Introduction
Prostate cancer (PC) is the second most frequently
diagnosed cancer in men worldwide. According to epidemiological
data, the estimated new cases will be over 900,000 and estimated
deaths over 250,000 each year (1).
PC originates from glandular epithelial cells mainly
from the peripheral zone of the gland (2–4).
During PC progression, normal tissue architecture is lost and
malignant cells acquire invasive characteristics (5,6). In
addition, PC is multifocal exhibiting different histopathological
patterns graded from 1 to 5 (Gleason grades). Diagnosis is
accompanied by Gleason score that considers the two predominant
patterns, giving a final value ranging from 2–10, where high
Gleason scores correspond to more undifferentiated tumors (7). This transformation involves
alterations in cell morphology and function, called
epithelial-mesenchymal transition (EMT) (8). During EMT many molecules change their
expression pattern. Transcription factors such as SNAIL, SLUG and
TWIST, and the mesenchymal markers N-cadherin and vimentin,
increase their expression. Some adhesion molecules such as
E-cadherin decrease their expression and others such as β-catenin
change their location from the plasma membrane to the nucleus
(9). It has been shown that the
decrease in E-cadherin is associated with poor prognosis in various
human tumors (10–13). In addition, E-cadherin
overexpression in cultured cells and in vivo tumor models
leads to a decrease of invasiveness and metastasis (14). Immunohistochemical studies on PC
tissue microarray showed that SNAIL staining is associated with
Gleason grade (15) with
increasing expression from benign prostatic hyperplasia (BPH) to PC
bone metastasis (16). SNAIL
transcription factor is a zinc finger protein that can mediate EMT
through downregulation of cell adhesion molecules such as
E-cadherin by binding to E-boxes located in the gene promoter
region. SNAIL can also lead to repression of tight junction
proteins like claudin, occluidin and zona occluden-1 (ZO-1)
(16).
Recently, syndecans, a heparan sulfate proteoglycan
family, have been shown to be involved in the PC progression
(17). In particular, syndecans 1
and 2 expression has been associated with the malignancy grade
rated by the Gleason score (18–21).
Transcriptional regulation of syndecans is poorly understood. A
complete characterization of syndecan 1 and 2 promoters has been
reported (22). In this regard,
Vihinen et al (1996) were able to map a highly active
syndecan 1 promoter region with binding capacity for Sp1 (22). No enhancer sites were found in
either the upstream region or the first intron (up to +15 kb),
while some repressor elements upstream of the promoter (−2.4 to −4
to 4 kb) were identified. In addition, 5 E-box sequences were found
in syndecan promoter to which SNAIL might bind, repressing this
syndecan in a direct way (23).
Previous in silico analysis performed in our laboratory
(unpublished data) revealed the presence of several putative
binding sites for SNAIL-1 in the promoter regions of syndecans 1
and 2. The aim of this study was to evaluate the presence of SNAIL
and its association with syndecans 1 and 2, and other EMT markers
in PC samples and cell lines. We propose that syndecans may be
regulated by SNAIL decreasing their expression during EMT in
PC.
Materials and methods
Biopsy samples
PC specimens were obtained from the biopsy archive
of the Pathological Anatomy Service, Clinic Hospital University of
Chile, with the corresponding authorization of the institutional
Ethics Committee. All samples were evaluated by an expert
pathologist (I.G.). For the immunohistochemical evaluation
specimens were grouped as BPH samples, a non-malignant control, and
PC samples with high and low histological Gleason grade.
Cell lines and culture conditions
Human PC cell lines (PC3 and LNCaP) were obtained
from the American Type Culture Collection (ATCC, Rockville, MD,
USA). Cells were cultured in Dulbecco’s modified Eagle’s medium
(DMEM) supplemented with 10% fetal bovine serum and 1% penicillin
and streptomycin. Cells were maintained under standard culture
conditions at 37°C and 5% CO2 in a humidified
environment.
Antibodies
Primary antibodies were obtained from Abcam (SNAIL,
N-cadherin; Cambridge, MA, USA), BD Transduction (E-cadherin;
Franklin Lakes, NJ, USA), Santa Cruz Biotechnology (syndecan-1,
Santa Cruz, CA, USA) and Contreras et al (24) (syndecan-2). Anti-rabbit secondary
fluorescein-conjugated antibody, anti-mouse and anti-rabbit
secondary peroxidase-conjugated antibodies were purchased from
Jackson ImmunoResearch (West Grove, PA, USA).
