actin bundles in
invasive foot structures termed invadopodia, and they suggested
that fascin1 is an integral component of invado-podia and it is
important for the stability of actin (5). Thus, fascin1 provides cells with
powerful invasive properties that may confer increased metastatic
potential. Fascin1 has recently received considerable attention as
a new biomarker or potential therapeutic target because it is
absent or at very low levels in epithelial cells (6), whereas the overexpression of fascin1
has been reported in tumors of the lung (7), esophagus (8), breast (9), colon (10), urinary bladder (11) and ovary (12) usually correlating with high grade,
extensive invasion, distance metastasis and poor prognosis. The
aberrant expression of fascin1 in these cancers has been indicated
to be linked to increased cell motility and tumor invasiveness.
Hashimoto et al hypothesized that fascin1 upregulation is
generally correlated with the aggressive behavior of cancer cells,
independent of the tissue origin (3). Depletion of fascin1 reduced
penetration into reconstituted matrix and greatly reduced the
spikeness of invading cells. Thus, fascin1 appears to provide
cancer cells with stable long lived invasive protrusion that allow
them to invade into the surrounding matrix. Previous studies of
fascin1 expression in ovarian cancer suggested that upregulation of
fascin1 in tumor tissue may promote invasion of ovarian carcinoma
(12). Similarly, fascin1
expression of advanced colorectal adenocarcinomas correlated with
shorter survival in stage III/IV patients (10). Although the hypothesis of
regulation by β-catenin signaling has received attention, how
fascin1 transcription is activated in carcinoma cells is largely
unknown. Dysregulation of the WNT/β-catenin signaling pathway has
been implicated in tumorigenesis at several sites including the
colon, rectum, breast, liver and ovary. Fascin1 has been shown to
bind with β-catenin at leading cell edges and cell-cell borders
supporting its role in modulating the functions of cell motility
and adhesion (13). Because
β-catenin has been reported as a second binding partner for
fascin1, we examined the expression of β-catenin in HGSOC and on
the possible association with fascin1. It was reported that in
ovarian cancer, nuclear localization of β-catenin was observed in
23% of serous tumors (14).
Knockdown of fascin1 in human colon carcinoma cells results in
decreased adhesion dynamics and subsequent reduction in cell
migration (15). In support of
this, a number of recent studies have shown clear roles for fascin1
in mouse models of tumor formation. Two reports demonstrated that
colon carcinoma cells stably expressing shRNA to knockdown fascin1
showed significantly decreased tumor growth and development in
xenograft mouse models (4,15). Animal studies have reported a
positive correlation between fascin1 expression and tumor
invasiveness (16). Expression of
fascin1 positively correlates with clinically aggressive tumors and
as such has recently received considerable attention as both a
potential prognostic marker and therapeutic target for treatment of
metastatic disease (17).
The aims of this study were to determine the
expression of fascin1 and β-catenin in HGSOC and to correlate its
expression with clinicopathologic parameters and demonstrate the
possibility of prognostic predictors. In addition, we investigated
the effects of fascin1 on cancer cell proliferation, migration and
invasion of ovarian cancer cells to determine the potential role of
fascin1 in ovarian cancer progression and utility of therapeutic
target. We propose that fascin1 promotes invasion and metastasis of
HGSOC cells and is associated with a more aggressive phenotype and
poor clinical outcome.
Materials and methods
Case selection and tissue preparation for
fascin1 and β-catenin immunohistochemistry
The cases were collected at the CHA Bundang Medical
Center, School of Medicine, CHA University from 1998 to 2011. A
total of 79 patients with HGSOC were enrolled in this study, and
their clinicopathological characteristics are summarized in
Table I. The ages ranged from 24
to 83 years (median age, 54 years); 37 patients (46.8%) were ≥55
years and 42 patients (53.2%) were <55 years old. The FIGO
stages at initial diagnosis were as follows: low stage (I/II) in 18
cases (22.8%) and high stage (III/IV) in 61 cases (77.2%). Lymph
node involvement and distant metastasis were detected in 45 (56.9%)
and 24 cases (30.4%), respectively. The mean follow-up interval was
75.9 months (range, 2–139 months). The patients were treated with a
first-line chemotherapeutic regimen consisting of paclitaxel and
cisplatin or carboplatin after radical surgery.
 | Table I.The association between fascin1
expression and clinicopathologic parameters of patients with
high-grade ovarian serous carcinoma. |
Table I.
The association between fascin1
expression and clinicopathologic parameters of patients with
high-grade ovarian serous carcinoma.
| Parameter | Case n=79 | FSCN1 expression
| P-value |
|---|
| Negative (%) | Positive (%) |
|---|
| Age, years | | | | |
| <55 | 43 | 26 (60.5) | 17 (20.1) | 0.116 |
| ≥55 | 36 | 16 (44.4) | 20 (55.6) | |
| FIGO stage | | | | |
| Low (I/II) | 18 | 14 (77.8) | 4 (22.2) | 0.021a |
| High
(III/IV) | 61 | 29 (47.5) | 32 (52.5) | |
| LN involvement | | | | |
| No | 34 | 23 (67.6) | 11 (32.4) | 0.034a |
| Yes | 45 | 20 (44.4) | 25 (55.6) | |
| Distant
metastasis | | | | |
| No | 55 | 34 (61.8) | 21 (38.2) | 0.040a |
| Yes | 24 | 9 (37.5) | 15 (62.5) | |
| Recurrence | | | | |
| No | 52 | 29 (55.8) | 23 (44.2) | 0.462 |
| Yes | 27 | 14 (51.9) | 13 (48.1) | |
All cases were reviewed by two pathologists. Tissue
cores for tissue microarray were collected from tumor (primary
site) and control sections (normal fallopian tube and benign serous
tumor). Tissue microarrays were constructed from archival
formalin-fixed, paraffin-embedded tissue blocks using a manual
tissue arrayer (Quick-Ray Manual Tissue Microarrayer; Unitma Co.
