Introduction
Although the incidence of gastric cancer has been
substantially declining for several decades, gastric cancer is
still the second most common cause of cancer-related death in the
world, which accounts for 738,000 deaths annually (1–3). Due
to its tendency to spread into the muscularis propria, the lymph
node, and the vessel and the relative asymptomatic progression of
early-stage, a number of cancer patients present with advanced
diseases and have a poor long-term prognosis (4). In the past decades, intensive efforts
have been made to identify specific markers to improve prognosis of
gastric cancer, there is still a lack of molecular markers for
targeting therapy. In clinical practice, clinicopathological
features are mostly relied on to predict patients’ outcome,
however, these prognostic factors do not fully predict individual
clinical outcome (5–7). As a result of these factors, there is
a continued need for identifying more prognostic markers in order
to guide clinical practice and improve the long-term prospect of
survival for gastric cancer patients.
A substantial body of evidence has shown that the
proliferation, invasion and metastasis of tumor cells, which are
highly associated with N-acetylglucosaminyltransferase V (GnT-V),
is one of the key factors reducing the effectiveness of cancer
treatment (8–14). GnT-V is a key enzyme that catalyses
the formation of 1, 6 N-acetylglucosamine (GlcNAc) through the
action of adding antennae branching structures on a common core
structure of Man3GlcNAc2 in the medial-Golgi apparatus (15–17).
A previous study proposed that post-translational modification of
cell surface glycoproteins through N-glycosylation, such as matrix
metalloproteinase-9 (MMP-9) and E-cadherin (18,19),
may have a role on the process of tumor invasion and metastasis,
damage to the surrounding tissues, such as the extracellular matrix
(ECM), the basement membrane and the vascular walls. The increased
levels of GnT-V in human breast cancer tissues were reported
(20,21). mRNA levels of GnT-V were elevated
during hepatocarcinogenesis of rats (22,23),
and increased expression of GnT-V in human hepatocellular carcinoma
tissues was also reported and positive correlation to tumor size
was observed (10,24). However, aberrantly expressed GnT-V
has been reported in a variety of tumors. In neuroblastomas and
colorectal cancer, the expression of GnT-V correlated significantly
with distant metastasis. In contrast, hepatocellular carcinoma
cases with low or no expression of GnT-V were more likely to show
recurrence than cases with high expression. Moreover, GnT-V
expression was inversely associated with prognosis and histology in
non-small cell lung cancers. Thus, its clinical role in gastric
cancer remains fragmentary. To explore its malignant influence and
prognostic significance, we examined the GnT-V protein expression
and localization of GnT-V in human cancer tissues as well as in
normal tissues. The correlation between GnT-V and other clinical
pathological parameters of gastric carcinomas including
growth-pattern, invasion and metastasis were also analyzed in this
study. Furthermore, we devised a knockdown approach, in which small
interfering RNA (siRNA)-directed against GnT-V mRNA was used to
downregulate GnT-V mRNA expression in two gastric cancer cell lines
SGC7901 and BGC823 cells. In additon, the biological behavior was
observed. The comparison of specific GnT-V expression in cancer
tissues and conventional clinicopathological analysis as well as
evaluation in gastric cancer lines raised a possibility of GnT-V
expression as a novel poor prognostic factor and a new targeting
marker for successful treatment of gastric cancer patients.
Materials and methods
Tumor samples and patient follow-up
Surgical specimens of gastric cancer patients
resected at surgery service in the Department of Surgery, Tongji
Hospital, School and Medicine and Shanghai Pudong New Area Gongli
Hospital were used as histological samples, with informed consent
from the patients. Samples were fixed with 20% formalin in PBS for
72 h and embedded with paraffin. The conventional
clinicopathological features and TNM staging were obtained by the
pathologists according to the criteria of the TNM classification
revised in 2003 by International Union against Cancer (UICC) and
American Joint Committee on Cancer (AJCC). The patients were
followed-up in outpatient clinic of our hospital for >36 months
after surgery.
Immunohistochemistry and pathology
review
Immunohistochemical analysis of cancer tissue and
adjacent non-cancerous mucosa was performed with a monoclonal
antibody against GnT-V (Abcam, USA). Incubation with the primary
antibody was followed by incubation with biotinylated goat
anti-mouse IgG antibody. The specimens were analyzed by SABC
method. The sections were examined by two independent observers
without prior knowledge of the clinical status of the patients.
RNA isolation from primary GCs
Fresh, frozen tumor tissues and adjacent
non-cancerous mucosa (different tissues from those used in
immunohistochemical analysis) were sent to the Department of
Gastroenterology, Tongji Hospital, Tongji University School of
Medicine from Shanghai Minhang Central Hospital and Shanghai Pudong
New Area Gongli Hospital with informed consent from the patients.
All samples were obtained by surgery and were stored at −80°C. The
RNA samples obtained from 43 patients with GC were subjected to
quantitative real-time reverse transcription-PCR (RT-PCR) analyses.
All of the patients were diagnosed clinically as well as
pathologically. The tumors were staged according to standard
methods, as stated above.
Quantitative real-time PCR analysis of
primary GCs
The level of GnT-V mRNA was detected by quantitative
real-time reverse transcription-PCR analysis (qRT-PCR). Reverse
transcription reactions were performed with the PrimeScript RT
Master Mix (Takara Biotechnology Co., Ltd.) and proceeded for 15
min at 37°C, followed by 5 sec at 85°C for complementary DNA (cDNA)
synthesis. Real-time reactions were performed using the SYBR
PrimeScript™ RT-PCR kit (Takara Biotechnology Co.) under
the following conditions: 30 sec at 95°C for 1 cycle, 5 sec at
95°C, 20 sec at 60°C for 40 cycles, 95°C for 0 sec, 65°C for 15 sec
and 95°C for 0 sec for melting curve analysis. The following primer
sets were used: GnT-V, 5′-GATGCTTCTGCACTTTAC-3′ and 5′-GCCTTG
ATGTACCTTTTG-3′; GAPDH, 5′-ATCACCATTGGCAAT GAG-3′ and
5′-AAGGTAGTTTCGTGGATG-3′. The relative mRNA expression level of
GnT-V in each sample was calculated using the comparative
expression level 2−ΔΔct method. All experiments were
carried out in triplicate for each data point.
Cell culture
SGC7901, BGC 823 cells and MKN45 cells were
generously provided by the cell division of center laboratory in
Tongji Hospital of Tongji University, Shanghai, China. Cells were
cultured in 90% RPMI-1640 (Gibco) supplemented with 100 U/ml
penicillin + streptomycin antibiotics (Gibco) and 10% fetal bovine
serum (Gibco) at 37°C with 5% CO2.
Construction of siRNA vector and
retroviral infection
Small interfering oligonucleotides specific for
GnT-V were designed on the Takara Bio website (http://www.takara-bio.co.jp/) and the oligonucleotide
sequences used in the construction of the siRNA vector were as
follows: 5′-TGCTGAAAGAAGGC TCGCAGATGAGCGTTTTGG CCACTGACTGACGCTCAT
CTGAGCCTTCTTT-3′ and 5′-CCTGAAAGAAGGCTCAG
ATGAGCGTCAGTCAGTGGCCAAAACGCTCATCTGCG AGCCTTCTTTC-3′. The
oligonucleotides were annealed and then ligated into HindIII
sites of the pcDNA6.2-GW/EmGFP-miR vector (Novo Bio). A retroviral
supernatant was obtained by transfection of human embryonic kidney
293 cells (HEK293) using a pL-MIG retrovirus packaging system (Novo
Bio). BGC823 and SGC7901 cells, two human GC cell lines, were
infected with the viral supernatant, and the cells were then sorted
by flow cytometry (BD). GFP-positive cells were confirmed by GnT-V
gene and protein expression.