Tissue microarray (TMA) construction
The PC TMA was constructed as follows: first, the
most representative tumor areas were carefully selected and marked
based on the matched hematoxylin and eosin (H&E) stained
slides. Altogether, 104 cores (1.5 mm diameter) of test tissue were
taken from the donor blocks with a tissue microarrayer (Beecher
Instruments, Silver Spring, MD, USA). Sections were stained with
H&E and then evaluated by the pathologist. The TMA contained a
mixture of tissue so that both benign and malignant samples of
different Gleason grades were represented on each block. Sections
of 4 μm were obtained with a microtome and transferred to
glass slides (SuperFrosts Plus, Menzel-Gläser, Braunschweig,
Germany). Finally, colon (3) and
tonsil (3) samples were included
as positive control for syndecans.
Immunohistochemistry
Tumor and control formalin-fixed and
paraffin-embedded samples (TMA) were cut into 4-μm sections,
mounted, deparaffinized and rehydrated in decreasing concentration
of ethanol and distilled water. The sections were washed with
phosphate-buffered saline (PBS) and antigen retrieval was performed
in a steam bath for 15 min at 90–95°C in 10 mM Citrate Buffer (pH
6.0). Endogenous peroxydase activity was inhibited by incubation in
3% H2O2. Later on, the sections were washed
and non-specific binding was blocked with 10% normal horse serum
solution (Vectastain). Then, sections were incubated with
corresponding primary antibodies overnight at 4°C or 1 h at 37°C.
Afterwards, samples were incubated with secondary antibody for 30
min at 37°C. Then, samples were washed and incubated with the
streptavidin-biotin system (Histostain®-Plus Bulk Kit).
After washing, the sections were incubated for 2 min at room
temperature with liquid 3,3’-diaminobenzidine substrate (DAB)
(Zymed®, LAB-SA Detection System and DAB-Plus Substrate
Kit) followed by counterstaining with hematoxylin. Finally, samples
were dehydrated in ethanol, cleared in xylene, coverslipped and
evaluated in a microscope Leica DM 2500 (18,21).
Immunocytochemistry
Cells were grown on 6-well tissue culture plates
over sterilized glass coverslips until 50–70% confluence was
reached. Then, cells were fixed with a solution containing 4% (v/v)
paraformaldehyde and sucrose in PBS for 30 min at room temperature
and stored in 0.02% (w/v) sodium azide in PBS at 4°C. Before
incubation with the antibodies, the coverslips were washed with a
20 mM PBS-glycine solution and then blocked with PBS-glycine (20
mM)-BSA (0.1%). The cells were incubated with the primary
antibodies overnight at 4°C or 1 h at 37°C, rinsed with 20 mM
PBS-glycine solution three times and incubated with a
FITC-conjugated secondary antibody (Jackson ImmunoResearch
Fluorescein-Conjugated AffiniPure Goat Anti-Rabbit) away from light
for 2 h. Finally, the coverslips were mounted and visualized under
a spinning disc microscope (Olympus BX61Wl).
Staining quantification
Photographs from immunohistochemistry and
immunocytochemistry were digitally processed to obtain the
integrated optical density (IOD). The average gray value of each
image was used to obtain the IOD. The IOD corresponds to absorption
of an optical element per unit distance for a given wavelength. The
staining and illumination conditions of the samples were
equivalent.
Western blot analysis
The cell culture medium was aspirated and the cells
were washed with PBS, trypsinized and centrifuged at 1,050 × g for
5 min. The resulting pellet was resuspended in a lysis buffer (50
nM Tris-HCl pH 7.4, 0.15 M NaCl, 1% sodium deoxycholate, 1% NP-40,
0.1% SDS, 5 nM EDTA), with a protease inhibitor cocktail (0.01
mg/ml benzamidine, 0.002 mg/ml antipain, 0.005 mg/ml leupeptin, 4
mM phenylmethylsulfonyl fluoride and 1 mM
Na3VO4, pH 7.4). Later, the cells were
scraped and the lysate was collected in a microfuge tube and passed
through a syringe to break up the cell aggregates. The cell lysate
was cleared by centrifugation at 15,000 × g for 15 min at 4°C, and
the supernatant was discarded. The protein pellet was collected for
protein quantification by the Bradford method at 570 nM using a Ray
Leigh spectrophotometer (UV-1600 model). For western blot analysis,
40 μg of protein were resolved over 10% polyacrylamide gels
and electrotransferred onto a nitrocellulose membrane (Bio-Rad,
Hercules, CA, USA). A molecular weight standard (Pierce, Rockford,
IL, USA) was also resolved for analyzing specific zones of the
gels. The efficiency of the process was measured staining the
membranes with Ponceau Red reactive. The non-specific sites on
membranes were blocked with blocking buffer [TBS-Tween-20 (100 mM
Tris-HCl, 0.9% NaCl, 0.1% Tween-20, pH 7.5) - 5% non-fat dry milk]
for 1 h at room temperature. Then, membranes were incubated with
the corresponding primary antibody in blocking buffer overnight at
4°C, followed by incubation with anti-mouse or anti-rabbit
secondary antibody peroxidase conjugated (in blocking buffer) and
detected by chemiluminescence (Biological Industries, Beit Haemek,
Israel) and autoradiography. The western blot analysis bands were
scanned and analyzed using the scientific software program
UN-SCAN-IT (Silk Scientific Corporation, Orem, UT, USA).