Ltd., Seoul, Korea). Tissue cylinders with a diameter of 3 mm were
punched from the tumor area of the donor block and were transferred
to a recipient paraffin block. Tissue microarrays were sectioned to
a 4-μm thickness.
Immunohistochemistry
Tissue microarray sections were dewaxed in xylene,
rehydrated in alcohol and immersed in 3% hydrogen peroxide for 10
min to suppress endogenous peroxidase activity. Antigen retrieval
was performed by heating (100°C) each section for 30 min in 0.01
mol/l sodium citrate buffer (pH 6.0). After three rinses in
phosphate-buffered saline (PBS) for 5 min, each section was
incubated for 1 h at room temperature with mouse monoclonal
antibodies to human fascin1 (Epitomics, Burlingame, CA, USA;
1:100), and human β-catenin (Novus Biologicals, Littleton, CO, USA)
all diluted in PBS; washed three times in PBS for 5 min; incubated
with horseradish peroxidase-labeled rabbit anti-mouse
immunoglobulin (DAKO; 1 h at room temperature); washed three times;
and incubated with a solution of diaminobenzidine (DAB) at room
temperature to visualize peroxidase activity.
Evaluation of immunostaining
Two experienced pathologists evaluated the
immunoreactivity and histological appearance of all tissue samples
in the microarray. The intensity of cytoplasmic tumor cell staining
was scored on a scale of 0 to 3: with 0 (no staining), 1 (weak
intensity), 2 (moderate intensity) and 3 (the strongest intensity),
and extent of tumor cells with cytoplasmic staining at each
intensity was estimated. The extent of staining was scored as 0
(0%), 1 (1–25%), 2 (26–50%), 3 (51–75%) or 4 (76–100%), according
to the percentages of positively stained areas in relation to the
whole carcinoma area. The final scores for fascin1 immunostaining
was obtained by mutiplying the staining intensity and the extent
scores, score ranging from 0 to 12. We designated as positive in
the cases with final staining score of ≥3.
Cell lines, media and culture
conditions
The human ovarian cancer cell lines (SKOV3, OVCAR3)
were purchased from the American Type Culture Collection
(Rockville, MD, USA). OVCAR3 cells were cultured in RPMI-1640
medium containing 10% fetal bovine serum (FBS), 100 U/ml penicillin
and 100 mg/ml streptomycin. SKOV3 cells were cultured in McCoy’s 5A
medium supplemented with 10% FBS, 100 U/ml penicillin and 100 mg/ml
streptomycin. Cell lines were incubated at 37°C in a humidified
atmosphere consisting of 5% CO2 and 90% humidity. These
cell lines grew in a monolayer and were passaged when cultures were
70–80% confluent.
Small interfering RNA preparation and
transfections
Cells were plated at 70% confluency in McCoy’s 5A,
RPMI-1640 containing 10% serum without antibiotics. We diluted 200
pmol/ml siRNA into 500 μl serum-starved media (Gibco, Grand
Island, NY, USA) without antibiotic-antimycotic (Invitrogen,
Carlsbad, CA, USA) solution. We diluted 10 μl Lipofectamine
2000 (Invitrogen) into 500 μl of the above described media,
incubated for 5 min at room temperature, and added 500 μl of
diluted transfection mixture containing the FSCN1 siRNA for another
20 min at room temperature. The transfection complex mixture was
added to the cells. Scrambled siRNA with Lipofectamine 2000 alone
was used as control. After 6 h, the medium was changed, and the
samples were assayed after 24, 48 and 72 h until ready for further
assay.
RNA isolation and quantitative real-time
PCR for FSCN1 expression
Total RNA was extracted from fresh tissues and the
ovarian cancer cell lines SKOV3, and OVCAR3 were homogenized in
TRIzol reagent (Invitrogen) in accordance with the manufacturer’s
instructions. RNA purity and concentration were confirmed by
spectrophotometry using a NanoDrop ND-1000 instrument (NanoDrop
Technologies, Wilmington, DE, USA). First-strand cDNA synthesis was
performed using a Superscript III kit (Invitrogen). cDNA samples
were analyzed in triplicate using the Bio-Rad CFX96 Real-Time PCR
Detection System. Briefly, 1 μg of total RNA was amplified
using the TaqMan Gene Expression Assay (Applied Biosystems,
Paisley, UK) for the analyses of GAPDH (ABI code: Hs99999905_m1),
and FSCN1 (ABI code: Hs00979631_g1), respectively. The PCR reaction
mix had a volume of 20 μl and contained 10 μl 2X
TaqMan master mix (Applied Biosystems), 1 μl primer and
probe kit (Applied Biosystems), 1 μl cDNA and 8 μl of
diethylenepyrocarbonate (DEPC) water. The reverse transcription
conditions used were as follows; 50°C for 2 min, 95°C for 10 min,
followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. RNA
levels were quantified at least three times. Transcript levels were
normalized versus GAPDH expression, and gene expression was
calculated using 2−ΔΔCt.
Western blot analysis
Cells were homogenized and extracted with protease
extraction buffer (Pro-Prep, iNtRON Biotechnology, Korea) and
centrifuged at 4°C, 13,000 rpm, for 15 min. Protein concentration
were determined by the Bradford assay. Total proteins were
electrophoresed on 10% Sodium Dodecyl Sulfate-Polyacrylamide gel.