Quantitative real-time PCR and western
blot analysis of gastric cell line
Quantitative real-time PCR analyses of GnT-V mRNA
expression in cell lines were performed as described above.
The expression of GnT-V protein was detected by
western blot assay. Cells (107) were harvested and lysed
with ice-cold lysis buffer (RIPA and a mixture of protease
inhibitors, Beyotime Institute of Biotechnology). Protein
concentration of the supernatant was determined by the BCA protein
assay procedure. Equal amount of proteins were separated by 10%
SDS-PAGE, respectively. Then, proteins were transferred to
polyvinylidene difluoride membrane using a semi-dry transfer
apparatus. The membrane was blocked in Tris-buffered saline (TBS)
with 5% non-fat milk for 1 h at room temperature, followed by
incubation with appropriate primary antibodies (1:500-diluted
antibody of GnT-V, Abcam and 1:2,000-diluted antibody of MMP-9,
Abcam) at 4°C overnight. After washing in TBS-Tween-20 buffer,
membranes were incubated for 2 h with the appropriate
peroxidase-conjugated secondary antibodies, following washing in
TBS-Tween-20 buffer, the protein bands on the membranes were
visualized using ECL kit (Beyotime Institute of Biotechnology). The
autographed film was scanned and processed with Odyssey Infrared
Imaging system. Protein bands were quantified by Quantity One. The
densitometric value of each protein band was normalized to
GAPDH.
Observation with transmission electron
microscopy (TEM)
BGC823 parent and KD cells were collected after
centrifuging (1500 rpm, 8 min), and fixed by 3%, pH 7.4
glutaraldehyde for 30 min at 4°C. The specimens were established
using conventional methods. Cells were observed by TEM after double
lead dyeing.
Cell proliferation assay (CCK-8)
Cells were seeded in 96-well plates at
2×103 cells/well. At the indicated times (d0–7), 10
μl Cell Counting Kit-8 (CCK-8, Beyotime Institute of
Biotechnology) solution and 100 μl RPMI-1640 + 10% FBS were
added to each well. The cells were incubated for 60 min and
absorbance at 450 nm was measured to calculate cell growth rates.
Growth rate = (absorbance at 450 nm at dx -absorbance at 450 nm at
d0) / (absorbance at 450 nm at d0).
Cell cycle analysis
Cells were harvested, washed with phosphate-buffered
saline (PBS) twice and then fixed with 75% cold ethanol for 12 h.
The fixed cells were spun down and re-suspended in PBS at
1×106 cells/ml. After incubation with ribonuclease A at
a final concentration of 3,000 U/ml at 37°C for 30 min, cells were
filtered through a 40-μm nylon mesh (BD Biosciences, USA).
The cell suspension was stained with propidium iodide before
analysis on a flow cytometer (BD Biosciences). Each test was
repeated in triplicate.
Cell apoptosis assay
Cells were harvested, washed twice in ice-cold PBS
solution, and re-suspended in binding buffer containing 7-AAD
(7-amino-actinomycin D) for 10 min, followed by the addition of
Annexin V-PE. Analysis of cell apoptosis was carried out using a
flow cytometer (BD Biosciences).
Cell invasion assay
Using 24-well transwell units with 8-μm pore
size polycarbonateinserts (Matrigel™ invasion chamber,
BD Biosciences). Cells that were suspended in RPMI-1640 + 10% FBS
were added to each upper compartment of the transwell units. After
being cultured for 24 h, cells migrating through the
matrigel-coated polycarbonate membrane were fixed by 4%
paraformaldehyde, then stained with Giemsa reagent and counted in
five different fields. These fields were selected randomly.
Statistical analysis
The associations between GnT-V expression and
clinical parameters were analyzed by the χ2 test or
Fisher’s exact test, as appropriate. The survival curves were
estimated using the Kaplan-Meier method and differences in survival
distributions were evaluated by the generalized Wilcoxon test.
Statistical comparisons of groups of cell lines were performed
using one-way analysis of variance (ANOVA) and statistical
significance was defined as P<0.05. All the analyses were
performed using SPSS13.0 software.
Results
Association between expressive levels of
GnT-V protein and clinicopathology in primary gastric cancer
tissues
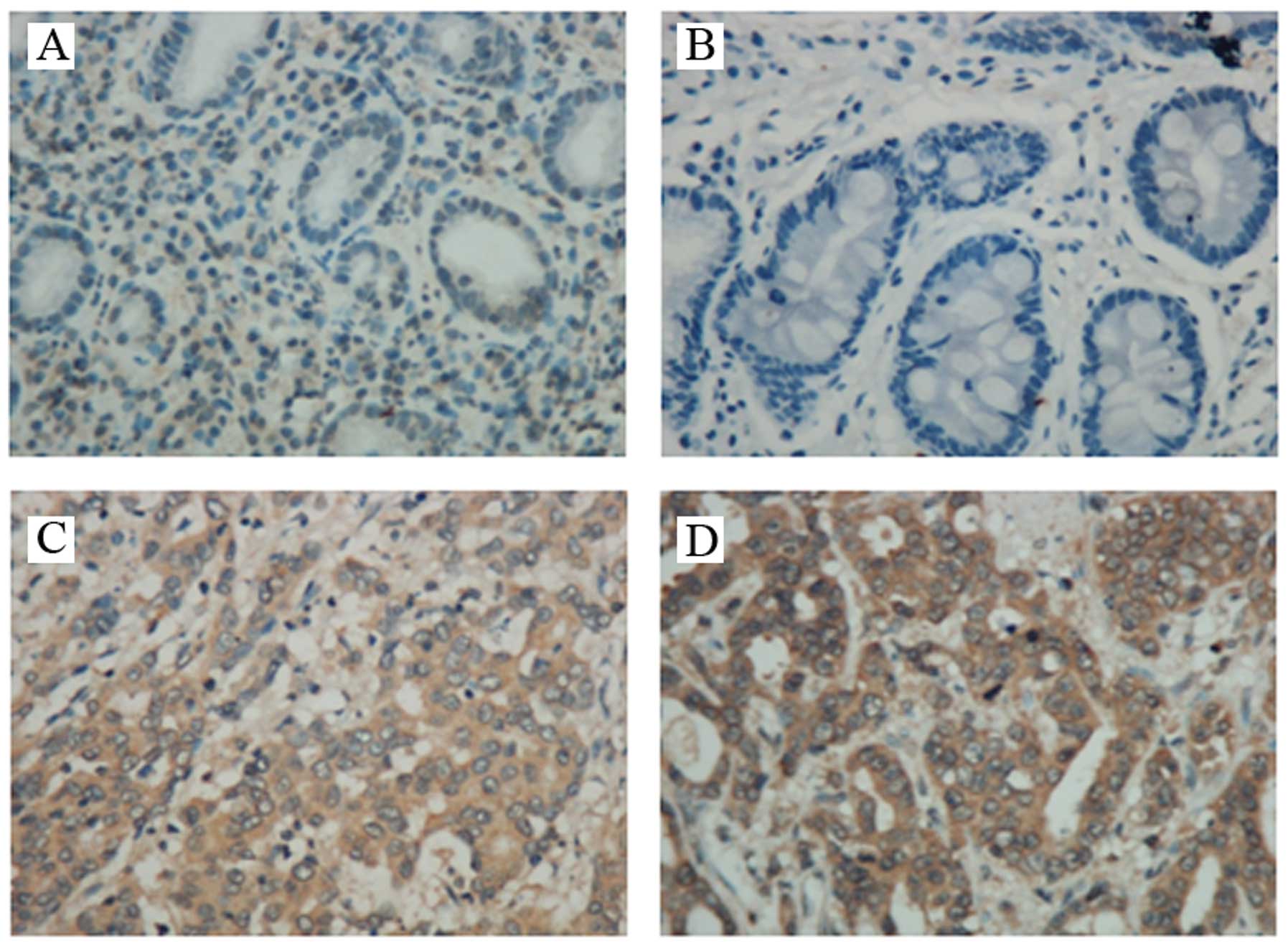
To assess the GnT-V protein expression of the
gastric cancer (GC) tissues, we first performed
immunohistochemistry analyses using 100 GC tissues and 100 chronic
gastritis (CG) tissues. Typical immunostaining patterns for GnT-V
in GC and CG tissue are shown in Fig.