Statistics analysis
Data were tabulated and analyzed using SPSS v17.0
software. Normal distribution was tested by Kolmogorov-Smirnov
test. Given the distribution of the data, a parametric test
(Pearson test) to calculate the correlation index was used. ANOVA
(Tukey’s test) was used to compare means. P<0.01 was considered
to indicate a statistically significant difference.
Results
TMA analysis
From the 98 samples of PC in the TMA (excluding
colon and tonsil controls), 4 spots containing prostatic stromal
tissue were ruled out. Samples used for analysis were classified
into 4 groups: non-tumoral control (BPH), and low, medium and high
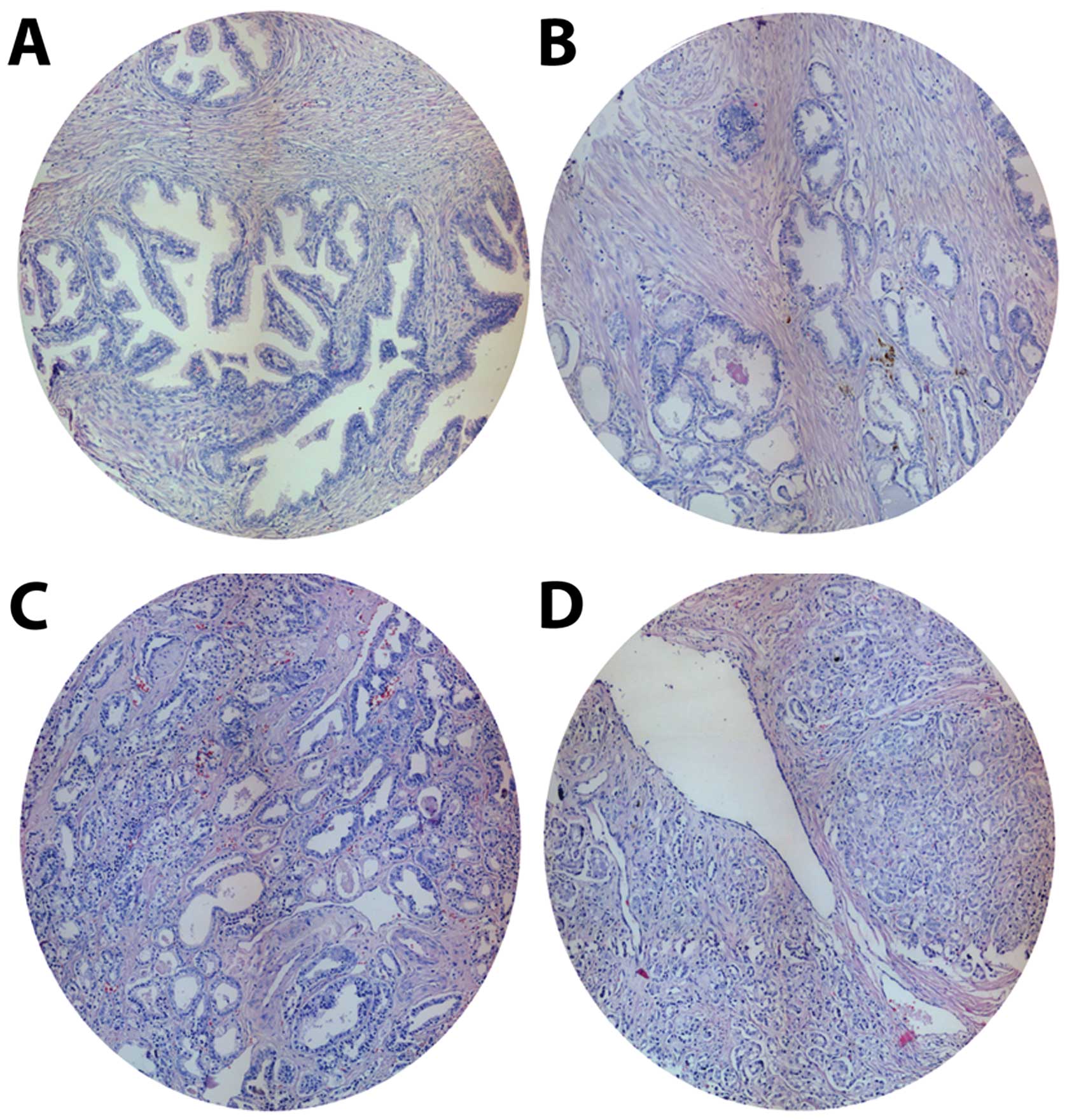
Gleason grade PC samples. The histological characteristics of the
TMA groups stained with H&E are presented in Fig. 1. TMA included 45 BPH and 47 PC
spots [9 corresponding to low (grade 1–2), 23 medium (grade 3) and
15 high Gleason grade (grade 4–5)], giving a total of 98
samples.