Separated proteins were transferred onto nitrocellulose membrane at
100 V for 2 h, and the membranes were blocked in 5% milk for 1 h.
The membrane was incubated with 1:1,000 dilution of rabbit
anti-FSCN1 (Epitomics), β-actin (1:10,000, Santa Cruz
Biotechnology, Santa Cruz, CA, USA) overnight at 4°C, washed with
TBST and incubated with 1:5,000 goat anti-rabbit IgG, 1:5,000
rabbit anti-mouse IgG (Santa Cruz Biotechnology) secondary
antibodies (1:5,000) for 1 h at room temperature. After the
membrane had been washed with TBST, the protein bands were
visualized using ECL reagent (iNtRON Biotechnology). The
quantification of protein was done by densitometric digital
analysis of protein bands using Quantity One® 1-D
Analysis Software version 4.6.7 (Bio-Rad Laboratories, Hercules,
CA, USA). Each protein band was normalized to the corresponding
β-actin band.
Wound-healing assay
Cell migration was measured using an in vitro
wound-healing assay. Cells were allowed to form a confluent
monolayer in a 96-well tissue culture plates coated with gelatin
before wounding. The wound was created by scraping monolayer cells
with a sterile pipette tip across the monolayer. The wounded
monolayers were washed twice with PBS to remove cell debris.
Monolayers were incubated in cell culture medium and imaged through
a microscope and photographed with a digital camera (CoolPix 950;
Nikon) at 24 h.
Colony-forming assay
Cells (SKOV3, OVCAR3) were seeded at
1×105 cells per well in 6-well plates. The next day,
cells were transfected with fascin1 siRNA and incubated for 48 h.
Transfected cells were then replated at 300 cells per well in
6-well culture dishes. After 14 days, colonies were visualized
using hematoxylin after fixation with 4% paraformaldehyde for 10
min and then counted. Groups of >50 cells were scored as
colonies.
Matrigel invasion assay
Cell invasion assay was carried out using Boyden
chambers containing Transwell (Corning costar #3422) membrane
filter inserts with pore size of 8 μm. The Transwell
membrane was coated with Matrigel at 3:7 dilution (BD Biocoat,
Bedford, MA, USA) for invasion assay, cells (1×104) in
McCoy’s 5A medium containing 0.1% BSA were seeded on Boyden
chambers (upper chamber). The lower chambers were filled with
McCoy’s 5A medium containing 10% FBS. After 48 h of invasion at
37°C, cells passing through the filters into bottom wells were
fixed in 100% ethanol and stained with hematoxylin and eosin
(Sigma-Aldrich, St. Louis, MO, USA).
Statistical analysis
SPSS 19.0 software was used for statistical analysis
(Chicago, IL, USA). The χ2 test and Fisher’s exact test
were used for comparison of the variables. Survival curves were
estimated by the Kaplan-Meier method and compared by the log-rank
test. Univariate and multivariate analyses were based on the Cox
proportional hazards model. Wound healing, colony forming, and
invasion assays were analyzed using a one-way analysis of variance.
For all analyses, P-values <0.05 were considered statistically
significant.
Results
The association between immunoreactivity
of fascin1 expression and clinical parameters in patients with
HGSOC and evaluation of β-catenin immunostaining
To investigate whether fascin1 expression is related
to HGSOC, we analyzed fascin1 expression in 79 HGSOC tissues.
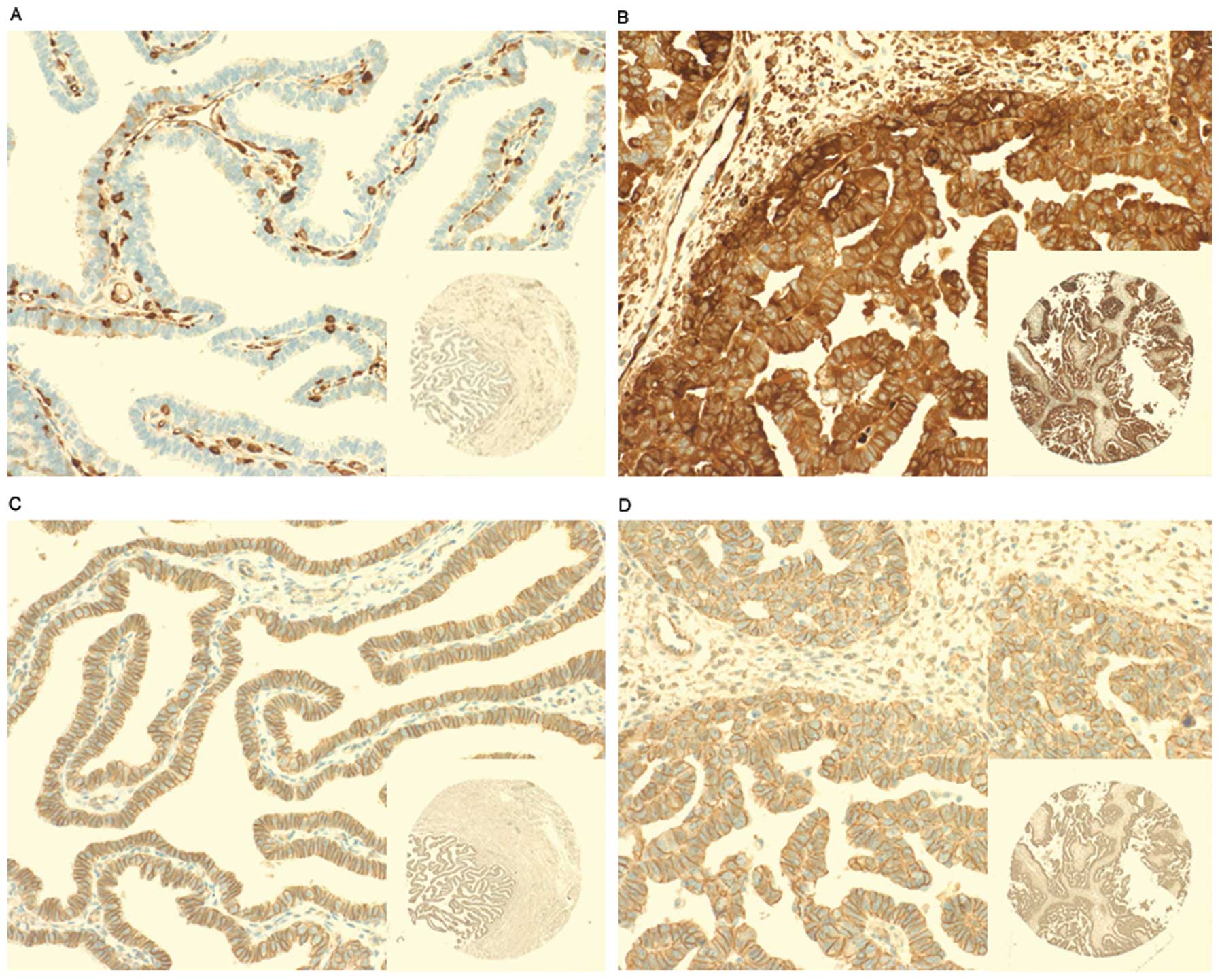
Fascin1 was immunonegative in the epithelial cells of fallopian
tube as a negative control and immunopositive in the endothelial
cells as a positive control (Fig.