1. As shown in Table I,
positive GnT-V expression was found in 61 (61%) GCs and 39 (39%)
CGs. Positive expression of GnT-V was significantly more prevalent
in tumors than CG tissues (P=0.0004). In cancer cells, GnT-V
expression was found diffusely in the cytoplasm or localized in the
Golgi apparatus, as reported previously for colon cancers (8).
 | Table I.GnT-V expression in gastric cancer
and chronic gastritis tissues. |
Table I.
GnT-V expression in gastric cancer
and chronic gastritis tissues.
| GnT-V expression
|
|---|
| Negative (%) | Positive (%) | χ2 | P-value |
|---|
| Chronic gastritis
tissues (n=100) | 65 (65.0) | 35 (35.0) | 12.520 | 0.0004 |
| Gastric cancer
(n=100) | 39 (39.0) | 61 (61.0) | | |
One hundred patients were analyzed in
this study
According to the standard of TNM classification
revised in 2003 by International Union against Cancer (UICC) and
American Joint Committee on Cancer (AJCC), lymph vessel invasion
and venous invasion were assessed. The TNM classification was
performed for the cases examined. As shown in Table II, GnT-V expression was associated
with pTNM classifications. There was a significant difference in
GnT-V-positive rate between TNM stages. For stage III and IV,
GnT-V-positive rate was 74.6% (44/59), which was significantly
higher than that (41.6%, 17/41) of stage I and II, P=0.0017.
Furthermore, there was a significant correlation, respectively,
between GnT-V and N factor (lymph node metastasis) and M factor
(distant metastasis), other than T factor (tumor depth). GnT-V
showed non-preferentially expressions in most of the tumors with
tumor size >5 cm (69.4%), P=0.2780. However, for T factor,
higher positive expression rate of GnT-V was significantly found in
T2-T4 phase (67.1%) compared to T1 phase tumors (38.1%), P=0.0300.
For N factor, there was a significant difference in tissues with
lymph node metastasis (76.3%) compared with non-lymph node
metastasis (39.0%), P=0.0004. For M factor, difference were
statistically found between M1 phase (89.2%) and M0 phase (50.0%),
P=0.0007. Additionally, in 50 tumor tissues with poorly
differentiated cells, 34 cases (68.0%) were GnT-V-positive, which
was significantly higher than in 50 well-moderately differentiated
cells (54.0%), P= 0.0219. Forty-three patients were followed-up in
outpatient clinic of our hospital for >36 months after surgery.
Positive GnT-V expression was found in 20 (90.1%) patients with
overall survival time <3 years, which was significant higher
than the patients with overall survival time >3 years, P=0.0009.
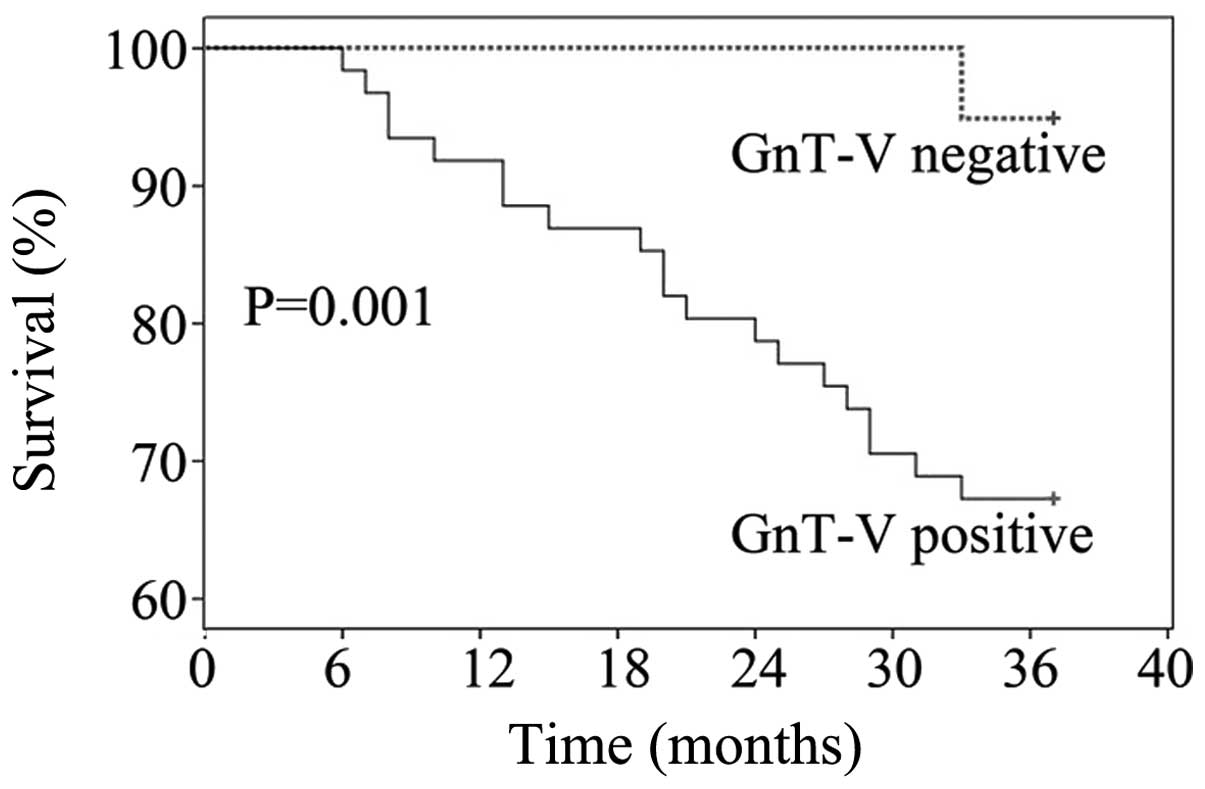
Fig. 2 showed that 3-year overall
survival rate for GnT-V-positive patients was 28.6%, which was
significantly lower than that for the GnT-V-negative (86.7%),
P=0.001.
 | Table II.Expression of GnT-V and
clinicopathological features as well as TNM classification in
gastric cancer. |
Table II.