SNAIL expression and distribution in
prostate samples
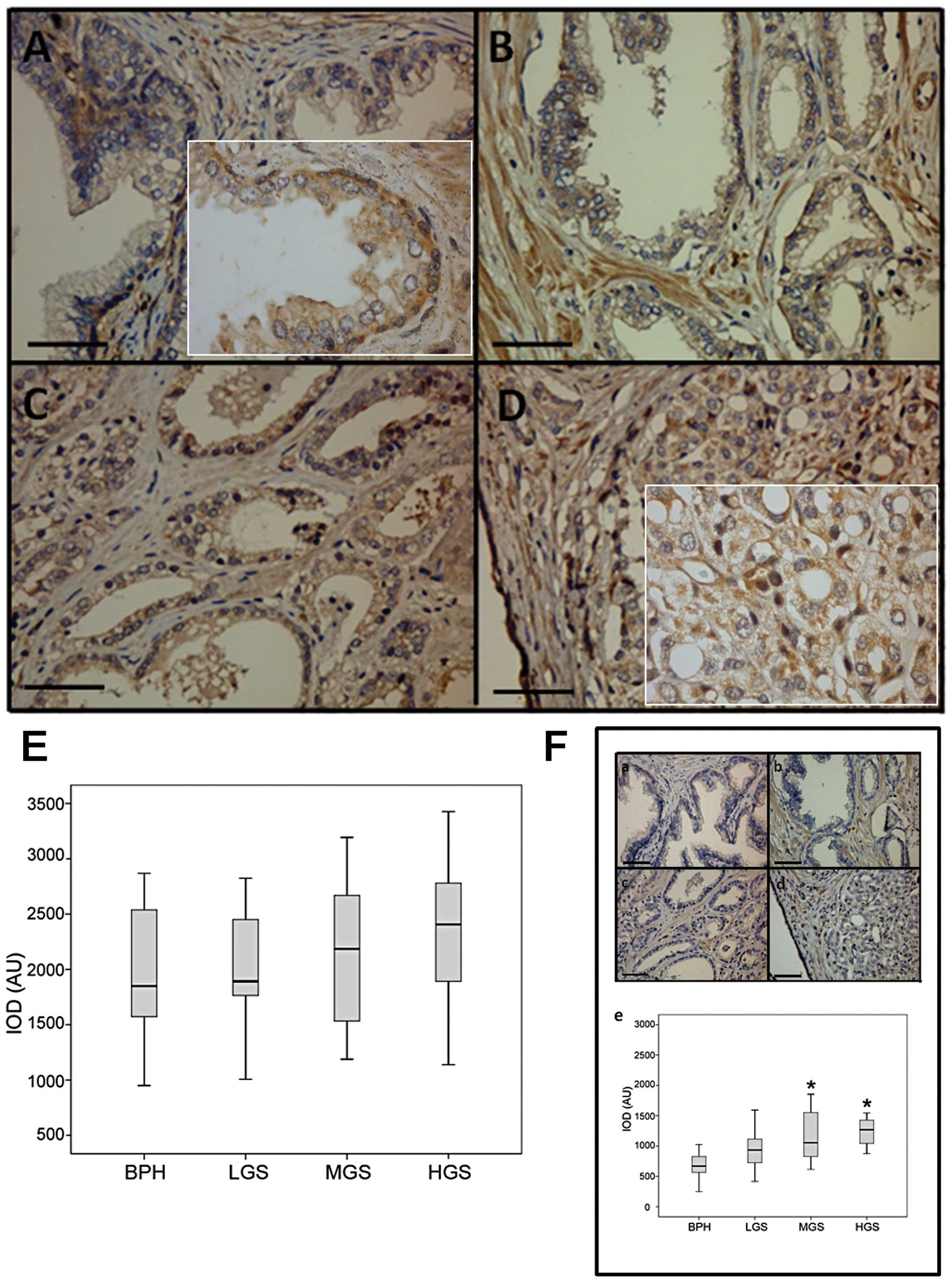
SNAIL staining is observed mainly in nuclei and
shows increased intensity in high Gleason compared to low grade
samples (Fig. 2A). H&E dyeing
was omitted to avoid interfering with SNAIL nuclear specific
staining. Average IOD for each sample showed normal distribution
(Kolmogorov-Smirnov P=0.689). Subsequently, the IOD means were
compared by ANOVA. Samples with a high Gleason grades show
SNAIL-staining IOD means significantly higher (P<0.01) than
samples with low Gleason grade and BPH (P<0.0001) (Fig. 2B). Given the normal distribution,
the Pearson test established a correlation coefficient of 0.734
between the IOD and the Gleason grade.