1A). Fascin1 positive immunostained cells were those containing
dark brown granules mainly distributed in the cytoplasm of HGSOC
cells (Fig. 1B). This finding
demonstrates upregulation of fascin1 as a phenotypic alteration in
HGSOC cells. Cells with positive β-catenin expression were defined
as those cells containing dark brown nuclei or cytoplasmic
staining, but there is no significant β-catenin positive staining
cells, whereas all cancer cells and fallopian tube epithelial cells
(normal control) in this study showed membrane staining (Fig. 1C and D). Table I shows the association between
fascin1 expression and clinicopathological parameters. We found
that fascin1 overexpression was significantly correlated with high
FIGO stage (III/IV) (P=0.021), lymph node involvement (P=0.034) and
distant metastasis (P=0.040). However, fascin1 expression was not
associated with other parameters such as age, and tumor
recurrence.
Fascin1 expression is an independent
prognostic marker in patients with high grade ovarian serous
carcinomas
We also evaluated whether fascin1 immunoreactivity
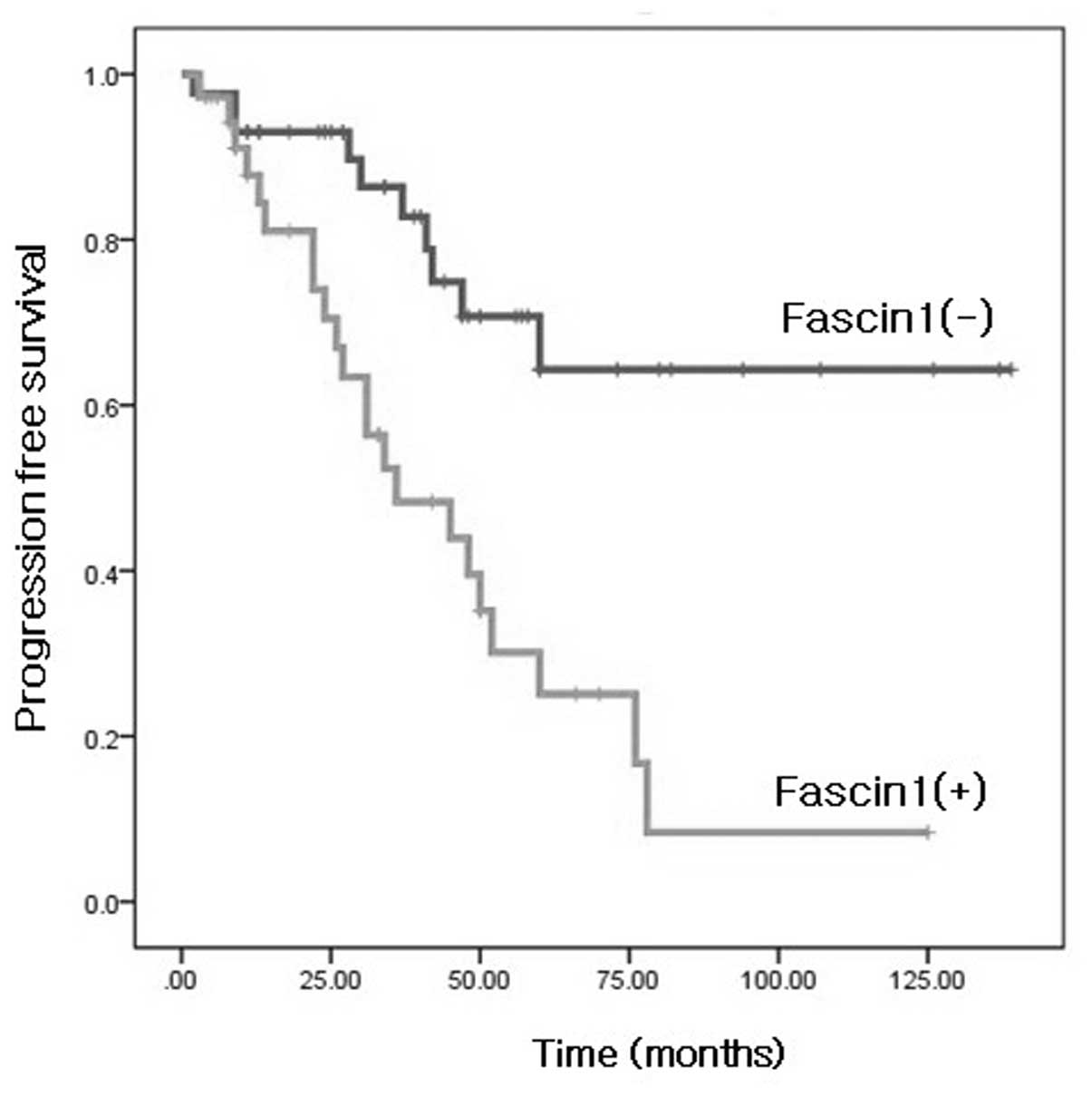
predicted survival of patients with HGSOC. A Kaplan-Meier survival
curve showed that the fascin1 expression group was significantly
associated with poor survival of the patients (Fig. 2). The univariate and multivariate
analyses results of progression-free survival for HGSOC are shown
in Table II. The results of
univariate analysis showed that age (P=0.012), FIGO stage
(P=0.010), distant metastasis (P<0.001), and fascin1 expression
(P<0.001) were correlated with progression-free survival. A
multivariate Cox regression analysis revealed that age (P=0.030),
and fascin1 expression (P=0.008) were independent prognostic
factors of progression-free survival.