Expression of GnT-V and
clinicopathological features as well as TNM classification in
gastric cancer.
|
Characteristics | n | Positive no.
(%) | P-value |
|---|
| Age | | | |
| >60 years | 27 | 17 (63.0) | 0.157 |
| ≤60 years | 73 | 44 (60.3) | |
| Sex | | | |
| Male | 61 | 37 (60.7) | 0.930 |
| Female | 39 | 24 (61.5) | |
| Tumor size | | | |
| <5 cm | 64 | 36 (56.3) | 0.278 |
| ≥5 cm | 36 | 25 (69.4) | |
| Depth of
infiltration | | | |
| T1 | 21 | 8 (38.1) | 0.030 |
| T2–T4 | 79 | 53 (67.1) | |
| T2–T3 | 65 | 41 (63.1) | 0.186 |
| T4 | 14 | 12 (85.7) | |
| Lymph node
metastasis | | | |
| No | 41 | 16 (39.0) | 0.0004 |
| Yes | 59 | 45 (76.3) | |
| Distant
metastasisa | | | |
| M0 | 72 | 36 (50.0) | 0.0007 |
| M1 | 28 | 25 (89.2) | |
| Histological
differentiation | | | |
|
Well-moderately | 50 | 27 (54.0) | 0.0219 |
| Poorly | 50 | 34 (68.0) | |
| TNM stages | | | |
| I–II | 41 | 17 (41.6) | 0.0017 |
| III–IV | 59 | 44 (74.6) | |
| Overall survival
time | | | |
| <3-year | 22 | 20 (90.1) | 0.0009 |
| ≥3-year | 21 | 8 (38.1) | |
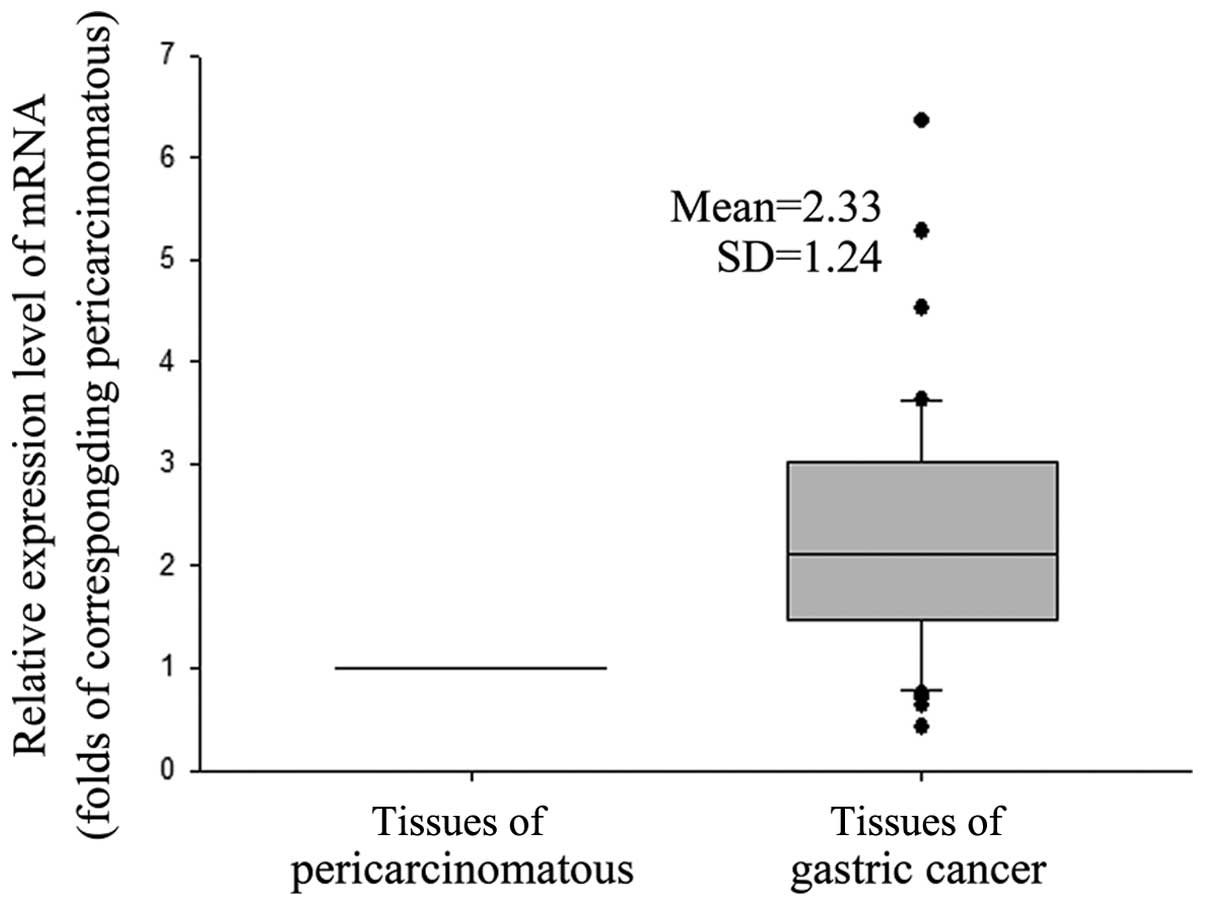
Higher expression levels of GnT-V mRNA in
primary GC tissues than pericarcinomatous tissues
We next analyzed the GnT-V mRNA expression in cancer
cells. As shown in Fig. 3, the
expression levels of gastric cancer tissues were 2.33±1.24-fold on
average that of the adjacent pericarcinomatous tissue, P=0.0000.
Furthermore, the clinical features of the 43 gastric cancer
patients were analyzed (Table
III). Among the tumors with size >5 cm / <5 cm, GnT-V
mRNA expression was elevated by 2.18±1.45- and 2.43±1.21-fold that
of pericarcinomatous tissues, respectively, P>0.05. GnT-V mRNA
expression was found in association with pTNM classifications,
which was consistent with the results of GnT-V protein detection by
IHC. For stage III and IV, GnT-V mRNA was elevated by
2.98±1.36-fold (n=21), which was significantly higher than of stage
I and II (1.70±0.69-fold, n=22), P=0.00018. Correlation between
GnT-V mRNA levels and T factor (tumor depth), N factor (lymph node
metastasis) and M factor (distant metastasis) had a significant
difference, respectively. For T factor, the levels of GnT-V mRNA
were elevated by 4.26±1.71-, 2.15±0.93- and 1.53±0.77-fold in T1
(n=5), T2–T3 (n=33) and T4 (n=5), respectively, with a significant
difference between T1–T3 and T4 group, P=7.91×10−5. For
N and M factor, GnT-V expression was significantly increased in
tumors with lymph node metastasis (P=0.0000) and with distant
metastasis (P=0.0000). However, no obvious difference in GnT-V
expression was found between poorly and well-moderately
differentiated GCs, P=0.1760.
 | Table III.Correlation of GnT-V mRNA expression
with clinic pathological features in gastric carcinoma. |
Table III.