Syndecan 1 expression and distribution in
prostate samples
The expression and distribution of syndecan 1 show a
very heterogeneous pattern within the groups studied. BPH spots
show a strong intensity localized mainly in the cytoplasm and
membrane of the basal cells. Furthermore, epithelial cells exhibit
a preferential localization in the baso-lateral region and
approximately 50% of the cytoplasmic localization is detected at
variable intensity (weak to moderate). This syndecan 1 distribution
is also found in the low Gleason group and, to a lesser extent, in
the medium Gleason group. However, in PC spots with high Gleason
grade, membrane localization is lost and a granular cytoplasmic
localization with low intensity is observed (Fig. 3). The main difference of this
marker among the groups is found in its location. For comparison,
E-cadherin (a validated epithelial marker) expression and
distribution was evaluated in PC TMA samples. This epithelial
marker shows an expected membrane location in most samples with
intensities varying from moderate to strong. In BPH spots,
E-cadherin shows mainly baso-lateral location in gland epithelial
cells and was absent in apical membrane. On the other hand, low
Gleason grade samples show syndecan 1 intensity and distribution
similar to BPH. However, in high Gleason grade spots, a loss of
intensity associated to gland architecture disorganization is
observed (Fig. 3E). In addition,
E-cadherin distribution shows a mixed pattern including cytoplasm
location (Fig. 3F). Significant
decrease in E-cadherin expression is observed only in medium and
high Gleason grade samples (Fig.
3F).
Syndecan 2 expression and distribution in
prostate samples
The expression of this marker is highly variable in
terms of location and immunostaining intensity. Similar to syndecan
1, syndecan 2 is found in both baso-lateral membrane and granular
cytoplasm. BPH specimens show a high intensity in basal cells and
basal lamina (Fig. 4A–D). No
significant difference in syndecan 2 expression is observed among
the different Gleason grade spots (Fig. 4E). However, cell location changes
as Gleason grade increases, switching from membrane-cytoplasmic to
cytoplasm-nucleus localization. Syndecan 2 is highly expressed in
fibroblast, therefore, its presence in stroma served as internal
positive control. For comparison, N-cadherin (a validated stromal
marker) expression and distribution was evaluated in PC TMA
samples. N-cadherin expression in BPH showed a mixed pattern
including both membrane and cytoplasm location in epithelial cells.
However, the immunostaining intensity, unlike E-cadherin, is weak
(Fig. 4Fa–d). The intensity of
N-cadherin staining is strong in stroma due to this molecule being
highly expressed in fibroblast and mesenchymal tissue. For this
reason, it serves also as an internal positive control (Fig. 4A). As expected, this marker is
increasing with the disorganization of prostate gland epithelium.
In low Gleason samples, N-cadherin is expressed mainly in
baso-lateral membrane of epithelial cells while in medium grade
spots the immunostaining of this molecule shows a decrease in
membrane and an increase in cytoplasm. Furthermore, in high Gleason
samples, N-cadherin shows a high expression of the membrane,
cytoplasmatic and even nuclear location. N-cadherin expression is
different only between BPH and medium/high Gleason grade samples
(Fig. 4Fe).
SNAIL expression in LNCaP and PC3 cell
lines
Considering that LNCaP and PC3 cell lines have been
widely used as in vitro model for PC, we studied the
location of the transcription factor SNAIL in these commercial cell
lines using fluorescent immunocytochemistry. Different cellular
SNAIL distribution is observed in these cell lines. LNCaP cells
(low tumorigenic capacity) show a homogeneous localization in the
nucleus and cytoplasm (Fig. 5A).
However, PC3 cells (high tumorigenic capacity) show an exclusively
nuclear localization (Fig. 5B).