 | Table II.Univariate log-rank analysis and
multivariate Cox regression analyses of progression-free survival
(months, mean ± standard deviation) in patients with high-grade
ovarian serous carcinoma. |
Table II.
Univariate log-rank analysis and
multivariate Cox regression analyses of progression-free survival
(months, mean ± standard deviation) in patients with high-grade
ovarian serous carcinoma.
| Variable | Case | No. of deaths | Progression-free
survival | P-value | Progression-free
survival hazard ratio (95%CI) | P-value |
|---|
| Age, years | | | | | | |
| <55 | 42 | 12 | 93.2±10.3 | 0.012a | 2.412
(1.086–5.354) | 0.030a |
| ≥55 | 37 | 20 | 49.7±6.7 | | | |
| LN involvement | | | | | | |
| Absent | 37 | 11 | 78.1±10.1 | 0.148 | 1.749
(0.731–4.185) | 0.209 |
| Present | 42 | 21 | 69.2±9.6 | | | |
| FIGO stage | | | | | | |
| I/II | 18 | 1 | 97.5±8.6 | 0.010a | 3.945
(0.475–32.792) | 0.204 |
| III/IV | 61 | 31 | 67.6±7.7 | | | |
| Distant
metastasis | | | | | | |
| Absent | 58 | 15 | 94.2±9.1 | <0.001a | 1.803
(0.879–3.737) | 0.113 |
| Present | 21 | 17 | 43.9±8.6 | | | |
| FSCN1
expression | | | | | | |
| Negative | 43 | 10 | 102.1±9.7 | <0.001a | 2.955
(1.32–6.60) | 0.008a |
| Positive | 36 | 22 | 46.3±6.7 | | | |
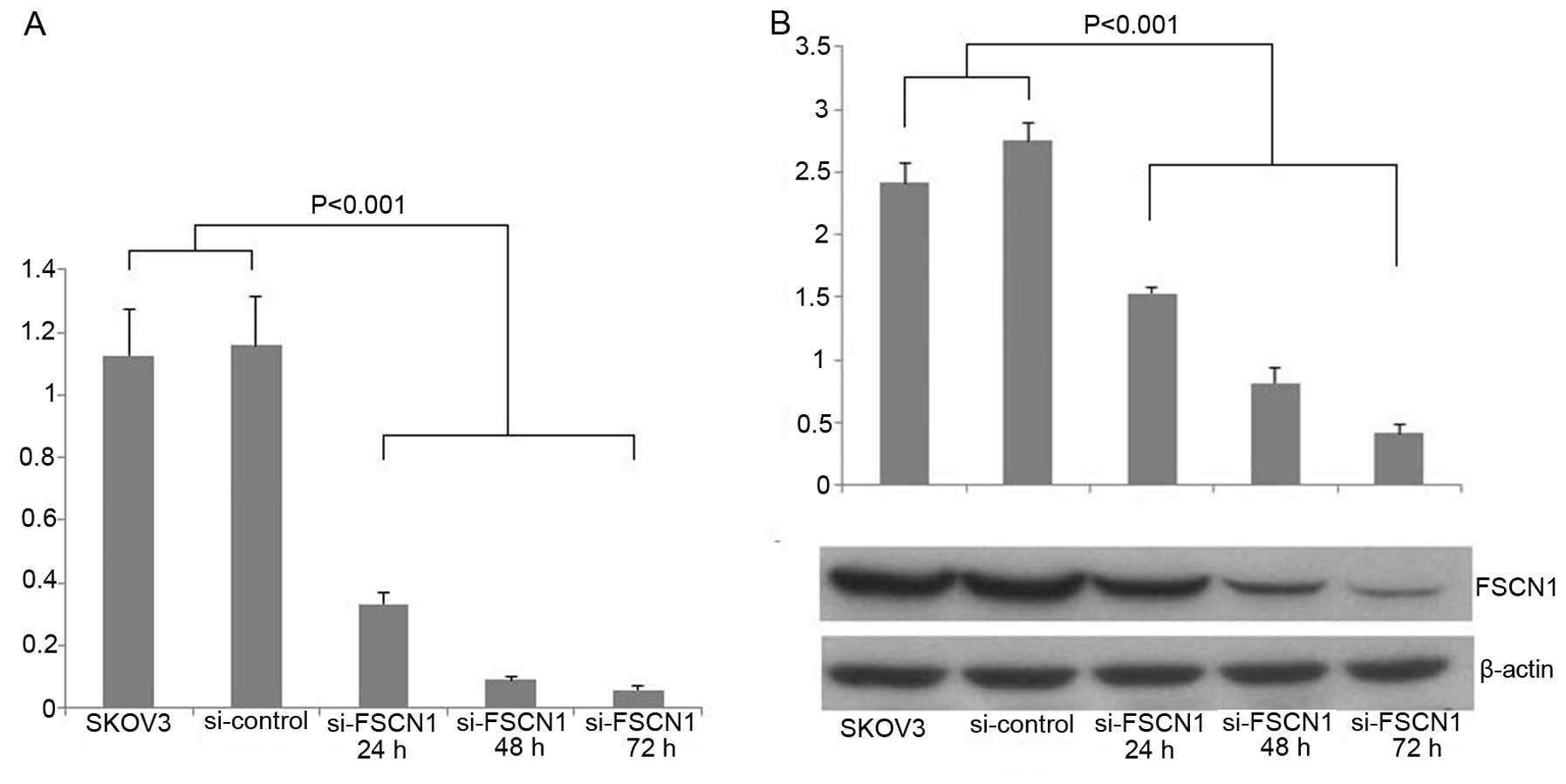
Fascin1 siRNA inhibits the expression of
fascin1 in ovarian cancer cell lines
We used siRNA against fascin1 to transfect the SKOV3
ovarian cancer cells. We examined fascin1 mRNA expression compared
with control cells after transfection. The result showed that
fascin1 mRNA expression was reduced by 71.3, 92.3 and 95% of the
transfected cells with 24, 48 and 72 h, respectively (P<0.001;
Fig. 3A). To demonstrate the
efficiency of fascin1 silencing at the protein expression level,
western blot analysis was used to detect the fascin1 protein
expression levels in 24, 48 and 72 h after transfection. We found
that fascin1 expression reduced by 44.3, 70.2 and 85.1% of the
transfected cells with 24, 48 and 72 h, respectively, compared with
control cell line (P<0.001; Fig.