Correlation of GnT-V mRNA expression
with clinic pathological features in gastric carcinoma.
| Clinicpathological
features | GnT-V mRNA
|
|---|
| n | Mean ± SD | P-value |
|---|
| Tumor size | | | |
| <5 cm | 18 | 2.18±1.45 | 0.265 |
| ≥5 cm | 25 | 2.43±1.21 | |
| Depth of
infiltration | | | |
| T1 | 5 | 1.53±0.77 | 0.065 |
| T2–T4 | 38 | 2.43±1.26 | |
| T2–T3 | 33 | 2.15±0.93 |
7.91×10−5 |
| T4 | 5 | 4.26±1.71 | |
| Lymph node
metastasis | | | |
| No | 24 | 1.55±0.64 |
4.98×10−8 |
| Yes | 19 | 3.30±1.12 | |
| Distant
metastasis | | | |
| M0 | 32 | 1.76±0.70 | 3.80 ×
10−9 |
| M1 | 11 | 3.80±1.11 | |
| Histological
differentiation | | | |
|
Well-moderately | 19 | 2.52±1.44 | 0.176 |
| Poorly | 24 | 2.16±1.04 | |
| TNM stages | | | |
| I–II | 22 | 1.70±0.69 | 0.00018 |
| III–IV | 21 | 2.98±1.36 | |
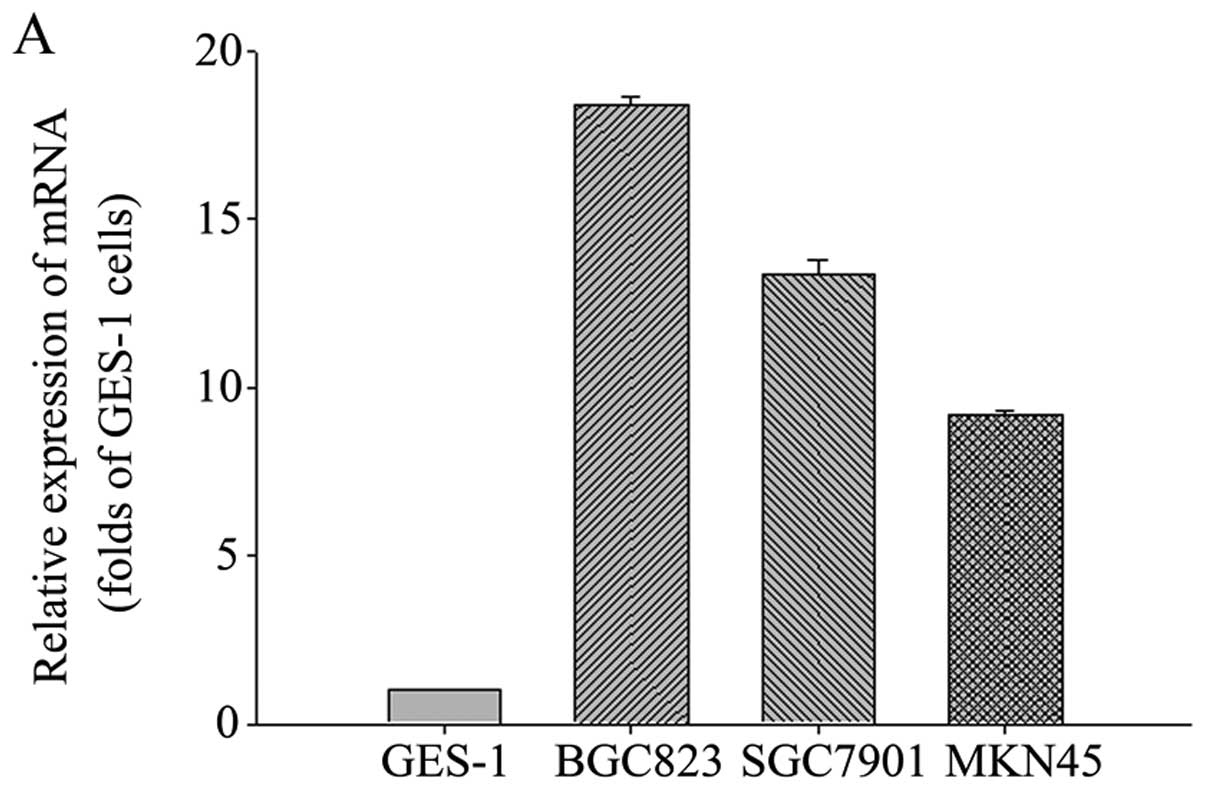
Expression of GnT-V in various human GC
cell lines
To further explore the underlying mechanism of GnT-V
in gastric cancer, we examined the expression of GnT-V in various
human GC cell lines and the gastric epithelial cell line GES-1. As
shown in Fig. 4, each cell line
expressed GnT-V at distinct levels. GnT-V expression was elevated
in gastric cancer cells compared to gastric normal epithelial
cells. The BGC823 cells showed the highest GnT-V mRNA and protein
expression among the three GC cell lines. It is known that the
BGC823 and MKN45 cell lines are poorly differentiated, and SGC7901
cell line is moderately differentiated. Hence, the levels of GnT-V
expression may not predict the degree of differentiation in cell
lines, which was consistent with previous results.
Downregulation of GnT-V expression in
BGC823 and SGC7901 cells
We next prepared a retroviral siRNA vector
containing a small hairpin construct capable of generating a duplex
RNAi oligonucleotide corresponding to human GnT-V. SGC7901 and
BGC823 cell lines were selected to further examination due to the
relatively higher expression of GnT-V mRNA than that of MKN45
cells. After retroviral infection, SGC7901 and BGC823cells were
sorted by flow cytometry. The GnT-V expression was effectively
downregulated by 38.93 and 88.07%, respectively, compared with
those in parent or mock cells (Fig.
5).