Furthermore, the SNAIL staining intensity is very high and
occasionally detected at the perinuclear region. Localization is
more evident when performing a merge between SNAIL staining (green)
and actin microfilaments (red). When comparing IOD, significant
differences in the SNAIL expression between the cell lines were
found (Fig. 5C). In addition,
protein extraction and western blot analysis were performed to
compare the SNAIL expression between the cell lines. Results show a
higher SNAIL protein expression in PC3 than LNCaP cells (Fig. 5D and E).
Syndecans 1 and 2 expression in LNCaP and
PC3 cell lines
Results obtained from syndecans 1 and 2 expression
are presented in Fig. 6. In LNCaP
and PC3 cells, expression of both syndecans is evident at plasma
membrane (Fig. 6B, C, E and F).
Comparison of IOD, in LNCaP cells (low tumorigenic capacity) show a
higher syndecans expression than PC3 cells (high tumorigenic
capacity) (Fig. 6G and H).
E-cadherin (control epithelial marker) shows a similar pattern
(Fig. 6A and D) and IOD (Fig. 6I).
 | Figure 6.Syndecans 1 and 2 and E-cadherin
ectodomain localization in LNCaP and PC3 cell lines. (A) E-cadherin
ectodomain, (B) syndecan 1 and (C) syndecan 2 in PC3 cell line. (D)
E-cadherin ectodomain, (E) syndecan 1 and (F) syndecan 2 in LNCaP
cell line. Inserts, negative controls. DAPI for nuclear staining.
Magnification, ×400. (G, H and I) Semi-quantification of syndecan
1, syndecan 2 and E-cadherin, immunofluorescence in LNCaP and PC3
cell lines, respectively. IOD, integrated optical density. AU,
arbitrary units. *P<0.01. |
Discussion
Searching for markers with diagnostic and prognostic
utility is a major challenge in cancer field. In this regard,
several markers of EMT such as SNAIL and TWIST, have recently been
associated with clinical variables in localized PC. In this
analysis, TWIST and vimentin, stand out as good predictors of
biochemical recurrence (25).
Recently, some roles for proteoglycans in PC have been reported.
Cellular changes and enzymatic activity in the developing tumor can
alter the composition and structure of proteoglycans modifying
their function (17). Our group
has reported that some heparan sulfate proteoglycans (syndecans 1
and 2) have a close association with malignancy and may also be
useful as markers of biochemical recurrence of PC (18,21).
Regarding syndecan 1, other studies have pointed out its utility as
a marker of malignancy with prognostic utility. In these studies
syndecan 1 is expressed in inverse relation to Gleason score
(26,27). The prognostic value of this
syndecan in patients treated with radical prostatectomy has been
also established (28). However,
other authors reported, despite the reduction of syndecan 1 in high
Gleason samples, that this syndecan is not a good predictor for
tumor recurrence or survival, reducing its clinical importance as a
marker (29). Regarding syndecan
2, changes from membrane to cytoplasm localization are associated
with increasing Gleason score. The syndecan 2 distribution is
observed mainly at the cytoplasm and nucleus in high Gleason
grades. Nuclear presence of this syndecan suggests its involvement
in transcriptional processes. Our results are consistent with
recent reports detecting nuclear localization of syndecans
(30). In addition, the
proteolytic cleavage of syndecan results in extracellular releasing
of its ectodomain. Multiple roles have been described for syndecan
shedding in health and disease (31,32).
The ectodomain may promote tumor growth and angiogenesis (33) and cytosolic domain might be
translocated to the nucleus regulating gene expression (30).
Recently, Smith and Odero-Marah (16) have reported the possible role of
SNAIL in PC and its potential utility as a therapeutic target.
Furthermore, it has been reported that the SNAIL1 increased
expression was positively correlated with PC de-differentiation,
but not with cancer progression or prognosis. There is evidence
indicating that SNAIL expression is upregulated from the early
stages of PC (15). The
association between increased expression of SNAIL and prostate
malignancy found in the present study is in agreement with other
previous works (8,34). Evidence provided by this work
support the hypothesis that SNAIL could be repressing the
expression of syndecan 1, in the same way as E-cadherin (35,36).
The decreased expression of syndecan 1 is associated with the loss
of basal cells and normal epithelial organization. Considering that
there are putative binding sites for SNAIL in both syndecans
promoters, it is reasonable to suggest an active role for SNAIL in
PC malignancy regulation. In our study, SNAIL was detected
preferentially localized in the nuclear region showing a gradually
increasing intensity with the Gleason grade. In addition, the high
SNAIL expression in PC3 cells (high tumorigenic capacity) compared
with LNCaP cells (low tumorigenic capacity), strongly suggest that
SNAIL could be favoring the tumorigenic process through different
cellular mechanisms. In PC cell lines, the expression of SNAIL,
using specific siRNA, has been shown to play a role by inhibiting
cellular aging (37). As a result,
such cells decreased their survival, presenting an increase in
caspase activity. Baritaki et al (38) studied the effects of a proteasome
inhibitor (NPI-0052) on metastatic PC cell lines showing that
treated cells decreased SNAIL levels and increased expression of
E-cadherin. In addition, these cells were unable to initiate EMT,
exhibiting a low degree of invasiveness.