3B).
Effects of fascin1 inactivation on cancer
cell migration, proliferation and invasion activity in fascin1
siRNA transfected ovarian cancer cell lines
We performed wound healing, colony forming and
Matrigel invasion assays after fascin1 siRNA transfection. Colony
numbers of transfected cancer cells decreased significantly to
95.7% (SKOV3), 78.1% (OVCAR3) compared with that of control cells
at 72 h (P<0.05; Fig. 4A). Cell
motility following wound generation showed less cell migration in
transfected cells compared with that of control cells (P<0.05;
Fig. 4B). After 16 h, we observed
that transfected cells resulted in 51.3% (SKOV3), and 55.3%
(OVCAR3) decreased migrating cell numbers in comparison with that
of the control. The Matrigel invasion assay was used to assess the
invasiveness of the cancer cells. The staining results are shown in
Fig. 4C at 48 h. The control cells
were more invasive and fascin1 siRNA transfected cells decreased
significantly to 35.8% (SKOV3), 31.1% (OVCAR3) compared with that
of control cells (P<0.05).
Discussion
Fascin1 has received great attention as a potential
therapeutic target among cytoskeletal proteins because multiple
clinical studies have implicated its expression correlates with
poor prognosis and metastasis in multiple carcinomas. This may be
because fascin1 is not normally expressed in some epithelial
tissues and when it is upregulated as a part of a mechanism of
cancer cell epithelial to mesenchymal progression, it confers
special motility and invasive properties on cancer cells (18). Given that fascin1 plays a key role
in assembly and stability of actin-rich bundles within protrusive
structures in cancer cells, it is possible that upregulation of
fascin1 in metastatic disease in vivo can assist in
promoting cell invasion through cytoskeletal assembly. A further
study identified fascin1 as being highly upregulated in a
subpopulation of circulating human breast tumor cells in a
xenograft model that undergo re-colonization of their tumors of
origin in a process termed ‘self-seeding’ (9). Upregulation of fascin1 in tumoral
tissue may promote invasion of ovarian carcinoma by cell-matrix
adhesion (19). It has been
reported that fascin1 was not expressed in epithelial cells of
normal fallopian tube and benign serous tumor but overexpressed in
ovarian serous carcinoma (12,20,21).
Therefore, the expression of fascin1 has been shown to be
associated with invasive phenotype and poor prognosis in ovarian
serous tumor. It was reported to be highly upregulated human
cancers suggesting that fascin1 may fundamentally contribute
towards disease progression (15).
This is one of the reasons that fascin1 has received considerable
attention recently as an emerging key prognostic marker of
metastatic disease. We are currently expanding our study to
evaluate the prognostic significance of fascin1 expression and its
effect of invasiveness in patients with HGSOC. We found that with
the exception of a few specimens, whereas fascin1 was not detected
in the normal fallopian tube and benign serous tumor, the
expression of fascin1 was significantly elevated in HGSOC tissue,
and this increase was FIGO stage-dependent. We also demonstrated
that fascin1 expression was higher in patients with lymph node
involvement and distant metastasis, this results showed that
fascin1 is a possible marker for predicting distant metastasis of
HGSOC. We conclude that fascin1 expression correlates with
invasiveness of HGSOC and the presence of fascin1 in primary tumors
has predictive value in determining the advanced clinical stage.
Consistent with our findings, Kabukcuoglu et al demonstrated
that fascin1 expression was correlated with clinical stage,
especially higher in tumors than normal samples (19). Our results also demonstrated
fascin1 expression have a strong influence on patients survival
outcome. Daponte et al reported that strong fascin1
expression is an independent prognostic factor for survival of
advanced ovarian serous carcinoma (22). Compatible with this report, our
study also demonstrated that fascin1 expressing group was
significantly associated with shorter progression-free survival. A
multivariate analysis showed that fascin1 expression was an
independent negative prognostic factor for progression-free
survival. Taken together, we suggest that fascin1 overexpression is
an indicator of poor prognosis in patients with HGSOC.