Observation of biological behavior in
BGC823 and SGC7901 KD cells
To confirm our clinical observations in
vitro, we examined the biological behavior in cells after GnT-V
gene silencing. We first used transmission electron microscopy
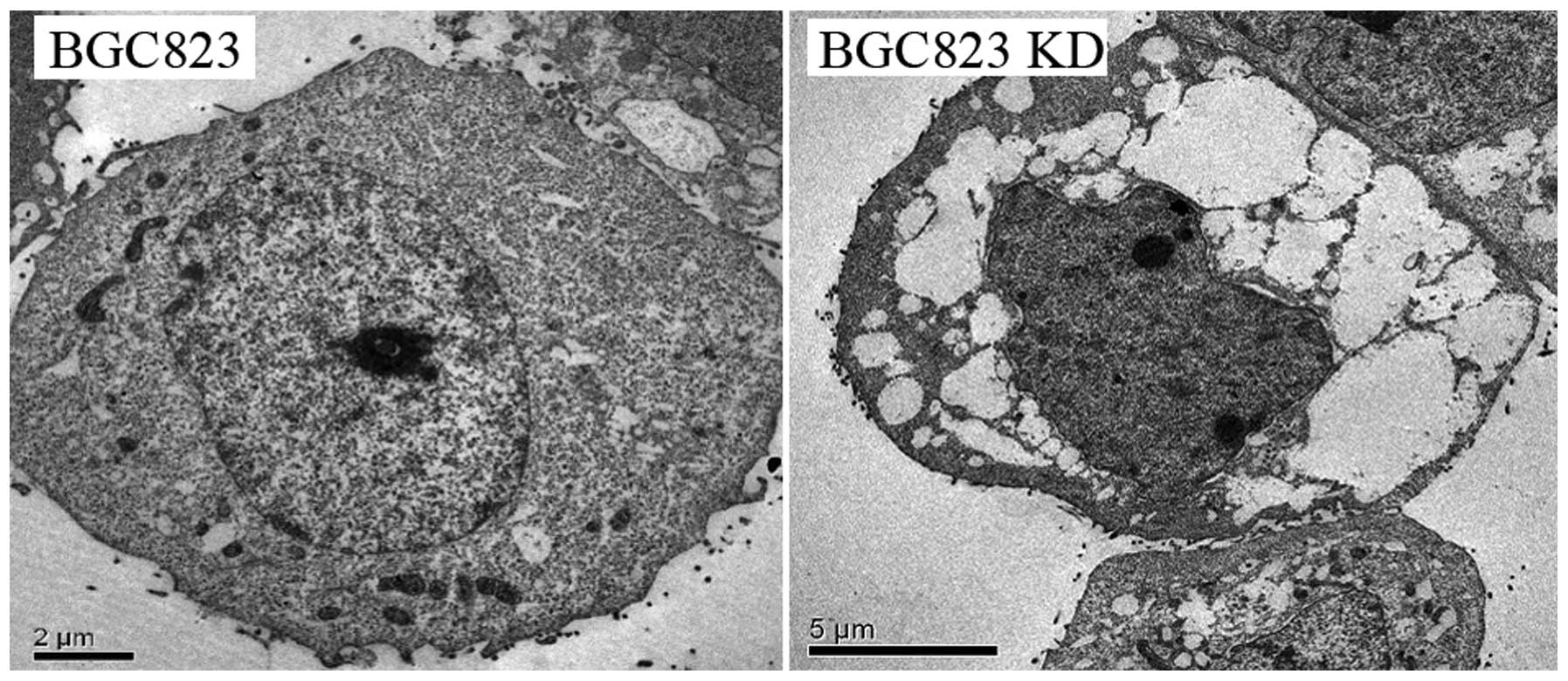
(TEM) to observe the BGC823 and KD cells. As shown in Fig. 6, the size of the nuclei was
decreased visibly, with image of shrinkage and irregularity in KD
cells. Moreover, cytoplasmic enrichment, nuclear heterochromatin
edge setting and reduction of organelles were present in KD cells,
in contrast, there were clear and complete nuclear membrane
structure and enriched cell organelles in cytoplasm of the BGC823
cells.
We investigated the cell growth ability using
proliferation assay by CCK-8 and cell cycle as well as apoptosis
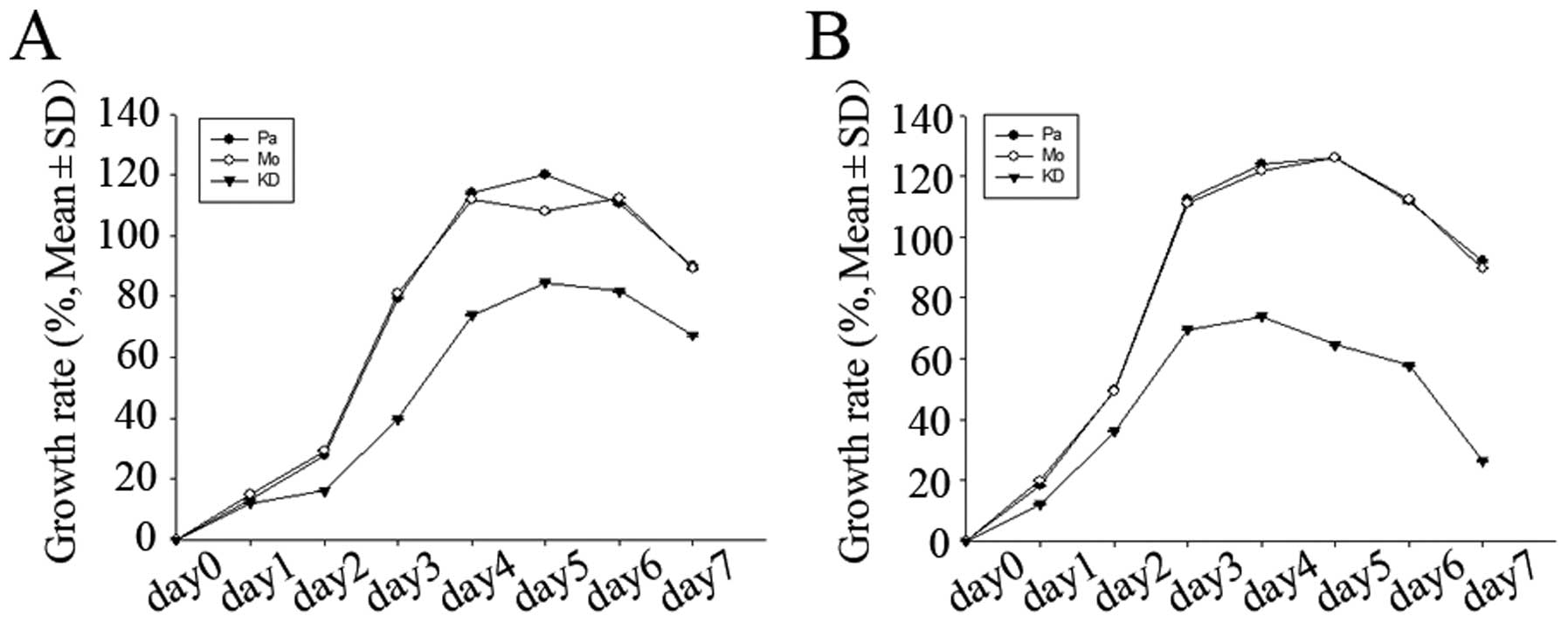
rate analysis by flow cytometry (FCM). As shown in Fig. 7, growth rates of KD cells were
lower than those of parent and mock cells over a 7-day period. Cell
cycle proportion in parent, mock and KD cells is presented in
Table IV. There was no significant
difference in G1, S and G2/M proportion between parent and mock
cells (p>0.05). Compared with parent and mock cells, G1
proportion in KD cells significantly increased (P<0.05),
whereas, the S and G2/M proportion decreased (P<0.05). This
indicated that downregulated GnT-V expression may decrease S, G2/M
proportion, and increase G1 proportion. The cell apoptosis rates
were all increased in KD cells compared with the parent and mock
cells (P<0.05) (Table IV).
 | Table IV.Cell cycle distribution and apoptosis
rate in parent, mock and KD cells. |
Table IV.
Cell cycle distribution and apoptosis
rate in parent, mock and KD cells.
| Cell cycle | Cell line
| Apoptosis rate
(%) |
|---|
| G1 | S | G2/M |
|---|
| SGC7901-Pa | 44.19±2.61 | 32.58±0.13 | 23.23±0.17 | 3.75±0.98 |
| SGC7901-Mo | 46.09±3.03 | 33.12±0.64 | 20.79±0.21 | 3.69±0.11 |
| SGC7901-KD | 75.47±0.31 | 14.33±2.47 | 10.20±0.67 | 18.12±2.08 |
| BGC823-Pa | 47.51±1.02 | 36.14±1.09 | 16.31±0.88 | 3.44±0.55 |
| BGC823-Mo | 46.87±1.38 | 36.41±1.04 | 16.72±1.02 | 3.70±0.47 |
| BGC823-KD | 79.81±1.45 | 14.83±1.08 | 5.36±1.24 | 23.54±0.87 |
Matrigel-coated transwell assay was applied to
detect cell invasion ability. Less cells penetrated the
matrigel-coated membrane in KD cells than in parental and mock
cells, P<0.05 (Fig. 8).