According to our results, the positive correlation
between high SNAIL expression and PC malignancy might be associated
with metalloproteinases induction (expression or activation). These
enzymes could be responsible for the proteolytic shedding of
syndecans explaining the decrease in their immunohistochemical
staining. Furthermore, the decreased expression of E-cadherin
(repressed by SNAIL) and the elevated expression of N-cadherin
would complete the model of PC progression.
On the contrary, it has been recently described in
PC, that TNFα can stabilize SNAIL level favoring EMT (39). Thus, EMT may involve the
coordinated upregulation of SNAIL and the downregulation of
syndecans during PC progression.
Acknowledgements
This study was supported by Fondo
Nacional de Ciencia y Tecnología (FONDECYT) projects 1110269 (H.C.)
and 1100183 (E.C.).
References
|
1.
|
Jemal A, Bray F, Center MM, et al: Global
Cancer Statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar
|
|
2.
|
Kufe DW, Pollock RE, Weichselbaum RR, Bast
RC Jr, Gansler TS, Holland JF and Frei E III: Neoplasms of the
prostate. Cancer Medicine. Decker BC: ISBN: ISBN
1-55009-113-12003
|
|
3.
|
Long RM, Morrissey C, Fitzpatrick JM and
Watson WG: Prostate epithelial cell differentiation and its
relevance to the understanding of prostate cancer therapies. Clin
Sci (Lond). 108:1–11. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
De Marzo AM, Platz EA, Sutcliffe S, et al:
Inflammation in prostate carcinogenesis. Nat Rev Cancer. 7:256–269.
2007.
|
|
5.
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Gos M, Miloszewska J and Przybyszewska M:
Epithelial-mesenchymal transition in cancer progression. Postepy
Biochem. 55:121–128. 2009.PubMed/NCBI
|
|
7.
|
Delahunt B, Miller RJ, Srigley JR, et al:
Gleason grading: past, present and future. Histopathology.
60:75–86. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Micalizzi DS, Farabaugh SM and Ford HL:
Epithelial-mesenchymal transition in cancer: Parallels between
normal development and tumor progression. J Mammary Gland Biol
Neoplasia. 15:117–134. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Pecina-Slaus N: Tumor suppressor gene
E-cadherin and its role in normal and malignant cells. Cancer Cell
Int. 3:17–18. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Chan AO, Lam SK, Chu KM, et al: Soluble
E-cadherin is a valid prognostic marker in gastric carcinoma. Gut.
48:808–811. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Mell LK, Meyer JJ, Tretiakova M, et al:
Prognostic significance of E-cadherin protein expression in
pathological stage I–III endometrial cancer. Clin Cancer Res.
10:5546–5553. 2004.PubMed/NCBI
|
|
12.
|
Gould Rothberg B and Bracken M: E-cadherin
immunohistochemical expression as a prognostic factor in
infiltrating ductal carcinoma of the breast: a systematic review
and meta-analysis. Breast Cancer Res Treat. 100:139–148.
2006.PubMed/NCBI
|
|
13.
|
Musial J, Sporny S and Nowicki A:
Prognostic significance of E-cadherin and ezrin immunohistochemical
expression in prostate cancer. Pol J Pathol. 58:235–243.
2007.PubMed/NCBI
|
|
14.
|
Zhou Q, Yan B, Hu X, et al: Luteolin
inhibits invasion of prostate cancer PC3 cells through E-cadherin.
Mol Cancer Ther. 8:1684–1691. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Heebøll S, Borre M, Ottosen PD, et al:
Snail1 is over-expressed in prostate cancer. APMIS. 117:196–204.
2009.
|
|
16.
|
Smith B and Odero-Marah V: The role of
Snail in prostate cancer. Cell Adh Migr. 6:433–441. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Edwards IJ: Proteoglycans in prostate
cancer. Nat Rev Urol. 21:196–206. 2012. View Article : Google Scholar
|
|
18.
|
Contreras HR, Ledezma RA, Vergara J, et
al: The expression of syndecan-1 and -2 is associated with Gleason
score and epithelial-mesenchymal transition markers E-cadherin and
beta-catenin, in prostate cancer. Urol Oncol. 28:534–540. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Shimada K, Nakamura M, De Velasco MA, et
al: Syndecan-1, a new target molecule involved in progression of
androgen-independent prostate cancer. Cancer Sci. 100:1248–1254.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Popović A, Demirović A, Spajić B, et al:
Expression and prognostic role of syndecan-2 in prostate cancer.