The precise mechanism of fascin1 has not been
clearly elucidated. In some cases, high fascin1 expression has been
correlated with low E-cadherin expression, indicating that as cells
progress through the epithelial to mesenchymal transition (EMT),
they gain fascin1 whilst losing E-cadherin. There is evidence that
fascin1 expression is regulated by two pathways, the WNT activated
TCF/LEF (T cell factor/lymphocyte enhancer-binding factor)
transcriptional signaling pathway that promotes EMT and cyclic-AMP
response element binding protein (CREB) and the arylhydrocarbon
receptor (AhR) (16,23). Hashitomoto et al suggest
that upregulation of fascin1 in aggressive human carcinomas appears
to have a multi-factorial basis but CREB and AhR as specific
regulators of fascin1 transcription do not support the hypothesis
that β-catenin signaling has a central role (23). The critical component of WNT
signaling pathway, β-catenin, plays an important role in this
process. The aberrant activation of β-catenin-TCF signaling
eventually leads to the accumulation of β-catenin in the nucleus,
where it forms transcriptionally active complexes with TCF/LEF
(24). It has been recognized that
fascin1 binds to β-catenin at leading cell edges and cell-cell
border as a novel target of β-catenin-TCF signaling, supporting its
role in modulating the functions of cell motility and adhesion and
fascin1 expression is tightly regulated during development of colon
cancer metastasis (16). However,
Jawhari et al demonstrated that fascin1 overexpressed cells
were not affected in their ability to localize E-cadherin and
β-catenin to cell-cell margin (25). It has also been reported that
fascin1 was a target of the β-catenin pathway in the invasive
progress of ovarian cancer. Gamallo et al reported that
β-catenin nuclear localization was correlated with improved
survival in early stage ovarian carcinomas, while Lee et al
revealed that 23% positivity of β-catenin nuclear localization in
HGSOC and they suggested that it was one of the mechanisms for
tumorigenicity in HGSOC possibly through activation of the
TCF/β-catenin pathway (14,26).
These results were not compatible with our findings. Otherwise, Cho
et al demonstrated that nuclear expression of β-catenin was
detected in only one case (0.6%) of serous ovarian tumor, membrane
staining was not different among benign, borderline, and malignant
tumors, and Kildal et al also reported that they observed
β-catenin nuclear localization in only one of 59 (1.7%) serous
adenocarcinoma cases (20,27). If ovarian serous tumor follows this
pathway, nuclear localization of β-catenin is increased due to the
decrease of contact inhibition of E-cadherin/β-catenin, but in this
study, nuclear localization of β-catenin was not observed. Whereas
all cases of HGSOC in our study demonstrated β-catenin membrane
staining, no detectable β-catenin nuclear or cytoplasmic staining
was found. Therefore, we suggest that β-catenin nuclear expression
may be an uncommon finding in HGSOC. Thus, due to a limited number
of studies and different results, further investigation including
evaluation of TCF activity of WNT signaling pathway in HGSOC is
required.
Fascin1 is currently the only actin bundling protein
localized along the entire length of filopodia and its depletion by
small interfering RNA (siRNA) leads to a substantially reduced
number of filopodia (4). The
fascin1 in invadopodia appears to stabilize the actin, as knockdown
of fascin1 increases the mobile fraction in invadopodia and
decreases the lifetime and size of invadopodia (5). Depletion of fascin1 reduced
penetration into reconstituted matrix and greatly reduced the
spikeness of invading cells. Thus, fascin1 appears to provide
efficient stable invasive protrusions that allow them to invade
into the matrix. Given the fact that there is a role for fascin1 in
cancer cell migration and proliferation, we examined the effects of
fascin1 on cell proliferation and invasion measured by colony
forming, wound healing and Matrigel-coated Transwell invasion
assays. To demonstrate the functional effect of fascin1 on
invasiveness of ovarian serous carcinoma, we performed fascin1
siRNA study, which provided us the functional consequences of
fascin1 inactivation. It has been shown that fascin1 downregulation
had inhibitory effects on tumor cell migration, proliferation, and
invasiveness of esophageal squamous cell carcinoma cell lines,
suggesting that fascin1 contributes to tumor progression and could
be a therapeutic molecular target (28). Hu et al have reported that
the expression of fascin1 in ovary tumor cell cultures is
significantly associated with the ability to grow and spread
intraperitoneally after intraperitoneal inoculation supporting the
role of fascin1 in ovarian metastasis (29). Consistent with this report, our
study further demonstrated that inactivation of fascin1 by siRNA
technique resulted in a decreased proliferation, motility and
invasiveness of ovarian cancer cells in vitro, suggesting
that fascin1 contributed to the invasion and metastasis of HGSOC.
Thus, these results imply that fascin1 expression in human ovarian
cancer cells plays an important role in their motile, invasive, and
metastatic capacities. Hashimoto et al suggested that
suppression of fascin1 expression using siRNA resulted in fewer
filopodia, altered cell protrusions, decreased Rac-dependent
migration on laminin, and decreased turnover of focal adhesions
(15). Chen et al has
reported that fascin1 as the primary target for the antitumor agent
migrastatin, macroketone in its inhibition of tumor cell migration,
invasion and metastasis (30). The
data demonstrated that migrastatin binds to the actin-binding site
in fascin1 and thus inhibits fascin1-dependent invasion in
vivo. The identification of such a specific inhibitor of
fascin1-actin binding will additionally provide new molecular
targets for cancer treatment.
In conclusion, we have demonstrated that fascin1
expression was significantly correlated with advanced clinical
stage and related to survival of the patients. Our results support
the concept that the blockage of fascin1 influences ovarian cancer
cell proliferation and invasive potential. Our findings highlight
the important role of fascin1 in aggressive progression of HGSOC
and imply that fascin1 is a potential prognostic marker for
patients with HGSOC and suggesting its use as a potential
therapeutic target for ovarian cancer treatment.
References
|
1.
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
2.
|
Park B, Park S, Kim TJ, et al:
Epidemiological characteristics of ovarian cancer in Korea. J
Gynecol Oncol. 21:241–247. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Hashimoto Y, Skacel M and Adams JC: Roles
of fascin in human carcinoma motility and signaling: prospects for
a novel biomarker? Int J Biochem Cell Biol. 37:1787–1804. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Vignjevic D, Kojima S, Aratyn Y, Danciu O,
Svitkina T and Borisy GG: Role of fascin in filopodial protrusion.