Discussion
Various mechanisms have been shown to underlie
elevated transcription of GnT-V in cancer, which is induced by
direct effects on the GnT-V promoter by the Ets family of
transcriptional activators, which are upregulated by a cellular
proliferation signaling pathway. This pathway begins with growth
factor receptors that activate tyrosine kinases at the cell surface
and proceeds through src, ras, raf and proto-Ha-ras
oncogenes (25–27). GnT-V shows a biological and
functional effect of invasiveness and metastatic potential in
vitro. The upregulating cell motility of 92-1, Mel202 and
IGR-39 compared to FM55P cells, melanoma cell lines, was found
directly associated with an increased level of β1–6 branched
N-oligosaccharides as the result of hyperactivity of GnT-V
(28). The mobility of Mv1Lu, mink
lung epithelial cells, was elevated by the transfection of the
GnT-V gene (29). A specific
increase in β1-6 branching due to an elevation in GnT-V expression
increases metastatic potential of mouse mammary lung cancer cells
(30). However, the role of GnT-V
in different cancers remains controversial. In the case of colon
cancer and hepatocarcinoma, for instance, high GnT-V expression is
associated with a poor prognosis (8,10).
In contrast, low GnT-V levels are linked to a poor prognosis in
lung, bladder carcinomas and neuroblastoma patients (11–13).
The function of GnT-V with gastric cancer and its invasion behavior
has scarcely been studied. Tian et al (14) reported that high GnT-V expression
was observed in 46% (23/50) gastric cancer tissues and was
significantly correlated with lymph node metastases, peritoneal
dissemination and liver metastases, respectively. Altogether, these
data supported the positive correlation in our report between
metastasis and GnT-V expression in gastric cancer patients.
The mechanisms underlying this relationship can be
demonstrated by recent advances in glycoprotein biology. GnT-V is a
Golgi located enzyme participating in the synthesis of
multi-antennary asparagines linked glycans (N-glycans) during the
processing of glycoproteins. It catalyses the transference of
GlcNAc residue from UDP-GlcNAc to the α1,6 mannoside of
C2C2 biantennary or
C2,4C2 triantennary N-glycans, and produce a
β1,6GlcNAc branching structure in the products,
C2C2,6 tri- or C2,4C2,6
tetra-antennary N-glycan (31–33).
Cancer invasion and metastasis is associated with changes in cell
growth control and morphology. For example, expression of epidermal
growth factor receptor (EGFR) family members in breast cancer
correlates with aggressive tumor behavior (34). EGFRs are generally N-glycosylated
transmembrane proteins, and the residency at the surface is
dependent in part on the dynamics of membrane remodeling.
Endogenous lectins, such as galectins, can cross-link glycoproteins
at the cell surface forming lattices that enhance residency time at
the cell surface (35). The level
of EGFR expression is correlated with poor survival in gastric
cancer patients and highly metastatic phenotype of gastric cancer
(36,37). Based on these points, GnT-V may
induce tumor metastasis through controling EGFR distribution on
tumor cell surface.
In line with the discrepancy of the relationship of
GnT-V activity and tumor size assessed by the TNM classification in
pancreatic carcinoma and hepatocellular carcinoma as well as colon
carcinoma (10,16,39),
our data demonstrated no significant relationship between tumor
size and GnT-V expression.
The overall survival rate of GnT-V-positive patients
was significantly less than that of GnT-V-negative patients. The
relationship between GnT-V expression and concomitant poor
prognosis in gastric cancer patients may provide insights into the
decision whether adjuvant chemotherapy is necessary or not for
those GnT-V-positive patients. The expression of GnT-V in resected
specimen will not only help such decision but also give information
on patient prognosis so that intensive follow-up may be done.
Based on the knowledge of histologic characteristics
of GnT-V in gastric cancer, we assumed that GnT-V is a valuable
targeting marker for inhibiting metastasis of gastric cancer. In
the present study, downregulation of GnT-V mRNA in gastric cancer
cell line SGC7901 and BGC823 support the hypotheses we assumed.
Intriguingly, ongoing clinical trials are proceeding to test the
possibility of swainsonine, an inhibitor for expression of β1-6
branched oligosaccharides, as an anti-cancer drug (38,39),
but not in gastric cancer yet. Screening of GnT-V expression in
resected cancer tissues may be able to identify post-operative
patients eligible for such an inhibitor.
Overall the present study provides the possibility
of GnT-V expression as a predictor for the prognosis of gastric
cancer, contributing therefore to improve the diagnosis, prognosis
and perhaps the therapeutic stratification of the patients.
Acknowledgements
We thank Duan Qianglin, Sun Xianghua,
Zhang Yi and Fang Xia for their technical assistance. This study
was supported by grant National Science Foundation of China, no.
81170333 and The Shanghai Committee of Science and Technology key
projects for basic research, no. 12JC1408402.
References
|
1.
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2.
|
Nagini S: Carcinoma of the stomach: a
review of epidemiology, pathogenesis, molecular genetics and
chemoprevention. World J Gastrointest Oncol. 4:156–169. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Danaei G, Vander Hoorn S, Lopez AD, Murray
CJ and Ezzati M; Comparative Risk Assessment collaborating group
(Cancers): Causes of cancer in the world: comparative risk
assessment of nine behavioural and environmental risk factors.
Lancet. 366:1784–1793. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Catalano V, Labianca R, Beretta GD, Gatta
G, de Braud F and Van Cutsem E: Gastric cancer. Crit Rev Oncol
Hematol. 71:127–164. 2009. View Article : Google Scholar
|
|
5.
|
Kim BS, Cho SW, Min SK and Lee BH:
Differences in prognostic factors between early and advanced
gastric cancer. Hepatogastroenterology. 58:1032–1040.
2011.PubMed/NCBI
|
|
6.
|
Seshadri RA, Jayanand SB and Ranganathan
R: Prognostic factors in patients with node-negative gastric
cancer: an Indian experience. World J Surg Oncol. 9:482011.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Qiu MZ, Wang ZQ, Luo HY, et al: Prognostic
analysis in node-negative gastric cancer patients in China. Tumour
Biol. 32:489–492. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Murata K, Miyoshi E, Kameyama M, et al:
Expression of N-acetylglucosaminyltransferase V in colorectal
cancer correlates with metastasis and poor prognosis. Clin Cancer
Res. 6:1772–1777. 2000.PubMed/NCBI
|
|
9.
|
Guo P, Chen HJ, Wang QY and Chen HL:
Downregulation of N-acetylglucosaminyltransferase V facilitates
all-trans retinoic acid to induce apoptosis of human
hepatocarcinoma cells. Mol Cell Biochem. 284:103–110. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Xu YY, Lu Y, Fan KY and Shen ZH: Apoptosis
induced by all-trans retinoic acid in
N-acetylglucosaminyltransferase V repressed human hepatocarcinoma
cells is mediated through endoplasmic reticulum stress. J Cell
Biochem. 100:773–782. 2007. View Article : Google Scholar
|
|
11.
|
Dosaka-Akita H, Miyoshi E, Suzuki O, Itoh
T, Katoh H and Taniguchi N: Expression of
N-acetylglucosaminyltransferase V is associated with prognosis and
histology in non-small cell lung cancers. Clin Cancer Res.