Prostate Cancer Prostatic Dis. 13:78–82. 2010.PubMed/NCBI
|
|
21.
|
Ledezma R, Cifuentes F, Gallegos I, et al:
Altered expression patterns of syndecan-1 and -2 predict
biochemical recurrence in prostate cancer. Asian J Androl.
13:476–480. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Vihinen T, Määttä A, Jaakkola P, et al:
Functional characterization of mouse syndecan-1 promoter. J Biol
Chem. 271:12532–12541. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Nackaerts K, Verbeken E, Deneffe G, et al:
Heparan sulfate proteoglycan expression in human lung-cancer cells.
Int J Cancer. 74:335–345. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Contreras HR, Fabre M, Granés F, et al:
Syndecan-2 expression in colorectal cancer-derived HT-29 M6
epithelial cells induces a migratory phenotype. Biochem Biophys Res
Commun. 286:742–751. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Behnsawy HM, Miyake H, Harada K and
Fujisawa M: Expression patterns of epithelial-mesenchymal
transition markers in localized prostate cancer: significance in
clinicopathological outcomes following radical prostatectomy. BJU
Int. 111:30–37. 2013. View Article : Google Scholar
|
|
26.
|
Kiviniemi J, Kallajoki M, Kujala I, et al:
Altered expression of syndecan-1 in prostate cancer. APMIS.
112:89–97. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Mennerich D, Vogel A, Klaman I, et al:
Shift of syndecan-1 expression from epithelial to stromal cells
during progression of solid tumours. Eur J Cancer. 40:1373–1382.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Shariat SF, Svatek RS, Kabbani W, et al:
Prognostic value of syndecan-1 expression in patients treated with
radical prostatectomy. BJU Int. 101:232–237. 2008.PubMed/NCBI
|
|
29.
|
Brimo F, Vollmer RT, Friszt M, et al:
Syndecan-1 expression in prostate cancer and its value as biomarker
for disease progression. BJU Int. 106:418–423. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Zong F, Fthenou E, Mundt F, et al:
Syndecan-1 domains regulate mesenchymal tumor cell adhesion,
motility and migration. PLoS One. 6:e148162011. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Manon-Jensen T, Itoh Y and Couchman JR:
Proteoglycans in health and disease: the multiple roles of syndecan
shedding. FEBS J. 277:3876–3889. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Choi S, Lee H, Choi JR and Oh ES:
Shedding; towards a new paradigm of syndecan function in cancer.
BMB Rep. 43:305–310. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Fears C, Gladson C and Woods A: Syndecan-2
is expressed in the microvasculature of gliomas and regulates
angiogenic processes in microvascular endothelial cells. J Biol
Chem. 281:14533–14536. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Talbot L, Bhattacharya S and Kuo P:
Epithelial-mesenchymal transition, the tumor microenvironment, and
metastatic behavior of epithelial malignancies. Int J Biochem Mol
Biol. 3:117–136. 2012.PubMed/NCBI
|
|
35.
|
Cano A, Pérez-Moreno MA, Rodrigo I, et al:
The transcription factor snail controls epithelial-mesenchymal
transitions by repressing E-cadherin expression. Nat Cell Biol.
2:76–83. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Nieto M and Cano A: The
epithelial-mesenchymal transition under control: Global programs to
regulate epithelial plasticity. Semin Cancer Biol. 22:361–368.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Emadi Baygi M, Soheili ZS, Schmitz I, et
al: Snail regulates cell survival and inhibits cellular senescence
in human metastatic prostate cancer cell lines. Cell Biol Toxicol.
26:553–567. 2010.PubMed/NCBI
|
|
38.
|
Baritaki S, Chapman A, Yeung K, et al:
Inhibition of epithelial to mesenchymal transition in metastatic
prostate cancer cells by the novel proteasome inhibitor, NPI-0052:
pivotal roles of Snail repression and RKIP induction. Oncogene.
28:3573–3585. 2009. View Article : Google Scholar
|
|
39.
|
Wang H, Fang R, Wang XF, et al:
Stabilization of Snail through AKT/GSK-3β signaling pathway is
required for TNF-α-induced epithelial-mesenchymal transition in
prostate cancer PC3 cells. Eur J Pharmacol. 714:48–55.
2013.PubMed/NCBI
|