J Cell Biol. 174:863–875. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Li A, Dawson JC, Forero-Vargas M, et al:
The actin-bundling protein fascin stabilizes actin in invadopodia
and potentiates protrusive invasion. Curr Biol. 20:339–345. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Kureishy N, Sapountzi V, Prag S, Anilkumar
N and Adams JC: Fascins, and their roles in cell structure and
function. Bioessays. 24:350–361. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Pelosi G, Pastorino U, Pasini F, et al:
Independent prognostic value of fascin immunoreactivity in stage I
non-small cell lung cancer. Br J Cancer. 88:537–547. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Zhang H, Xu L, Xiao D, et al: Fascin is a
potential biomarker for early-stage oesophageal squamous cell
carcinoma. J Clin Pathol. 59:958–964. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Yoder BJ, Tso E, Skacel M, et al: The
expression of fascin, an actin-bundling motility protein,
correlates with hormone receptor-negative breast cancer and a more
aggressive clinical course. Clin Cancer Res. 11:186–192. 2005.
|
|
10.
|
Hashimoto Y, Skacel M, Lavery IC,
Mukherjee AL, Casey G and Adams JC: Prognostic significance of
fascin expression in advanced colorectal cancer: an
immunohistochemical study of colorectal adenomas and
adenocarcinomas. BMC Cancer. 6:2412006. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Tong GX, Yee H, Chiriboga L, Hernandez O
and Waisman J: Fascin-1 expression in papillary and invasive
urothelial carcinomas of the urinary bladder. Hum Pathol.
36:741–746. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Kabukcuoglu S, Oner U, Ozalp SS, Bildirici
K, Yalcin OT and Colak E: The role of actin bundling protein fascin
in the progression of ovarian neoplasms. Eur J Gynaecol Oncol.
27:171–176. 2006.PubMed/NCBI
|
|
13.
|
Tao YS, Edwards RA, Tubb B, Wang S, Bryan
J and McCrea PD: beta-catenin associates with the actin-bundling
protein fascin in a noncadherin complex. J Cell Biol.
134:1271–1281. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Lee CM, Shvartsman H, Deavers MT, et al:
beta-catenin nuclear localization is associated with grade in
ovarian serous carcinoma. Gynecol Oncol. 88:363–368. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Hashimoto Y, Parsons M and Adams JC: Dual
actin-bundling and protein kinase C-binding activities of fascin
regulate carcinoma cell migration downstream of Rac and contribute
to metastasis. Mol Biol Cell. 18:4591–4602. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Vignjevic D, Schoumacher M, Gavert N, et
al: Fascin, a novel target of beta-catenin-TCF signaling, is
expressed at the invasive front of human colon cancer. Cancer Res.
67:6844–6853. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Hashimoto Y, Ito T, Inoue H, et al:
Prognostic significance of fascin overexpression in human
esophageal squamous cell carcinoma. Clin Cancer Res. 11:2597–2605.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Machesky LM and Li A: Fascin: Invasive
filopodia promoting metastasis. Commun Integr Biol. 3:263–270.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Kabukcuoglu S, Ozalp SS, Oner U, et al:
Actin bundling protein fascin expression in ovarian neoplasms:
comparison of histopathologic features of tumors obtained by the
first and secondary cytoreduction surgeries. Eur J Gynaecol Oncol.
27:123–128. 2006.
|
|
20.
|
Cho EY, Choi Y, Chae SW, Sohn JH and Ahn
GH: Immunohistochemical study of the expression of adhesion
molecules in ovarian serous neoplasms. Pathol Int. 56:62–70. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Wen YH, Yee H, Goswami S and Shukla PS:
Fascin expression in serous tumors of ovary correlates with
aggressiveness of malignancy. Int J Gynecol Pathol. 28:187–192.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Daponte A, Kostopoulou E, Papandreou CN,
et al: Prognostic significance of fascin expression in advanced
poorly differentiated serous ovarian cancer. Anticancer Res.
28:1905–1910. 2008.PubMed/NCBI
|
|
23.
|
Hashimoto Y, Loftis DW and Adams JC:
Fascin-1 promoter activity is regulated by CREB and the aryl
hydrocarbon receptor in human carcinoma cells. PLoS One.
4:e51302009. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Clevers H and Nusse R: Wnt/beta-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Jawhari AU, Buda A, Jenkins M, et al:
Fascin, an actin-bundling protein, modulates colonic epithelial
cell invasiveness and differentiation in vitro. Am J Pathol.
162:69–80. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Gamallo C, Palacios J, Moreno G, Calvo de
Mora J, Suarez A and Armas A: beta-catenin expression pattern in
stage I and II ovarian carcinomas: relationship with beta-catenin
gene mutations, clinicopathological features, and clinical outcome.
Am J Pathol. 155:527–536. 1999. View Article : Google Scholar
|
|
27.
|
Kildal W, Risberg B, Abeler VM, et al:
beta-catenin expression, DNA ploidy and clinicopathological
features in ovarian cancer: a study in 253 patients. Eur J Cancer.
41:1127–1134. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Xie JJ, Xu LY, Zhang HH, et al: Role of
fascin in the proliferation and invasiveness of esophageal
carcinoma cells. Biochem Biophys Res Commun. 337:355–362. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Hu W, McCrea PD, Deavers M, Kavanagh JJ,
Kudelka AP and Verschraegen CF: Increased expression of fascin,
motility associated protein, in cell cultures derived from ovarian
cancer and in borderline and carcinomatous ovarian tumors. Clin Exp
Metastasis. 18:83–88. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Chen L, Yang S, Jakoncic J, Zhang JJ and
Huang XY: Migrastatin analogues target fascin to block tumour
metastasis. Nature. 464:1062–1066. 2010. View Article : Google Scholar : PubMed/NCBI
|