10:1773–1779. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Ishimura H, Takahashi T, Nakagawa H, et
al: N-acetylglucosaminyltransferase V and beta1-6 branching
N-linked oligosaccharides are associated with good prognosis of
patients with bladder cancer. Clin Cancer Res. 12:2506–2511. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Inamori K, Gu J, Ohira M, et al: High
expression of N-acetylglucosaminyltransferase V in favorable
neuroblastomas: involvement of its effect on apoptosis. FEBS Lett.
580:627–632. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Tian H, Miyoshi E, Kawaguchi N, et al: The
implication of N-acetylglucosaminyltransferase V expression in
gastric cancer. Pathobiology. 75:288–294. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Pinho SS, Figueiredo J, Cabral J, et al:
E-cadherin and adherens-junctions stability in gastric carcinoma:
functional implications of glycosyltransferases involving N-glycan
branching biosyn-thesis, N-acetylglucosaminyltransferases III and
V. Biochim Biophys Acta. 1830:2690–2700. 2013. View Article : Google Scholar
|
|
16.
|
Wei T, Liu Q, He F, Zhu W, Hu L, Guo L and
Zhang J: The role of N-acetylglucosaminyltransferases V in the
malignancy of human hepatocellular carcinoma. Exp Mol Pathol.
93:8–17. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Yang HM, Yu C and Yang Z:
N-acetylglucosaminyltransferase V negatively regulates integrin
α5β1-mediated monocyte adhesion and transmigration through vascular
endothelium. Int J Oncol. 41:589–598. 2012.PubMed/NCBI
|
|
18.
|
Pinho SS, Seruca R, Gärtner F, Yamaguchi
Y, Gu J, Taniguchi N and Reis CA: Modulation of E-cadherin function
and dysfunction by N-glycosylation. Cell Mol Life Sci.
68:1011–1020. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Kim YS, Hwang SY, Kang HY, et al:
Functional proteomics study reveals that
N-Acetylglucosaminyltransferase V reinforces the
invasive/metastatic potential of colon cancer through aberrant
glycosylation on tissue inhibitor of metalloproteinase-1. Mol Cell
Proteomics. 7:1–14. 2008. View Article : Google Scholar
|
|
20.
|
Abbott KL, Aoki K, Lim JM, et al: Targeted
glycoproteomic identification of biomarkers for human breast
carcinoma. J Proteome Res. 7:1470–1480. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Guo HB, Randolph M and Pierce MJ:
Inhibition of a specific N-glycosylation activity results in
attenuation of breast carcinoma cell invasiveness-related
phenotypes: inhibition of epidermal growth factor-induced
dephosphorylation of focal adhesion kinase. Biol Chem.
282:22150–22162. 2007. View Article : Google Scholar
|
|
22.
|
Miyoshi E, Nishikawa A, Ihara Y, et al:
N-acetylglucosaminyltransferase III and V messenger RNA levels in
LEC rats during hepatocarcinogenesis. Cancer Res. 53:3899–3902.
1993.PubMed/NCBI
|
|
23.
|
Miyoshi E, Ihara Y, Nishikawa A, et al:
Gene expression of N-acetylglucosaminyltransferases III and V: a
possible implication for liver regeneration. Hepatology.
22:1847–1855. 1995.PubMed/NCBI
|
|
24.
|
Yao M, Zhou DP, Jiang SM, Wang QH, Zhou
XD, Tang ZY and Gu JX: Elevated activity of
N-acetylglucosaminyltransferase V in human hepatocellular
carcinoma. J Cancer Res Clin Oncol. 124:27–30. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Buckhaults P, Chen L, Fregien N and Pierce
M: Transcriptional regulation of N-acetylglucosaminyltransferase V
by the src oncogene. J Biol Chem. 272:19575–19581. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Lu Y and Chaney W: Induction of
N-acetylglucosaminyltran sferase V by elevated expression of
activated or proto-Ha-ras oncogenes. Mol Cell Biochem. 122:85–92.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Pierce M, Buckhaults P, Chen L and Fregien
N: Regulation of N-acetylglucosaminyltransferase V and Asn-linked
oligosaccharide beta(1,6) branching by a growth factor signaling
pathway and effects on cell adhesion and metastatic potential.
Glycoconj J. 14:623–630. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Przybyło M, Pocheć E, Link-Lenczowski P
and Lityńska A: Beta 1-6 branching of cell surface glycoproteins
may contribute to uveal melanoma progression by up-regulating cell
motility. Mol Vis. 14:625–636. 2008.PubMed/NCBI
|
|
29.
|
Demetriou M, Nabi IR, Coppolino M, Dedhar
S and Dennis JW: Reduced contact-inhibition and substratum adhesion
in epithelial cells expressing GlcNAc-transferase V. J Cell Biol.
130:383–392. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Seberger PJ and Chaney WG: Control of
metastasis by Asnlinked, β-6 branched oligosaccharides in mouse
mammary cancer cells. Glycobiology. 9:235–241. 1999.
|
|
31.
|
Zhao Y, Sato Y, Isaji T, et al: Branched
N-glycans regulate the biological functions of integrins and
cadherins. FEBS J. 275:1939–1948. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Gu J and Taniguchi N: Potential of
N-glycan in cell adhesion and migration as either a positive or
negative regulator. Cell Adh Migr. 2:243–245. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Isaji T, Kariya Y, Xu Q, Fukuda T,
Taniguchi N and Gu J: Functional roles of the bisecting GlcNAc in
integrin-mediated cell adhesion. Methods Enzymol. 480:445–459.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Ye Q, Kantonen S and Gomez-Cambronero J:
Serum deprivation confers the MDA-MB-231 breast cancer line with an
EGFR/JAK3/PLD2 system that maximizes cancer cell invasion. J Mol
Biol. 425:755–766. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Lau KS and Dennis JW: N-Glycans in cancer
progression. Glycobiology. 18:750–760. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Atmaca A, Werner D, Pauligk C, et al: The
prognostic impact of epidermal growth factor receptor in patients
with metastatic gastric cancer. BMC Cancer. 12:5242012. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Nan BC, Shao DM, Chen HL, Huang Y, Gu JX,
Zhang YB and Wu ZG: Alteration of N-acetylglucosaminyltransferases
in pancreatic carcinoma. Glycoconj J. 15:1033–1037. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Shaheen PE, Stadler W, Elson P, Knox J,
Winquist E and Bukowski RM: Phase II study of the efficacy and
safety of oral GD0039 in patients with locally advanced or
metastatic renal cell carcinoma. Invest New Drugs. 23:577–581.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Taylor JB, Strickland J, May T and Hawkins
DE: Effect of subacute swainsonine (locoweed; Oxytropis
sericea) consumption on immunocompetence and serum constituents
of sheep in a nutrient-restricted state. Vet Hum Toxicol.
42:199–204. 2000.PubMed/NCBI
|